Syneresis Behavior of Polymer Gels Aged in Different Brines from Gelants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelling Behavior of Different Polymers
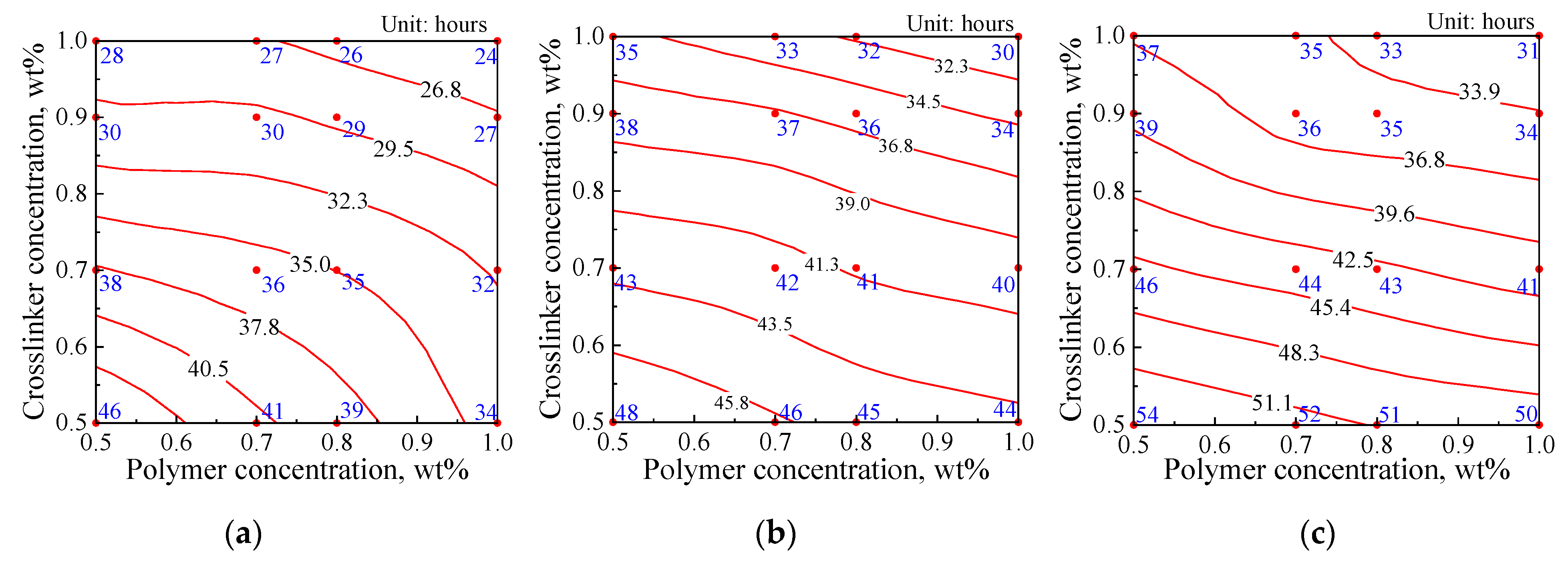
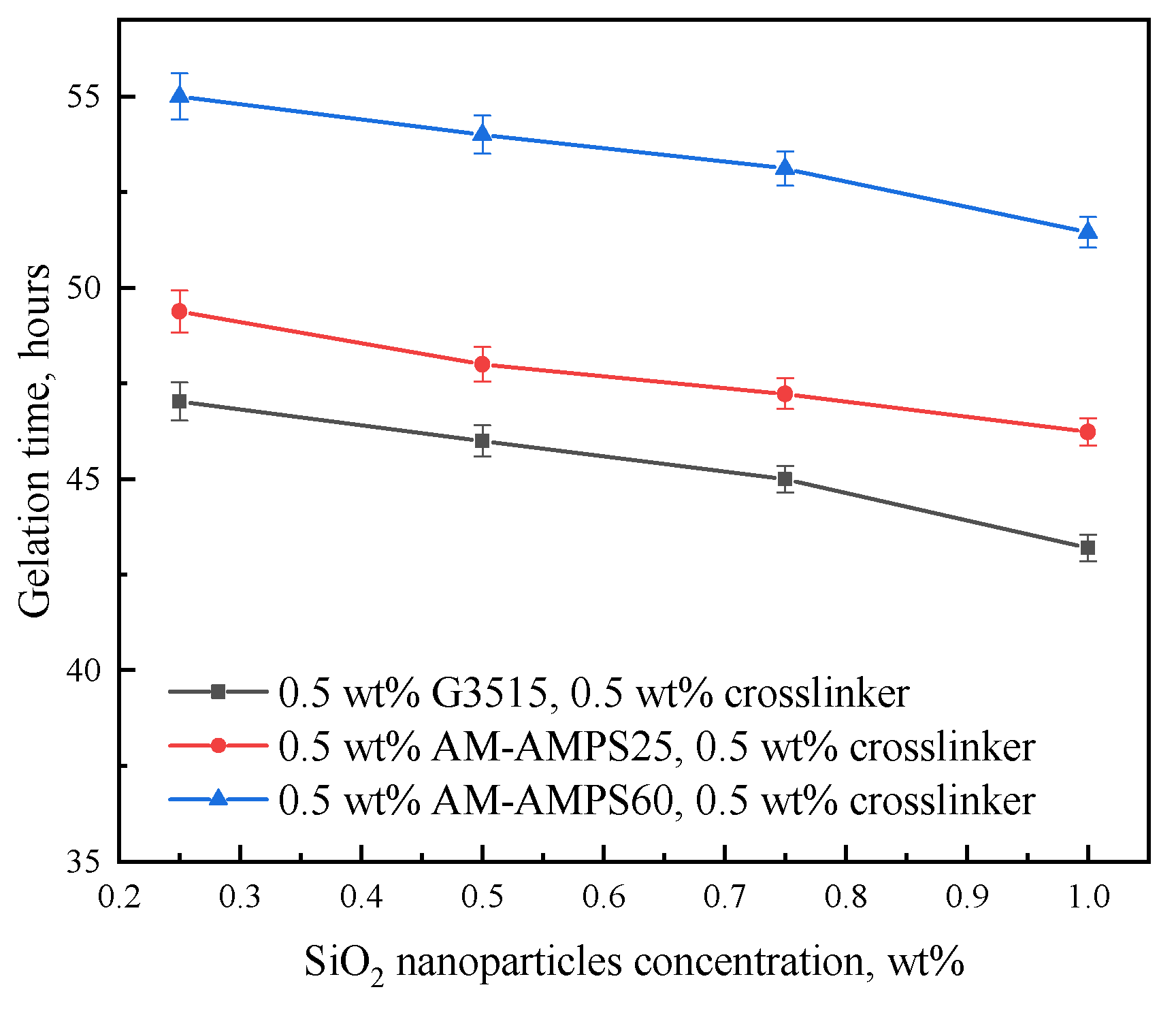
2.1.1. Gelation Time
