pH-Responsive, Thermo-Resistant Poly(Acrylic Acid)-g-Poly(boc-L-Lysine) Hydrogel with Shear-Induced Injectability
Abstract
1. Introduction
2. Results and Discussion
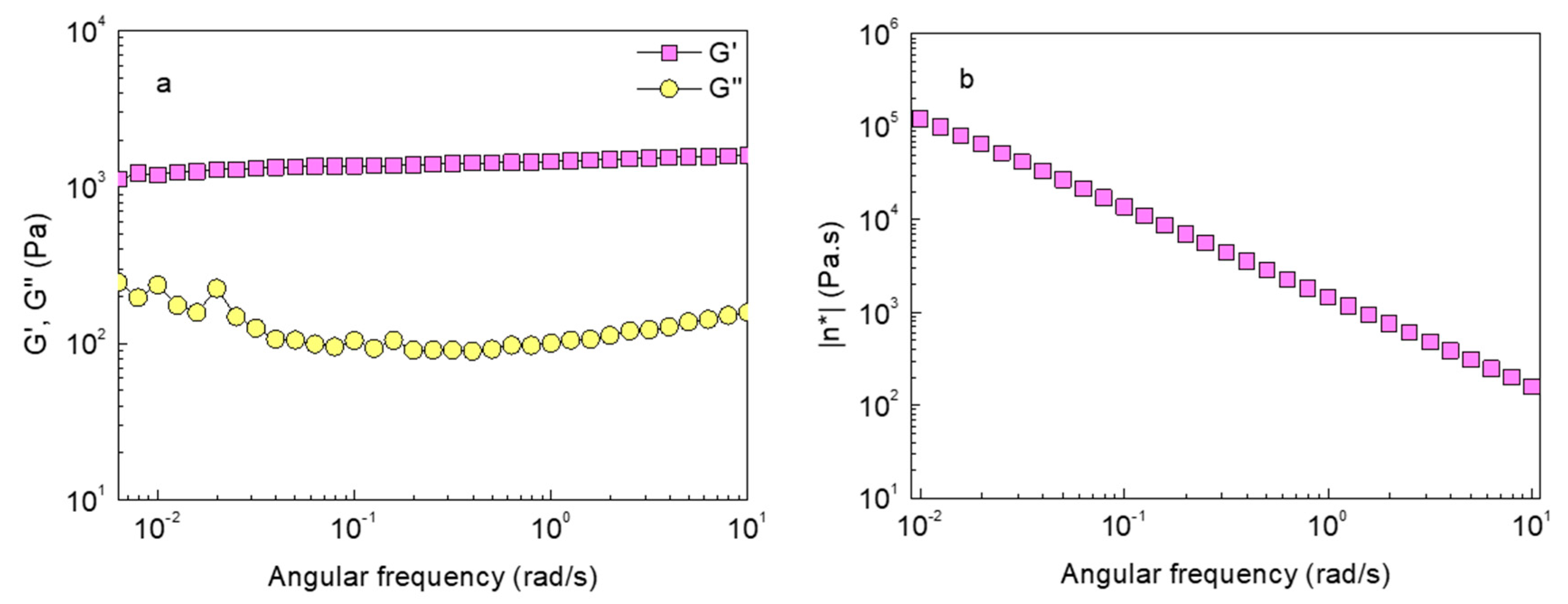
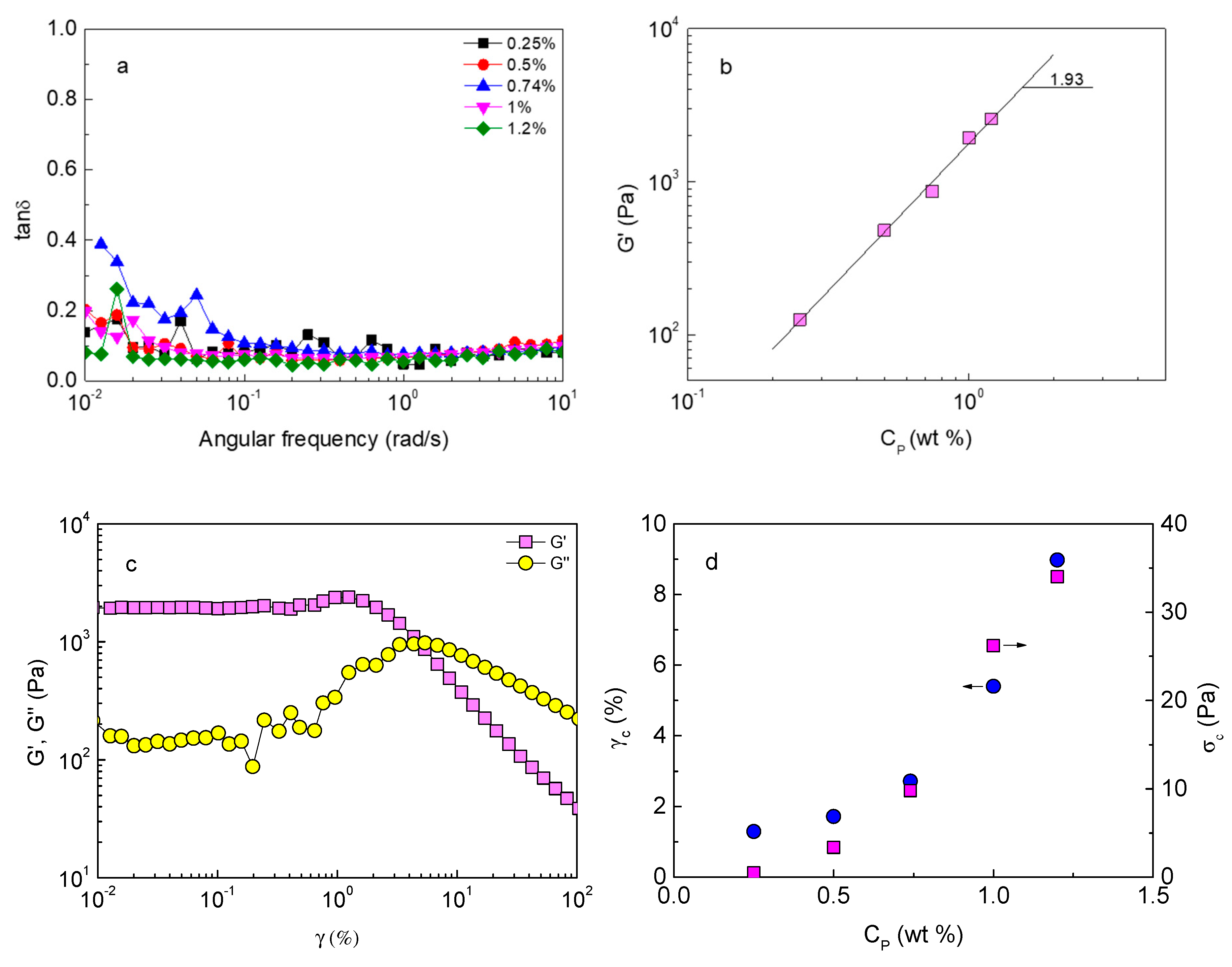
2.1. Gelation and Polymer Concentration Dependence
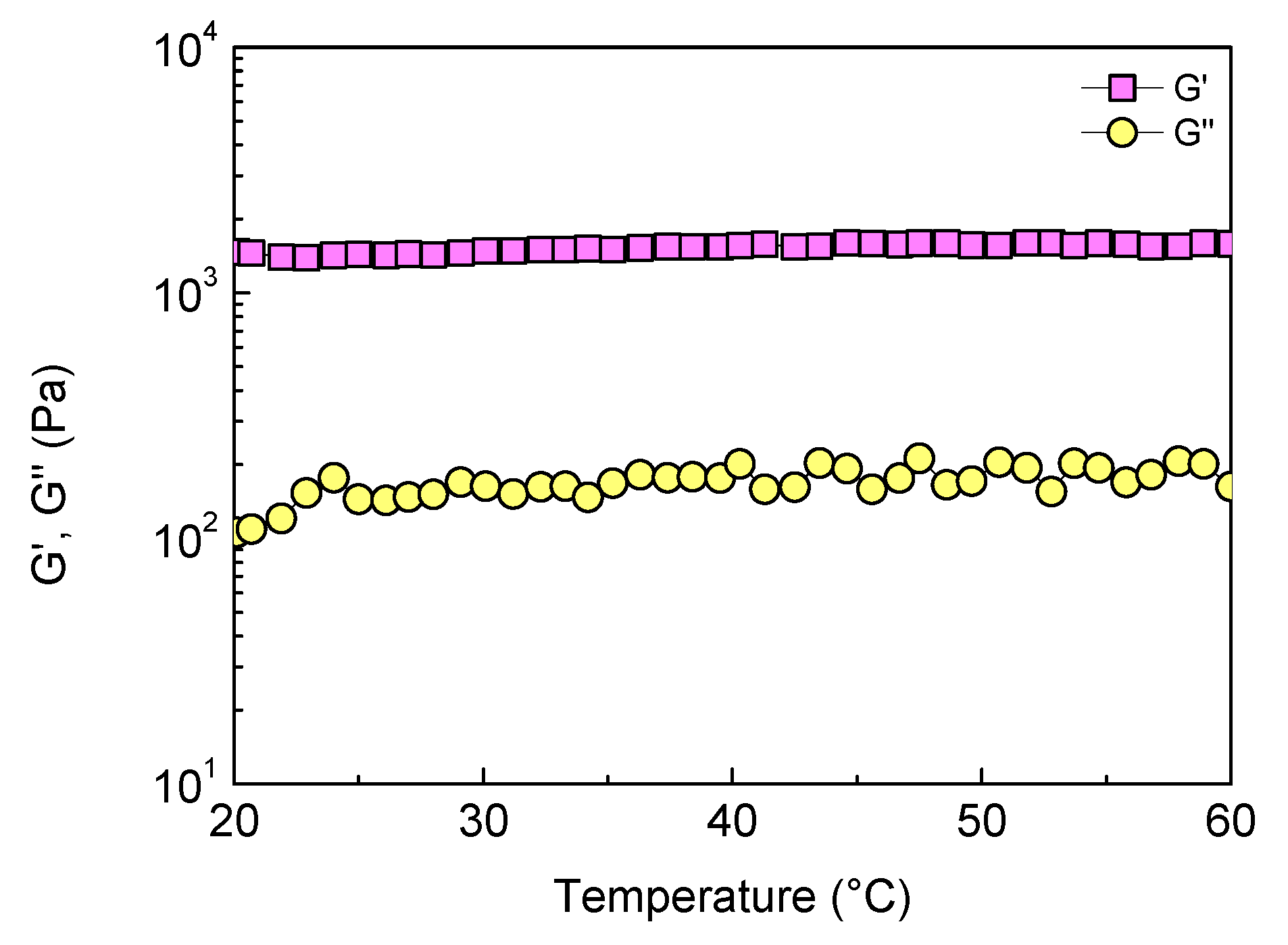
2.2. Temperature Effect
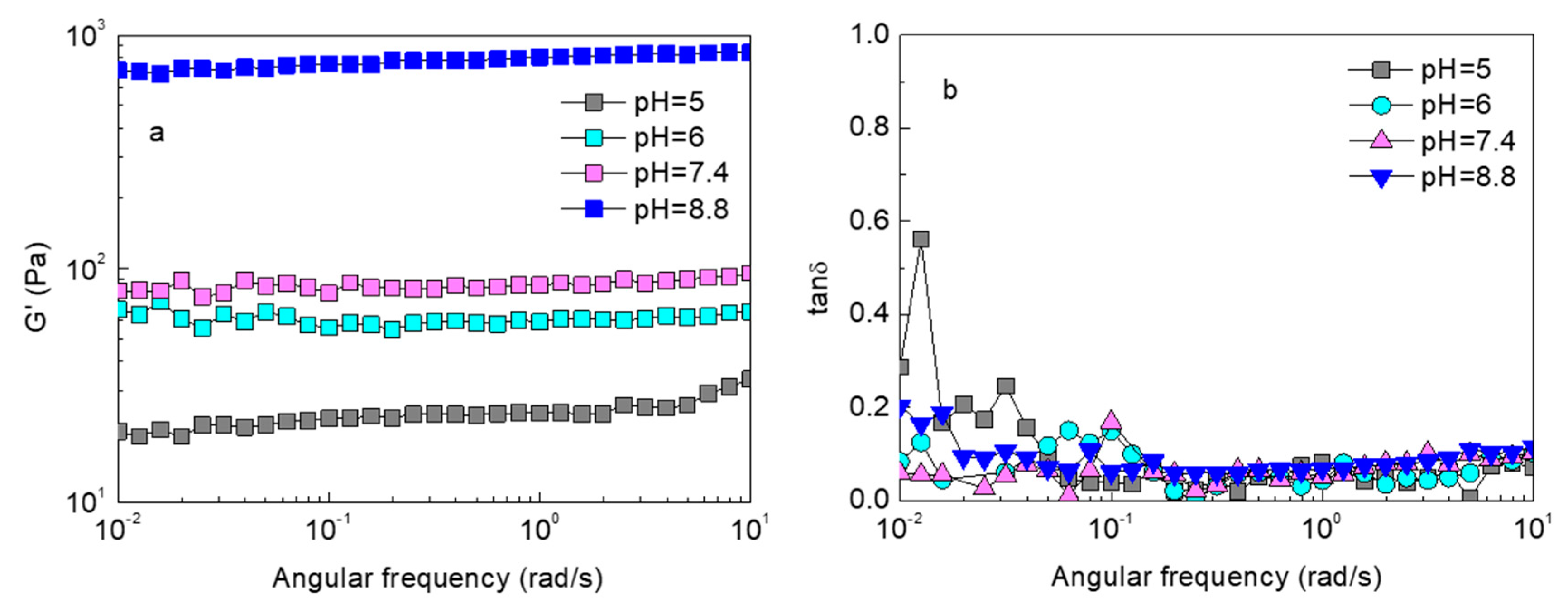
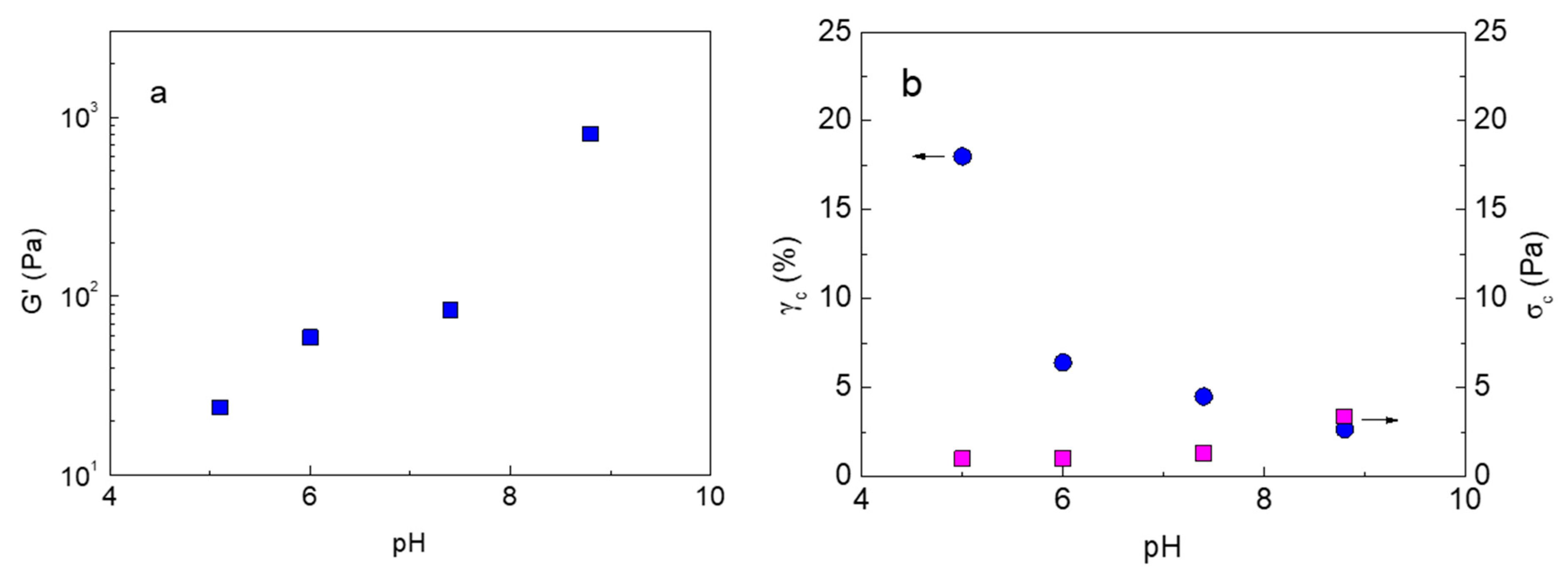
2.3. pH Responsiveness
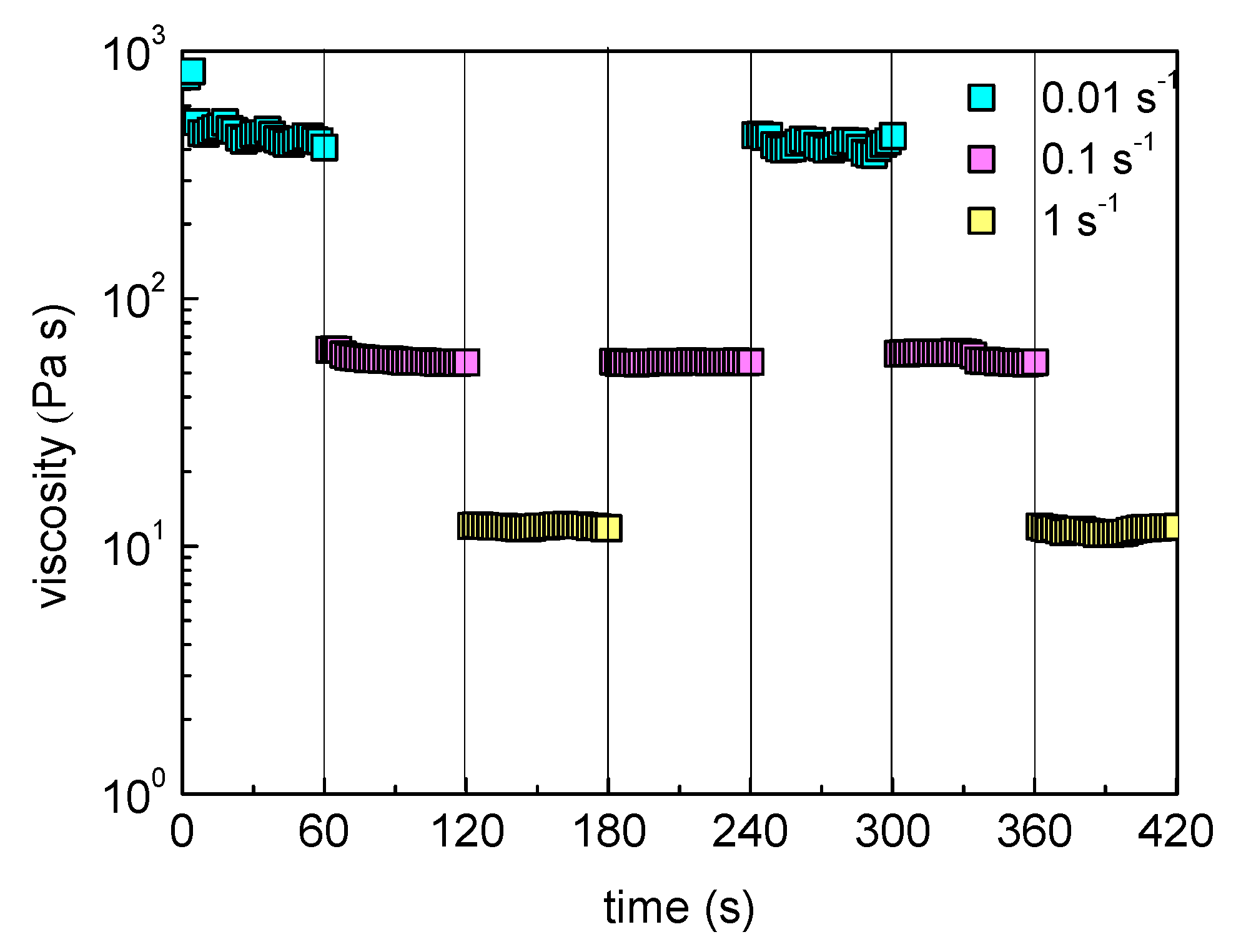
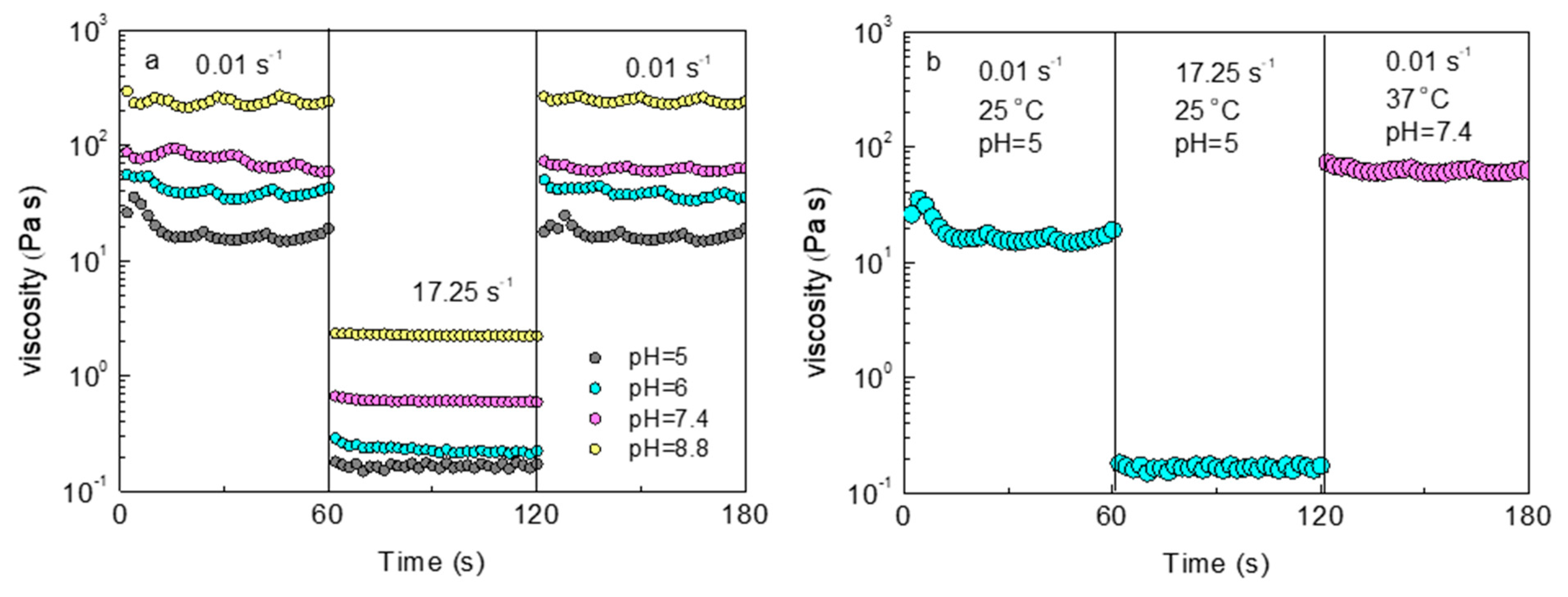
2.4. Shear Responsiveness and Self-Healing
2.5. Hydrogel after Deprotection of P(b-LL)
3. Conclusions
4. Materials and Methods
4.1. Materials
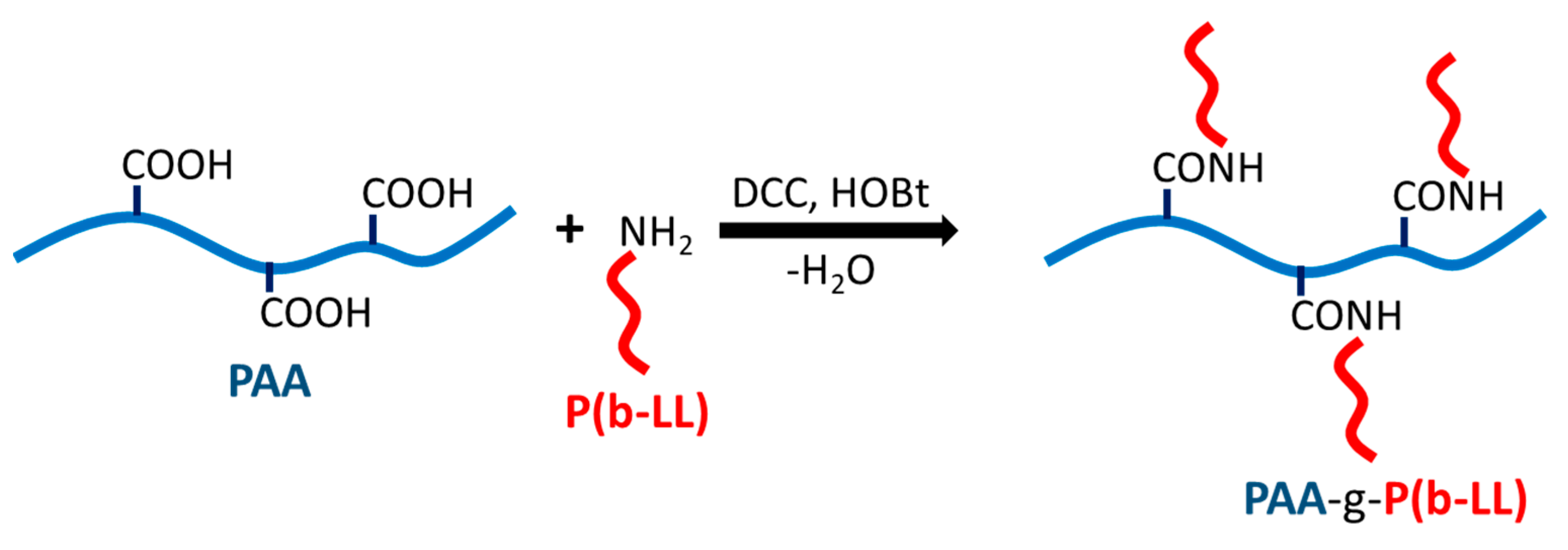
4.2. Synthesis and Characterization of PAA-g-P(b-LL)
4.3. Preparation of Samples for 1H-NMR Spectroscopy
4.4. Hydrogel Sample Preparation
4.5. Rheology
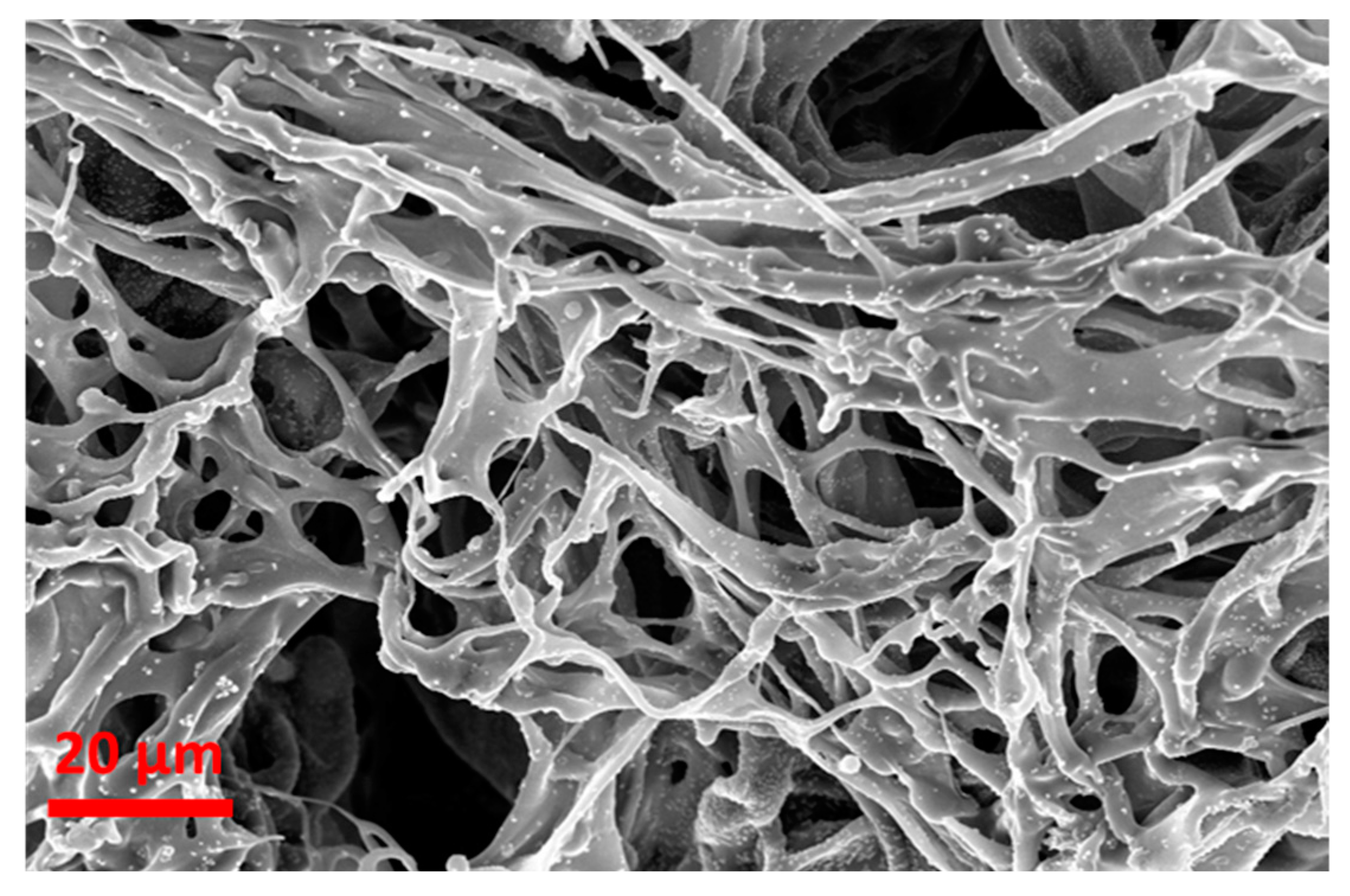
4.6. Scanning Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.P.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Iliopoulos, I.; Audebert, R. Viscometric behaviour of hydrophobically modified poly(sodium acrylate). Polym. Bull. 1988, 20, 577–582. [Google Scholar] [CrossRef]
