Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction
Abstract
1. Introduction
2. Results and Discussion
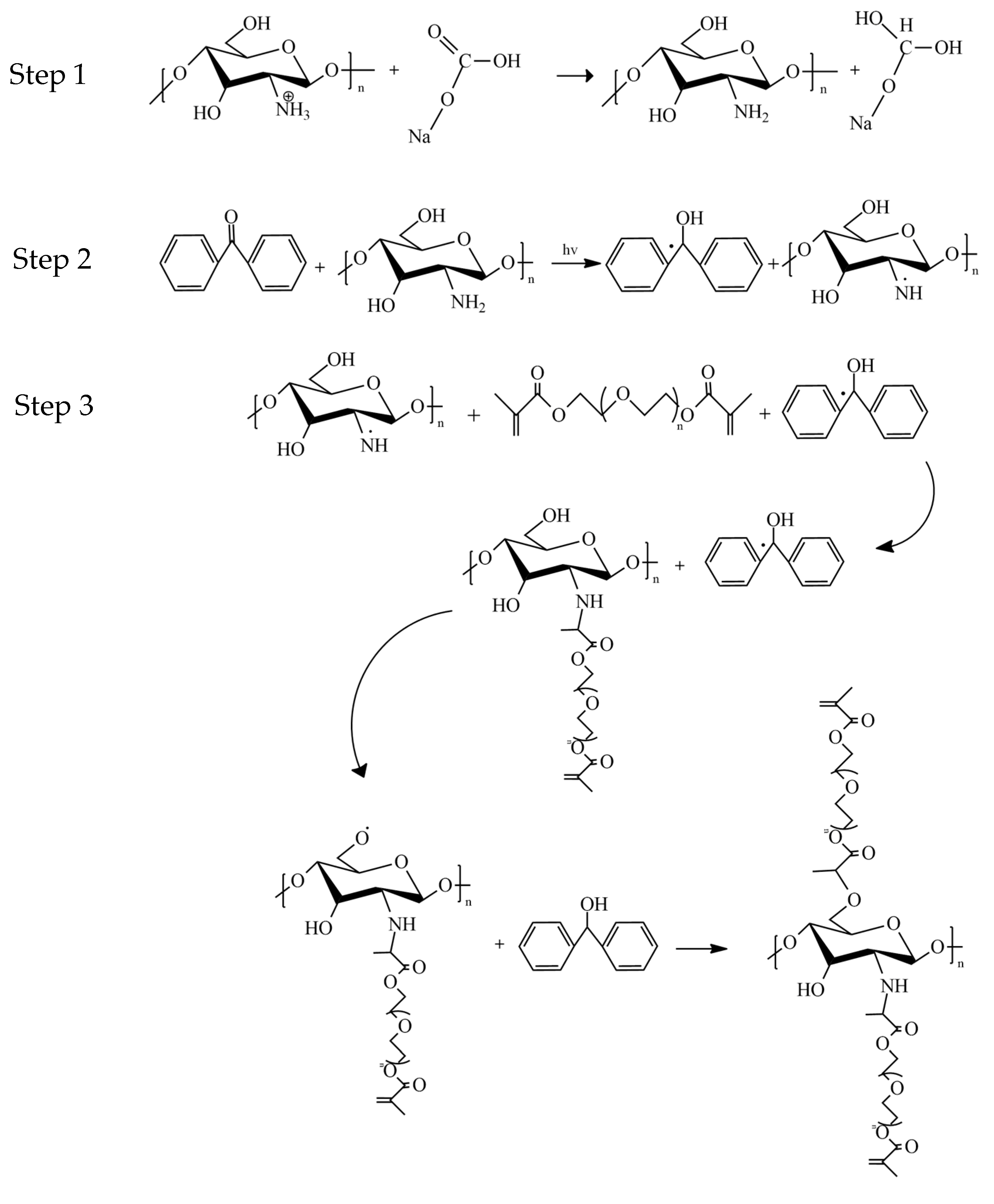
2.1. Analysis of Crosslinkage Formation Following UV-Photocrosslinking Procedure
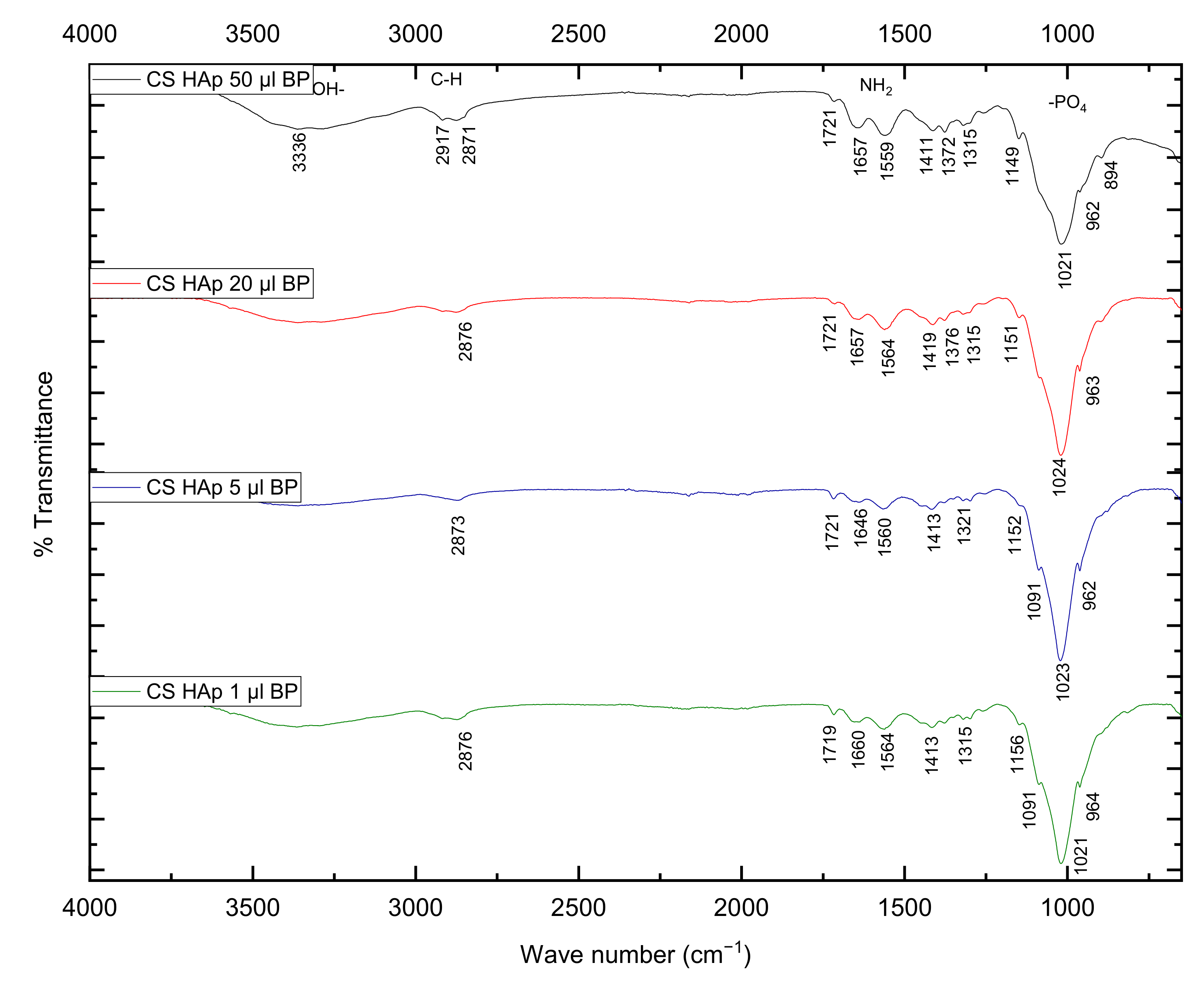
2.2. Fourier-Transform Infrared Spectroscopy Analysis
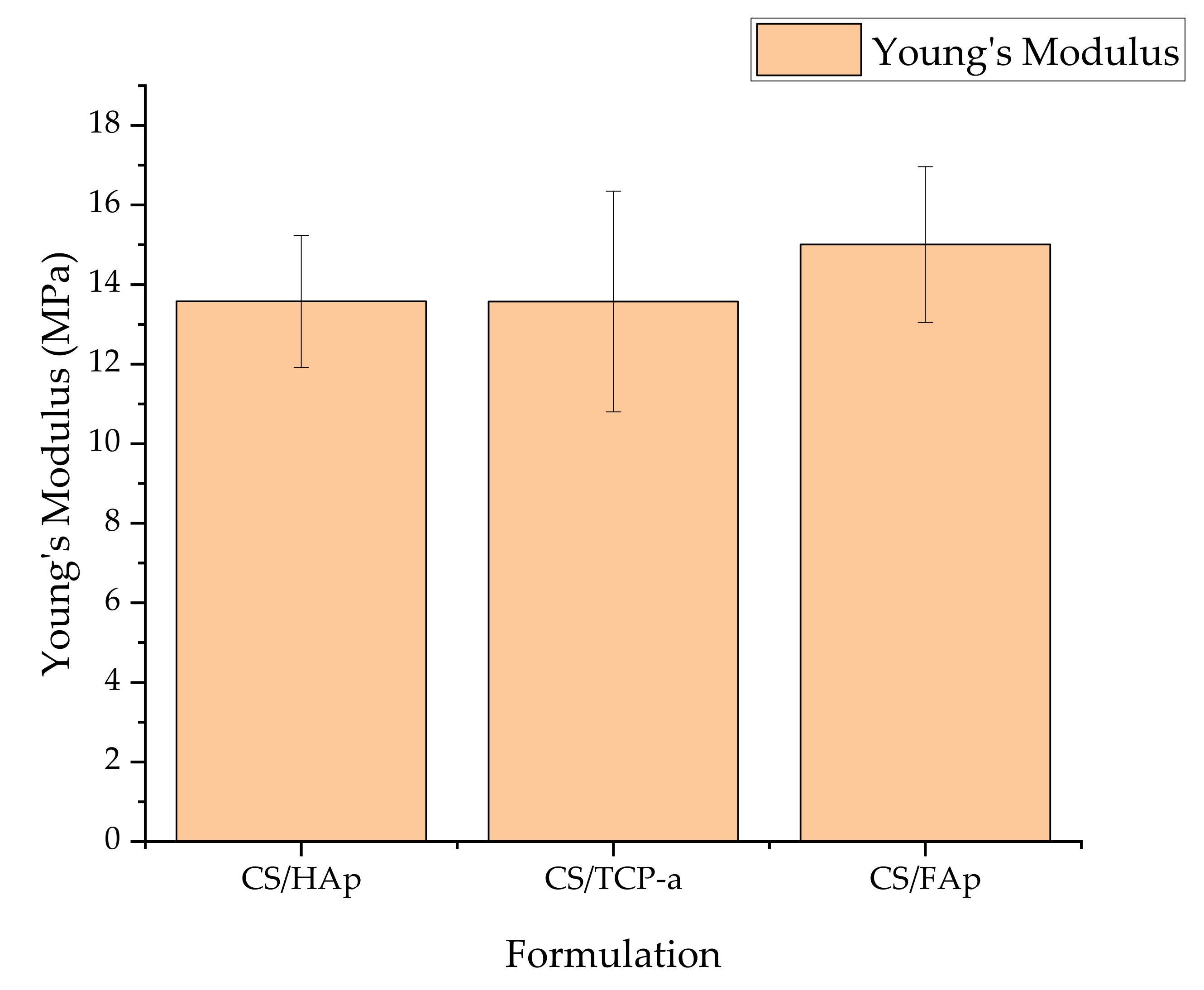
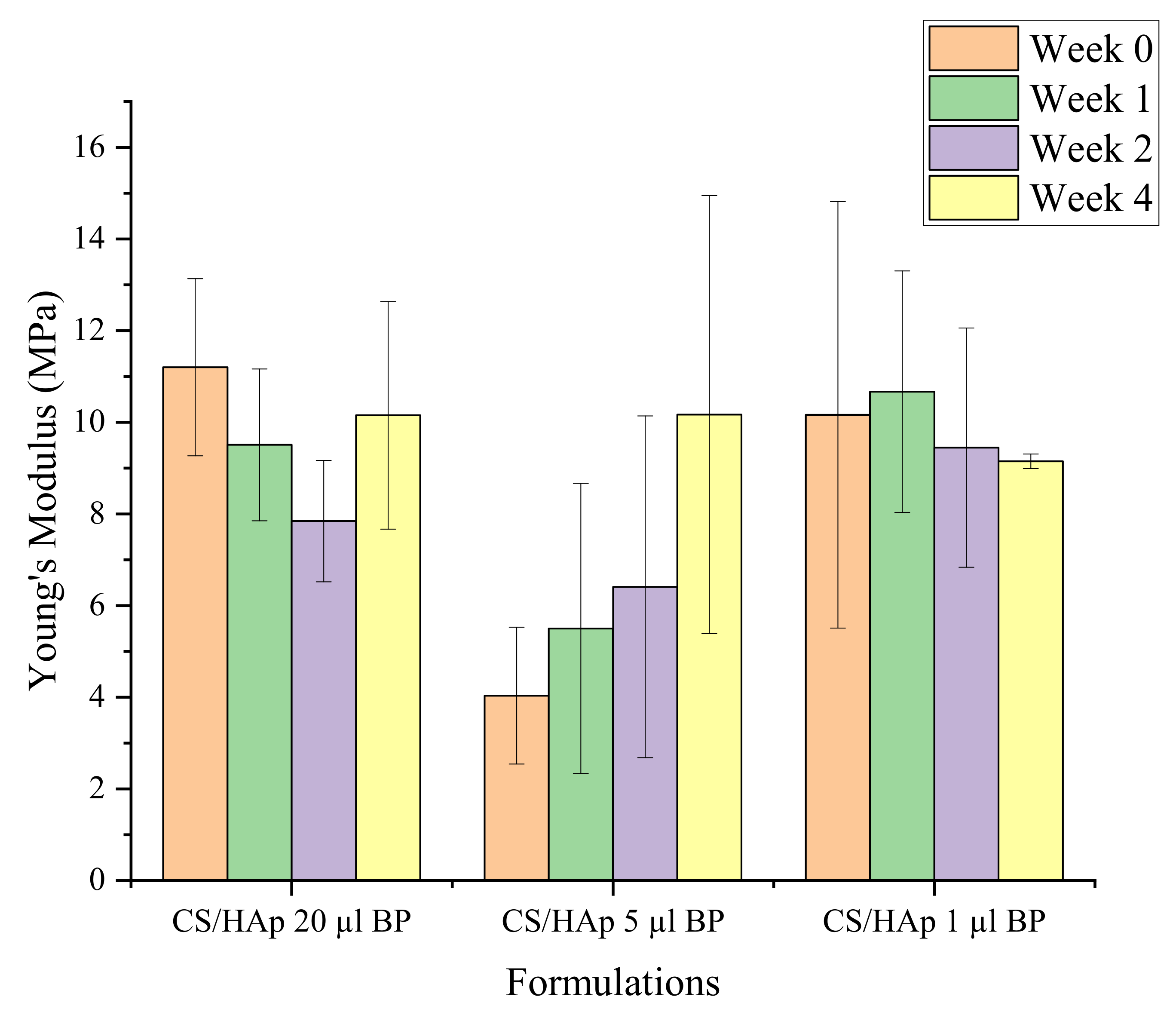
2.3. Mechanical Assessment
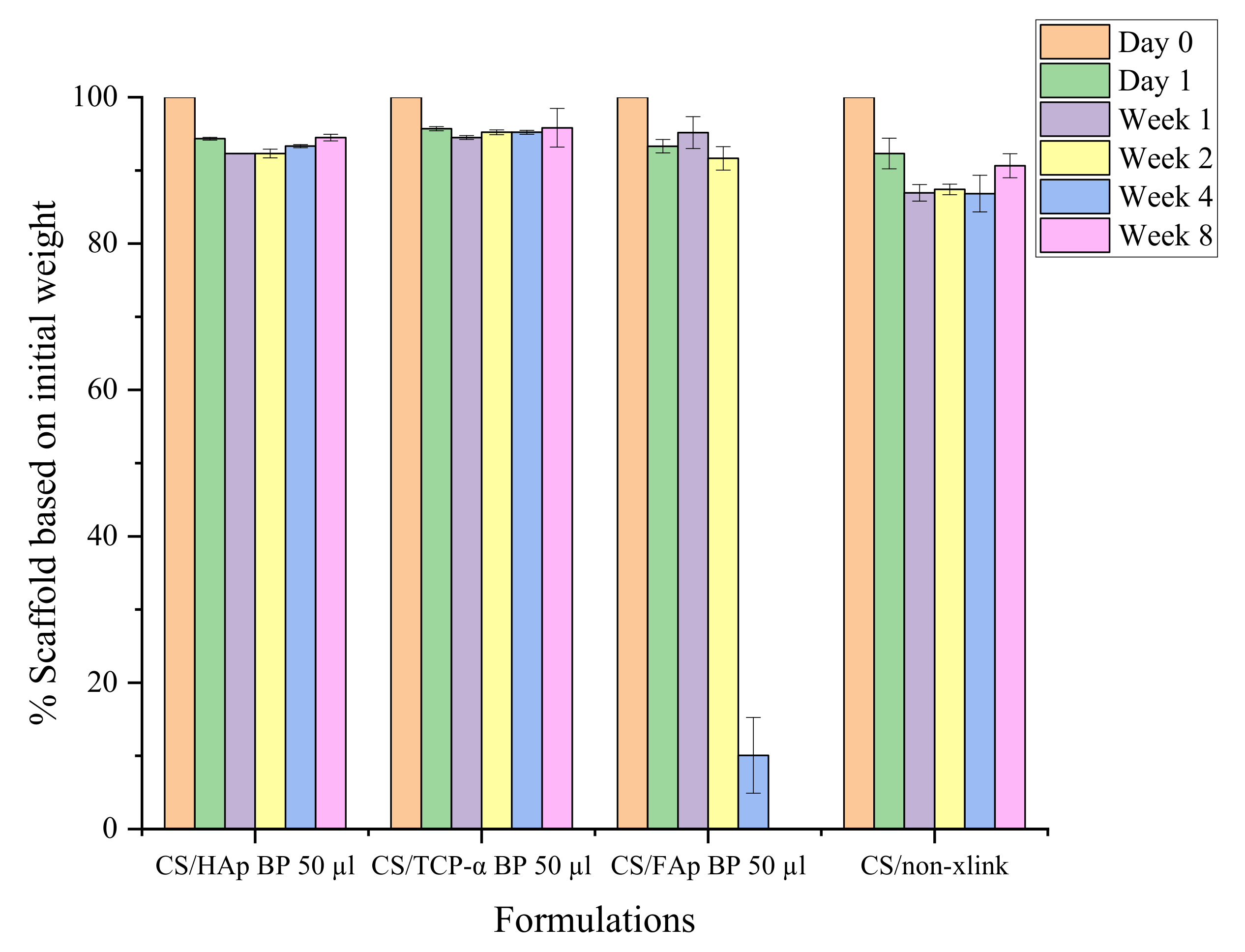
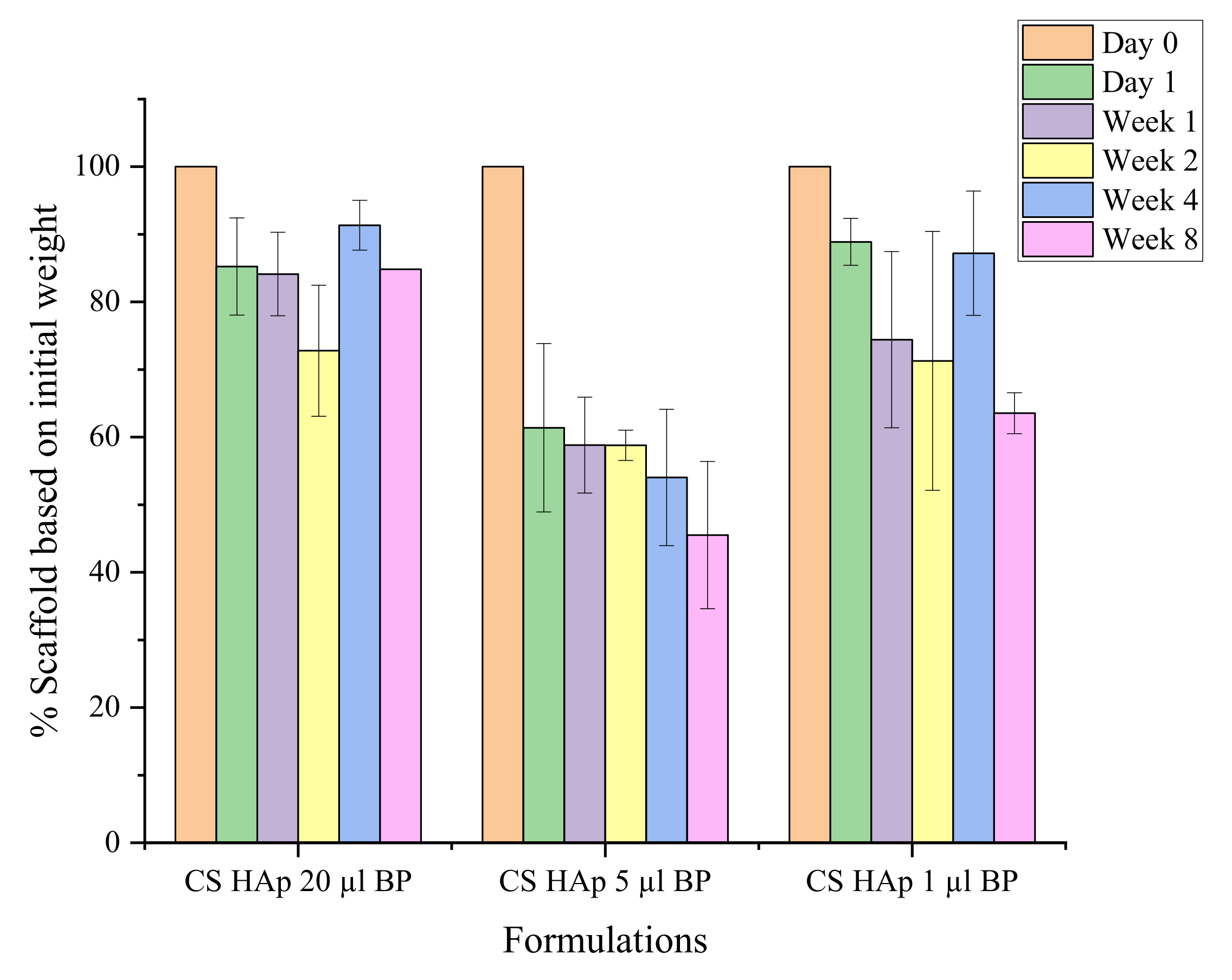
2.4. In Vitro Biodegradation Assessment
Mechanical Stability during Biodegradation
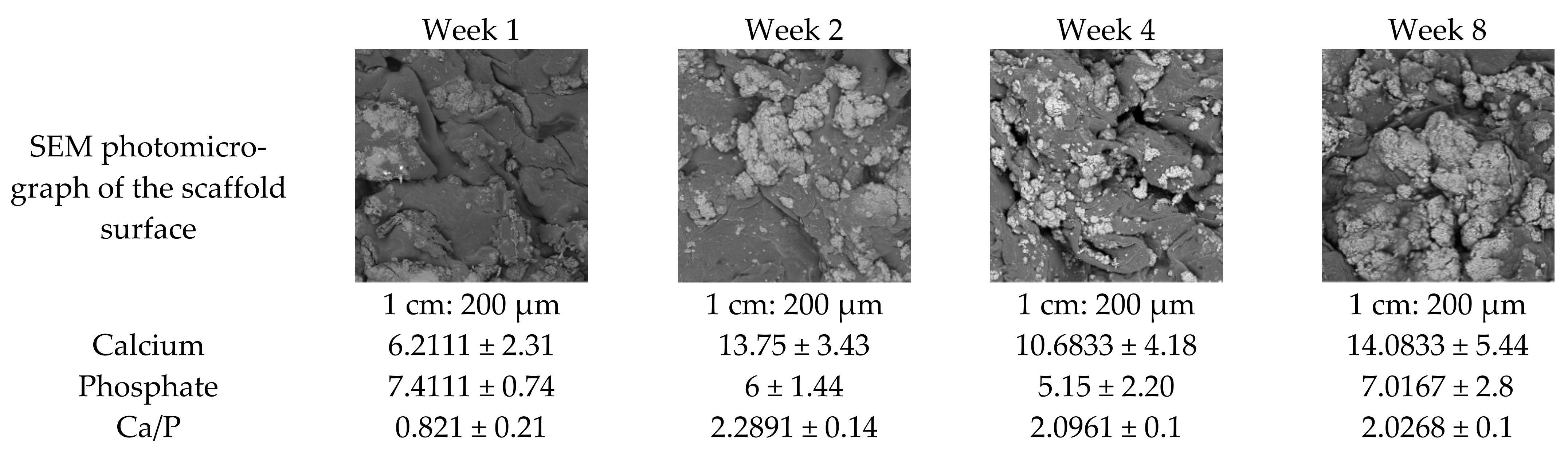
