Preliminary Investigation of Linum usitatissimum Mucilage-Based Hydrogel as Possible Substitute to Synthetic Polymer-Based Hydrogels for Sustained Release Oral Drug Delivery
Abstract
:1. Introduction
2. Results & Discussion
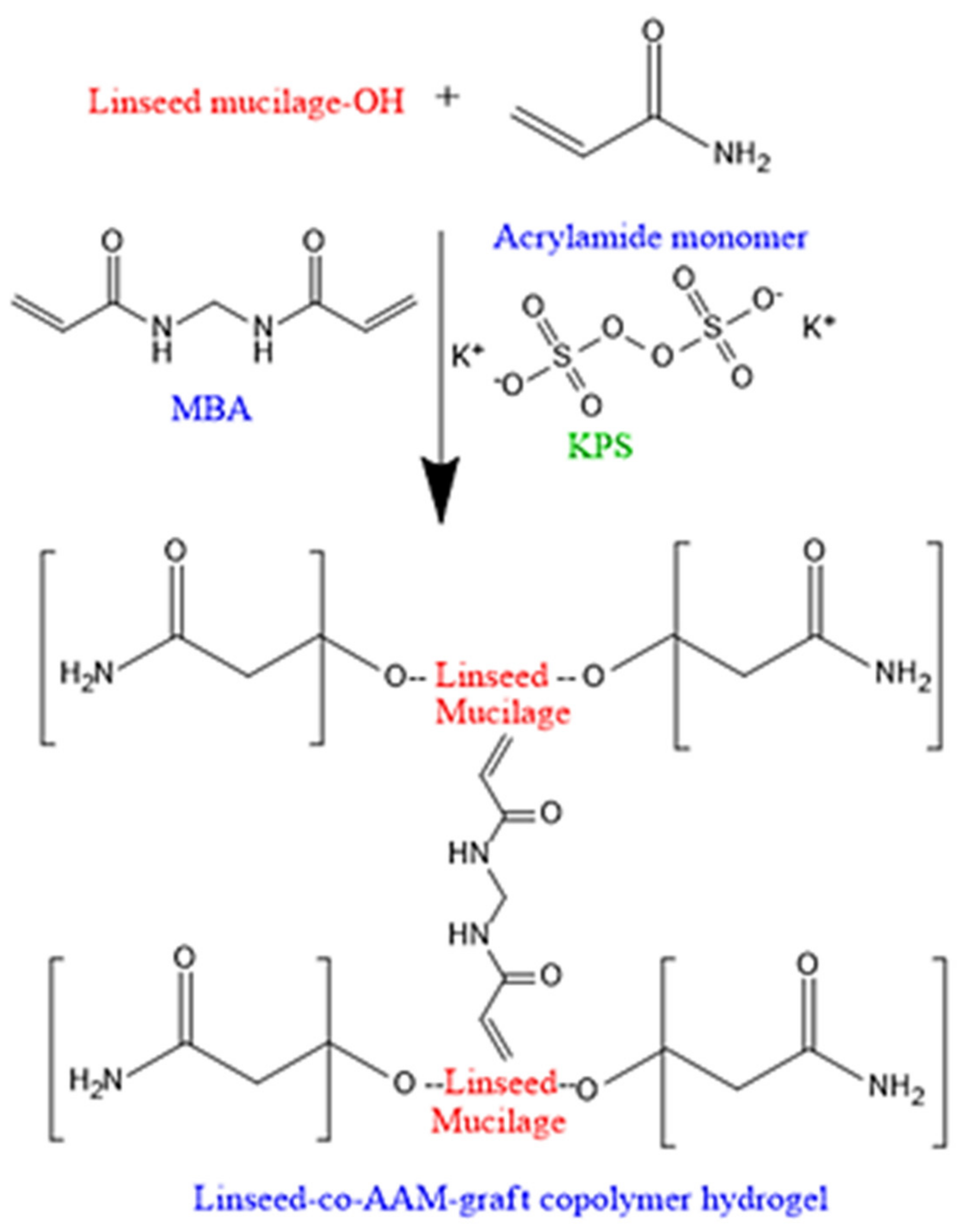
2.1. Characterization of Linseed-Co-AAM Graft Copolymeric Hydrogels
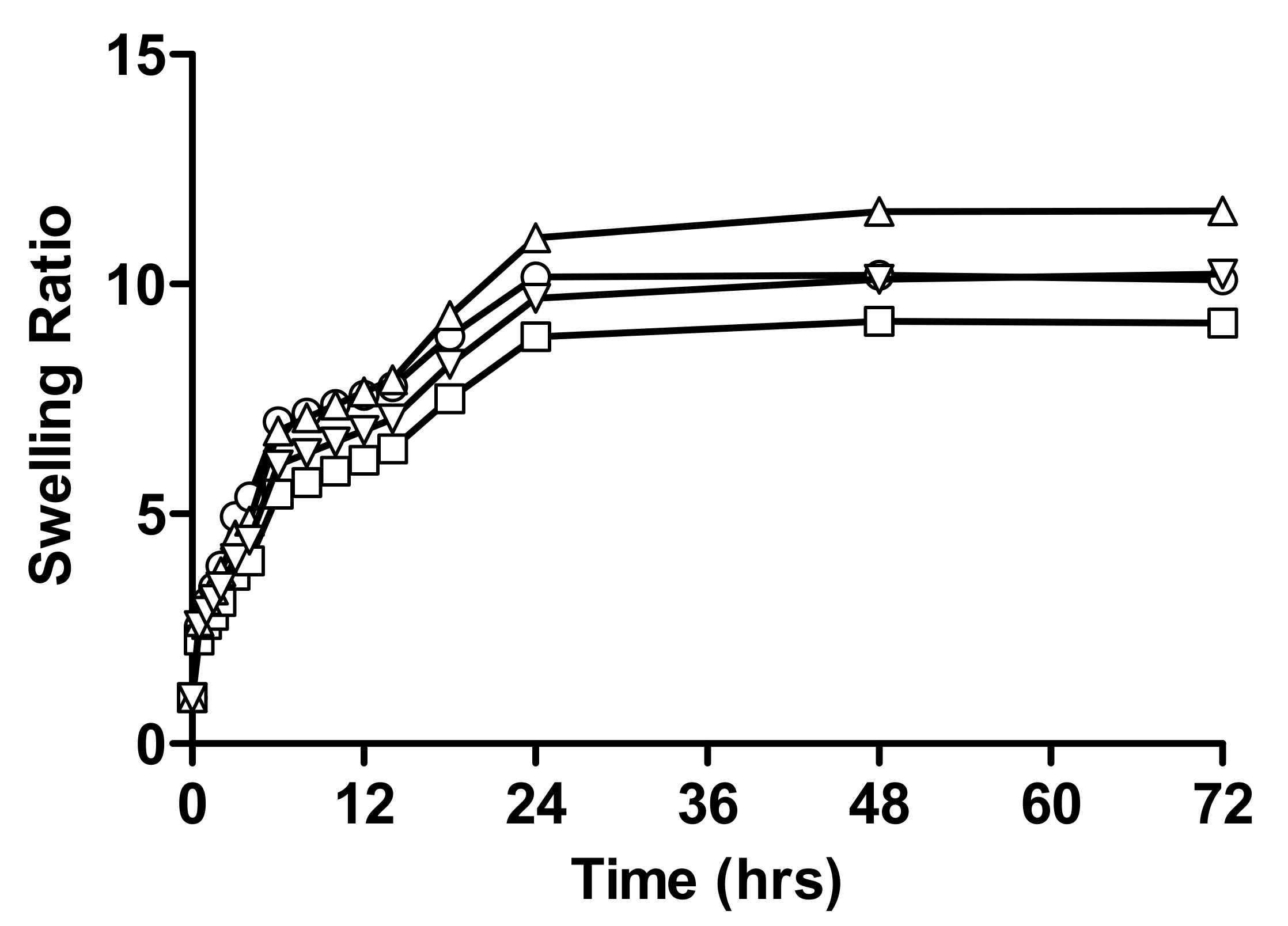
2.1.1. Swelling Studies
Effect of Varying Concentration of Monomer on Swelling Behaviour of Hydrogel
Effect of Varying Concentration of Linseed Mucilage on Swelling Behaviour of Hydrogel
Effect of Varying Concentration of Crosslinker
2.1.2. Percent Equilibrium Swelling (%ES) of All Formulations of Linseed-Co-AAM
2.2. Determination of Drug Loading
2.3. Instrumental Analysis
2.3.1. Fourier Transforms Infrared (FTIR) Analysis
2.3.2. Scanning Electron Microscopy (SEM)
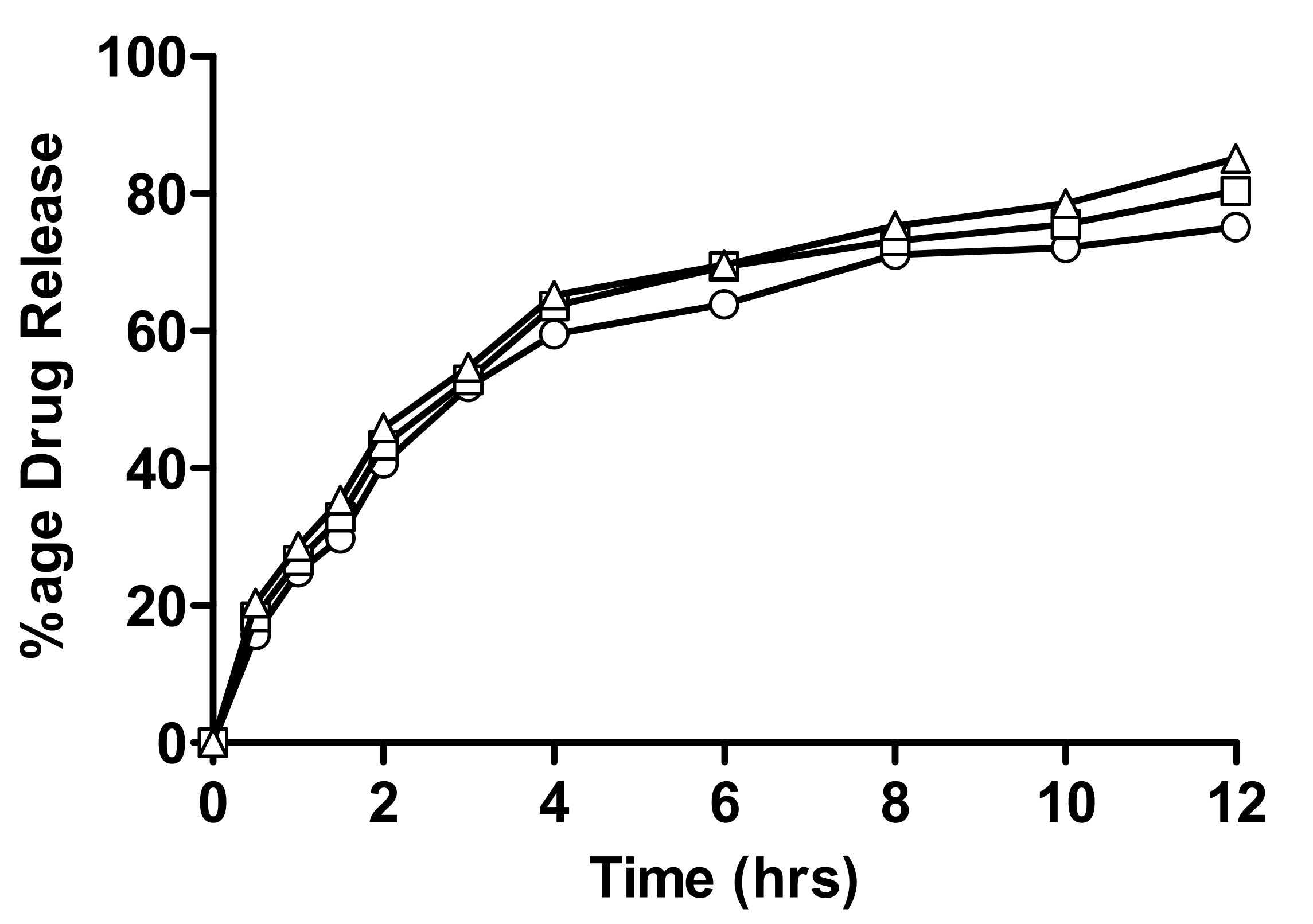
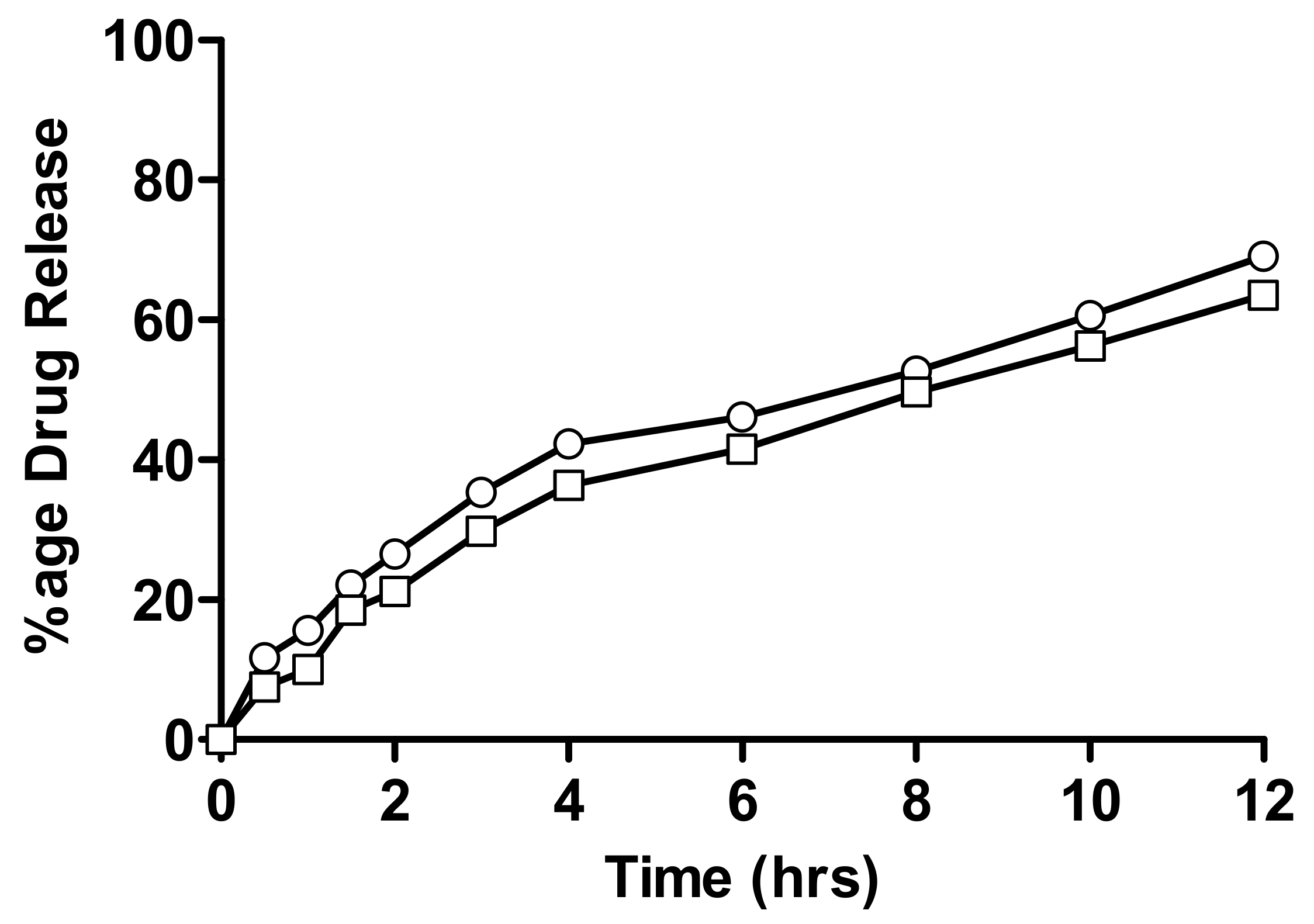
2.4. In-Vitro Drug Release Measurement
2.4.1. In-Vitro Drug Release of Linseed-Co-AAM Formulations
2.4.2. Evaluation of Drug Release Kinetics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Linseed Mucilage Extraction
4.2.2. Preparation of Linseed-Based Hydrogels
4.2.3. Characterization of Linseed-Co-AAM Graft Copolymeric Hydrogels
Swelling Studies
Percentage of Equilibrium Swelling/Equilibrium Water Content
Drug Loading
4.2.4. Instrumental Analysis
Fourier Transform Infrared (FTIR) Analysis
Scanning Electron Microscopy (SEM) Analysis
4.2.5. In-Vitro Drug Release Study