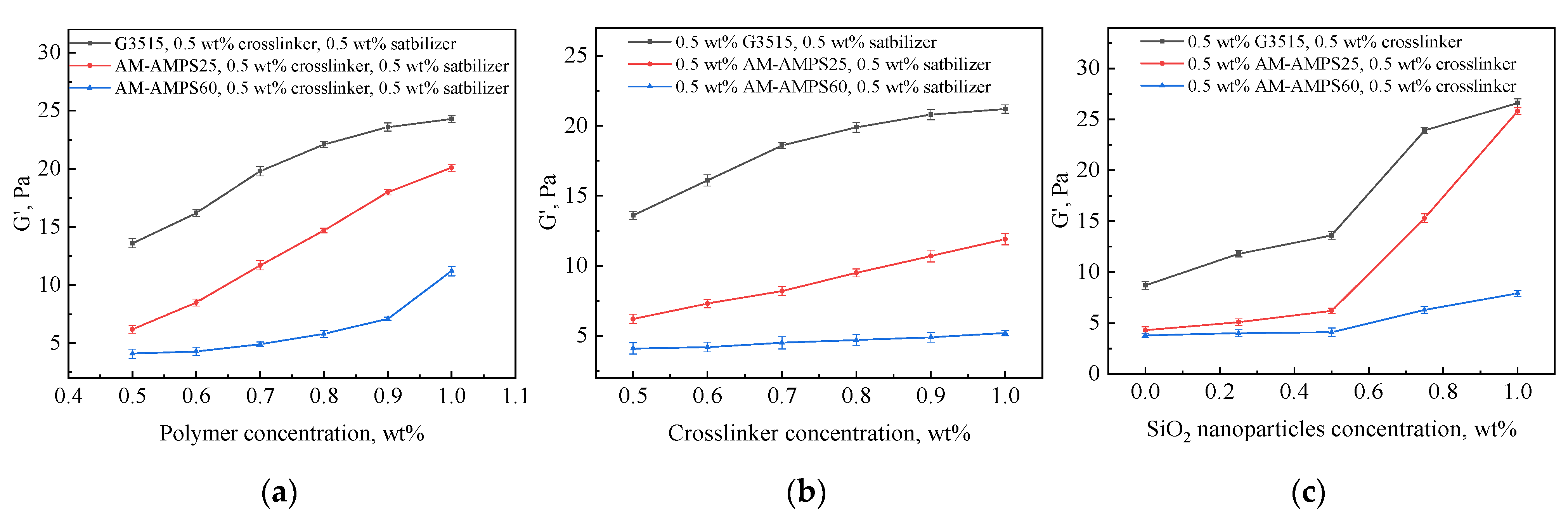
2.1.2. Gel Strength
2.1.3. Thermal Stability of Gels
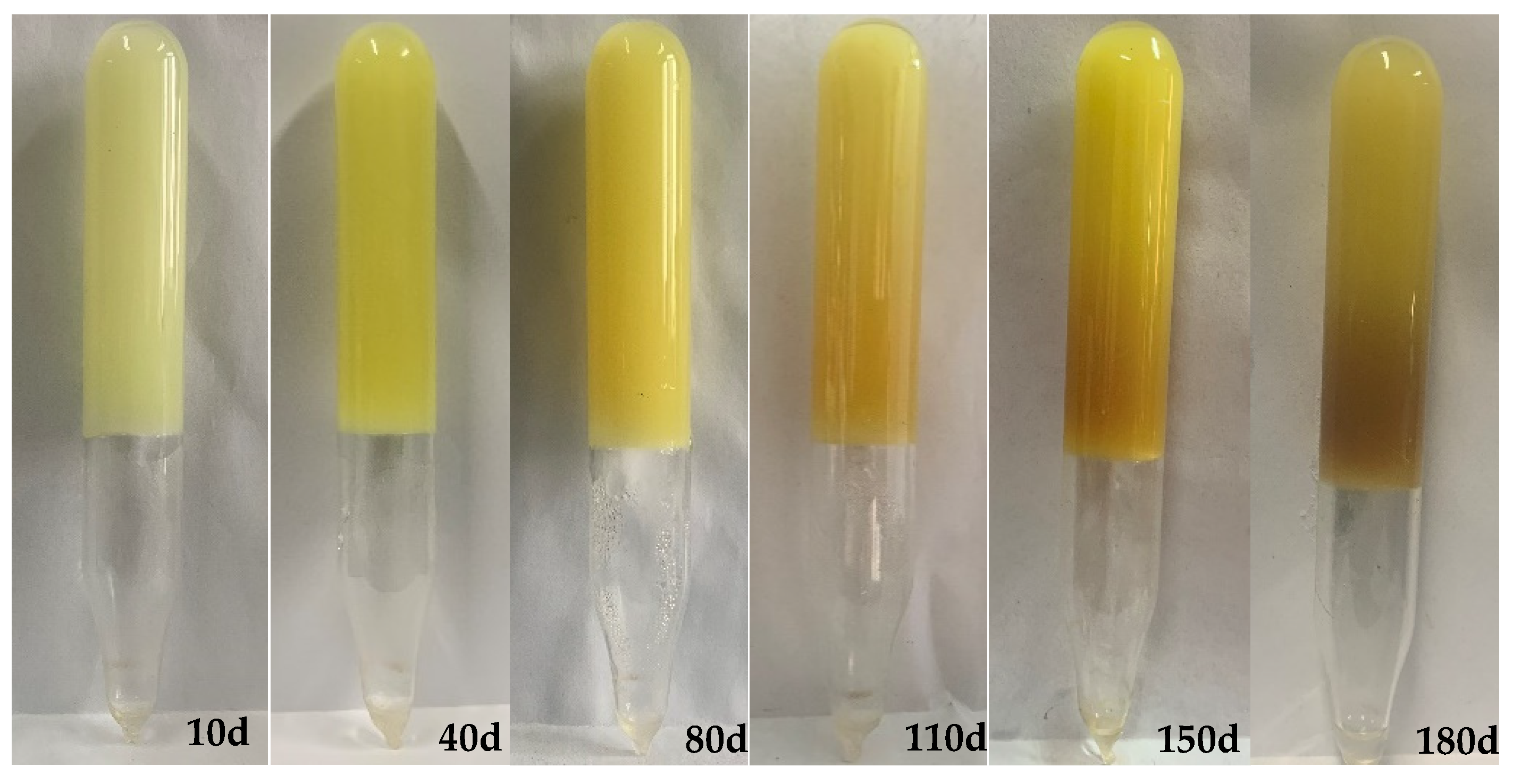
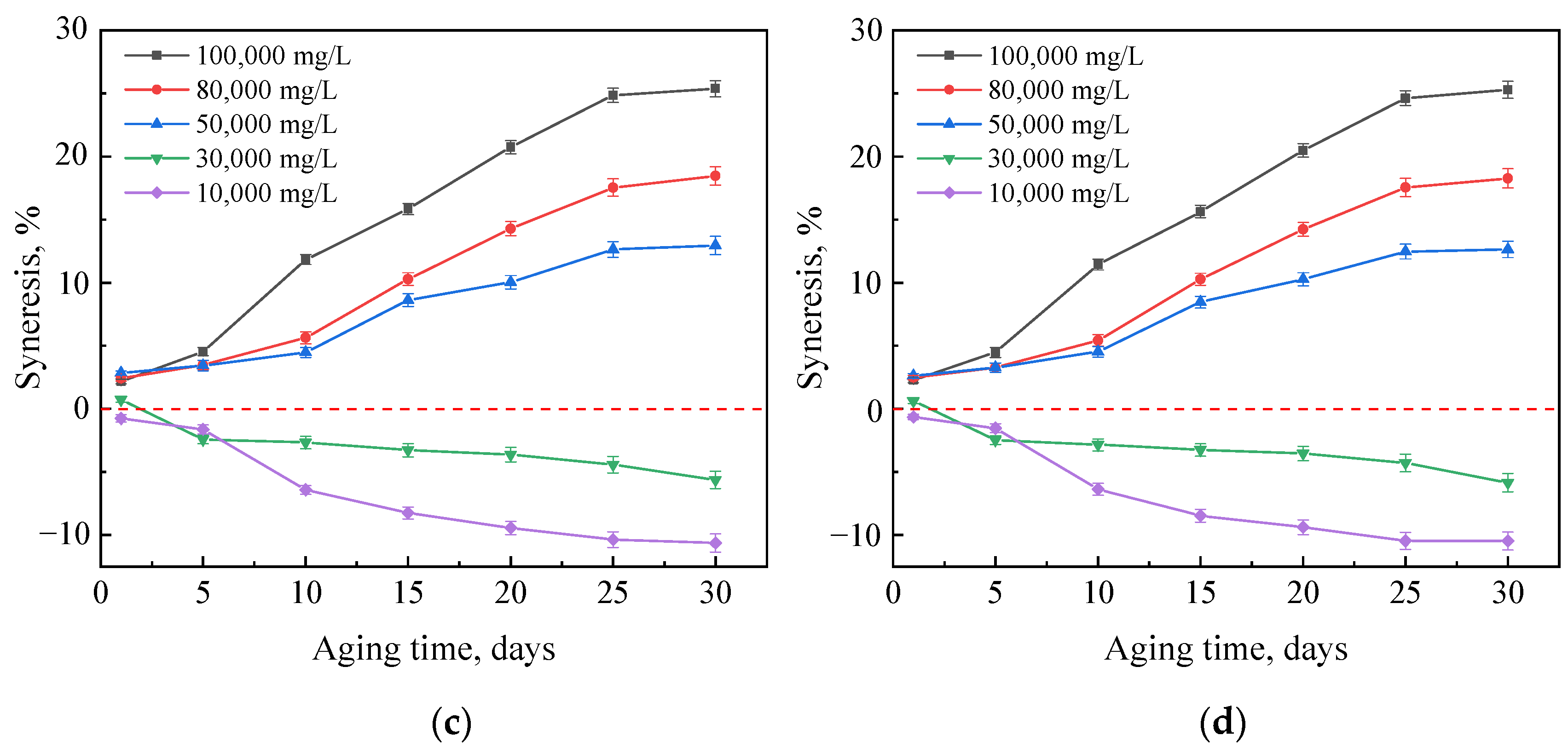
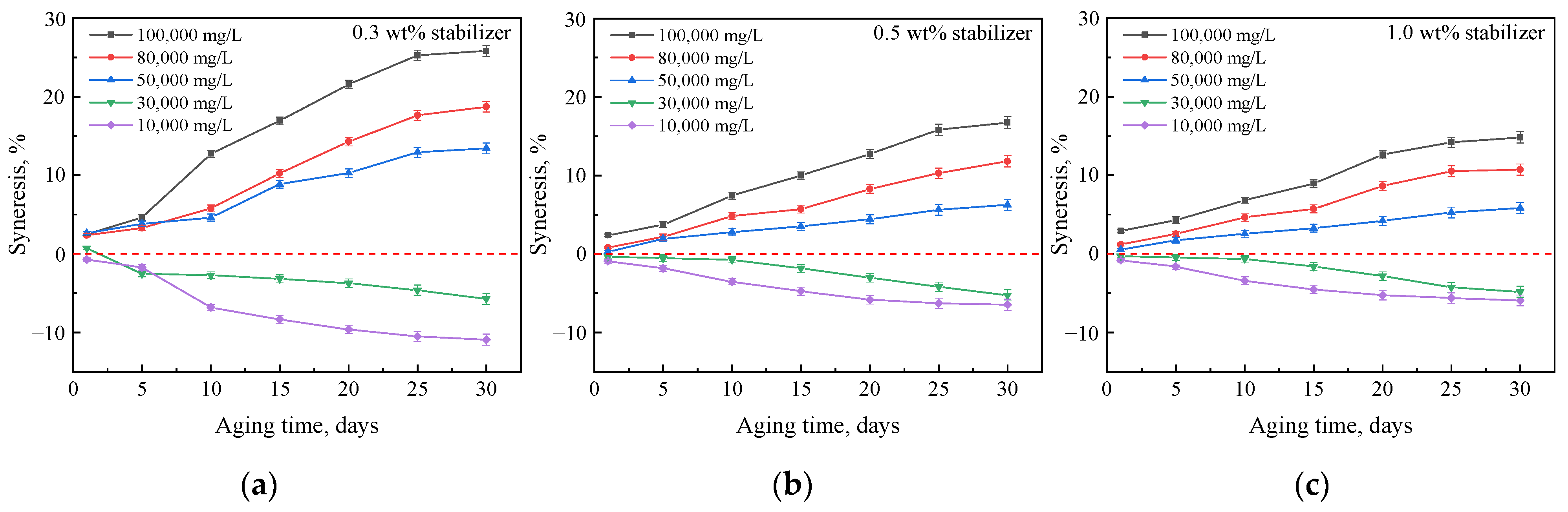
2.2. Mass Changes of Gels Aged in Hard Brines
2.2.1. Effects of Crosslinker and Polymer Concentrations
