2D Materials (WS2, MoS2, MoSe2) Enhanced Polyacrylamide Gels for Multifunctional Applications
Abstract
1. Introduction
2. Results and Discussion
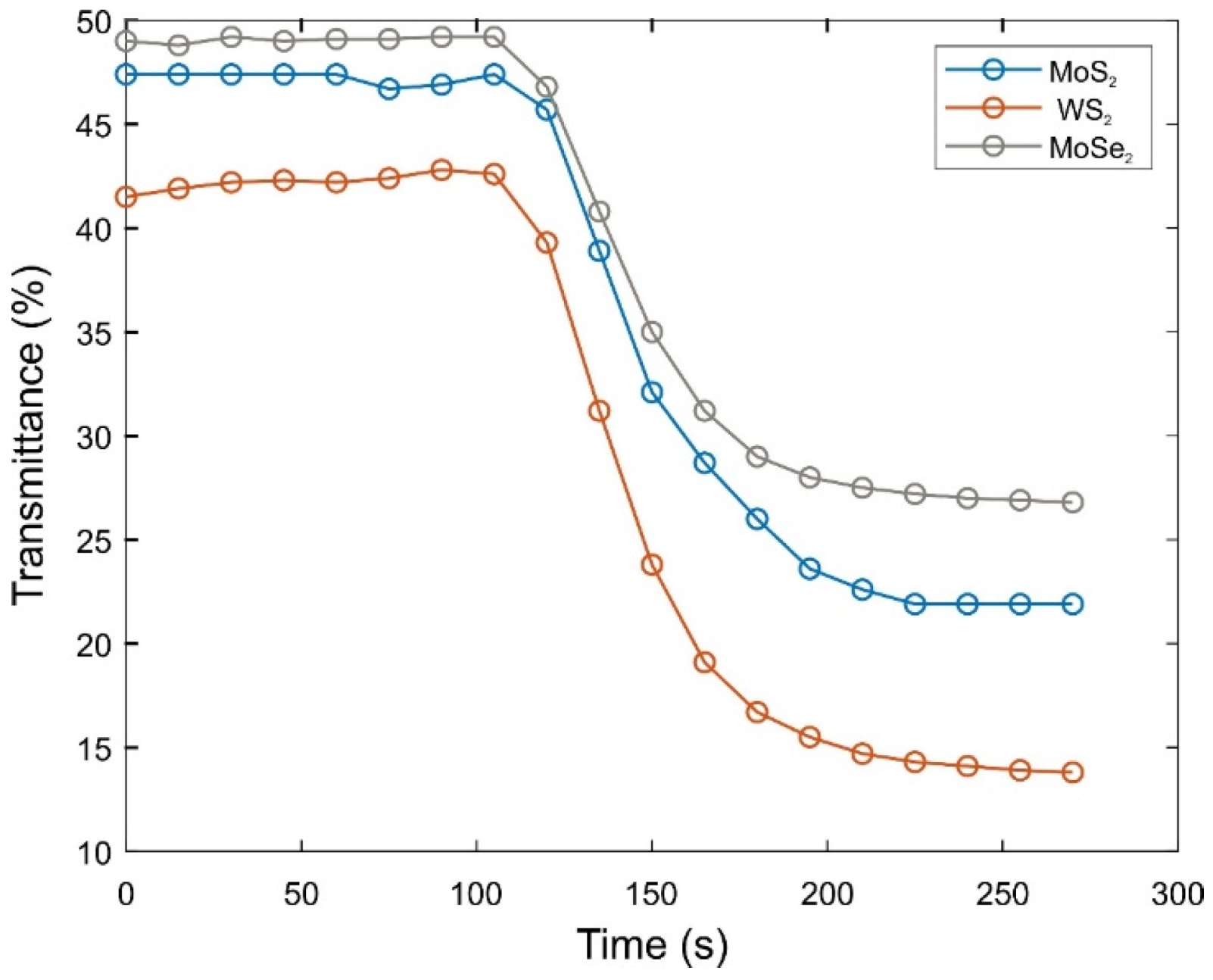
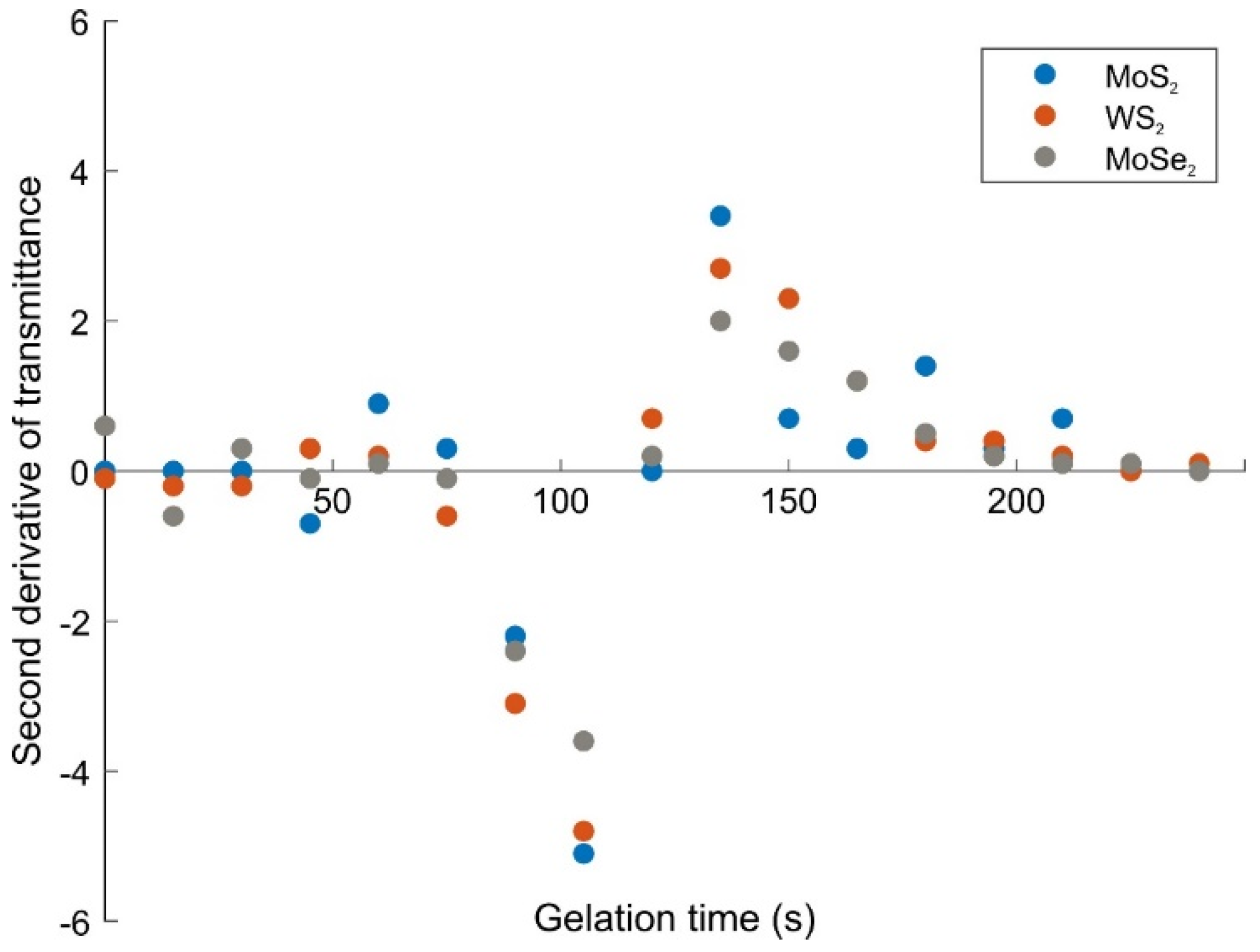
2.1. Gelation Process and Drying of Composite Gels
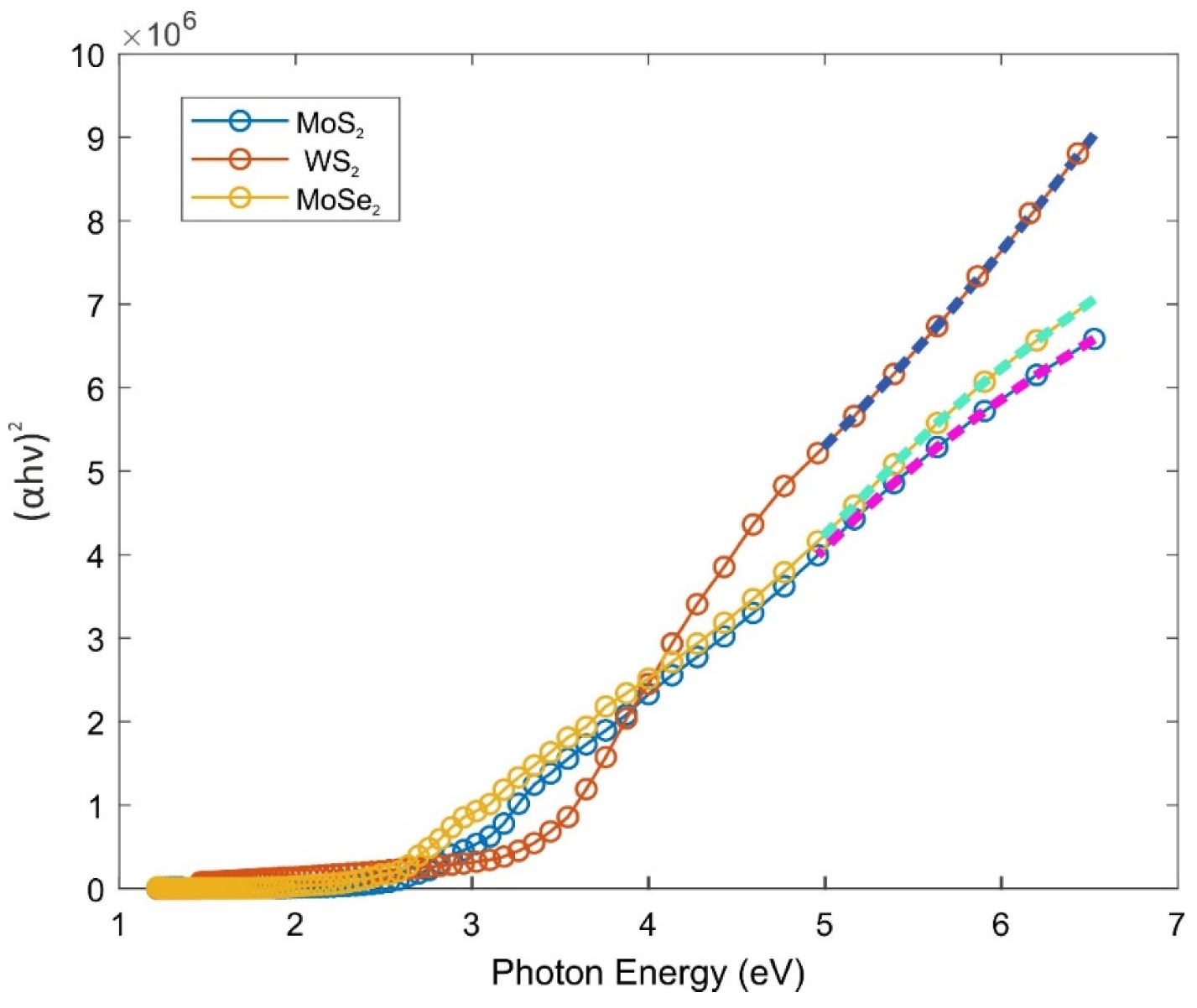
2.2. Optical and Electronic Properties
3. Conclusions
4. Materials and Methods
4.1. Preparation of WS2, MoS2, and MoSe-Doped PAAm Composite Gels
4.2. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Liu, J.; Guo, T.; Wang, J.J.; Tang, X.; Nicolosi, V. Multifunctional Ti3C2Tx MXene Composite Hydrogels with Strain Sensitivity toward Absorption-Dominated Electromagnetic-Interference Shielding. ACS Nano 2021, 15, 1465–1474. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Qin, Z.; Zhang, L.; Ye, Y.; Cao, M.; Gao, L.; Jiao, T. Preparation of PdNPs doped chitosan-based composite hydrogels as highly efficient catalysts for reduction of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125889. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Li, Y.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.; Heinz, T.F.; et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature 2014, 514, 470–474. [Google Scholar] [CrossRef]
- Beal, A.R.; Hughes, H.P. Kramers-Kronig analysis of the reflectivity spectra of 2H-MoS2, 2H-MoSe2 and 2H-MoTe2. J. Phys. C Solid State Phys. 1979, 12, 881–890. [Google Scholar] [CrossRef]
- Su, S.-H.; Hsu, Y.-T.; Chang, Y.-H.; Chiu, M.-H.; Hsu, C.-L.; Hsu, W.-T.; Chang, W.-H.; He, J.-H.; Li, L.-J. Band gap-tunable molybdenum sulfide selenide monolayer alloy. Small 2014, 10, 2589–2594. [Google Scholar] [CrossRef]