4.3. Mathematical Models of Drug Release Kinetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyholm, D.; Lennernäs, H.; toxicology. Irregular gastrointestinal drug absorption in Parkinson’s disease. Expert Opin. Drug Metab. 2008, 4, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, Y.; Chen, W.; Li, J.; Wang, X.; Chen, Y.; Yu, Y.; Shen, Z.; Zhang, Y. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J. Mater. Chem. B 2020, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Deen, G.R.; Loh, X.J. Stimuli-responsive cationic hydrogels in drug delivery applications. Gels 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Zhang, J.; Chen, X.; Zhang, J.; Chen, G.; Zhang, K.; Lin, J.; Guo, C.; Liu, J. Trigger-Detachable Hydrogel Adhesives for Bioelectronic Interfaces. Adv. Funct. Mater. 2021, 31, 2106446. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Wang, Z.; Wang, Z.; Lan, Y.; Ge, Q.A.M. Anisotropically Fatigue-Resistant Hydrogels. Adv. Mater. 2021, 33, 2102011. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Lan, Y.; Liu, J. Bioinspired 2D Isotropically Fatigue-Resistant Hydrogels. Adv. Mater. 2022, 34, 2270064. [Google Scholar] [CrossRef]
- Chen, W.; Kouwer, P.H. Combining Mechanical Tuneability with Function: Biomimetic Fibrous Hydrogels with Nanoparticle Crosslinkers. Adv. Funct. Mater. 2021, 31, 2105713. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Controlled drug release from hydrogel-based matrices: Experiments and modeling. Int. J. Pharm. 2015, 486, 144–152. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Hashemi, S.; Zohuriaan-Mehr, M. Synthesis of fast-swelling superabsorbent hydrogels: Effect of crosslinker type and concentration on porosity and absorption rate. Eur. Polym. J. 2003, 39, 1341–1348. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, S.A.; Noreen, S.; tul Muntaha, S. Linum usitatissimum seed mucilage-alginate mucoadhesive microspheres of metformin HCl: Fabrication, characterization and evaluation. Int. J. Biol. Macromol. 