2.5. Scanning Electron Microscopy and Energy Dispersive X-ray Spectrometry
3. Conclusions
4. Materials and Methods
4.1. Fabrication of Chitosan-Based Bone Regeneration Scaffold
4.1.1. Incorporation of Various Bioceramic Compositions
4.1.2. Modification of Chitosan-Based Scaffold Grafting Properties through Various Photoinitiator Composition
4.2. Crosslinking Test in 1% Acetic Acid
4.3. Swelling Behaviour
4.4. Fourier-Transform Infrared Spectroscopy (FTIR)
4.5. Compression Test
4.6. Degradation Test in Simulated Body Fluid
4.7. Scanning Electron Microscopy and Energy Dispersive X-ray Spectrometry (SEM-EDX)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Solomon, L.; Warwick, D.J.; Nayagam, S. Fractures and Joint Injury. In Apley’s Concise System of Orthopaedics and Fractures, 3rd ed.; Hodder Education: London, UK, 2005; pp. 266–280. [Google Scholar]
- Rupp, M.; Biehl, C.; Budak, M.; Thormann, U.; Heiss, C.; Alt, V. Diaphyseal long bone nonunions—Types, aetiology, economics, and treatment recommendations. Int. Orthop. 2018, 42, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Devine, D.M.; Hoctor, E.; Hayes, J.S.; Sheehan, E.; Evans, C.H. Extended release of proteins following encapsulation in hydroxyapatite/chitosan composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2018, 84, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zheng, F.; Zhao, W.; Zhang, Y.; Yuan, J.; Zhang, B.; Li, L. Prevalence and influencing factors of nonunion in patients with tibial fracture: Systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 377. [Google Scholar] [CrossRef]
- Ercal, P.; Pekozer, G.G. A current overview of scaffold-based bone regeneration strategies with dental stem cells. Adv. Exp. Med. Biol. 2020, 1288, 61–85. [Google Scholar]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Svedbom, A.; Hernlund, E.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 137. [Google Scholar] [CrossRef]
- Bone Nonunion. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554385/ (accessed on 25 May 2022).