- Yao, W.; Kang, Z.; Deng, J.; Chen, Y.; Song, Q.; Ding, X.L.; Lu, F.; Wang, W. Synthesis of 2D MoS2(1-x)Se2x semiconductor alloy by chemical vapor deposition. RSC Adv. 2020, 10, 42172–42177. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Ouyang, G. The effect of alloying on the band engineering of two-dimensional transition metal dichalcogenides. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 105, 90–96. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumbhakar, P.; Krishnamoorthy, A.; Nakano, A.; Sadasivuni, K.K.; Vashishta, P.; Roy, A.K.; Kochat, V.; Tiwary, C.S. Review of strategies toward the development of alloy two-dimensional (2D) transition metal dichalcogenides. iScience 2021, 24, 103532. [Google Scholar] [CrossRef] [PubMed]
- Yerga, R.M.N.; Alvarez-Galvan, M.C.; Del Valle, F.; De La Mano, J.A.V.; Fierro, J.L.G. Water Splitting on Semiconductor Catalysts under Visible-Light Irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, J.-K.; Jin, L.; Hsu, Y.-T.; Yu, S.F.; Li, L.-J.; Yang, H.Y. Selective decoration of Au nanoparticles on monolayer MoS2 single crystals. Sci. Rep. 2013, 3, 1839. [Google Scholar] [CrossRef] [PubMed]
- Notley, S.M. High yield production of photoluminescent tungsten disulphide nanoparticles. J. Colloid Interface Sci. 2013, 396, 160–164. [Google Scholar] [CrossRef]
- Dey, B.; Mondal, R.K.; Mukherjee, S.; Satpati, B.; Mandal, A.; Senapati, D.; Babu, S.P.S. A supramolecular hydrogel for generation of a benign DNA-hydrogel. RSC Adv. 2015, 5, 105961–105968. [Google Scholar] [CrossRef]