- Xu, C.; Kopeček, J. Self-assembling hydrogels. Polym. Bull. 2007, 58, 53–63. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, T.; An, L.; Sun, Z.; Tong, T. Conformational study on sol-gel transition in telechelic polyelectrolytes. J. Phys. Chem. B 2010, 114, 3449–3456. [Google Scholar] [CrossRef]
- Ghelichi, M.; Qazvini, N.T. Self-organization of hydrophobic-capped triblock copolymers with polyelectrolyte midblock: A coarse-grained molecular dynamics simulation study. Soft Matter 2016, 12, 4611–4620. [Google Scholar] [CrossRef]
- Chassenieux, C.; Tsitsilianis, C. Recent trends on pH/thermo-responsive self-assembling hydrogels: From polyions to peptide-based polymeric gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef]
- Tsitsilianis, C. Self-assembling Hydrogels from pH-Responsive Ionic Block Copolymers. In Hydrogels; Gels Horizons: From Science to Smart Materials; Thakur, V.K., Thakur, M.K., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Nicolai, T.; Colombani, O.; Chassenieux, C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter 2010, 6, 3111–3118. [Google Scholar] [CrossRef]
- Tsitsilianis, C.; Iliopoulos, I.; Ducouret, G. An associative polyelectrolyte end-capped with short polystyrene chains. Synthesis and rheological behavior. Macromolecules 2000, 33, 2936–2943. [Google Scholar] [CrossRef]
- Katsampas, I.; Roiter, Y.; Minko, S.; Tsitsilianis, C. Multifunctional stimuli responsive ABC terpolymers: From 3-compartment micelles to 3-dimentional Network. Macromol. Rapid Commun. 2005, 26, 1371–1376. [Google Scholar] [CrossRef]
- Hietala, S.; Monomen, P.; Strandman, S.; Järvi, P.; Torkkeli, M.; Jankova, K.; Hvilsted, S.; Tenhu, H. Synthesis and rheological properties of an associative star polymers in aqueous solutions. Polymer 2009, 48, 4087–4096. [Google Scholar] [CrossRef]
- Hourdet, D.; L’Alloret, F.; Audebert, R. Reversible thermothickening of aqueous polymer solutions. Polymer 1994, 35, 2624–2630. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Tsitsilianis, C. Responsive polymer hydrogels: Physical gelling through polyion complexation. Gels 2017, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Tsitsilianis, C.; Katsampas, I.; Sfika, V. ABC Heterotelechelic Associative Polyelectrolytes. Rheological Behavior in Aqueous Media. Macromolecules 2000, 33, 9054–9059. [Google Scholar] [CrossRef]
- Stavrouli, N.; Aubry, T.; Tsitsilianis, C. Rheological properties of ABA telechelic polyelectrolyte and ABA polyampholyte reversible hydrogels: A comparative study. Polymer 2008, 49, 1249–1256. [Google Scholar] [CrossRef]
- Hietala, S.; Strandman, S.; Järvi, P.; Torkkeli, M.; Jankova, K.; Hvilsted, S.; Tenhu, H. Rheological properties of associative star polymers in aqueous solutions: Effect of hydrophobe length and polymer topology. Macromolecules 2009, 42, 1726–1732. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Song, J.-Y.; Lee, E.-J.; Park, S.-K. Rheological properties and microstructures of Carbopol gel network system. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Di Giuseppe, E.; Corbi, F.; Funiciello, F.; Massmeyer, A.; Santimano, T.N.; Rosenaub, M.; Davaille, A. Characterization of Carbopol hydrogel rheology for experimental tectonics and geodynamics. Tectonophysics 2015, 642, 29–45. [Google Scholar] [CrossRef]
- Bossard, F.; Aubry, T.; Gotzamanis, G.T.; Tsitsilianis, C. pH-Tunable rheological properties of a telechelic cationic polyelectrolyte reversible hydrogel. Soft Matter 2006, 2, 510–516. [Google Scholar] [CrossRef]
- Popescu, M.-T.; Tsitsilianis, C.; Papadakis, C.M.; Adelsberger, J.; Balog, S.; Busch, P.; Hadjiantoniou, N.A.; Patrickios, C.S. Stimuli-responsive Amphiphilic Polyelectrolyte Heptablock Copolymer Physical Hydrogels: An Unusual pH-response. Macromolecules 2012, 45, 3523–3530. [Google Scholar] [CrossRef]
- Taktak, F.F.; Butun, V. Synthesis and Physical Gels of pH- and Thermo-responsive Tertiary Amine Methacrylate based ABA Triblock Copolymers and Drug Release Studies. Polymer 2010, 51, 3618–3626. [Google Scholar] [CrossRef]