- Nicholson, J.A.; Makaram, N.; Simpson, A.H.R.W.; Keating, J.F. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Yang, L.; Wang, K.; Sun, T.; Ji, Y.; Liu, L.; Yu, W.; Qu, Y.; Wang, J.; et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 211–221. [Google Scholar] [CrossRef]
- Elakkiya, S.; Ramesh, A.S.; Prabhu, K. Systematic analysis on the efficacy of bone enhancement methods used for success in dental implants. J. Indian Prosthodont. Soc. 2017, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhuo, Y.; Sun, Y.; Fu, H.; Han, Z. Microstructure design and degradation performance in vitro of three-dimensional printed bioscaffold for bone tissue engineering. Adv. Mech. Eng. 2019, 11, 1687814019883784. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Fourie, J.; Taute, F.; du Preez, L.; de Beer, D. Chitosan Composite Biomaterials for Bone Tissue Engineering—A Review. Regen. Eng. Transl. Med. 2022, 8, 1–21. [Google Scholar] [CrossRef]
- Polo-Corrales, J.; Latorre-Esteves, L.; Ramirez-Vick, M. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2013, 31, 1713–1723. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; Qin, K.H.; Hu, D.A.; Wang, E.J.; Li, A.J.; et al. Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef]
- López-Lacomba, J.L.; García-Cantalejo, J.M.; Casado, J.V.S.; Abarrategi, A.; Magaña, V.C.; Ramos, V. Use of rhBMP-2 Activated Chitosan Films to Improve Osseointegration. Biomacromolecules 2006, 7, 792–798. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Wei, Y.; Leach, J.B. Protein–Hydrogel Interactions in Tissue Engineering: Mechanisms and Applications. Tissue Eng. Part B Rev. 2013, 19, 160–171. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, W.; Lee, P.; Wang, Y.; Li, J.; Kumbar, S.G.; Yu, X. Polymer-ceramic spiral structured scaffolds for bone tissue engineering: Effect of hydroxyapatite composition on human fetal osteoblasts. PLoS ONE 2014, 9, e85871. [Google Scholar]
- Choi, S.M.; Chaudhry, P.; Zo, S.M.; Han, S.S. Advances in Protein-Based Materials: From Origin to Novel Biomaterials. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; Volume 1078, pp. 161–210. [Google Scholar]
- Dixon, D.T.; Gomillion, C.T. Conductive scaffolds for bone tissue engineering: Current state and future outlook. J. Funct. Biomater. 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Giannoudis, P.V. Enhancement of fracture healing with the diamond concept: The role of the biological chamber. Injury 2011, 42, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Henkel, J. Bone Tissue Engineering in Two Preclinical Ovine Animal Models. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2017. [Google Scholar]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial bone grafts claimed as an alternative to autografts: Current trends for clinical applications in orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Raftery, R.; O’Brien, F.J.; Cryan, S.A. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng.—Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic peptides and attachment methods determine tissue regeneration in modified bone graft substitutes. J. Funct. Biomater. 2021, 12, 22. [Google Scholar] [CrossRef]
- Durham, E.L.; Kishinchand, R.; Grey, Z.J.; Cray, J.J. rhBMP2 alone does not induce macrophage polarization towards an increased inflammatory response. Mol. Immunol. 2020, 117, 94–100. [Google Scholar] [CrossRef]
- Alves, A.; Wancket, L.; Metz, A. Current considerations in medical device pathology. In Biocompatibility and Performance of Medical Devices, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 491–543. [Google Scholar]
- Fournet, M.E.B.; Azaman, F.A.; Gunbay, S.; Chen, Y.Y.; Devine, D.M. Orthopedic 3D Printing in Orthopedic Medicine. In Polymer-Based Additive Manufaturing: Biomedical Application; Devine, D.M., Ed.; Springer: Cham, Switzerland, 2019; pp. 121–142. [Google Scholar]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and Evaluation of Gelatin-Chitosan-Nanobioglass 3D Porous Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2016, 2016, 9825659. [Google Scholar] [CrossRef]
- Khouri, J.; Penlidis, A.; Moresoli, C. Viscoelastic properties of crosslinked chitosan films. Processes 2019, 7, 157. [Google Scholar] [CrossRef]
- Lan Levengood, S.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B Mater. Biol. Med. 2015, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef] [PubMed]
- Yadav, L.R.; Chandran, S.V.; Lavanya, K.; Selvamurugan, N. Chitosan-based 3D-printed scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 108, 197–211. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan grafted/cross-linked with biodegradable polymers: A review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef]
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.A.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable Chitosan-graft-Poly(L-lactide) Copolymers for Bone Tissue Engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Shi, B.; Cheng, X. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials 2007, 28, 1515–1522. [Google Scholar] [CrossRef]
- Meeremans, M.; Van Damme, L.; De Spiegelaere, W.; Van Vlierberghe, S.; De Schauwer, C. Equine tenocyte seeding on gelatin hydrogels improves elongated morphology. Polymers 2021, 13, 747. [Google Scholar] [CrossRef]
- Nokoorani, Y.D.; Shamloo, A.; Bahadoran, M.; Moravvej, H. Fabrication and characterization of scaffolds containing different amounts of allantoin for skin tissue engineering. Sci. Rep. 2021, 11, 16164. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-chemical characterization and biological tests of collagen/silk fibroin/chitosan scaffolds cross-linked by dialdehyde starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Kurniati, M.; Nuraini, I.; Winarti, C. Investigation of Swelling Ratio and Textures Analysis of Acrylamide-Nanocellulose Corncobs Hydrogel. J. Phys. Conf. Ser. 2021, 1805, 012036. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Amir, L.R.; Suhardi, P.; Abas, B. Scaffold degradation during bone tissue reconstruction in Macaca nemestrina mandible. Interv. Med. Appl. Sci. 2016, 8, 77–81. [Google Scholar] [PubMed]
