Experimental Study on Profile Control of Polymer and Weak Gel Molecules in Porous Media
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials and Instruments
2.2. Experimental Methods
3. Results and Discussion
3.1. Molecular Coil Size of Polymer and Weak Gel before and after Flowing through Sand-Packed Cores
- (1)
- When the core permeability is less than 5.8 D, the equivalent sphere diameter of the molecular coil of the polymer solution is basically stable. When the core permeability continues to decrease, the molecular coil size is no longer reduced. This indicates that although the polymer solution is sheared in the core, molecules are weakly sheared, because they are not cross linked and have good monomolecular activity, allowing the polymer to easily migrate, and upon a certain extent of shear, the molecular coil size can remain stable. Therefore, under the condition of permeability from 1 D to 10 D, the polymer molecules after shearing can migrate to deep reservoirs to improve the sweep efficiency and oil displacement effect.
- (2)
- When the phenolic gel flows through the core with a permeability higher than 8.5 D, the reduction in the equivalent sphere diameter of the molecular coil is less than 40%, indicating that the molecules of phenolic gel are just weakly cross linked and still have good monomolecular activity, allowing the phenolic gel to easily migrate. Therefore, the phenolic gel has good profile control and oil displacement capability in the deep core with a permeability higher than 8.5 D. However, when the core permeability is reduced to 5.6 D and 1 D, the molecular coil size of phenolic gel is lower than that of polymer solution, indicating that under these permeability conditions, the phenolic gel is greatly sheared and suffers a great viscosity loss, significantly reducing its capability to improve the sweep efficiency in deep reservoir.
- (3)
- The initial equivalent sphere diameter of the molecular coil of the aluminum gel is the largest. However, when flowing through cores with different permeabilities, the absolute value of the equivalent sphere diameter of molecular coil of the aluminum gel is lower than that of the phenolic gel. This indicates that the aluminum gel has weaker monomolecular activity than the phenolic gel, and it is more greatly sheared in cores, so its profile control and oil displacement capability in deep reservoir is weaker than that of the phenolic gel. Similar to the phenolic gel, when the permeability is reduced to 5.6 D and 1 D, the molecular coil size of the aluminum gel is also lower than that of the polymer solution, indicating that under these permeability conditions, the capability of aluminum gel to improve the sweep efficiency in deep reservoir is significantly reduced.
3.2. Viscoelasticity of Polymer and Weak Gel before and after Flowing through Sand-Packed Cores
- (1)
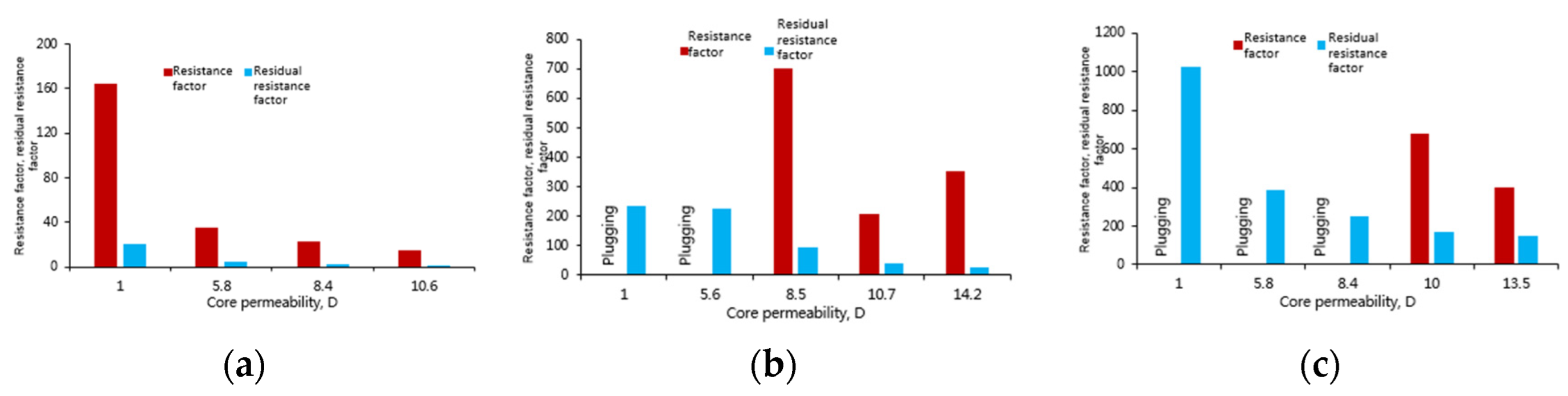
- When the core permeability is 10.6 D and 8.4 D, the elastic modulus and viscous modulus of the polymer solution decrease greatly. When the core permeability is less than 5.8 D, the viscoelastic modulus is basically stable. This indicates that the molecular size and aggregation morphology of the polymer solution no longer changes after it encounters a shear force. Ultimately, when the core permeability is 1 D, the elastic modulus is 10.9 MPa, indicating that the polymer remains a non-Newtonian viscoelastic fluid, which can still be capable of improving the sweep efficiency in deep reservoirs by virtue of the viscoelasticity under the condition of low permeability.