2020, 155, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K. Isolation and characterization of Linum usitatisimum polysaccharide to prepare mucoadhesive beads of diclofenac sodium. Int. J. Biol. Macromol. 2018, 116, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.T.; Hussain, M.A.; Yuk, S.H.; Bashir, S.; Nauman, M. Polysaccharides based superabsorbent hydrogel from Linseed: Dynamic swelling, stimuli responsive on–off switching and drug release. Carbohydr. Polym. 2016, 136, 750–756. [Google Scholar] [CrossRef]
- Nerkar, P.P.; Gattani, S. In vivo, in vitro evaluation of linseed mucilage based buccal mucoadhesive microspheres of venlafaxine. Drug Deliv. 2011, 18, 111–121. [Google Scholar] [CrossRef]
- Nerkar, P.P.; Gattani, S.G. Oromucosal delivery of venlafaxine by linseed mucilage based gel: In vitro and in vivo evaluation in rabbits. Arch. Pharmacal Res. 2013, 36, 846–853. [Google Scholar] [CrossRef]
- Kurra, P.; Narra, K.; Puttugunta, S.B.; Kilaru, N.B.; Mandava, B.R. Development and optimization of sustained release mucoadhesive composite beads for colon targeting. Int. J. Biol. Macromol. 2019, 139, 320–331. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Hussain, M.A.; Ashraf, M.U.; Haseeb, M.T.; Farid-ul-Haq, M. Linseed hydrogel based floating drug delivery system for fluoroquinolone antibiotics: Design, in vitro drug release and in vivo real-time floating detection. Saudi Pharm. J. 2020, 28, 538–549. [Google Scholar] [CrossRef]
- Haseeb, M.T.; Hussain, M.A.; Bashir, S.; Ashraf, M.U.; Ahmad, N. Evaluation of superabsorbent linseed-polysaccharides as a novel stimuli-responsive oral sustained release drug delivery system. Drug Dev. 2017, 43, 409–420. [Google Scholar] [CrossRef]