- Dhibar, S.; Dey, A.; Ghosh, D.; Mandal, A.; Dey, B. Mechanically tuned Molybdenum dichalogenides (MoS2 and MoSe2) dispersed supramolecular hydrogel scaffolds. J. Mol. Liq. 2019, 276, 184–193. [Google Scholar] [CrossRef]
- Byron, M.L.; Variano, E.A. Refractive-index-matched hydrogel materials for measuring flow-structure interactions. Exp. Fluids 2013, 54, 1456. [Google Scholar] [CrossRef]
- Chen, X.; Yan, H.; Bao, C.; Zhu, Q.; Liu, Z.; Wen, Y.; Li, Z.; Zhang, T.; Lin, Q. Fabrication and evaluation of homogeneous alginate/polyacrylamide–chitosan–gelatin composite hydrogel scaffolds based on the interpenetrating networks for tissue engineering. Polym. Eng. Sci. 2022, 62, 116–128. [Google Scholar] [CrossRef]
- Kalshetti, P.P.; Rajendra, V.B.; Dixit, D.N.; Parekh, P.P. Hydrogels as a drug delivery system and applications: A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 1–7. [Google Scholar]
- Voronova, M.I.; Surov, O.V.; Afineevskii, A.V.; Zakharov, A.G. Properties of polyacrylamide composites reinforced by cellulose nanocrystals. Heliyon 2020, 6, e05529. [Google Scholar] [CrossRef] [PubMed]
- Rukmanikrishnan, B.; Ramalingam, S.; Lee, J. Quaternary ammonium silane-reinforced agar/polyacrylamide composites for packaging applications. Int. J. Biol. Macromol. 2021, 182, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.M.; Liu, Y.H.; Dong, G.X.; Zhao, D.J. Fabrication and Properties of Composites of Linear Polyacrylamide and Functionalized Carbon Nanotubes. Adv. Mater. Res. 2014, 936, 12–16. [Google Scholar] [CrossRef]