- Oustadi, F.; Imani, R.; Haghbin Nazarpak, M.; Sharifi, A.M. Genipin-crosslinked gelatin hydrogel incorporated with PLLA-nanocylinders as a bone scaffold: Synthesis, characterization, and mechanical properties evaluation. Polym. Adv. Technol. 2020, 31, 1783–1792. [Google Scholar] [CrossRef]
- Qiu, Y. Chitosan Derivatives for Tissue Engineering. Ph.D. Thesis, Clemson University, Clemson, UK, 2008. [Google Scholar]
- Taschner, R.; Gauss, P.; Knaack, P.; Liska, R. Biocompatible Photoinitiators Based on Poly-α-ketoesters. J. Polym. Sci. 2019, 58, 242–253. [Google Scholar] [CrossRef]
- Surowiec, R.K.; Allen, M.R.; Wallace, J.M. Bone hydration: How we can evaluate it, what can it tell us, and is it an effective therapeutic target? Bone Rep. 2022, 16, 101161. [Google Scholar] [CrossRef]
- Granke, M.; Does, M.D.; Nyman, J.S. The Role of Water Compartments in the Material Properties of Cortical Bone. Calcif. Tissue Int. 2015, 97, 292–307. [Google Scholar] [CrossRef]
- Tao, L.; Zhonglong, L.; Ming, X.; Zezheng, Y.; Zhiyuan, L.; Xiaojun, Z.; Jinwu, W. In vitro and in vivo studies of a gelatin/carboxymethyl chitosan/LAPONITE® composite scaffold for bone tissue engineering. RSC Adv. 2017, 7, 54100–54110. [Google Scholar] [CrossRef]
- Nga, N.K.; Thanh Tam, L.T.; Ha, N.T.; Hung Viet, P.; Huy, T.Q. Enhanced biomineralization and protein adsorption capacity of 3D chitosan/hydroxyapatite biomimetic scaffolds applied for bone-tissue engineering. RSC Adv. 2020, 10, 43045–43057. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; El-Betany, A.; Menzel, H.; Chen, X. Influence of photoinitiator concentration and irradiation time on the crosslinking performance of visible-light activated pullulan-HEMA hydrogels. Int. J. Biol. Macromol. 2018, 120, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Han, J.; Mo, J.; Dong, P.; Zhuo, Y.; Feng, Y. Biocompatiable silk fibroin/carboxymethyl chitosan/strontium substituted hydroxyapatite/cellulose nanocrystal composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 136, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Hasirci, V.; Huri, P.Y.; Tanir, T.E.; Eke, G.; Hasirci, N. Polymer fundamentals: Polymer synthesis. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 478–506. [Google Scholar]
- Riga, E.K.; Saar, J.S.; Erath, R.; Hechenbichler, M.; Lienkamp, K. On the limits of benzophenone as cross-linker for surface-attached polymer hydrogels. Polymers 2017, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-collagen-hydroxyapatite membranes for tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef]
- Liao, Y.; Li, H.; Shu, R.; Chen, H.; Zhao, L.; Song, Z.; Zhou, W. Mesoporous Hydroxyapatite/Chitosan Loaded with Recombinant-Human Amelogenin Could Enhance Antibacterial Effect and Promote Periodontal Regeneration. Front. Cell. Infect. Microbiol. 2020, 10, 180. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccarides with use of IR spectra deconvolution. Biopolym.—Biospectrosc. Sect. 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Fern, H.W.; Salimi, M.N. Hydroxyapatite nanoparticles produced by direct precipitation method: Optimization and characterization studies. AIP Conf. Proc. 2021, 2339, 020215. [Google Scholar]
- Nazeer, M.A.; Yilgör, E.; Yilgör, I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, characterization, and antimicrobial activity of magnesium-doped hydroxyapatite suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Vargas, F.; Ruiz, J.; López, M.E. Solid-state synthesis of alpha tricalcium phosphate for cements used in biomedical applications. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 193–200. [Google Scholar] [CrossRef]
- Thaitalay, P.; Srakaew, N.L.O.; Rattanachan, S.T. Comparison among alpha-tricalcium phosphate synthesized by solid state reaction and wet chemical reaction for calcium phosphate cements. Chiang Mai J. Sci. 2018, 45, 2123–2131. [Google Scholar]
- Szurkowska, K.; Szeleszczuk, Ł.; Kolmas, J. Effects of synthesis conditions on the formation of Si-substituted alpha tricalcium phosphates. Int. J. Mol. Sci. 2020, 21, 9164. [Google Scholar] [CrossRef] [PubMed]
- Canillas, M.; De Lima, G.G.; Rodríguez, M.A.; Nugent, M.J.D.; Devine, D.M. Bioactive composites fabricated by freezing-thawing method for bone regeneration applications. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 761–773. [Google Scholar] [CrossRef]
- Sinusaite, L.; Antuzevics, A.; Popov, A.I.; Rogulis, U.; Misevicius, M.; Katelnikovas, A.; Kareiva, A.; Zarkov, A. Synthesis and luminescent properties of Mn-doped alpha-tricalcium phosphate. Ceram. Int. 2021, 47, 5335–5340. [Google Scholar] [CrossRef]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jozefaciuk, G.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Ginalska, G. Fluorapatite ceramics for bone tissue regeneration: Synthesis, characterization and assessment of biomedical potential. Mater. Sci. Eng. C 2020, 116, 111211. [Google Scholar] [CrossRef]
- Vidal, C.; Alves, P.; Alves, M.M.; Carmezim, M.J.; Fernandes, M.H.; Inácio, P.L.; Ferreira, F.B.; Santos, T.G.; Santos, C. Fabrication of a biodegradable and cytocompatible magnesium/nanohydroxyapatite/fluorapatite composite by upward friction stir processing for biomedical applications. J. Mech. Behav. Biomed. Mater. 2022, 129, 105137. [Google Scholar] [CrossRef]
- Anastasiou, A.D.; Nerantzaki, M.; Gounari, E.; Duggal, M.S.; Giannoudis, P.V.; Jha, A.; Bikiaris, D. Antibacterial properties and regenerative potential of Sr2+ and Ce3+ doped fluorapatites; a potential solution for peri-implantitis. Sci. Rep. 2019, 9, 14469. [Google Scholar] [CrossRef]
- Burke, G.; Cao, Z.; Devine, D.M.; Major, I. Preparation of biodegradable polyethylene glycol dimethacrylate hydrogels via thiol-ene chemistry. Polymers 2019, 11, 1339. [Google Scholar] [CrossRef]
- Kasgoz, H.; Heydarova, S. Styrene-PEG (600) DMA crosslinked polymers for absorption of oil derivatives. J. Macromol. Sci. Part A Pure Appl. Chem. 2011, 48, 556–561. [Google Scholar] [CrossRef]