- (2)
- When the core permeability is ≥8.5 D, the viscoelastic modulus of the phenolic gel is higher than that of the polymer solution, indicating that under the condition of high permeability, the capability of phenolic gel to improve the sweep efficiency in the formation is higher than that of polymer solution. However, when the core permeability is ≤5.6 D, the elastic modulus is reduced to 0, and the viscous modulus is also significantly reduced to below 100 MPa, making the phenolic gel become a pure viscous non-Newtonian fluid. Therefore, when the core permeability is low, the phenolic gel is less capable than the polymer solution to improve the sweep efficiency in the formation.
- (3)
- When the core permeability is ≥10 D, the viscoelastic modulus of the aluminum gel is significantly higher than that of the polymer solution, indicating that under this condition, the capability of aluminum gel to improve the sweep efficiency in the formation is higher than that of polymer solution. However, when the core permeability is ≤8.4 D, the viscoelastic modulus of aluminum gel is smaller than that of polymer solution, indicating that under this condition, aluminum gel is less capable than polymer solution to improve the sweep efficiency in the formation.
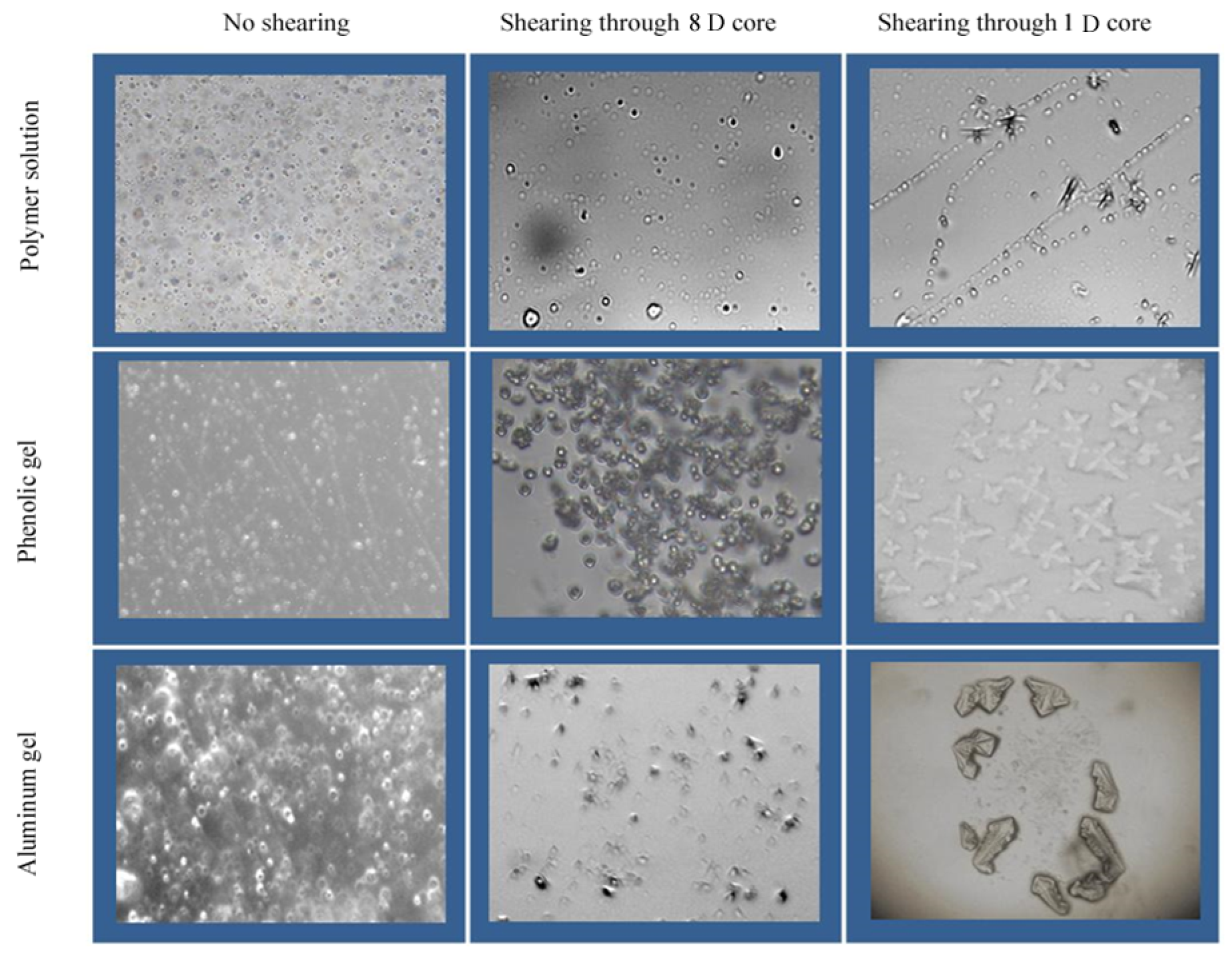
3.3. Microscopic Aggregation Morphology in Water Solution of Polymer and Weak Gel before and after Flowing through Sand-Packed Cores
- (1)
- The stock solution of the polymer shows a tight molecular aggregation state before flowing through the sand-packed core. After flowing through the 8.4 D core, the polymer molecules still exhibit aggregation state, but the solution becomes thin, which also explains the reason for the decrease in molecular coil size and viscoelasticity of polymer solution. After flowing through a 1.0 D core, the molecular structure of the polymer is further destroyed, the degree of intermolecular aggregation is further reduced, but the solution is still in a continuous state, and there is still an interwound active force between molecules, so the solution still has a certain viscoelasticity to improve the sweep efficiency in deep formation.
