Robust SiO2–Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Adding Water Volume to ACG on Gel Process
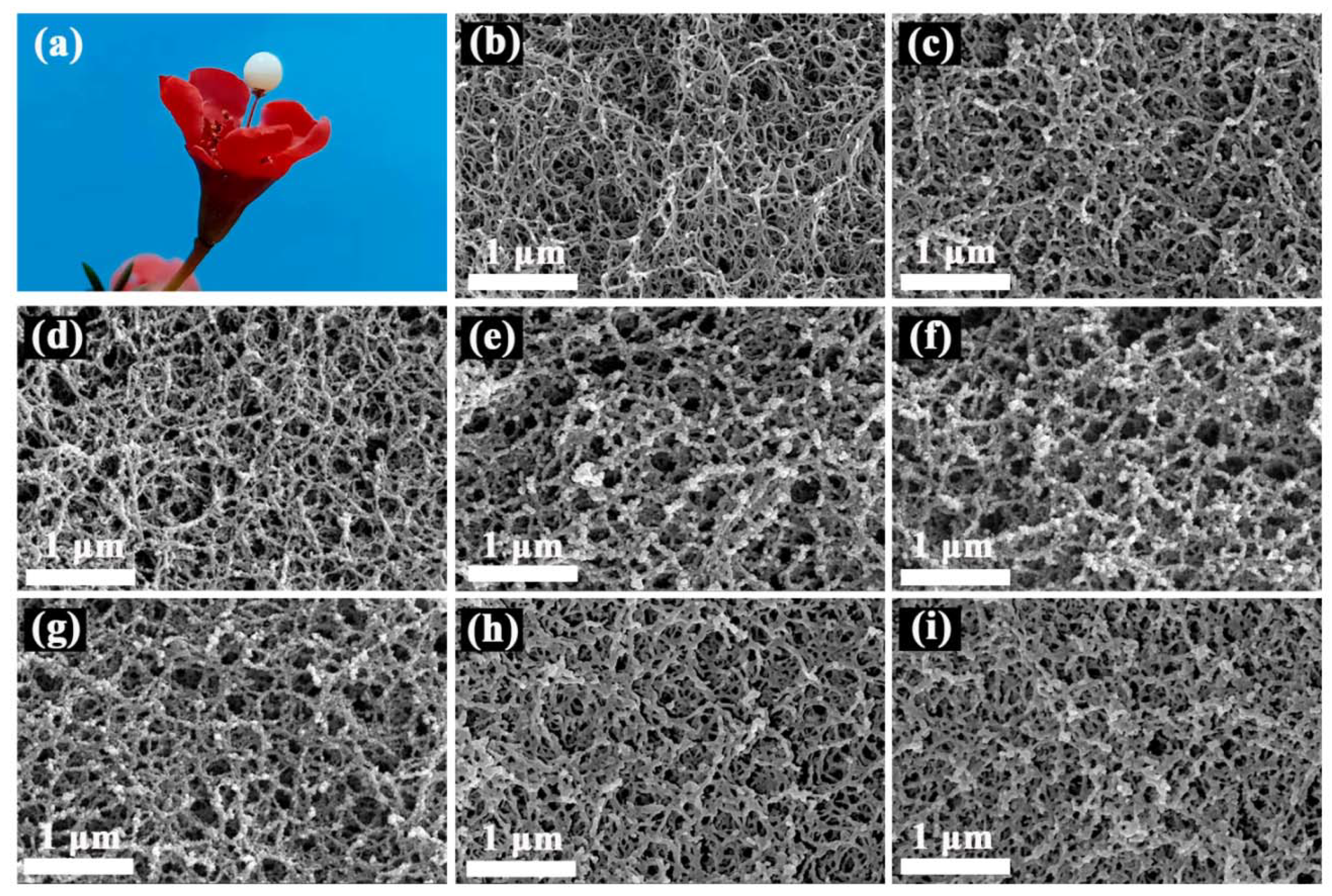
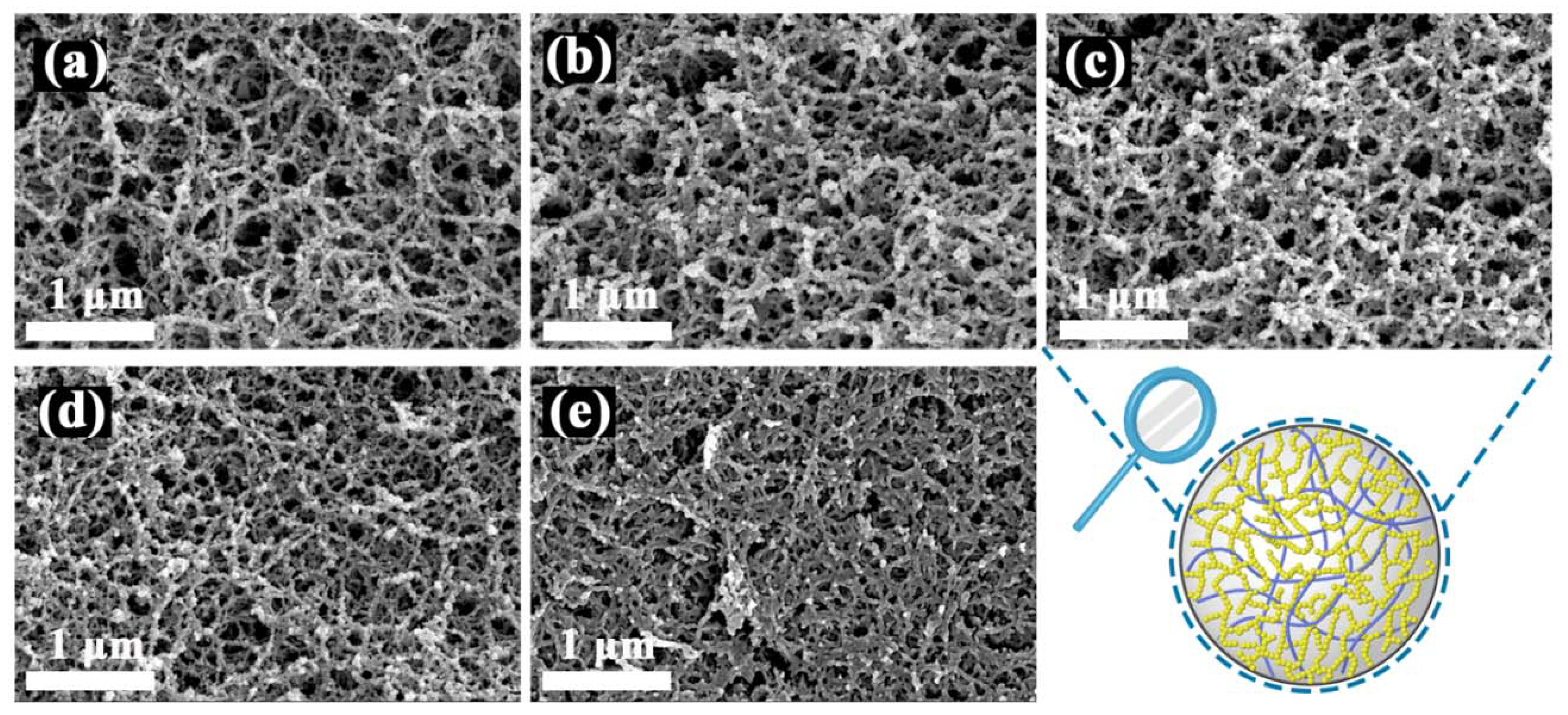
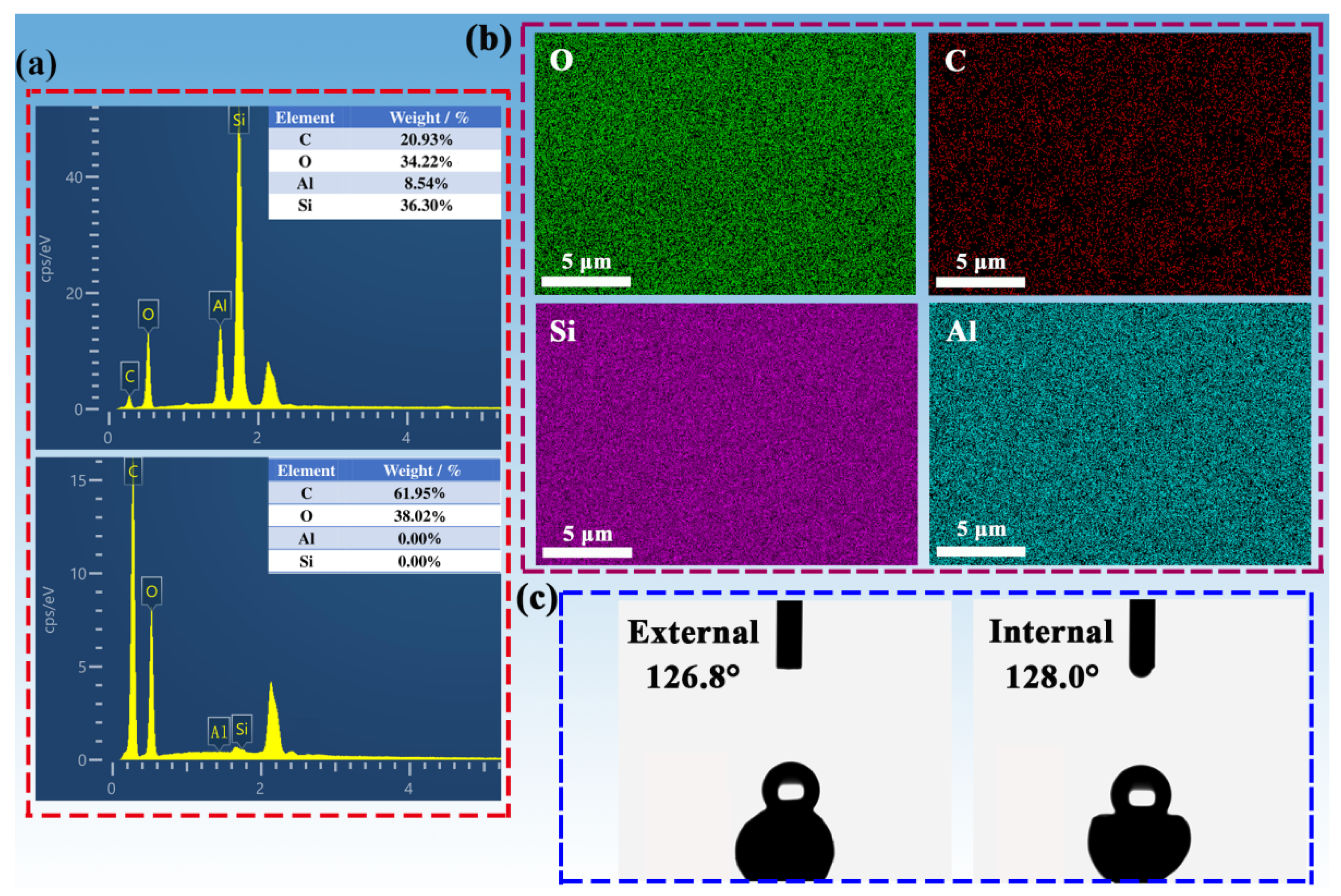
2.2. Structural Determination of SCABs
2.3. Nitrogen Adsorption–Desorption Test of SCABs
2.4. Compression Test
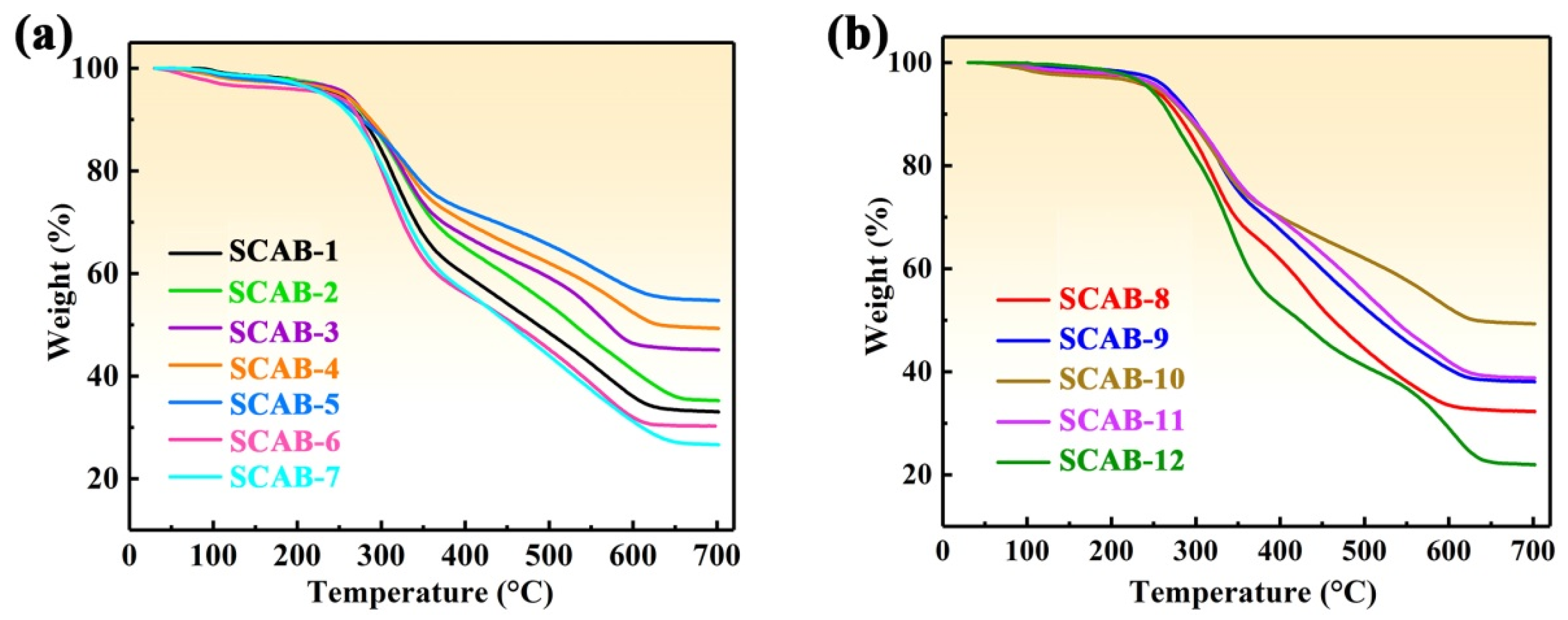
2.5. Thermal Stability
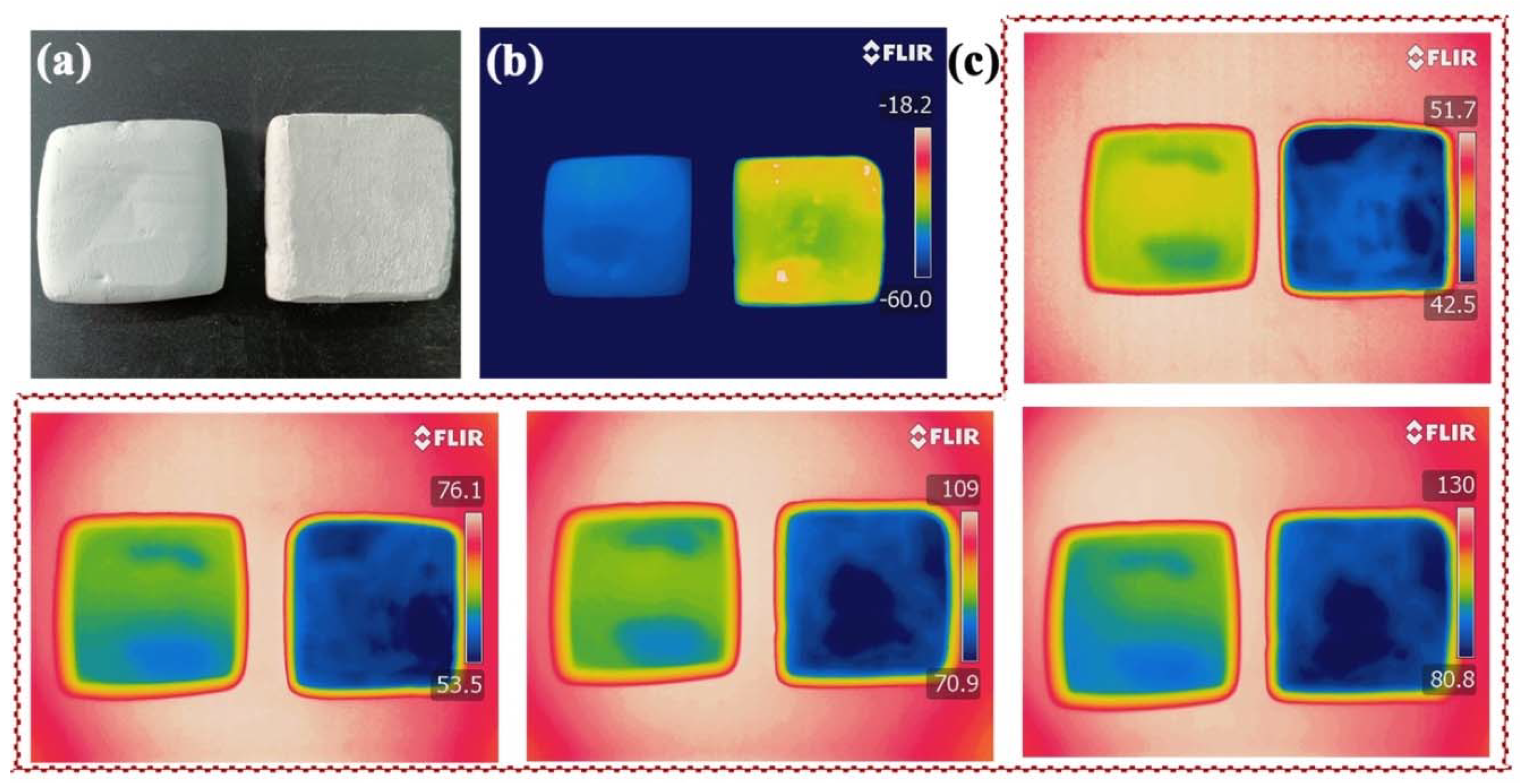
2.6. Thermal Transport Properties of SCABs
3. Conclusions
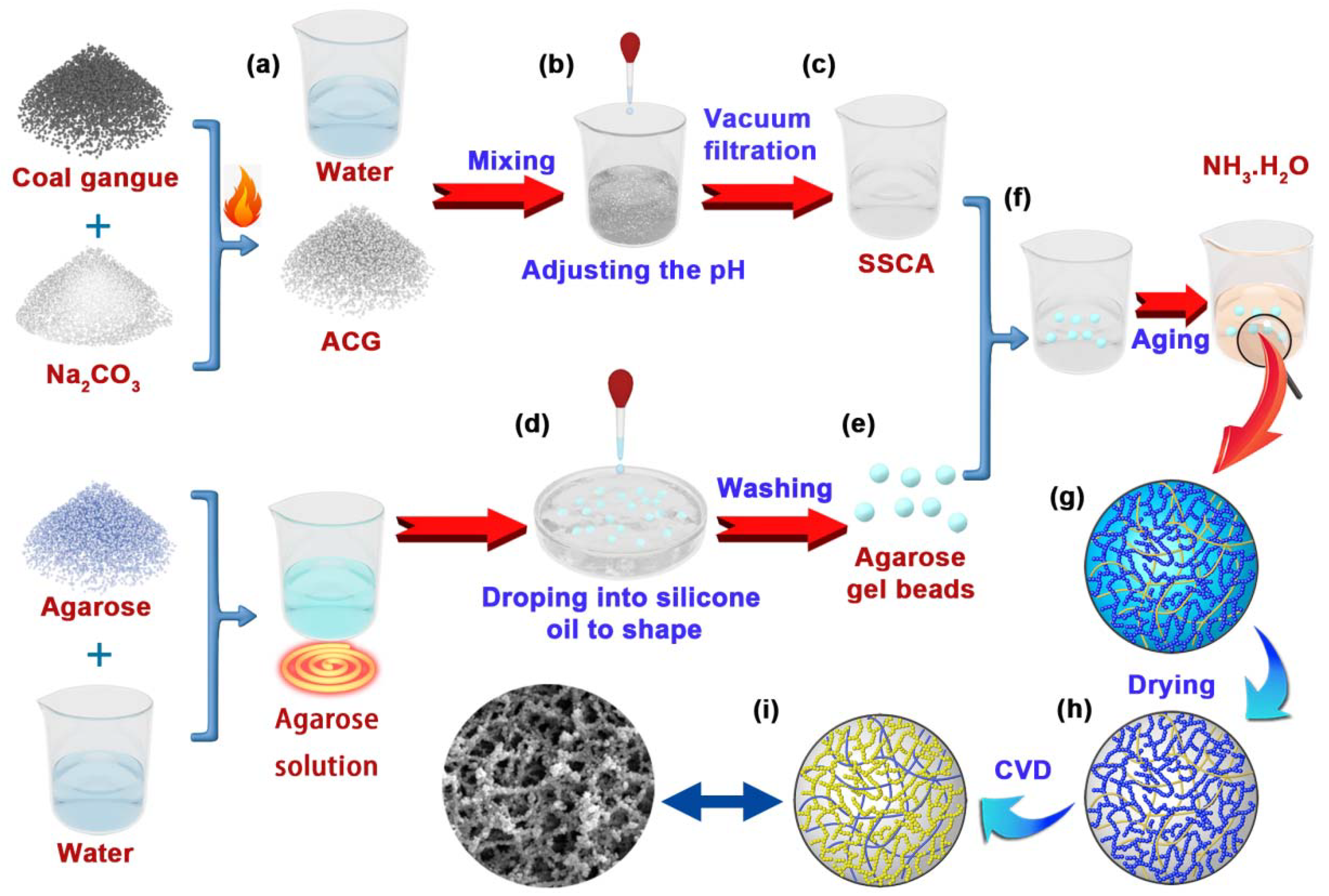
4. Materials and Methods
4.1. Materials
4.2. Activation of the Coal Gangue
4.3. Preparation of the SSCA
4.4. Preparation of the Agarose Gel Beads
4.5. Preparation of the SCABs
4.6. Preparation of the PBCS
4.7. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Li, Y.; Zhang, R. Silica aerogels having high flexibility and hydrophobicity prepared by sol-gel method. Ceram. Int. 2018, 44, 21262–21268. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Morgado, A.; Soares, A.; Flores-Colen, I.; Veiga, M.D.R.; Gomes, M.G. Durability of Thermal Renders with Lightweight and Thermal Insulating Aggregates: Regranulated Expanded Cork, Silica Aerogel and Expanded Polystyrene. Gels 2021, 7, 35. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, X.; Xu, T.; Dai, C.; Gu, Y.; Yun, S.; Hu, T.; Guan, G.; Chen, J. Ultralight and Hydrophobic Palygorskite-based Aerogels with Prominent Thermal Insulation and Flame Retardancy. ACS Appl. Mater. Interfaces 2020, 12, 11815–11824. [Google Scholar] [CrossRef] [PubMed]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.H.; Ni, X.X.; Li, C.X.; You, B.; Sun, G. Co-gel strategy for preparing hierarchically porous silica/polyimide nanocomposite aerogel with thermal insulation and flame retardancy. J. Mater. Chem. A 2020, 8, 9701–9712. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Yilmaz, E.; Deveci, H. Sustainable nanocomposites of epoxy and silica xerogel synthesized from corn stalk ash: Enhanced thermal and acoustic insulation performance. Compos. Part B Eng. 2018, 150, 1–6. [Google Scholar] [CrossRef]
- Mohammadi, R.; Azadmehr, A.; Maghsoudi, A. Fabrication of the alginate-combusted coal gangue composite for simultaneous and effective adsorption of Zn(II) and Mn(II). J. Environ. Chem. Eng. 2019, 7, 103494. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Yang, L.; Wang, M. Effective Removal of Calcium and Magnesium Ions from Water by a Novel Alginate-Citrate Composite Aerogel. Gels 2021, 7, 125. [Google Scholar] [CrossRef]
- Hu, H.L. Recent advances of polymeric phase change composites for flexible electronics and thermal energy storage system. Compos. Part B Eng. 2020, 195, 108094. [Google Scholar] [CrossRef]