- Jung, F.A.; Schweins, R.; Rikkou-Kalourkoti, M.; Patrickios, C.S.; Papadakis, C.M.; Tsitsilianis, C. Effect of pH on the Dynamics and Structure of Thermoresponsive Telechelic Polyelectrolyte Networks: Impact on Hydrogel Injectability. ACS Appl. Polym. Mater. 2021, 3, 819–829. [Google Scholar]
- Lamch, Ł.; Ronka, S.; Moszyńska, I.; Warszyński, P.; Wilk, K.A. Hydrophobically Functionalized Poly(Acrylic Acid) Comprising the Ester-Type Labile Spacer: Synthesis and Self-Organization in Water. Polymers 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Colombani, O.; Lejeune, E.; Charbonneau, C.; Chassenieux, C.; Nicolai, T. Ionization Of Amphiphilic Acidic Block Copolymers. J. Phys. Chem. B 2012, 116, 7560–7565. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning injectable hydrogels. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Wu, M.; Chen, J.; Huang, W.; Yan, B.; Peng, Q.; Liu, J.; Chen, L.; Zeng, H. Injectable and Self-Healing Nanocomposite Hydrogels with Ultrasensitive pH-Responsiveness and Tunable Mechanical Properties: Implications for Controlled Drug Delivery. Biomacromolecules 2020, 21, 2409–2420. [Google Scholar] [CrossRef]