- (2)
- Before flowing through the sand-packed core, the phenolic gel shows a denser network aggregate than the polymer solution, and its viscoelasticity and molecular coil size are larger than those of the stock solution of polymer because it is a weak gel formed by chemical crosslinking of molecules, having a stronger intermolecular interaction. After flowing through the 8.5 D core, the intermolecular network structure is partially destroyed, and the intermolecular interaction becomes poor; however, the gel still shows a network aggregation morphology as a whole, so its viscoelasticity and molecular coil size are still higher than those of the polymer solution. After flowing through 1.0 D core, the phenolic gel, with large monomolecular diameter, is sheared into gel particles, so the network aggregate cannot be formed, and the viscoelasticity is significantly reduced, making the gel’s deep migration ability reduce correspondingly.
- (3)
- Before flowing through the sand-packed cores, the aluminum gel is a weak gel formed by crosslinking of coordination bonds, so the intermolecular interaction is also strengthened, the network aggregates formed are dense, and the viscoelasticity and molecular coil size are higher than those of the stock solution of polymer. After flowing through 8.4 D core, because the monomolecular activity of the aluminum gel is weaker than that of the phenolic gel, its intermolecular network structure is more seriously destroyed than the phenolic gel, making the molecules of the aluminum gel impossible to form network aggregates, so the viscoelasticity and molecular coil size of the aluminum gel are significantly reduced. After flowing through 1.0 D core, the molecular structure of the aluminum gel is further broken, and the viscoelasticity is greatly reduced, reducing the gel’s deep migration ability.