- Kausar, A. Properties of Polyacrylamide and Functional Multi-walled Carbon Nanotube Composite. Am. J. Nanosci. Nanotechnol. Res. 2016, 4, 1–9. [Google Scholar]
- Narimani, A.; Kordnejad, F.; Kaur, P.; Bazgir, S.; Hemmati, M.; Duong, A. Rheological and thermal stability of interpenetrating polymer network hydrogel based on polyacrylamide/hydroxypropyl guar reinforced with graphene oxide for application in oil recovery. J. Polym. Eng. 2021, 41, 788–798. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Bobji, M.S.; Srivastava, C. Nanoparticle-reinforced polyacrylamide hydrogel composites for clinical applications: A review. J. Mater. Sci. 2022, 57, 8041–8063. [Google Scholar] [CrossRef]
- Aktaş, D.K.; Evingür, G.A.; Pekcan, Ö. Critical exponents of gelation and conductivity in Polyacrylamide gels doped by multiwalled carbon nanotubes. Compos. Interfaces 2010, 17, 301–318. [Google Scholar] [CrossRef]
- Kara, S.; Arda, E.; Dolastir, F.; Pekcan, O. Electrical and optical percolations of polystyrene latex–multiwalled carbon nanotube composites. J. Colloid Interface Sci. 2010, 344, 395–401. [Google Scholar] [CrossRef]
- Arda, E.; Mergen, Ö.B.; Pekcan, Ö. Electrical and optical percolations in PMMA/GNP composite films. Phase Transit. 2018, 91, 546–557. [Google Scholar] [CrossRef]
- Uğur, Ş.; Yargı, Ö.; Pekcan, Ö. Percolation and Film Formation Behaviors of MWNT/PS Nanocomposites. Procedia Eng. 2011, 1709–1717. [Google Scholar] [CrossRef][Green Version]
- Zhai, L.; Shi, T.; Wang, H. Preparation of polyvinylpyrrodione microspheres by dispersion polymerization. Front. Chem. China 2009, 4, 83–88. [Google Scholar] [CrossRef]