- Tsitsilianis, C.; Serras, G.; Ko, C.-H.; Jung, F.; Papadakis, C.-M.; Rikkou-Kalourkoti, M.; Patrickios, C.S.; Schweins, R.; Chassenieux, C. Thermoresponsive Hydrogels Based on Telechelic Polyelectrolytes: From Dynamic to “Frozen” Networks. Macromolecules 2018, 51, 2169–2179. [Google Scholar] [CrossRef]
- Panzade, P.; Puranik, P.K. Carbopol Polymers: A Versatile Polymer for Pharmaceutical Applications. Res. J. Pharm. Technol. 2010, 3, 672–675. [Google Scholar]
- Stamou, A.; Iatrou, H.; Tsitsilianis, C. NIPAm-based Modification of Poly(L-lysine): A pH-dependent LCST-type Thermoresponsive biodegradable polymer. Polymers 2022, 14, 802. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Hourdet, D. Synthesis and thermoassociative properties in aqueous solution of graft copolymers containing poly(N-isopropylacrylamide) side chains. Polymer 1999, 40, 4941–4951. [Google Scholar] [CrossRef]

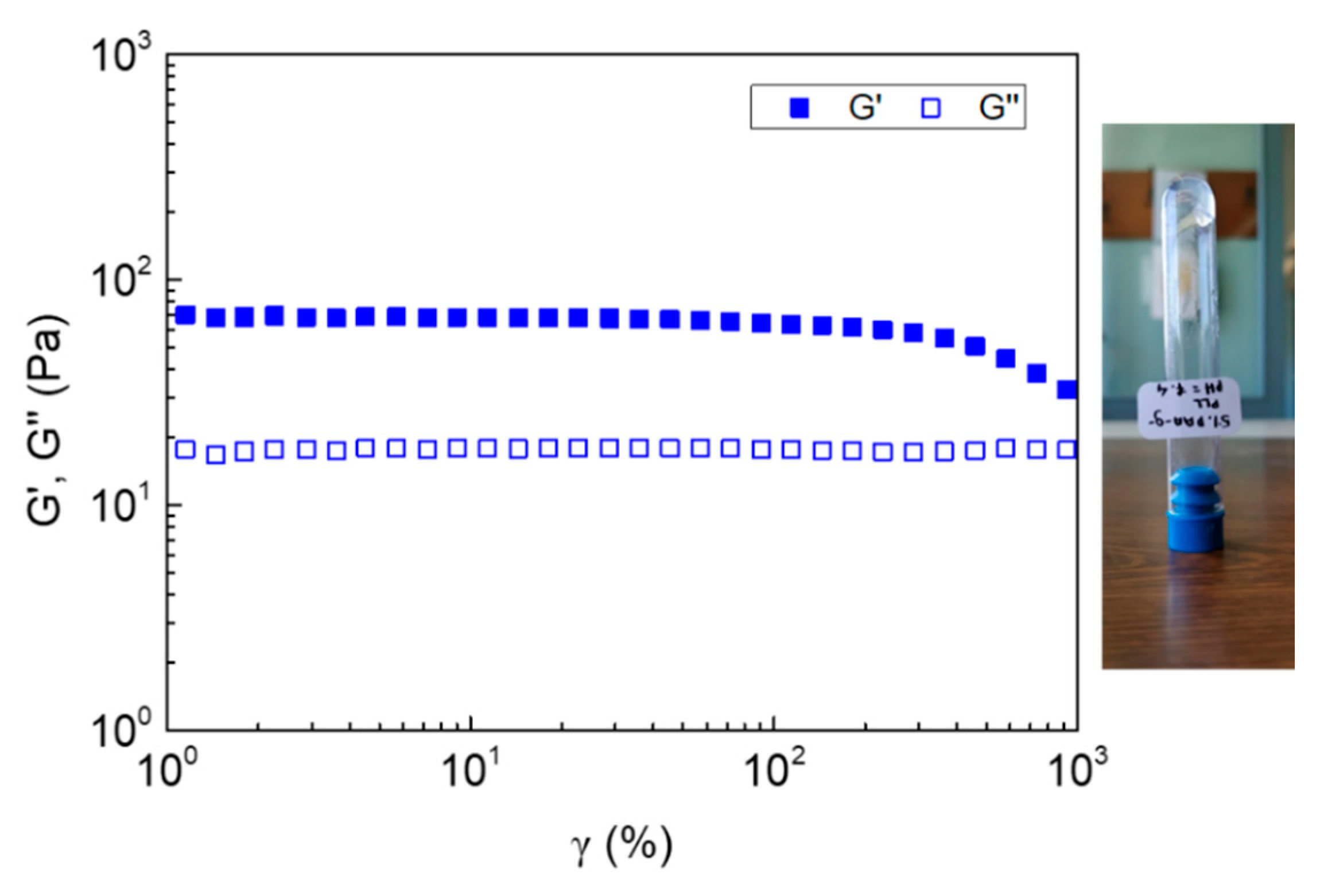

| Sample | Concentration (wt%) | pH |
|---|---|---|
| S1 | 1.2 | 8.8 |
| S2 | 1 | 8.8 |
| S3 | 0.74 | 8.8 |
| S4 | 0.5 | 8.8 |
| S5 | 0.25 | 8.8 |
| S6 | 0.5 | 7.4 |