2.2.2. Effect of Nanoparticle Concentration
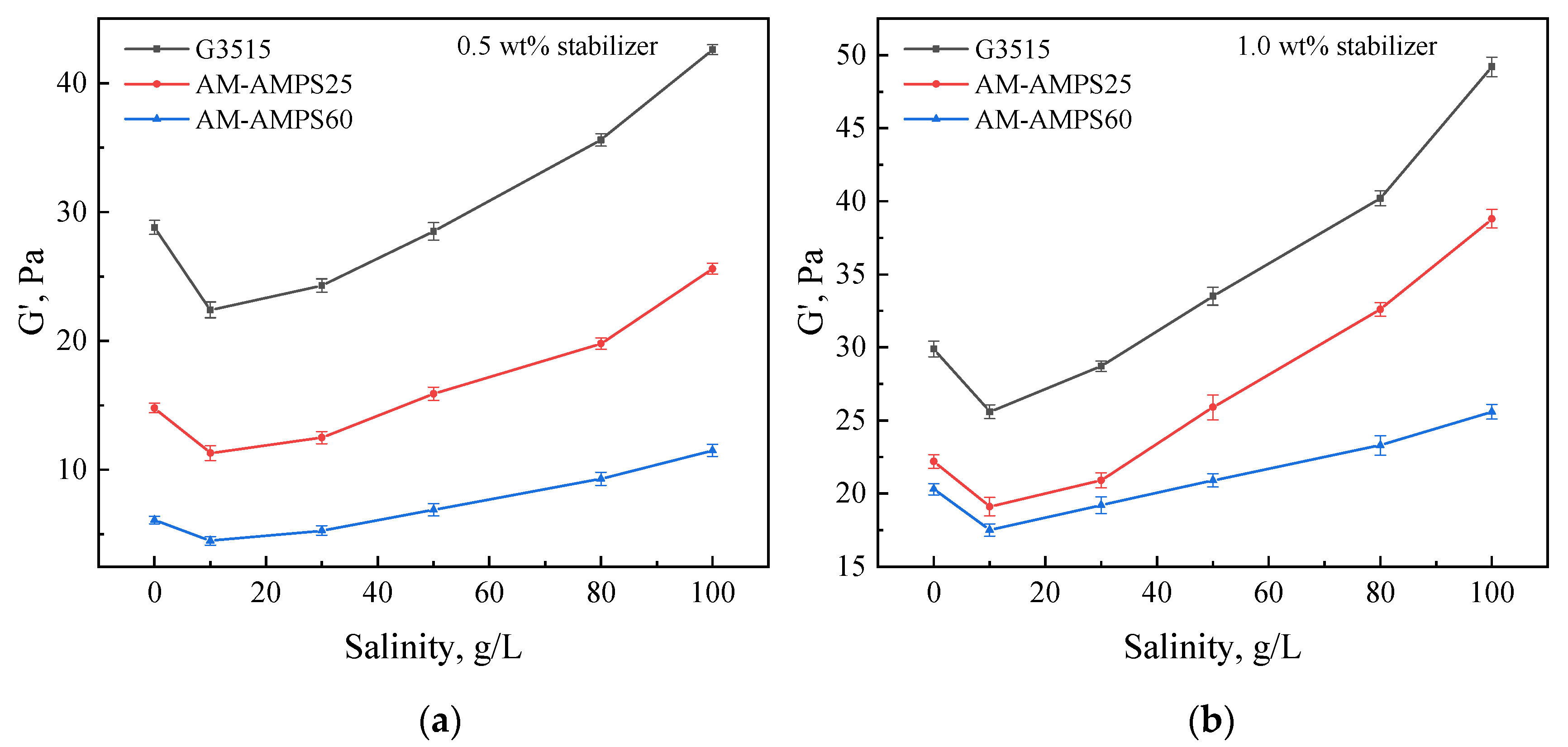
2.2.3. Effect of Polymer Type
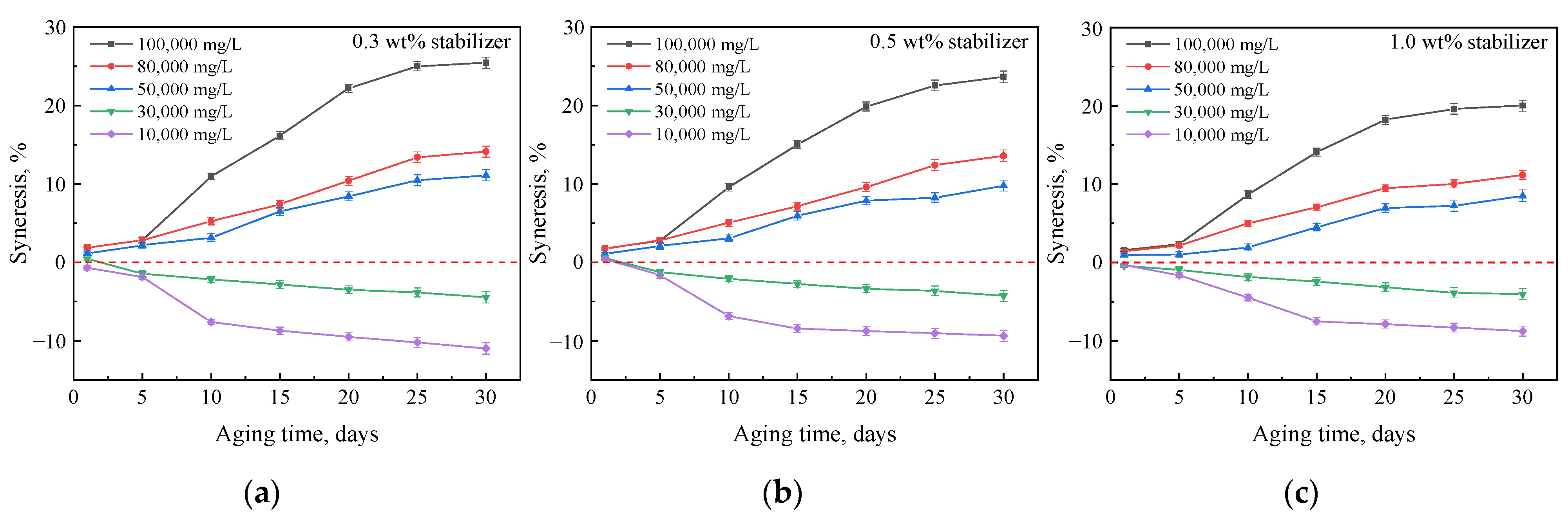
2.3. Strength Changes of Gels Aged in Hard Brines
3. Conclusions
- Using an AM-AMPS copolymer or HPAM as a gelatinizer, water-soluble phenolic resin as a crosslinker, and SiO2 nanoparticles as a stabilizer, a gel stabilized at 70 °C for more than 180 days was prepared with low salinity water.
- When gels formed in the soft brine were aged in hard brines with salinities less than 30,000 mg/L, they absorbed water and swelled, reducing the gel strength. However, when the salinity was higher than 50,000 mg/L, the gel exhibited syneresis.
- Optimizing the polymer structure and adding nanoparticles effectively overcame gel syneresis and enhanced the gel stability. The AM-AMPS copolymer with a high AMPS group content was more suitable for preparing salt-resistant gels.
- The type of polymer should be flexibly selected based on the salinity of the formation water, with HPAM for salinities below 30,000 mg/L and AM-AMPS for salinities above 50,000 mg/L.