- Murray, P.G.; Ramesh, M. Chapter 4—Water Soluble Polymers Produced by Homogeneous Dispersion Polymerization. In Specialty Monomers and Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Wang, G.-X.; Lu, M.; Hou, Z.-H.; Liu, L.-C.; Liang, E.-X.; Wu, H. Living radical polymerization of polyacrylamide with submicrometer size by dispersion polymerization. e-Polymers 2015, 15, 75–79. [Google Scholar] [CrossRef]
- Umaña, E.; Ougizawa, T.; Inoue, T. Preparation of new membranes by complex formation of itaconic acid-acrylamide copolymer with polyvinylpyrrolidone: Studies on gelation mechanism by light scattering. J. Membr. Sci. 1999, 157, 85–96. [Google Scholar] [CrossRef]
- Cho, K.; Pak, J.; Chung, S.; Lee, T. Recent Advances in Interface Engineering of Transition-Metal Dichalcogenides with Organic Molecules and Polymers. ACS Nano 2019, 13, 9713–9734. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, Z.; Cao, X.; Lu, G.; Wang, L.-H.; Fan, Q.-L.; Huang, W.; Zhang, H. Preparation of MoS2 -Polyvinylpyrrolidone Nanocomposites for Flexible Nonvolatile Rewritable Memory Devices with Reduced Graphene Oxide Electrodes. Small 2012, 8, 3517–3522. [Google Scholar] [CrossRef]
- Voyutsky, S. Colloid Chemistry; Mir Publishers: Moscow, Russia, 1978; pp. 38–43. [Google Scholar]
- Young, R.J. Introduction to Polymers; Chapman and Hall: New York, NY, USA, 1983. [Google Scholar]
- Tanaka, T. Collapse of Gels and the Critical Endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Stockmayer, W.H. Theory of Molecular Size Distribution and Gel Formation in Branched—Chain Polymers. J. Chem. Phys. 1943, 11, 45–55. [Google Scholar] [CrossRef]