- Wan, W.C.; Zhang, R.Y.; Ma, M.Z.; Zhou, Y. Monolithic aerogel photocatalysts: A review. J. Mater. Chem. A 2018, 6, 754–775. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Liu, L.; Zhang, Z.; Shen, J. Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation. Gels 2021, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ye, C.; Wang, B. Novel Al2O3–SiO2 aerogel/porous zirconia composite with ultra-low thermal conductivity. J. Porous Mater. 2017, 25, 171–178. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, X.; Zhang, M.; Guo, M. Synthesis of SiO2–Al2O3 composite aerogel from fly ash: A low-cost and facile approach. J. Sol-Gel Sci. Technol. 2019, 93, 281–290. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Ghica, M.E.; Duraes, L. An overview on alumina-silica-based aerogels. Adv. Colloid Interface Sci. 2020, 282, 102189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, S.; Li, X. Facile preparation of a SiO2–Al2O3 aerogel using coal gangue as a raw material via an ambient pressure drying method and its application in organic solvent adsorption. RSC Adv. 2015, 5, 103656–103661. [Google Scholar] [CrossRef]
- Han, L.; Ren, W.; Wang, B.; He, X.; Ma, L.; Huo, Q.; Wang, J.; Bao, W.; Chang, L. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water. Fuel 2019, 253, 1184–1192. [Google Scholar] [CrossRef]