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Gel Preparation
4.2.2. Bottle Testing
4.2.3. Determination of Gel Strength
4.2.4. Gel Aged in Hard Brines
4.2.5. Differential Scanning Calorimetry (DSC) Measurement
4.2.6. Microstructure of Gels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aldhaheri, M.; Wei, M.; Zhang, N.; Bai, B. A review of field oil-production response of injection-well gel treatments. SPE Reserv. Evaluation Eng. 2019, 22, 597–611. [Google Scholar] [CrossRef]
- Moradiaraghi, A. A review of thermally stable gels for fluid diversion in petroleum production. J. Pet. Sci. Eng. 2000, 26, 1–10. [Google Scholar] [CrossRef]
- Zaitoun, A.; Tabary, R.; Rousseau, D.; Pichery, T.R.; Braun, O. Using Microgels to Shut Off Water in a Gas Storage Well. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar] [CrossRef]
- El-Karsani, K.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer systems for water shutoff and profile modification: A review over the last decade. SPE J. 2014, 19, 135–149. [Google Scholar] [CrossRef]
- Al-Maamari, R.S.; Al-Hashami, A.R.; Al-Sharji, H.H.; Dupuis, G.; Bouillot, J.; Templier, A.; Zaitoun, A. Development of Thermo-Gels for Depth Conformance Control. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar] [CrossRef]
- Dupuis, G.; Lesuffleur, T.; Desbois, M.; Bouillot, J.; Zaitoun, A. Water Conformance Treatment using SMG Microgels: A Successful Field Case. In Proceedings of the SPE EOR Conference at Oil & Gas West Asia, Muscat, Oman, 31 March–2 April 2016. [Google Scholar] [CrossRef]
- Johnson, L.M.; Norton, C.A.; Huffman, N.D.; Mecham, J.B.; Rothrock, G.D. Nanocapsules for Controlled Release of Waterflood Agents for Improved Conformance. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 26–28 September 2016. [Google Scholar] [CrossRef]
- Liang, K.; Han, P.; Chen, Q.; Su, X.; Feng, Y. Comparative Study on Enhancing Oil Recovery under High Temperature and High Salinity: Polysaccharides Versus Synthetic Polymer. ACS Omega 2019, 4, 10620–10628. [Google Scholar] [CrossRef]
- Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous Hybrids of Silica Nanoparticles and Hydrophobically Associating Hydrolyzed Polyacrylamide Used for EOR in High-Temperature and High-Salinity Reservoirs. Energies 2014, 7, 3858–3871. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Huang, Z.; Chen, Q.; Wang, J.; Feng, Y. Application of copolymeric particulates for oilwell in-depth performance control. J. Appl. Polym. Sci. 2006, 102, 5330–5335. [Google Scholar] [CrossRef]
- Aldhaheri, M.; Wei, M.; Zhang, N.; Bai, B. Field design guidelines for gel strengths of profile-control gel treatments based on reservoir type. J. Pet. Sci. Eng. 2020, 194, 107482. [Google Scholar] [CrossRef]
- Seright, R.; Brattekas, B. Water shutoff and conformance improvement: An introduction. Pet. Sci. 2021, 18, 450–478. [Google Scholar] [CrossRef]
- Bryant, S.L.; Rabaioli, M.R.; Lockhart, T.P. Influence of syneresis on permeability reduction by polymer gels. SPE Prod. Facil. 1996, 11, 209–215. [Google Scholar] [CrossRef]
- Dawe, R.A.; Zhang, Y. Mechanistic study of the selective action of oil and water penetrating into a gel emplaced in a porous medium. J. Pet. Sci. Eng. 1994, 12, 113–125. [Google Scholar] [CrossRef]
- Al-Sharji, H.H.; Grattoni, C.A.; Da We, R.A.; Zimmerman, R.W. Pore-scale study of the flow of oil and water through polymer gels. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999. [Google Scholar] [CrossRef]
- Krishnan, P.; Asghari, K.; Willhite, G.P.; Mccool, C.S.; Green, D.W.; Vossoughi, S. Dehydration and permeability of gels used in in situ permeability modification treatments. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000. [Google Scholar] [CrossRef]
- Brattekas, B.; Haugen, A.; Graue, A.; Seright, R. Gel dehydration by spontaneous imbibition of brine from aged polymer gel. SPE J. 2013, 19, 122–134. [Google Scholar] [CrossRef]
- Karimi, S.; Kazemi, S.; Kazemi, N. Syneresis measurement of the HPAM-Cr (III) gel polymer at different conditions: An experimental investigation. J. Nat. Gas Sci. Eng. 2016, 34, 1027–1033. [Google Scholar] [CrossRef]
- Ding, H.; Geng, J.; Lu, Y.; Zhao, Y.; Bai, B. Impacts of crosslinker concentration on nanogel properties and enhanced oil recovery capability. Fuel 2020, 267, 117098. [Google Scholar] [CrossRef]
- Digiacomo, P.M.; Schramm, C.M. Mechanism of Polyacrylamide Gel Syneresis Determined by C-13 NMR. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Denver, CO, USA, 1–3 June 1983. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Ge, J.; Jiang, P.; Zhu, X. Experimental research of syneresis mechanism of HPAM/Cr3+ gel. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 96–103. [Google Scholar] [CrossRef]