- Navarro-Verdugo, A.L.; Goycoolea, F.M.; Romero-Meléndez, G.; Higuera-Ciapara, I.; Argüelles-Monal, W. A modified Boltzmann sigmoidal model for the phase transition of smart gels. Soft Matter 2011, 7, 5847. [Google Scholar] [CrossRef]
- Pekcan, Ö.; Kara, S. Gelation Mechanisms. Mod. Phys. Lett. B 2012, 26, 1230019. [Google Scholar] [CrossRef]
- Ziff, R.M.; Stell, G. Kinetics of polymer gelation. J. Chem. Phys. 1980, 73, 3492–3499. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. (Eds.) Polymer Gels; Gels Horizons: From Science to Smart Materials; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Nayır, Ş.; Kıvrak, S.; Kara, İ.; Uysal, B.Ö.; Pekcan, Ö. Tungsten disulfide (WS2) Doped Polyacrylamide (PAAm) Composites: Gelation and Optical Studies. Optik Int. J. Light Electron Optik 2021, 245, 167673. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Aziz, S.B.; Mamand, S.M.; Saed, S.R.; Abdullah, R.M.; Hussein, S.A. New Method for the Development of Plasmonic Metal-Semiconductor Interface Layer: Polymer Composites with Reduced Energy Band Gap. J. Nanomater. 2017, 2017, 8140693. [Google Scholar] [CrossRef]
- Yu, L.; Li, D.; Zhao, S.; Li, G.; Yang, K. First Principles Study on Electronic Structure and Optical Properties of Ternary GaAs:Bi Alloy. Materials 2012, 5, 2486–2497. [Google Scholar] [CrossRef]