| S7 | 0.5 | 6 |
| S8 | 0.5 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karga, M.-E.; Kargaki, M.-E.; Iatrou, H.; Tsitsilianis, C. pH-Responsive, Thermo-Resistant Poly(Acrylic Acid)-g-Poly(boc-L-Lysine) Hydrogel with Shear-Induced Injectability. Gels 2022, 8, 817. https://doi.org/10.3390/gels8120817
Karga M-E, Kargaki M-E, Iatrou H, Tsitsilianis C. pH-Responsive, Thermo-Resistant Poly(Acrylic Acid)-g-Poly(boc-L-Lysine) Hydrogel with Shear-Induced Injectability. Gels. 2022; 8(12):817. https://doi.org/10.3390/gels8120817
Chicago/Turabian StyleKarga, Maria-Eleni, Maria-Eleni Kargaki, Hermis Iatrou, and Constantinos Tsitsilianis. 2022. "pH-Responsive, Thermo-Resistant Poly(Acrylic Acid)-g-Poly(boc-L-Lysine) Hydrogel with Shear-Induced Injectability" Gels 8, no. 12: 817. https://doi.org/10.3390/gels8120817
APA StyleKarga, M.-E., Kargaki, M.-E., Iatrou, H., & Tsitsilianis, C. (2022). pH-Responsive, Thermo-Resistant Poly(Acrylic Acid)-g-Poly(boc-L-Lysine) Hydrogel with Shear-Induced Injectability. Gels, 8(12), 817. https://doi.org/10.3390/gels8120817