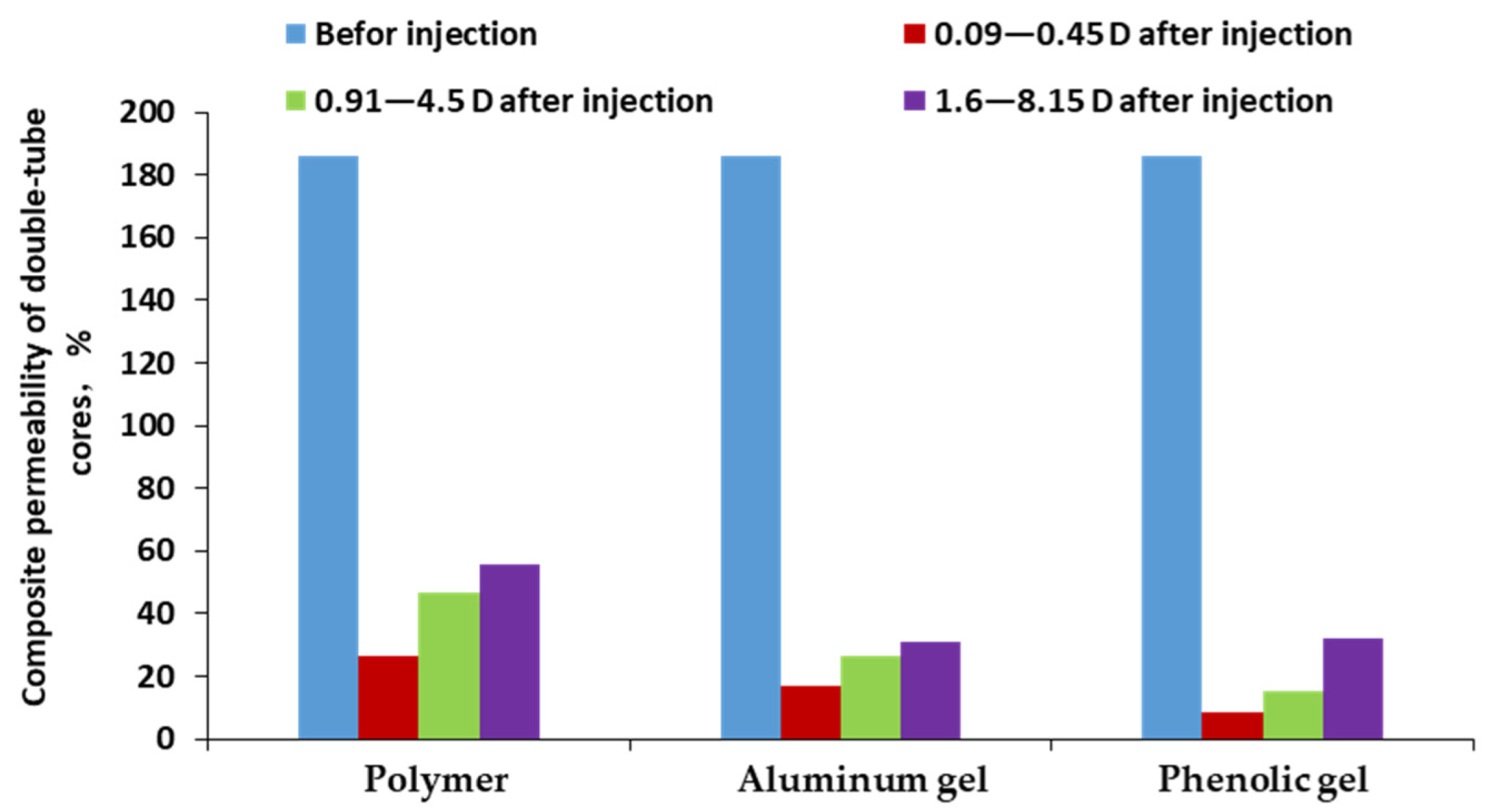
3.4. Matching of Polymer and Weak Gel in Porous Media
3.5. Profile Control and Oil Displacement Capability of Polymer and Weak Gel
4. Conclusions
- (1)
- Polymer solution does not create intermolecular crosslinking and only forms intermolecular winding, with good flexibility of the molecular chain and strong monomolecular activity. Although the molecular coil size and viscoelasticity of polymer suffers a certain loss when it flows through cores, they tend to be stable when the permeability is lowered to a certain level. Therefore, the polymer solution is capable of deep profile control and oil displacement for reservoirs with permeability of 1–10 D. However, when the permeability is higher than 8.2 D, the resistance factor produced by the polymer becomes lower, making the capability of the polymer for deep profile control and oil displacement weaker.