- Xu, H.; Song, W.; Cao, W.; Shao, G.; Lu, H.; Yang, D.; Chen, D.; Zhang, R. Utilization of coal gangue for the production of brick. J. Mater. Cycles Waste Manag. 2016, 19, 1270–1278. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Sai, H.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Zhao, C.; Li, Z.; Li, F. Flexible aerogels based on an interpenetrating network of bacterial cellulose and silica by a non-supercritical drying process. J. Mater. Chem. A 2013, 1, 7963–7970. [Google Scholar] [CrossRef]
- Boonrawd, C.; Yodyingyong, S.; Benyahia, L.; Triampo, D. Novel Solvent–Latex Mixing: Thermal Insulation Performance of Silica Aerogel/Natural Rubber Composite. Gels 2021, 8, 7. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Zhang, Z.; Sebe, G.; Wu, R.; Virtudazo, R.V.R.; Tingaut, P.; Koebel, M.M. Multiscale Assembly of Superinsulating Silica Aerogels within Silylated Nanocellulosic Scaffolds: Improved Mechanical Properties Promoted by Nanoscale Chemical Compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Yan, M.; Fu, Y.; Pan, Y.; Cheng, X.; Gong, L.; Zhou, Y.; Ahmed, H.; Zhang, H. Highly elastic and fatigue resistant wood/silica composite aerogel operated at extremely low temperature. Compos. Part B Eng. 2022, 230, 109496. [Google Scholar] [CrossRef]