- Maciejewska, M.; Rogulska, M.; Józwicki, M.; Głodowska, N. Synthesis and characterization of porous copolymers of 2-hydroxyethyl methacrylate with ethylene glycol dimethacrylate. Polym. Adv. Technol. 2021, 32, 2566–2575. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical behavior of bioactive poly(ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. Biomed. Eng. Online 2020, 19, 69. [Google Scholar] [CrossRef]
- Patel, P.P.; Buckley, C.; Taylor, B.L.; Sahyoun, C.C.; Patel, S.D.; Mont, A.J.; Mai, L.; Patel, S.; Freeman, J.W. Mechanical and biological evaluation of a hydroxyapatite-reinforced scaffold for bone regeneration. J. Biomed. Mater. Res.—Part A 2019, 107, 732–741. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kang, J.H. Mechanical properties of compact bone defined by the stress-strain curve measured using uniaxial tensile test: A concise review and practical guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jojczuk, M.; Lukasiewicz, P.; Nogalski, A.; Ginalska, G. Highly porous fluorapatite/β-1,3-glucan composite for bone tissue regeneration: Characterization and in vitro assessment of biomedical potential. Int. J. Mol. Sci. 2021, 22, 10414. [Google Scholar] [CrossRef]
- Choi, G.; Cha, H.J. Recent advances in the development of nature-derived photocrosslinkable biomaterials for 3D printing in tissue engineering. Biomater. Res. 2019, 23, 18. [Google Scholar] [CrossRef]
- Augat, P.; Hollensteiner, M.; von Rüden, C. The role of mechanical stimulation in the enhancement of bone healing. Injury 2021, 52, S78–S83. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Feng, Y.; Liu, Y.; Willie, B.M.; Yang, H. The combined effects of dynamization time and degree on bone healing. J. Orthop. Res. 2022, 40, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Evans, C.H.; Tetsworth, K. Reverse dynamisation: A modern perspective on stephan perren’s strain theory. Eur. Cells Mater. 2021, 41, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [CrossRef]
- Barcik, J.; Ernst, M.; Balligand, M.; Dlaska, C.E.; Drenchev, L.; Zeiter, S.; Epari, D.R.; Windolf, M. Short-term bone healing response to mechanical stimulation—A case series conducted on sheep. Biomedicines 2021, 9, 988. [Google Scholar] [CrossRef]
- Thompson, C.L.; Fu, S.; Knight, M.M.; Thorpe, S.D. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front. Bioeng. Biotechnol. 2020, 8, 602646. [Google Scholar] [CrossRef]
- Perren, S.M. Evolution of the internal fixation of long bone fractures. J. Bone Jt. Surg.—Ser. B 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Blackwood, K.A.; Bock, N.; Dargaville, T.R.; Ann Woodruff, M. Scaffolds for growth factor delivery as applied to bone tissue engineering. Int. J. Polym. Sci. 2012, 2012, 174942. [Google Scholar] [CrossRef]
- Mahmood, S.K.; Abdul Razak, I.S.; Ghaji, M.S.; Mohamed Yusof, L.; Mahmood, Z.K.; Rameli, M.A.B.P.; Zakaria, Z.A.B. In vivo evaluation of a novel nanocomposite porous 3D scaffold in a rabbit model: Histological analysis. Int. J. Nanomed. 2017, 12, 8587–8598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; De Sá, M.J.C.; Dalton, M.; Devine, D.M. Biodegradable medical implants. In Bioresorbable Polymers and Their Biomedical Applications, 1st ed.; Perale, G., Hilborn, J., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 17–46. [Google Scholar]
- De Mori, A.; Hafidh, M.; Mele, N.; Yusuf, R.; Cerri, G.; Gavini, E.; Tozzi, G.; Barbu, E.; Conconi, M.; Draheim, R.R.; et al. Sustained release from injectable composite gels loaded with silver nanowires designed to combat bacterial resistance in bone regeneration applications. Pharmaceutics 2019, 11, 116. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Petersen, J.; Fielding, G.; Banerjee, S.; Bose, S. ZnO, SiO2, and SrO doping in resorbable tricalcium phosphates: Influence on strength degradation, mechanical properties, and in vitro bone-cell material interactions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2203–2212. [Google Scholar] [CrossRef]
- El-fiqi, A.; Kim, J.; Bang, S.; El-fiqi, A.; Kim, H. Fabrication of nanofibrous macroporous scaffolds of poly (lactic acid) incorporating bioactive glass nanoparticles by camphene-assisted phase separation. Mater. Chem. Phys. 2013, 143, 1092–1101. [Google Scholar]
- Seyedmajidi, S.; Seyedmajidi, M. Fluorapatite: A Review of Synthesis, Properties and Medical Applications vs. Hydroxyapatite. Iran. J. Mater. Sci. Eng. 2022, 19, 1–20. [Google Scholar]
- Seyedmajidi, S.; Rajabnia, R.; Seyedmajidi, M. Evaluation of antibacterial properties of hydroxyapatite/bioactive glass and fluorapatite/bioactive glass nanocomposite foams as a cellular scaffold of bone tissue. J. Lab. Physicians 2018, 10, 265–270. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, K.; Huang, X.; Liu, J.; Liu, H.; Boccaccini, A.R.; Wan, Y.; Guo, X.; Shao, Z. Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 2019, 91, 60–71. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef]
- Hernandez, E.; Robles-Vazquez, O.; Orozco-Avila, I.; Sánchez-Díaz, J. An Overview of Mechanical Tests for Polymeric Biomaterial Scaffolds Used in Tissue Engineering. J. Res. Updates Polym. Sci. 2016, 4, 168–178. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, J.; Hou, J.; Guo, H.; Liu, C. Bone regeneration using cell-mediated responsive degradable PEG-based scaffolds incorporating with rhBMP-2. Biomaterials 2013, 34, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Karthick, K.; Arumugam, K.P. Properties of Biodegradable Polymers and Degradation for Sustainable Development. Int. J. Chem. Eng. Appl. 2011, 2, 164–167. [Google Scholar] [CrossRef]
- Chen, H.; Sun, K.; Tang, Z.; Law, R.V.; Mansfield, J.F.; Clarkson, B.H. Synthesis of fluorapatite nanorods and nanowires by direct precipitation from solution. Cryst. Growth Des. 2006, 6, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, K.; Okudera, T.; Okudera, H.; Nordquist, W.D.; Krutchkoff, D.J. Part I: Crystalline fluorapatite-coated hydroxyapatite, physical properties. J. Oral Implantol. 2011, 37, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.S.; Syame, S.M.; Mohamed, W.S.; Hakim, A.S. Incorporation of Plant Extracted Hydroxyapatite and Chitosan Nanoparticles on the Surface of Orthodontic Micro-Implants: An In-Vitro Antibacterial Study. Microorganisms 2022, 10, 581. [Google Scholar] [CrossRef]