- (2)
- Phenolic gel and aluminum gel are weak gels formed by intermolecular chemical and physical crosslinking, so their molecular coil size and viscoelasticity are apparently greater than the polymer solution. They are highly capable of deep profile control and oil displacement in cores with permeability ˃8.5 D, and more capable than polymer solution. Since the monomolecular activity of weak gels is lower than that of the polymer, in cores with low permeability, the weak gel systems are more susceptible to shearing action; they may plug the cores during injection, meaning that their molecular coil size and viscoelasticity are seriously lost. Therefore, the two weak gel systems can only work for profile control near wellbore but cannot interfere with deep formations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Qu, Z.; Zhao, B. Study on the relationship among oil displacement efficiency and variation coefficient, rush coefficient and differential of permeability in Sanjianfang reservoir of the Shanshan oilfield. J. Xi’an Eng. Univ. 1999, 21, 39–43. [Google Scholar]
- Wang, D.; Cheng, J.; Wu, J.; Wang, G. Application of polymer flooding technology in Daqing Oilfield. Acta Pet. Sin. 2005, 26, 74–78. [Google Scholar]
- Hou, J.; Guo, L.; Yuan, F.; Du, Q.; Yu, B. Quantitative characterization for production performance of polymer flooding in different types of reservoirs in Shengli Oilfield. Acta Pet. Sin. 2008, 29, 577–581. [Google Scholar]
- Liu, L.; Li, H. Numerical simulation of the permeability variation coefficient effect on polymer flooding. Oilfield Chem. 2011, 28, 414–418. [Google Scholar]
- Smith, J.E. Performance of 18 polymers in aluminium citrate colloidal dispersion gels. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14 February 1995. [Google Scholar]
- Sydansk, R.D.; Southwell, G.P. More than 12 years’ experience with a successful conformance-control polymer-gel technology. SPE Prod. Facil. 2000, 15, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.E.; Liu, H.; Guo, S. Laboratory studies of in-depth colloidal dispersion gel technology for Daqing oil field. In Proceedings of the SPE/AAPG Western Regional Meetings, Long Beach, CA, USA, 19 June 2000; pp. 533–545. [Google Scholar]
- Lei, G.; Li, L.; Nasr-El-Din, H.A. New gel aggregates to improve sweep efficiency during waterflooding. SPE Reserv. Eval. Eng. 2011, 14, 120–128. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Cao, B. Applicability evaluation and application of hydrophobic association polymer weak gel. Petrochem. Ind. Appl. 2018, 1, 65–69. [Google Scholar]
- Zhong, W.; Zhao, B.; Han, S. Performance evaluation and application of deep profile control system in high temperature and high salinity reservoirs. Oilfield Chem. 2020, 1, 29–34. [Google Scholar]
- Zaitoun, A.; Makakou, P.; Blin, N.; Al-Maamari, R.S.; Al-Hashmi, A.R.; Abdel-Goad, M.; Al-Sharji, H.H. Shear stability of EOR polymers. SPE J. 2012, 17, 35–339. [Google Scholar] [CrossRef]
- Al Hashmi, A.R.; Al Maamari, R.S.; Al Shabibi, I.S.; Mansoor, A.M.; Zaitoun, A.; Al Sharji, H.H. Rheology and mechanical degradation of high-molecular-weight partially hydrolyzed polyacrylamide during flow through capillaries. J. Pet. Sci. Eng. 2013, 105, 100–106. [Google Scholar] [CrossRef]