- Wen, S.; Ren, H.; Zhu, J.; Bi, Y.; Zhang, L. Fabrication of Al2O3 aerogel-SiO2 fiber composite with enhanced thermal insulation and high heat resistance. J. Porous Mater. 2018, 26, 1027–1034. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Yang, Z.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Mechanically Robust and Flame-Retardant Silicon Aerogel Elastomers for Thermal Insulation and Efficient Solar Steam Generation. ACS Omega 2020, 5, 8638–8646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wang, Q.; Wang, T.; Liang, Y. Facile one-step precursor-to-aerogel synthesis of silica-doped alumina aerogels with high specific surface area at elevated temperatures. J. Porous Mater. 2016, 24, 889–897. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, J.; Ni, X.Y.; Li, Y.; Wang, B.; Wu, G.M.; Zhou, B. Preparation and characterization of hydrophobic silica aerogels doped with fibers. Rare Met. Mat. Eng. 2008, 37, 16–19. [Google Scholar]
- Li, X.F.; Feng, J.Z.; Yin, J.; Jiang, Y.G.; Feng, J. Preparation and Properties of SiBCO Aerogel and Its Composites. Nanomaterials 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, B.; Hong, C.Q.; Wang, A.Z.; Zhou, S.T.; Qu, Q.; Zhou, S.B.; Zhang, X.H. Preparation and structural evolution of SiOC preceramic aerogel during high-temperature treatment. Ceram. Int. 2018, 44, 563–570. [Google Scholar] [CrossRef]
