Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis
Abstract
:1. Introduction
2. Results and Discussion
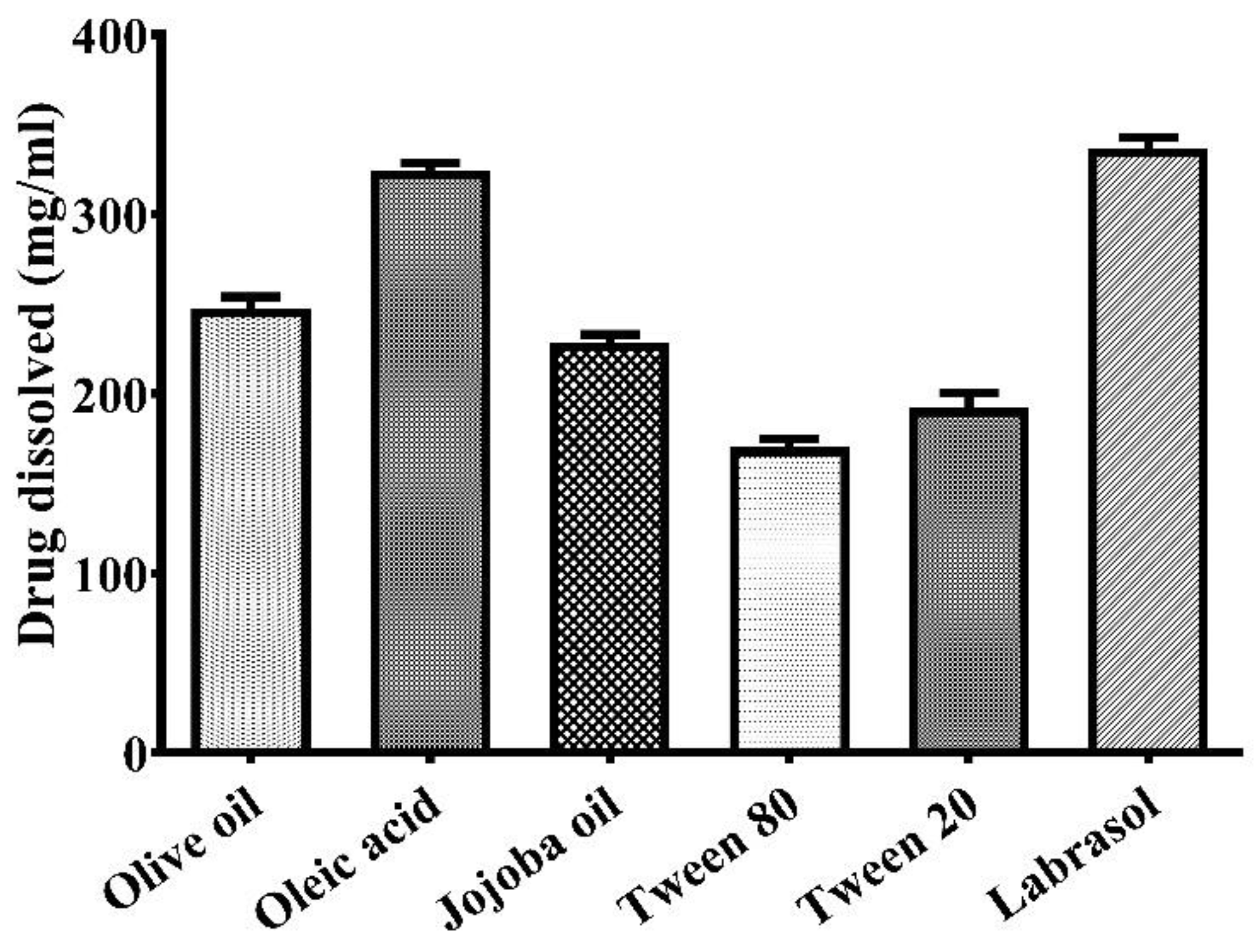
2.1. Selection of Suitable Excipients
2.2. Optimization and Statistical Analysis of Variables
2.2.1. Effect of Independent Variables on Particle Size of Nanoemulsion
2.2.2. Effect of Independent Variables on PDI of Nanoemulsion
2.2.3. Effect of Independent Variables on Percent Transmittance
2.2.4. Selection of Optimized Formulation
2.3. Evaluation of Optimized Nanoemulsion
2.4. Evaluation of Nanogel
2.4.1. In Vitro Drug Release Study
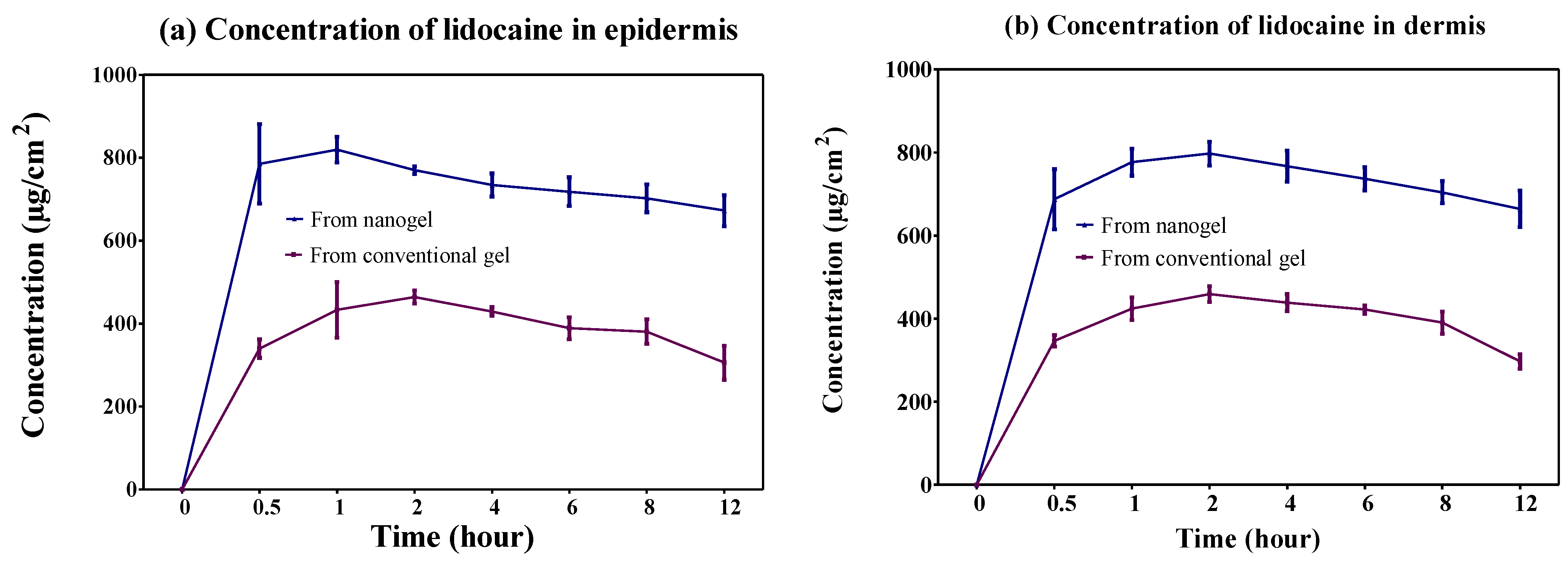
2.4.2. Dermatokinetic Study
2.4.3. In Vivo Skin Safety Study
2.4.4. Stability Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Solubility Studies for the Selection of Suitable Excipients
4.3. Fabrication and Optimization of Lidocaine Nanoemulsion
4.4. Characterization of Optimized Nanoemulsion
4.4.1. Determination of particle Size, PDI and Percent Transmittance
4.4.2. Determination of Thermodynamic Stability
4.4.3. Determination of Refractive Index
4.4.4. Determination of Zeta Potential
4.5. Development of Drug-Loaded Nanogel
4.6. Evaluation of Nanogel
4.6.1. Morphological and pH Determination
4.6.2. Determination of Spreadability and Extrudability
4.6.3. Determination of Drug Content
4.6.4. In Vitro Drug Release Study
4.6.5. Dermatokinetic Study
4.6.6. In Vivo Skin Safety Study
4.6.7. Stability Studies
4.7. Statistical Analysis
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial lipid-nanosystem for dermal delivery of 5-fluorouracil and resveratrol against skin cancer: Delineation of improved dermatokinetics and epidermal drug deposition enhancement analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef]
- Qi, Y.; Yao, X.; Du, X.; An, S. Local anesthetic lidocaine-encapsulated polymyxin–chitosan nanoparticles delivery for wound healing: In vitro and in vivo tissue regeneration. Drug Deliv. 2021, 28, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naskar, A.; Kim, K.S. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.Y. Exploring the effects of pain and stress on wound healing. Adv. Ski. Wound Care 2012, 25, 38–44. [Google Scholar] [CrossRef]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqubal, M.K.; Saleem, S.; Iqubal, A.; Chaudhuri, A.; Pottoo, F.H.; Ali, J.; Baboota, S. Natural, synthetic and their combinatorial nanocarriers based drug delivery system in the treatment paradigm for wound healing via dermal targeting. Curr. Pharm. Des. 2020, 26, 4551–4568. [Google Scholar] [CrossRef] [PubMed]
- Jae, M.L.; Jung, K.S.; Ji, S.J.; Sang, Y.C.; Dong, W.K. Antioxidant effect of lidocaine and procaine on reactive oxygen species-induced endothelial dysfunction in the rabbit abdominal aorta. Korean J. Anesthesiol. 2010, 59, 104–110. [Google Scholar]
- Lenfant, F.; Lahet, J.-J.; Courderot-Masuyer, C.; Freysz, M.; Rochette, L. Lidocaine has better antioxidant potential than ropivacaine and bupivacaine: In vitro comparison in a model of human erythrocytes submitted to an oxidative stress. Biomed. Pharmacother. 2004, 58, 248–254. [Google Scholar] [CrossRef]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2021, 410, 471–475. [Google Scholar] [CrossRef]
- Gordon, S.M.; Chuang, B.P.; Wang, X.M.; Hamza, M.A.; Rowan, J.S.; Brahim, J.S.; Dionne, R.A. The differential effects of bupivacaine and lidocaine on prostaglandin E2 release, cyclooxygenase gene expression and pain in a clinical pain model. Anesth. Analg. 2008, 106, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, J.; Sinclair, R.; Bonderovic, M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol. Scand. 2006, 50, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Kunze, H.; Nahas, N.; Traynor, J.R.; Wurl, M. Effects of local anaesthetics on phospho-lipases. Biochim. Biophys. Acta 1976, 441, 93–102. [Google Scholar] [CrossRef]
- Vasseur, P.B.; Paul, H.A.; Dybdal, N.; Crumley, L. Effects of local anesthetics on healing of abdominal wounds in rabbits. Am. J. Vet. Res. 1984, 45, 2385–2388. [Google Scholar]
- Abrão, J.; Fernandes, C.R.; White, P.F.; Shimano, A.C.; Okubo, R.; Lima, G.B.; Bachur, J.A.; Garcia, S.B. Effect of local anaesthetic infiltration with bupivacaine and ropivacaine on wound healing: A placebo-controlled study. Int. Wound J. 2014, 11, 379–385. [Google Scholar] [CrossRef]