- Liu, P.; Peng, J.; Li, J.; Wu, J. Radiation crosslinking of CMC-Na at low dose and its application as substitute for hydrogel. Radiat. Phys. Chem. 2005, 72, 635–638. [Google Scholar] [CrossRef]
- Baker, J.P.; Hong, L.H.; Blanch, H.W.; Prausnitz, J.M. Effect of initial total monomer concentration on the swelling behavior of cationic acrylamide-based hydrogels. Macromolecules 1994, 27, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- YÜRÜKSOY, B.I. Swelling behavior of acrylamide-2-hydroxyethyl methacrylate hydrogels. Turk. J. Chem. 2000, 24, 147–156. [Google Scholar]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, W.; Wang, H.; Qi, W.; Yue, L.; Ye, Q. Synthesis and characterisation of starch grafted superabsorbent via 10 MeV electron-beam irradiation. Carbohydr. Polym. 2014, 101, 798–803. [Google Scholar] [CrossRef]
- Chavda, H.; Patel, C. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, G.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M. Modified chitosan 4. Superabsorbent hydrogels from poly (acrylic acid-co-acrylamide) grafted chitosan with salt-and pH-responsiveness properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and effect of structure on swelling properties of hydrogels based on high methylated pectin and acrylic polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Karadaǧ, E.; Saraydin, D.; Çetinkaya, S.; Güven, O. In vitro swelling studies and preliminary biocompatibility evaluation of acrylamide-based hydrogels. Biomaterials 1996, 17, 67–70. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A. Characterization of poly (vinyl alcohol)/poly (ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Torres, R.; Usall, J.; Teixido, N.; Abadias, M.; Vinas, I. Liquid formulation of the biocontrol agent Candida sake by modifying water activity or adding protectants. J. Appl. Microbiol. 