- Chandradass, J.; Kang, S.; Bae, D.S. Synthesis of silica aerogel blanket by ambient drying method using water glass based precursor and glass wool modified by alumina sol. J. Non-Cryst. Solids 2008, 354, 4115–4119. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saito, T.; Isogai, A. Aerogels with 3D Ordered Nanofiber Skeletons of Liquid-Crystalline Nanocellulose Derivatives as Tough and Transparent Insulators. Angew. Chem. Int. Ed. 2014, 53, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, Y.; Li, C.; Sun, G.; You, B. Superhydrophobic and Multifunctional Aerogel Enabled by Bioinspired Salvinia Leaf-Like Structure. Adv. Funct. Mater. 2021. [Google Scholar] [CrossRef]
- Kucharek, M.; MacRae, W.; Yang, L. Investigation of the effects of silica aerogel particles on thermal and mechanical properties of epoxy composites. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106108. [Google Scholar] [CrossRef]
- Sai, H.Z.; Wang, M.J.; Miao, C.Q.; Song, Q.Q.; Wang, Y.T.; Fu, R.; Wang, Y.X.; Ma, L.T.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef]
- Gu, H.; Gao, C.; Zhou, X.; Du, A.; Naik, N.; Guo, Z. Nanocellulose nanocomposite aerogel towards efficient oil and organic solvent adsorption. Adv. Compos. Hybrid Mater. 2021, 4, 459–468. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Reverchon, E.; Cardea, S. Production of Porous Agarose-Based Structures: Freeze-Drying vs. Supercritical CO2 Drying. Gels 2021, 7, 198. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, C.; Qiao, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous geopolymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106629. [Google Scholar] [CrossRef]