- Hancı, V.; Hakimoğlu, S.; Özaçmak, H.; Bektaş, S.; Özaçmak, H.S.; Özdamar, Ş.O.; Yurtlu, S.; Turan, I.Ö. Comparison of the effects of bupivacaine, lidocaine, and tramadol infiltration on wound healing in rats. Rev. Bras. Anestesiol. 2012, 62, 804–810. [Google Scholar] [CrossRef]
- Caielli, S.; Banchereau, J.; Pascual, V. Neutrophils come of age in chronic inflammation. Curr. Opin. Immunol. 2012, 24, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.; Rossaint, J.; Van Aken, H.; Westphal, M.; Hahnenkamp, K.; Zarbock, A. Lidocaine reduces neutrophil recruitment by abolishing chemokine-induced arrest and transendothelial migration in septic patients. J. Immunol. 2014, 192, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Peck, S.L.; Johnston, R.B.; Horwitz, L.D. Reduced neutrophil superoxide anion release after prolonged infusions of lidocaine. J. Pharmacol. Exp. Ther. 1985, 235, 418–422. [Google Scholar]
- Drucker, M.; Cardenas, E.; Arizti, P.; Valenzuela, A.; Gamboa, A. Experimental studies on the effect of lidocaine on wound healing. World J. Surg. 1998, 22, 394–398. [Google Scholar] [CrossRef]
- Waite, A.; Gilliver, S.C.; Masterson, G.R.; Hardman, M.J.; Ashcroft, G.S. Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br. J. Anaesth. 2010, 104, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, R.; Farhan, A.; Abu-Huwaij, R.; Mahmoud, N.N.; Kamal, A. Lidocaine microemulsion-laden organogels as lipid-based systems for topical delivery. J. Pharm. Innov. 2020, 15, 521–534. [Google Scholar] [CrossRef]
- da Silva Marques, T.Z.; Santos-Oliveira, R.; de Siqueira, L.B.D.O.; da Silva Cardoso, V.; de Freitas, Z.M.F.; da Silva Ascencao Barros, R.D.C.; Villa, A.L.V.; de Bustamante Monteiro, M.S.D.S.; Dos Santos, E.P.; Ricci-Junior, E. Development and characterization of a nanoemulsion containing propranolol for topical delivery. Int. J. Nanomed. 2018, 13, 2827–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Ali, J.; Baboota, S. Design Expert® supported optimization and predictive analysis of selegiline nanoemulsion via the olfactory region with enhanced behavioural performance in Parkinson’s disease. Nanotechnology 2016, 27, 435101. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Formulation of nanoemulsion: A comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; Alfaleh, M.A.; Asfour, H.Z. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in A549 lung cancer cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
- Ahmed, S.; Gull, A.; Alam, M.; Aqil, M.; Sultana, Y. Ultrasonically tailored, chemically engineered and “QbD” enabled fabrication of agomelatine nanoemulsion; optimization, characterization, ex-vivo permeation and stability study. Ultrason. Sonochem. 2018, 41, 213–226. [Google Scholar] [CrossRef]
- Bali, V.; Ali, M.; Ali, J. Study of surfactant combinations and development of a novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. Coll.Surf. B Biointerf. 2010, 76, 410–420. [Google Scholar] [CrossRef]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Katare, O.P. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimization, dermatokinetics and in vivo evaluation. J. Microencapsul. 2015, 32, 419–431. [Google Scholar] [CrossRef]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA parkinson’s disease model. BioMed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B.; Abualsunun, W.A.; Almehmady, A.M.; Khames, A.; Rizg, W.Y.; et al. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Ashhar, M.U.; Kumar, S.; Ali, J.; Baboota, S. CCRD based development of bromocriptine and glutathione nanoemulsion tailored ultrasonically for the combined anti-parkinson effect. Chem. Phys. Lipids 2021, 235, 105035. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.S.; Algahtani, M.S.; Ahmad, J.; Kohli, K.; Shafiq-Un-Nabi, S.; Warsi, M.H.; Ahmad, M.Z. Formulation design and evaluation of aceclofenac nanogel for topical application. Ther. Deliv. 2020, 11, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Aiyalu, R.; Govindarjan, A.; Ramasamy, A. Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Braz. J. Pharm. Sci. 2016, 52, 493–507. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Anjum, H.; Gupta, M.M.; Ali, J.; Baboota, S. Determination of in vivo virtue of dermal targeted combinatorial lipid nanocolloidal based formulation of 5-fluorouracil and resveratrol against skin cancer. Int. J. Pharm. 