- Mazalan, E.; Chaudhary, K.; Haider, Z.; Abd Hadi, S.F.; Ali, J. Determination of calcium to phosphate elemental ratio in natural hydroxypatite using LIBS. J. Phys. Conf. Ser. 2018, 1027, 012013. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Abdulkareem, B.; Nwannenna, C.S. Synthesis techniques, characterization and mechanical properties of natural derived hydroxyapatite scaffolds for bone implants: A review. SN Appl. Sci. 2021, 3, 822. [Google Scholar] [CrossRef]
- Iga, C.; Pawel, S.; Marcin, L.; Justyna, K.L. Polyurethane composite scaffolds modified with the mixture of gelatin and hydroxyapatite characterized by improved calcium deposition. Polymers 2020, 12, 410. [Google Scholar]
- Miculescu, F.; Lută, C.; Constantinescu, A.E.; Maidaniuc, A.; Mocanu, A.C.; Miculescu, M.; Voicu, S.I.; Ciocan, L.T. Considerations and Influencing Parameters in EDS Microanalysis of Biogenic Hydroxyapatite. J. Funct. Biomater. 2020, 11, 82. [Google Scholar] [CrossRef]
- Fernández, M.P.R.; Gehrke, S.A.; Martinez, C.P.A.; Guirado, J.L.C.; de Aza, P.N. SEM-EDX study of the degradation process of two xenograft materials used in sinus lift procedures. Materials 2017, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Redondo, F.L.; Giaroli, M.C.; Ciolino, A.E.; Ninago, M.D. Preparation of porous poly(Lactic acid)/tricalcium phosphate composite scaffolds for tissue engineering. Biointerface Res. Appl. Chem. 2022, 12, 5610–5624. [Google Scholar]
- Choy, C.S.; Lee, W.F.; Lin, P.Y.; Wu, Y.F.; Huang, H.M.; Teng, N.C.; Pan, Y.H.; Salamanca, E.; Chang, W.J. Surface Modified β-Tricalcium phosphate enhanced stem cell osteogenic differentiation in vitro and bone regeneration in vivo. Sci. Rep. 2021, 11, 9234. [Google Scholar] [CrossRef] [PubMed]
- Ma, G. Three common preparation methods of hydroxyapatite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 688, 033057. [Google Scholar] [CrossRef]
- Guo, T.; Li, Y.; Cao, G.; Zhang, Z.; Chang, S.; Czajka-Jakubowska, A.; Nör, J.E.; Clarkson, B.H.; Liu, J. Fluorapatite-modified scaffold on dental pulp stem cell mineralization. J. Dent. Res. 2014, 93, 1290–1295. [Google Scholar] [CrossRef]
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732. [Google Scholar]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Shemshad, S.; Kamali, S.; Khavandi, A.; Azari, S. Synthesis, characterization and in-vitro behavior of natural chitosan-hydroxyapatite-diopside nanocomposite scaffold for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 516–526. [Google Scholar] [CrossRef]
- Wu, M.; Wu, P.; Xiao, L.; Zhao, Y.; Yan, F.; Liu, X.; Xie, Y.; Zhang, C.; Chen, Y.; Cai, L. Biomimetic mineralization of novel hydroxyethyl cellulose/soy protein isolate scaffolds promote bone regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2020, 162, 627–1641. [Google Scholar] [CrossRef]
- Lim, K.T.; Patel, D.K.; Choung, H.W.; Seonwoo, H.; Kim, J.; Chung, J.H. Evaluation of Bone Regeneration Potential of Long-Term Soaked Natural Hydroxyapatite. ACS Appl. Bio Mater. 2019, 2, 5535–5543. [Google Scholar] [CrossRef]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Farrell, H.; Higginbotham, C.L. Compressive strength and bioactivity properties of photopolymerizable hybrid composite hydrogels for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 641–650. [Google Scholar] [CrossRef]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Kennedy, J.E.; Higginbotham, C.L. Mechanical properties and thermal behaviour of PEGDMA hydrogels for potential bone regeneration application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; TAKADAMA, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]






| Various Ceramic Compositions | Various BP Content | |||||
|---|---|---|---|---|---|---|
| Sample | CS/HAp | CS/TCP-α | CS/FAp | CS/HAp/20 µL BP | CS/HAp/10 µL BP | CS/HAp/5 µL BP |
| Gel fraction in 1% v/v acetic acid ± SD | 55.437 ± 6.37 | 67.1465 ± 7.93 | 49.0943 ± 4.01 | 53.4653 ± 4.79 | 56.5475 ± 2.46 | 51.1598 ± 4.16 |
| Sample | Equilibrium Water Content, EWC ± SD | Water Uptake, WU ± SD | % Swelling ± SD | Gel Fraction PBS ± SD |
|---|---|---|---|---|
| CS/HAp | 65.74 ± 0.79 | 191.97 ± 6.84 | 291.97 ± 6.84 | 99.28 ± 0.59 |
| CS/TCP-α | 59.29 ± 0.95 | 145.74 ± 5.68 | 245.74 ± 5.68 | 99.72 ± 0.88 |
| CS/FAp | 87.71 ± 1.92 | 726.65 ± 127.2 | 826.65 ± 127.22 | 94.55 ± 1.03 |
| CS/HAp/20 µL BP | 64.47 ± 0.08 | 181.43 ± 0.63 | 281.43 ± 0.63 | 96.34 ± 0.18 |
| CS/HAp/10 µL BP | 64.60 ± 0.5 | 182.51 ± 4.04 | 282.51 ± 4.04 | 96.83 ± 0.27 |
| CS/HAp/5 µL BP | 63.61 ± 0.43 | 174.85 ± 3.28 | 274.85 ± 3.28 | 97.40 ± 1.86 |
| Weight (g) | Volume (µL) | Volume (mL) | ||||
|---|---|---|---|---|---|---|
| CS | HAp | TCP-α | BP | PEGDMA600 | Acetic acid | |
| 1:1:0 | 1.5 | 1.5 | 0 | 50 | 100 | 12.5 |
| 1:0:1 | 1.5 | 0 | 1.5 | 50 | 100 | 12.5 |
| Weight (g) | Volume (µL) | Volume (mL) | |||
|---|---|---|---|---|---|
| CS | HAp | BP | PEGDMA600 | Acetic acid | |
| CS/HAp 1:1 | 1.5 | 1.5 | 20 | 100 | 12.5 |
| 1.5 | 1.5 | 5 | 100 | 12.5 | |
| 1.5 | 1.5 | 1 | 100 | 12.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azaman, F.A.; Zhou, K.; Blanes-Martínez, M.d.M.; Brennan Fournet, M.; Devine, D.M. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels 2022, 8, 696. https://doi.org/10.3390/gels8110696
Azaman FA, Zhou K, Blanes-Martínez MdM, Brennan Fournet M, Devine DM. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels. 2022; 8(11):696. https://doi.org/10.3390/gels8110696
Chicago/Turabian StyleAzaman, Farah Alwani, Keran Zhou, María del Mar Blanes-Martínez, Margaret Brennan Fournet, and Declan M. Devine. 2022. "Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction" Gels 8, no. 11: 696. https://doi.org/10.3390/gels8110696
APA StyleAzaman, F. A., Zhou, K., Blanes-Martínez, M. d. M., Brennan Fournet, M., & Devine, D. M. (2022). Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels, 8(11), 696. https://doi.org/10.3390/gels8110696