- Biskri, Z.E.; Rached, H.; Bouchear, M.; Rached, D.; Aida, M.S. A Comparative Study of Structural Stability and Mechanical and Optical Properties of Fluorapatite (Ca5(PO4)3F) and Lithium Disilicate (Li2Si2O5) Components Forming Dental Glass–Ceramics: First Principles Study. J. Electron. Mater. 2016, 45, 5082–5083. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, A.M.; Ahmed, H.M. From Insulating PMMA Polymer to Conjugated Double Bond Behavior: Green Chemistry as a Novel Approach to Fabricate Small Band Gap Polymers. Polymers 2017, 9, 626. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Ahmed, H.M. Synthesis of Polymer Nanocomposites Based on [Methyl Cellulose] (1−x):(CuS)x (0.02 M ≥ x ≥ 0.08 M) with Desired Optical Band Gaps. Polymers 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Wiley Publishing: Hoboken, NJ, USA, 2004. [Google Scholar]
- Rawat, A.; Mahavar, H.K.; Chauhan, S.; Tanwar, A.; Singh, P.J. Optical band gap of Polyvinylpyrrolidone/Polyacrylamide blend thin films. Indian J. Pure Appl. Phys. 2012, 50, 100–104. [Google Scholar]
- Uysal, B.Ö.; Evingür, G.A.; Pekcan, Ö. Polyacrylamide mediated polyvinyl pyrrolidone composites incorporated with aligned molybdenum disulfide. J. Appl. Polym. Sci. 2022, 139, 52061. [Google Scholar] [CrossRef]
- Evingür, G.A.; Sağlam, N.A.; Çimen, B.; Uysal, B.Ö.; Pekcan, Ö. The WS2 dependence on the elasticity and optical band gap energies of swollen PAAm composites. J. Compos. Mater. 2021, 55, 71–76. [Google Scholar] [CrossRef]

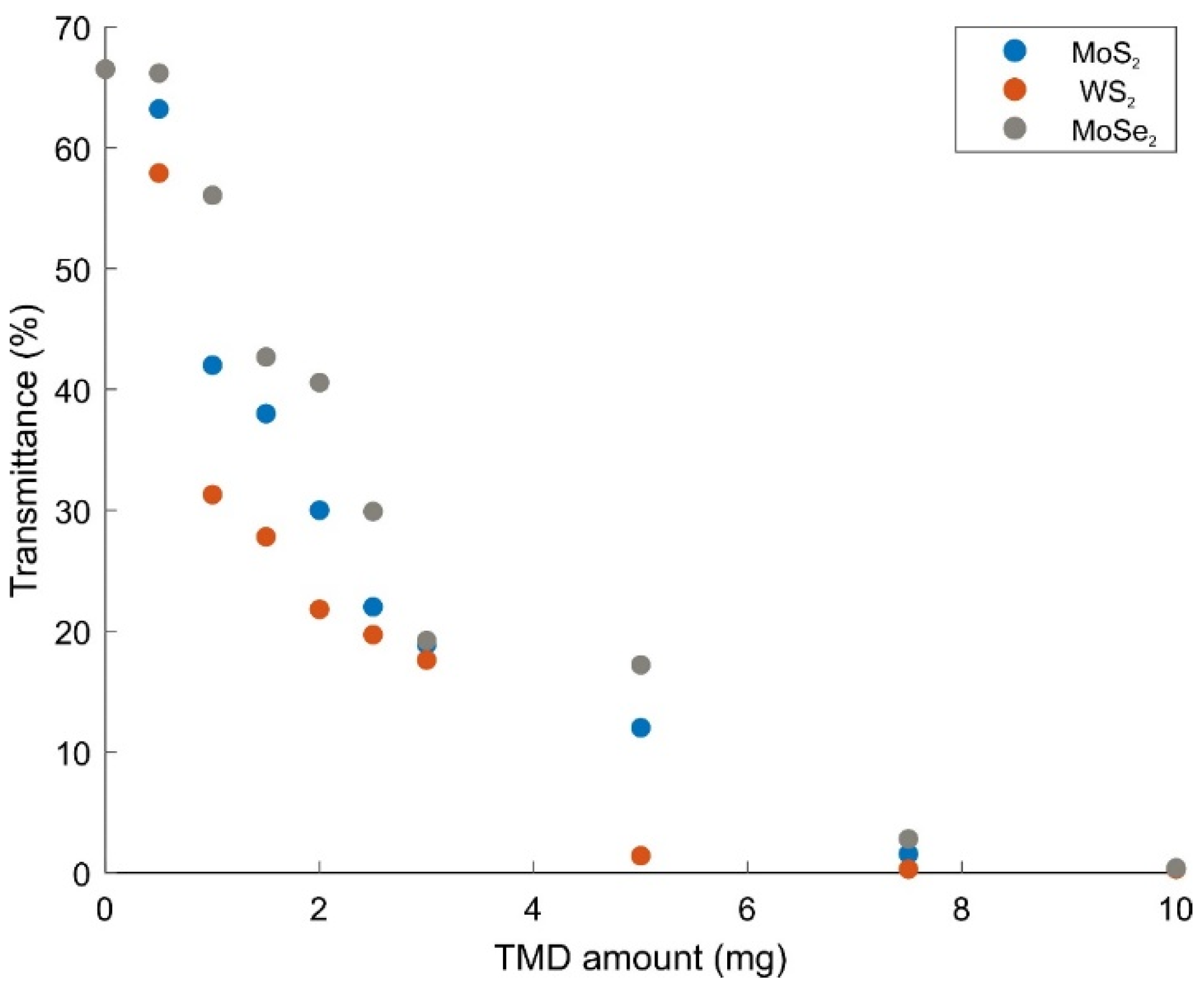
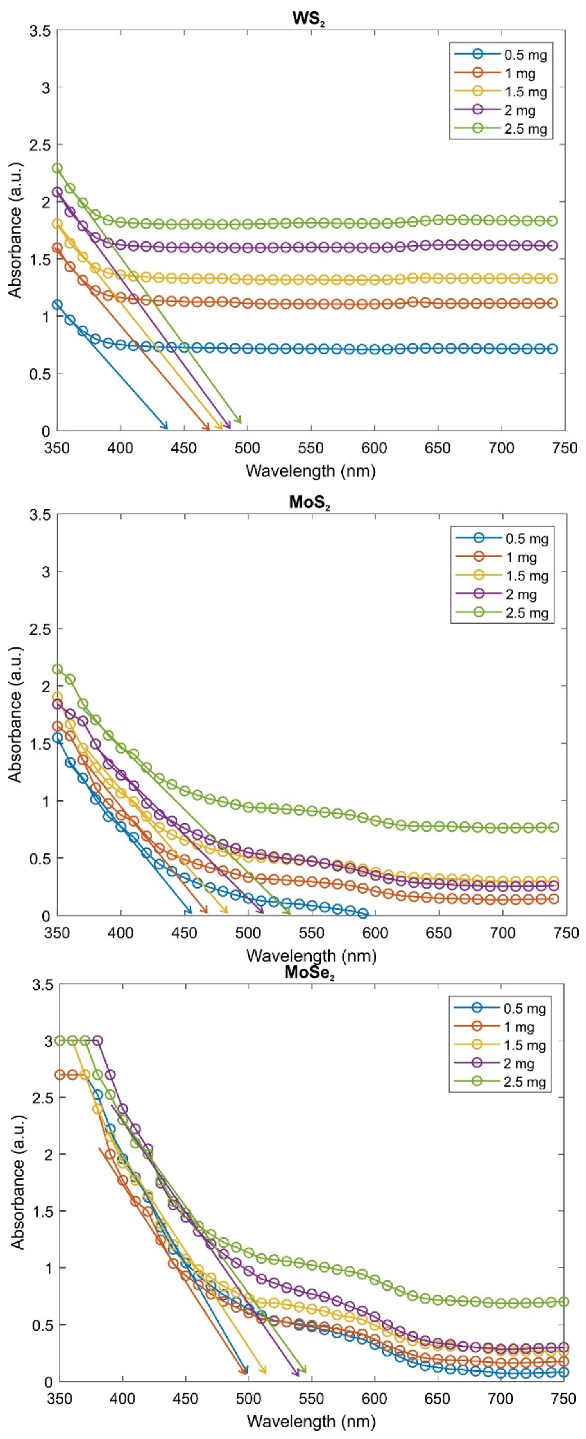
| TMD Amount in WS2/MoS2/MoSe2 + PAAm Composite Gel (mg) | Bandgap Energy of WS2 + PAAm (eV, from Absorbance Edge) | Bandgap Energy of MoS2 + PAAm (eV, from Absorbance Edge) | Bandgap Energy of MoSe2 + PAAm (eV, from Absorbance Edge) |