2021, 610, 121179. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, G. Das. Formulation development and evaluation of anti-inflammatory potential of cordia obliqua topical gel on animal model. Pharmacogn. J. 2017, 9, s93–s98. [Google Scholar] [CrossRef] [Green Version]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured lipidic carriers for dual drug delivery in the management of psoriasis: Systematic optimization, dermatokinetic and preclinical evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Uprit, S.; Kumar Sahu, R.; Roy, A.; Pare, A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm. J. 2013, 21, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Ozkan, Y.; Savaser, A.; Baykara, T. In-vitro release studies of chlorpheniramine maleate from gels prepared by different cellulose derivatives. Farmaco 2003, 58, 605–611. [Google Scholar] [CrossRef]



| Factors | Level Used | |
|---|---|---|
| Independent variables | Low | High |
| Oil (%w/w) | 2 | 4 |
| Emulsifier (%w/w) | 2 | 6 |
| Dependent variables | Constraints | |
| Particle size (nm) | Minimum | |
| PDI | Minimum | |
| Transmittance (%) | Maximum | |
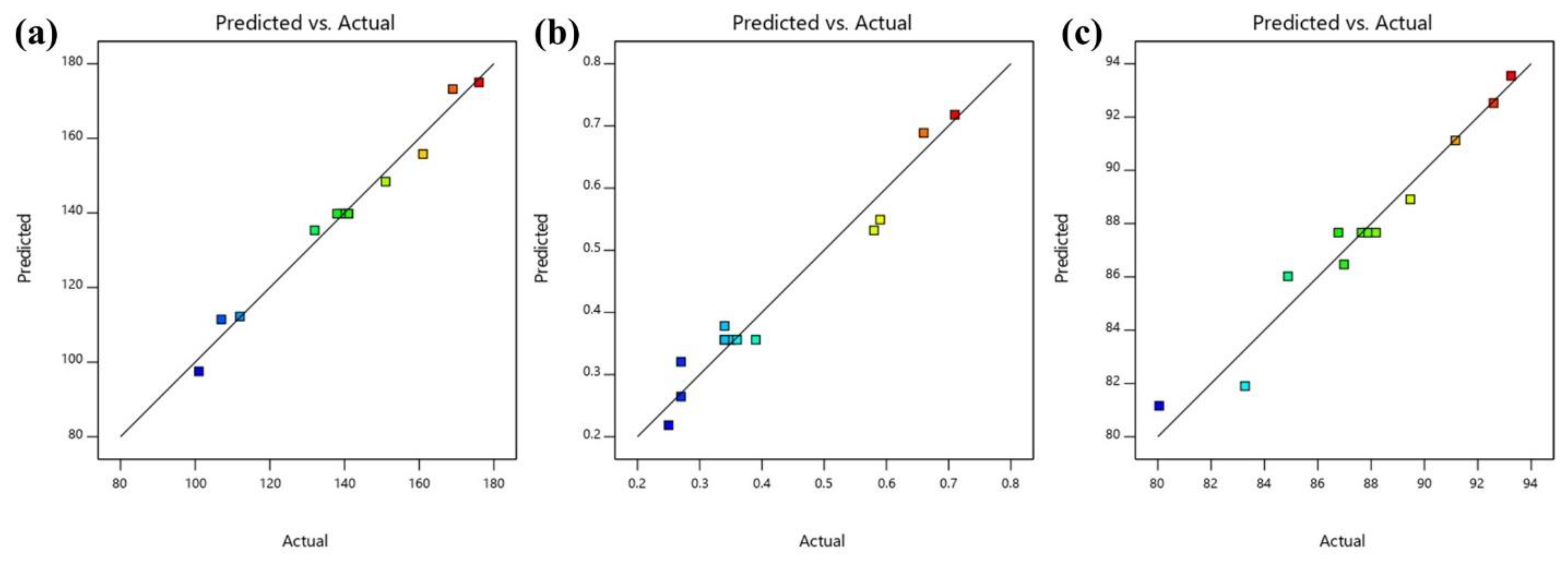
| Run | Factor 1 A: Oil (% w/w) | Factor 2 B: Emulsifier (% w/w) | Response 1 Particle Size (nm) | Response 2 PDI | Response 3 Transmittance (%) | |||
|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | Actual | Predicted | |||
| 1 | 3 | 6.82843 | 112 | 112.25 | 0.27 | 0.2646 | 91.16 | 91.12 |
| 2 | 3 | 1.17157 | 176 | 175.00 | 0.71 | 0.7179 | 80.05 | 81.16 |
| 3 | 3 | 4 | 141 | 139.80 | 0.35 | 0.3560 | 87.64 | 87.66 |
| 4 | 3 | 4 | 140 | 139.80 | 0.34 | 0.3560 | 87.82 | 87.66 |
| 5 | 4 | 2 | 169 | 173.23 | 0.66 | 0.6888 | 83.27 | 81.90 |
| 6 | 4.41421 | 4 | 161 | 155.79 | 0.58 | 0.5320 | 84.88 | 86.02 |
| 7 | 2 | 2 | 151 | 148.39 | 0.59 | 0.5492 | 86.98 | 86.47 |
| 8 | 2 | 6 | 101 | 97.52 | 0.25 | 0.2187 | 93.25 | 93.55 |
| 9 | 4 | 6 | 132 | 135.36 | 0.34 | 0.3783 | 89.47 | 88.91 |
| 10 | 3 | 4 | 138 | 139.80 | 0.34 | 0.3560 | 88.18 | 87.66 |