- Halim, Z.A.A.; Awang, N.; Yajid, M.A.M.; Ahmad, N.; Hamdan, H. A comparison between the effects of hydrophobic and hydrophilic silica aerogel fillers on tensile and thermal properties of unsaturated polyester composites. Polym. Bull. 2021, 78, 1–19. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Y.; Jiang, J.; Wang, L.; Song, M.; Bi, R.; Zhu, P.; Jiang, F. Multifunctional Superelastic Cellulose Nanofibrils Aerogel by Dual Ice-Templating Assembly. Adv. Funct. Mater. 2021, 31, 2106269. [Google Scholar] [CrossRef]
- Hu, W.; Li, M.; Chen, W.; Zhang, N.; Li, B.; Wang, M.; Zhao, Z. Preparation of hydrophobic silica aerogel with kaolin dried at ambient pressure. Colloids Surf. A 2016, 501, 83–91. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef]



| Added Water Volume (mL) | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 |
| Gelation Time (h) at Room Temperature | 0.6 | 2.8 | 7.2 | 14.1 | 26.0 | 39.7 | — | — |
| Gelation Time (h) at 60 °C | 0.30 | 1.6 | 2.0 | 2.9 | 3.8 | 5.0 | — | — |
| Residue Rate (%) | — | 4.41 | 4.49 | 4.50 | 4.33 | 4.47 | 4.36 | 4.48 |
| Sample (SCAB-) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average pore size (nm) | 14.6 | 15.4 | 16.2 | 17.6 | 18.0 | 14.3 | 13.4 | 15.6 | 16.5 | 16.8 | 15.5 | 13.5 |