|---|---|---|---|
| 0.5 | 2.91 | 2.73 | 2.56 |
| 1 | 2.73 | 2.66 | 2.53 |
| 1.5 | 2.69 | 2.55 | 2.49 |
| 2 | 2.59 | 2.40 | 2.46 |
| 2.5 | 2.50 | 2.35 | 2.43 |
| Type of the Composite Gel | Linear Fitting Equations with R2 Values | Bandgap Energy (eV, from Tauc Plot) |
|---|---|---|
| MoS2 + PAAm | y = (1.650 × 106)x − 4.087 × 106 R2 = 0.994 | 2.48 |
| WS2 + PAAm | y = (2.434 × 106)x − 6.923 × 106 R2 = 0.998 | 2.84 |
| MoSe2 + PAAm | y = (1.867 × 106)x − 5.028 × 106 R2 = 0.996 | 2.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özuğur Uysal, B.; Nayır, Ş.; Açba, M.; Çıtır, B.; Durmaz, S.; Koçoğlu, Ş.; Yıldız, E.; Pekcan, Ö. 2D Materials (WS2, MoS2, MoSe2) Enhanced Polyacrylamide Gels for Multifunctional Applications. Gels 2022, 8, 465. https://doi.org/10.3390/gels8080465
Özuğur Uysal B, Nayır Ş, Açba M, Çıtır B, Durmaz S, Koçoğlu Ş, Yıldız E, Pekcan Ö. 2D Materials (WS2, MoS2, MoSe2) Enhanced Polyacrylamide Gels for Multifunctional Applications. Gels. 2022; 8(8):465. https://doi.org/10.3390/gels8080465
Chicago/Turabian StyleÖzuğur Uysal, Bengü, Şeyma Nayır, Melike Açba, Betül Çıtır, Sümeyye Durmaz, Şevval Koçoğlu, Ekrem Yıldız, and Önder Pekcan. 2022. "2D Materials (WS2, MoS2, MoSe2) Enhanced Polyacrylamide Gels for Multifunctional Applications" Gels 8, no. 8: 465. https://doi.org/10.3390/gels8080465
APA StyleÖzuğur Uysal, B., Nayır, Ş., Açba, M., Çıtır, B., Durmaz, S., Koçoğlu, Ş., Yıldız, E., & Pekcan, Ö. (2022). 2D Materials (WS2, MoS2, MoSe2) Enhanced Polyacrylamide Gels for Multifunctional Applications. Gels, 8(8), 465. https://doi.org/10.3390/gels8080465