| 11 | 1.58579 | 4 | 107 | 111.46 | 0.27 | 0.3205 | 92.59 | 92.52 |
| 12 | 3 | 4 | 139 | 139.80 | 0.36 | 0.3560 | 87.89 | 87.66 |
| 13 | 3 | 4 | 141 | 139.80 | 0.39 | 0.3560 | 86.77 | 87.66 |
| Response | Mean Square | Standard Deviation | R2 | Adjusted R2 | Predicted R2 | Suggested Model |
|---|---|---|---|---|---|---|
| Particle size (nm) | 1209.97 | 3.84 | 0.9832 | 0.9713 | 0.8870 | Quadratic |
| PDI | 0.0574 | 0.0407 | 0.9612 | 0.9334 | 0.7558 | Quadratic |
| Parameters | Nanogel | Conventional Gel |
|---|---|---|
| Colour | Creamy | White |
| Appearance | Translucent | Translucent |
| Washability | Good washability | Good washability |
| Homogeneity | Good | Good |
| pH | 6.87 ± 0.51 | 6.93 ± 0.32 |
| Spreadability (gm.cm/sec) | 73.32 ± 4.59 | 70.42 ± 4.69 |
| Extrudability (gm/cm2) | 107.41 ± 6.42 | 114.81 ± 6.42 |
| Drug content (Percent) | 99.94 ± 1.70 | 100.33 ± 2.08 |
| Dermatokinetic Parameters | Nanogel | Conventional Gel | ||
|---|---|---|---|---|
| Epidermis | Dermis | Epidermis | Dermis | |
| Cmax (mg/cm2) | 2.64 ± 0.02 | 2.53 ± 0.06 | 1.48 ± 0.02 | 1.46 ± 0.04 |
| Tmax (h) | 0.83 ± 0.29 | 1.33 ± 0.58 | 1.33 ± 0.58 | 1.67 ± 0.58 |
| AUC0–12 h (mg/cm2h) | 26.15 ± 0.92 | 26.39 ± 0.91 | 14.13 ± 0.49 | 14.40 ± 0.18 |
| Ke (h−1) | 0.016 ± 0.004 | 0.010 ± 0.001 | 0.02 ± 0.01 | 0.021 ± 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Ali, A.; Rahman, M.A.; Warsi, M.H.; Yusuf, M.; Alam, P. Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis. Gels 2022, 8, 466. https://doi.org/10.3390/gels8080466
Ali A, Ali A, Rahman MA, Warsi MH, Yusuf M, Alam P. Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis. Gels. 2022; 8(8):466. https://doi.org/10.3390/gels8080466
Chicago/Turabian StyleAli, Amena, Abuzer Ali, Mohammad Akhlaquer Rahman, Musarrat Husain Warsi, Mohammad Yusuf, and Prawez Alam. 2022. "Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis" Gels 8, no. 8: 466. https://doi.org/10.3390/gels8080466
APA StyleAli, A., Ali, A., Rahman, M. A., Warsi, M. H., Yusuf, M., & Alam, P. (2022). Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis. Gels, 8(8), 466. https://doi.org/10.3390/gels8080466