| Sample | SCAB-1 | SCAB-2 | SCAB-3 | SCAB-4 | SCAB-5 | SCAB-6 | SCAB-7 | SCAB-8 | SCAB-9 | SCAB-10 | SCAB-11 | SCAB-12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Added water volume (mL) | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 40 | 40 | 40 | 40 | 40 |
| Agarose (wt. %) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1.5 | 2 | 2.5 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Ji, C.; Fu, R.; Yang, X.; Wan, Z.; Wen, L.; Song, Q.; Liu, Y.; Wang, Y.; Sai, H. Robust SiO2–Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue. Gels 2022, 8, 165. https://doi.org/10.3390/gels8030165
Gu J, Ji C, Fu R, Yang X, Wan Z, Wen L, Song Q, Liu Y, Wang Y, Sai H. Robust SiO2–Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue. Gels. 2022; 8(3):165. https://doi.org/10.3390/gels8030165
Chicago/Turabian StyleGu, Jie, Chao Ji, Rui Fu, Xin Yang, Zhichen Wan, Lishuo Wen, Qiqi Song, Yinghui Liu, Yaxiong Wang, and Huazheng Sai. 2022. "Robust SiO2–Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue" Gels 8, no. 3: 165. https://doi.org/10.3390/gels8030165
APA StyleGu, J., Ji, C., Fu, R., Yang, X., Wan, Z., Wen, L., Song, Q., Liu, Y., Wang, Y., & Sai, H. (2022). Robust SiO2–Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue. Gels, 8(3), 165. https://doi.org/10.3390/gels8030165