2003, 94, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrand, J.; Michaud, P.; Picton, L.; Muller, G.; Courtois, B.; Ralainirina, R.; Courtois, J. Flax (Linum usitatissimum) seed cake: A potential source of high molecular weight arabinoxylans? J. Agric. Food Chem. 2005, 53, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, S.; Okay, O. Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: Synthesis and characterization. Polymer 2000, 41, 3693–3704. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, P.; Zhang, J.; Wang, A. Study on superabsorbent composite XVI. Synthesis, characterization and swelling behaviors of poly (sodium acrylate)/vermiculite superabsorbent composites. Eur. Pol. J. 2007, 43, 1691–1698. [Google Scholar] [CrossRef]
- Sorour, M.; El-Sayed, M.; Moneem, N.A.E.; Talaat, H.A.; Shalaan, H.; Marsafy, S.E. Characterization of hydrogel synthesized from natural polysaccharides blend grafted acrylamide using microwave (MW) and ultraviolet (UV) techniques. Starch-Stärke 2013, 65, 172–178. [Google Scholar] [CrossRef]
- AHMED, A.B.; NATH, L.K. Drug-excipients compatibility studies of nicorandil in controlled release floating tablet. Int. J. Pharm. Pharm. Sci 2014, 6, 468–475. [Google Scholar]
- Rashid, A.; Tulain, U.R.; Iqbal, F.M.; Malikd, N.S.; Erum, A. Synthesis, characterization and in vivo evaluation of ph sensitive hydroxypropyl methyl cellulose-graft-acrylic acid hydrogels for sustained drug release of model drug nicorandil. Gomal J. Med. Sci. 2020, 18, 99–106. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Mechanisms of solute and drug transport in relaxing, swellable, hydrophilic glassy polymers. Polymer 1999, 40, 3383–3398. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J. Flaxseed gum solution functional properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Rocha, M.S.; Rocha, L.C.; da Silva Feijó, M.B.; dos Santos Marotta, P.L.L.; Mourao, S.C. Effect of pH on the flaxseed (Linum usitatissimum L. seed) mucilage extraction process. Acta Scientiarum. Technol. 2021, 43, e50457. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Katime, I.; Velada, J.; Novoa, R.; de Apodaca, E.D.; Puig, J.; Mendizabal, E. Swelling kinetics of poly (acrylamide)/poly (mono-n-alkyl itaconates) hydrogels. Polym. Int. 1996, 40, 281–286. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; IntechOpen: London, UK, 2011; pp. 117–150. [Google Scholar]
- Sharma, R.; Walker, R.B.; Pathak, K. Evaluation of the kinetics and mechanism of drug release from econazole nitrate nanosponge loaded carbapol hydrogel. Indian J. Pharm. Educ. Res. 2011, 45, 25–31. [Google Scholar]
- Reddy, K.R.; Mutalik, S.; Reddy, S. Once-daily sustained-release matrix tablets of nicorandil: Formulation and in vitro evaluation. AAPS Pharmscitech 2003, 4, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.; Panchal, D.R.; Patel, U.; Brahmbhatt, T.; Suthar, M. Matrix type drug delivery system: A review. JPSBR 2011, 1, 143–151. [Google Scholar]
- Xu, Y.; Cui, B.; Ran, R.; Liu, Y.; Chen, H.; Kai, G.; Shi, J. Risk assessment, formation, and mitigation of dietary acrylamide: Current status and future prospects. Food Chem. Toxicol. 2014, 69, 1–12. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kurdtabar, M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Ali, L.; Sohail, M. Synthesis of chemically cross-linked polyvinyl alcohol-co-poly (methacrylic acid) hydrogels by copolymerization; a potential graft-polymeric carrier for oral delivery of 5-fluorouracil. DARU J. Pharm. Sci. 2013, 21, 44. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.B.; Nath, L.K. Fabrication and in vitro evaluation of floating matrix tablet of nicorandil using factorial design. J. Pharm. Res. 2011, 4, 1950–1954. [Google Scholar]





| Hydrogel Code | % Equilibrium Swelling (% ES) | |
|---|---|---|
| pH 1.2 | pH 4.5 | |
| F1 | 91.89 | 91.54 |
| F2 | 90.26 | 90.53 |
| F3 | 93.50 | 93.21 |
| F4 | 93.72 | 93.26 |
| F5 | 94.29 | 94.88 |
| F6 | 90.09 | 91.37 |
| F7 | 89.08 | 90.22 |
| Hydrogel Code | Nicorandil-Loaded |
|---|---|
| mg ± S.E.M | |
| F1 | 69 ± 1.4 |
| F2 | 65 ± 1.7 |
| F3 | 58 ± 1.1 |
| F4 | 60 ± 1.3 |
| F5 | 66 ± 1.5 |
| F6 | 40 ± 2.1 |
| F7 | 30 ± 1.9 |
| Hydrogel Code | Zero Order Model | First Order Model | Higuchi Model | Korsmeyer–Peppas Model | Hixson–Crowell Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | KO | R2 | K1 | R2 | kH | R2 | kKP | N | R2 | kHC | |
| F1 | 0.825 | 4.310 | 0.968 | 0.087 | 0.988 | 17.894 | 0.991 | 15.913 | 0.54 | 0.954 | 0.024 |
| F2 | 0.824 | 3.928 | 0.976 | 0.074 | 0.992 | 16.329 | 0.995 | 14.541 | 0.54 | 0.952 | 0.020 |
| F3 | 0.377 | 4.171 | 0.848 | 0.100 | 0.924 | 17.988 | 0.979 | 26.002 | 0.45 | 0.752 | 0.027 |
| F4 | 0.284 | 4.288 | 0.828 | 0.111 | 0.898 | 18.578 | 0.975 | 28.427 | 0.45 | 0.724 | 0.030 |
| F5 | 0.287 | 4.477 | 0.849 | 0.123 | 0.906 | 19.374 | 0.986 | 29.821 | 0.45 | 0.757 | 0.033 |
| F6 | 0.707 | 3.385 | 0.899 | 0.058 | 0.994 | 14.243 | 0.996 | 15.641 | 0.46 | 0.852 | 0.016 |
| F7 | 0.752 | 3.174 | 0.916 | 0.052 | 0.986 | 13.307 | 0.986 | 13.318 | 0.50 | 0.875 | 0.015 |