- Tu, T.N.; Wisup, B. Investigating the Effect of Polymer Gels Swelling Phenomenon under Reservoir Conditions on Polymer Conformance Control Process. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 15–17 November 2011. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Zhang, J.; Bai, B. Gel composition and brine concentration effect on hydrogel dehydration subjected to uniaxial compression. J. Pet. Sci. Eng. 2019, 182, 106358. [Google Scholar] [CrossRef]
- Bai, B.; Zhang, H. Preformed-particle-gel transport through open fractures and its effect on water flow. SPE J. 2010, 16, 388–400. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Ni, T.; Huang, G.; Zheng, J.; Gao, P.; Chen, M. Simulation of the crosslinking mechanism of polyacrylamide/water-soluble phenolic aldehyde. J. Chem. Coll. Univ. 2010, 31, 577–582. (In Chinese) [Google Scholar]
- Sang, Q.; Li, Y.; Yu, L.; Li, Z.; Dong, M. Enhanced oil recovery by branched-preformed particle gel injection in parallel-sandpack models. Fuel 2014, 136, 295–306. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Wang, K.; Zou, C.; Gao, M.; Fang, Y.; Zhao, M.; Wu, Y.; You, Q. Study on a Novel Cross-Linked Polymer Gel Strengthened with Silica Nanoparticles. Energy Fuels 2017, 31, 9152–9161. [Google Scholar] [CrossRef]
- Wu, Q.; Ge, J. Experimental investigation of the entanglement network and nonlinear viscoelastic behavior of a nano-SiO2 strengthened polymer gel. J. Mol. Liq. 2021, 339, 117288. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Giraldo, M.A.; Llanos, S.; Maya, G.; Zabala, R.D.; Nassar, N.N.; Franco, C.A.; Alvarado, V.; Cortés, F.B. The effects of SiO2 nanoparticles on the thermal stability and rheological behavior of hydrolyzed polyacrylamide based polymeric solutions. J. Pet. Sci. Eng. 2017, 159, 841–852. [Google Scholar] [CrossRef]
- He, S.Q.; Bai, Y.Q.; Yu, T.T.; Dai, C.; Zhang, H. Strength and temperature tolerance improvement by diatomite doped polyacrylamide gel for water shut-off. Sci. Technol. Eng. 2018, 018, 65–71. (In Chinese) [Google Scholar]
- Dai, C.; Chen, W.; You, Q.; Wang, H.; Yang, Z.; He, L.; Jiao, B.; Wu, Y. A novel strengthened dispersed particle gel for enhanced oil recovery application. J. Ind. Eng. Chem. 2016, 41, 175–182. [Google Scholar] [CrossRef]
- Flory, P.L. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Hermans, J.J. Statistical thermodynamics of swollen polymer networks. J. Polym. Sci. Part A Polym. Chem. 2010, 59, 191–208. [Google Scholar] [CrossRef]
- Gales, J.R.; Young, T.; Willhite, G.P.; Green, D.W. Equilibrium swelling and syneresis properties of xanthan gum-cr(iii) gels. SPE Adv. Technol. 1994, 2, 190–198. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, M.; Gao, M.; Song, X.; Dai, C. The experimental study of silica nanoparticles strengthened polymer gel system. J. Dispers. Sci. Technol. 2021, 42, 298–305. [Google Scholar] [CrossRef]
- Moradi-Araghi, A.; Cleveland, D.H.; Westerman, I.J. Development and Evaluation of EOR Polymers Suitable for Hostile Environments: II-Copolymers of Acrylamide and Sodium AMPS. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 1 February 1987. [Google Scholar] [CrossRef]
- Sydansk, R.D. A newly developed chromium(III) gel technology. SPE Reserv. Eng. 1990, 5, 346–352. [Google Scholar] [CrossRef]







| Sample | a | b |
|---|---|---|
| ΔH (J/g) | 330.8 | 321.9 |
| wf (%) | 99.19 | 96.52 |
| wb (%) | 0.81 | 3.48 |
| Ion | Na+ | Mg2+ | Ca2+ | Cl− | SO42− | HCO3− | Total |
|---|---|---|---|---|---|---|---|
| Ion content (mg·L−1) | 285 | 5 | 9 | 123 | 175 | 375 | 972 |
| Ion | Na+ | Mg2+ | Ca2+ | Cl− | SO42− | HCO3− | Total |
|---|---|---|---|---|---|---|---|
| Ion content (mg·L−1) | 21,093 | 8141.8 | 8762.2 | 61,524 | 259.4 | 173.6 | 99,954 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Ge, J.; Wu, Q.; He, Z.; Wang, W.; Cao, G. Syneresis Behavior of Polymer Gels Aged in Different Brines from Gelants. Gels 2022, 8, 166. https://doi.org/10.3390/gels8030166
Guo H, Ge J, Wu Q, He Z, Wang W, Cao G. Syneresis Behavior of Polymer Gels Aged in Different Brines from Gelants. Gels. 2022; 8(3):166. https://doi.org/10.3390/gels8030166
Chicago/Turabian StyleGuo, Hongbin, Jijiang Ge, Qianhui Wu, Ziyu He, Wei Wang, and Guojuan Cao. 2022. "Syneresis Behavior of Polymer Gels Aged in Different Brines from Gelants" Gels 8, no. 3: 166. https://doi.org/10.3390/gels8030166
APA StyleGuo, H., Ge, J., Wu, Q., He, Z., Wang, W., & Cao, G. (2022). Syneresis Behavior of Polymer Gels Aged in Different Brines from Gelants. Gels, 8(3), 166. https://doi.org/10.3390/gels8030166