- Lin, M.; Xin, J.; Li, M.; Dong, Z. Study on shear stability of low concentration partially hydrolyzed polyacrylamide/aluminum citrate crosslinking system. Acta Polym. Sin. 2008, 1, 8–12. [Google Scholar] [CrossRef]
- Zhang, K. Polymeric Physics; Chemical Industry Press: Beijing, China, 1981. [Google Scholar]
- Han, P. Variation law of seepage field and oil displacement effect of alternate injection polymer flooding. Pet. Geol. Oilfield Dev. Daqing 2014, 33, 101–104. [Google Scholar]
- Liu, Z.; Li, Y.; Gao, W.; Xue, X.; Wang, S. Experimental study on variable resistance seepage law of polymer in vertical heterogeneous reservoirs. Xinjiang Pet. Geol. 2017, 38, 331–336. [Google Scholar]
- Zhang, N.; Lu, X.; Xie, K. Seepage characteristics and influential factors of polymer weak gel under high salt and medium-low permeability conditions. Oilfield Chem. 2020, 37, 254–259. [Google Scholar]
- Liu, Z.; Cheng, H.; Xu, C.; Chen, Y.; Chen, Y.; Li, Y. Effect of lithologyon pore-scale residual oil displacement in chemical flooding usingnuclear magnetic resonance experiments. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26 March 2018. [Google Scholar]
- Zhao, S.; Pu, W.; Li, K. Study on profile control ability of polymer microspheres to heterogeneous formation. Pet. Reserv. Eval. Dev. 2019, 9, 51–56. [Google Scholar]
- Li, J.; Liu, Y.; Gao, Y.; Cheng, B.; Fanle, M.E.N.G.; Huaimin, X.U. Effects of microscopic pore structure heterogeneity on the distribution and morphology of remaining oil. Pet. Explor. Dev. 2018, 45, 1043–1052. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Gao, Y.; Cheng, B.Y.; Jiang, H.Q. Pore-scale study of the pressure-sensitive effect of sandstone and its influence on multiphase flows. Pet. Sci. 2019, 16, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lu, X.; Liu, J.; Shuqiong, H.U. Mechanism and gelling effects of linked polymer solution in the core. Pet. Explor. Dev. 2013, 40, 507–513. [Google Scholar] [CrossRef]
- Luo, X.R.; Zhao, B.; Ren, X.J. Effect of mechanical properties of pre-crosslinked gel particles on micro migration and plugging. Lithol. Reserv. 2021, 33, 176–184. [Google Scholar]
- Sun, Z. Research on the Evaluation Method of Polymer Microsphere Reservoir Adaptability and the Profile Control and Displacement Mechanism; Northeast Petroleum University: Daqing, China, 2017. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wei, J.; Zhao, L.; Ni, J.; Fu, L.; Wang, J. Experimental Study on Profile Control of Polymer and Weak Gel Molecules in Porous Media. Gels 2022, 8, 467. https://doi.org/10.3390/gels8080467
Li X, Wei J, Zhao L, Ni J, Fu L, Wang J. Experimental Study on Profile Control of Polymer and Weak Gel Molecules in Porous Media. Gels. 2022; 8(8):467. https://doi.org/10.3390/gels8080467
Chicago/Turabian StyleLi, Xuanran, Jing Wei, Lun Zhao, Jun Ni, Libing Fu, and Jincai Wang. 2022. "Experimental Study on Profile Control of Polymer and Weak Gel Molecules in Porous Media" Gels 8, no. 8: 467. https://doi.org/10.3390/gels8080467
APA StyleLi, X., Wei, J., Zhao, L., Ni, J., Fu, L., & Wang, J. (2022). Experimental Study on Profile Control of Polymer and Weak Gel Molecules in Porous Media. Gels, 8(8), 467. https://doi.org/10.3390/gels8080467