| Formulation Code | Linseed Mucilage | Acrylamide | Initiator | Crosslinker |
|---|---|---|---|---|
| (g/100g) | (g/100g) | (g/100g) | (g/100g) | |
| F1 | 1.0 | 12.5 | 0.2 | 0.2 |
| F2 | 1.0 | 17.5 | 0.2 | 0.2 |
| F3 | 1.0 | 15 | 0.2 | 0.2 |
| F4 | 1.5 | 15 | 0.2 | 0.2 |
| F5 | 2.0 | 15 | 0.2 | 0.2 |
| F6 | 1.0 | 15 | 0.2 | 0.3 |
| F7 | 1.0 | 15 | 0.2 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, A.; Erum, A.; Mumtaz, S.; Tulain, U.R.; Malik, N.S.; Alqahtani, M.S. Preliminary Investigation of Linum usitatissimum Mucilage-Based Hydrogel as Possible Substitute to Synthetic Polymer-Based Hydrogels for Sustained Release Oral Drug Delivery. Gels 2022, 8, 170. https://doi.org/10.3390/gels8030170
Mahmood A, Erum A, Mumtaz S, Tulain UR, Malik NS, Alqahtani MS. Preliminary Investigation of Linum usitatissimum Mucilage-Based Hydrogel as Possible Substitute to Synthetic Polymer-Based Hydrogels for Sustained Release Oral Drug Delivery. Gels. 2022; 8(3):170. https://doi.org/10.3390/gels8030170
Chicago/Turabian StyleMahmood, Arshad, Alia Erum, Sophia Mumtaz, Ume Ruqia Tulain, Nadia Shamshad Malik, and Mohammed S. Alqahtani. 2022. "Preliminary Investigation of Linum usitatissimum Mucilage-Based Hydrogel as Possible Substitute to Synthetic Polymer-Based Hydrogels for Sustained Release Oral Drug Delivery" Gels 8, no. 3: 170. https://doi.org/10.3390/gels8030170
APA StyleMahmood, A., Erum, A., Mumtaz, S., Tulain, U. R., Malik, N. S., & Alqahtani, M. S. (2022). Preliminary Investigation of Linum usitatissimum Mucilage-Based Hydrogel as Possible Substitute to Synthetic Polymer-Based Hydrogels for Sustained Release Oral Drug Delivery. Gels, 8(3), 170. https://doi.org/10.3390/gels8030170






