Photocatalytic Hydrogen Production Using Porous 3D Graphene-Based Aerogels Supporting Pt/TiO2 Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
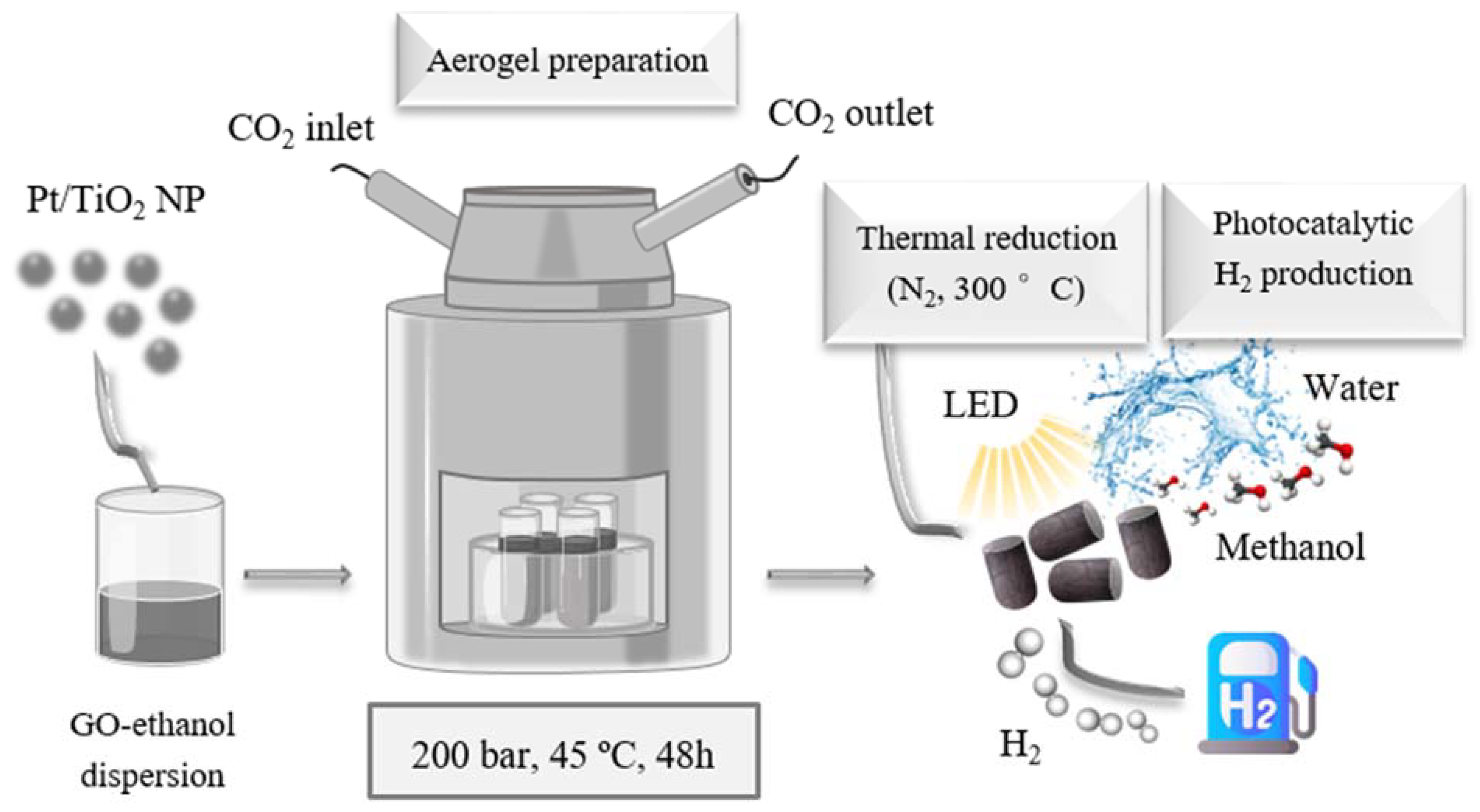
2.1. Aerogel Synthesis
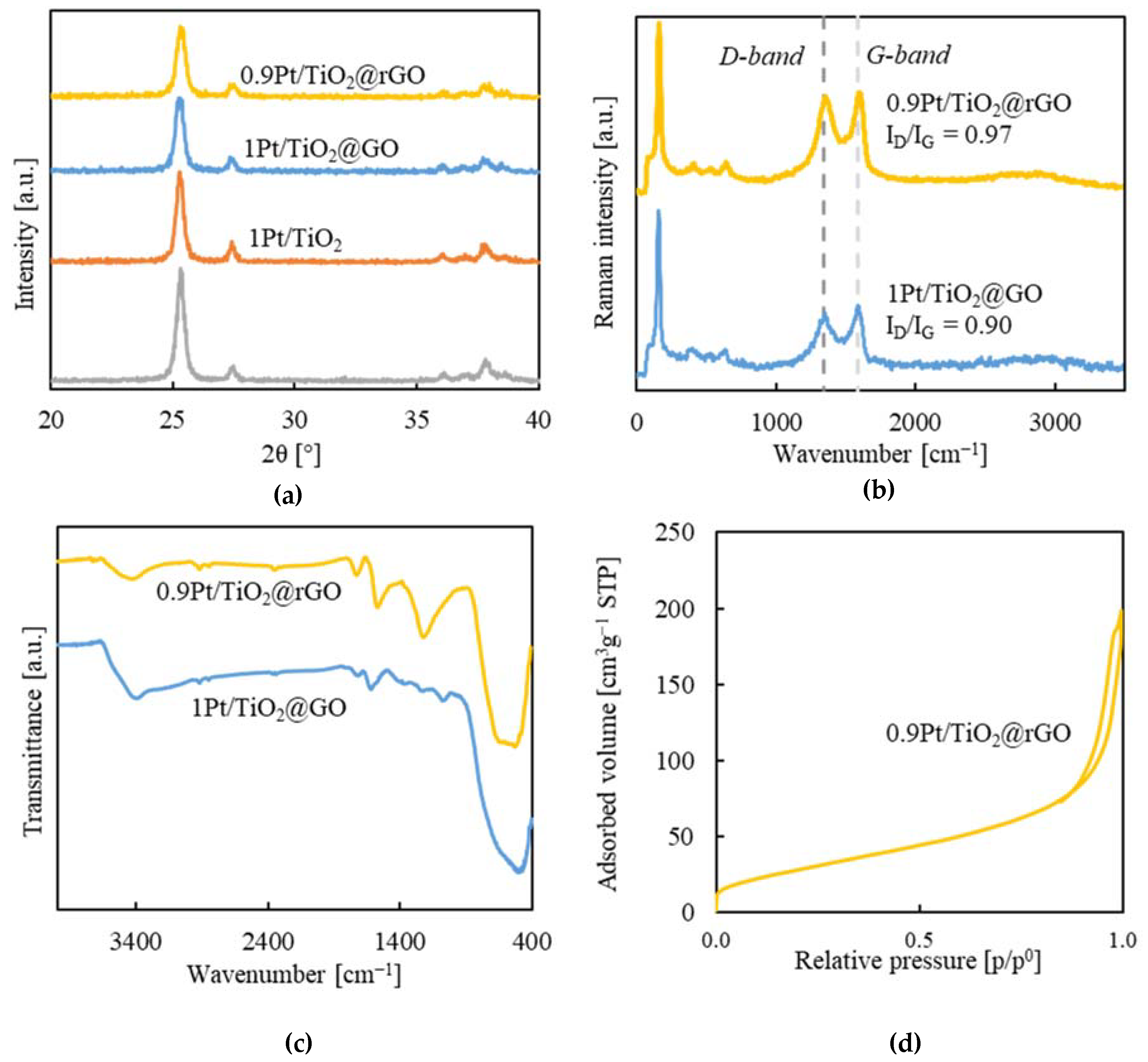
2.2. Aerogels Structure
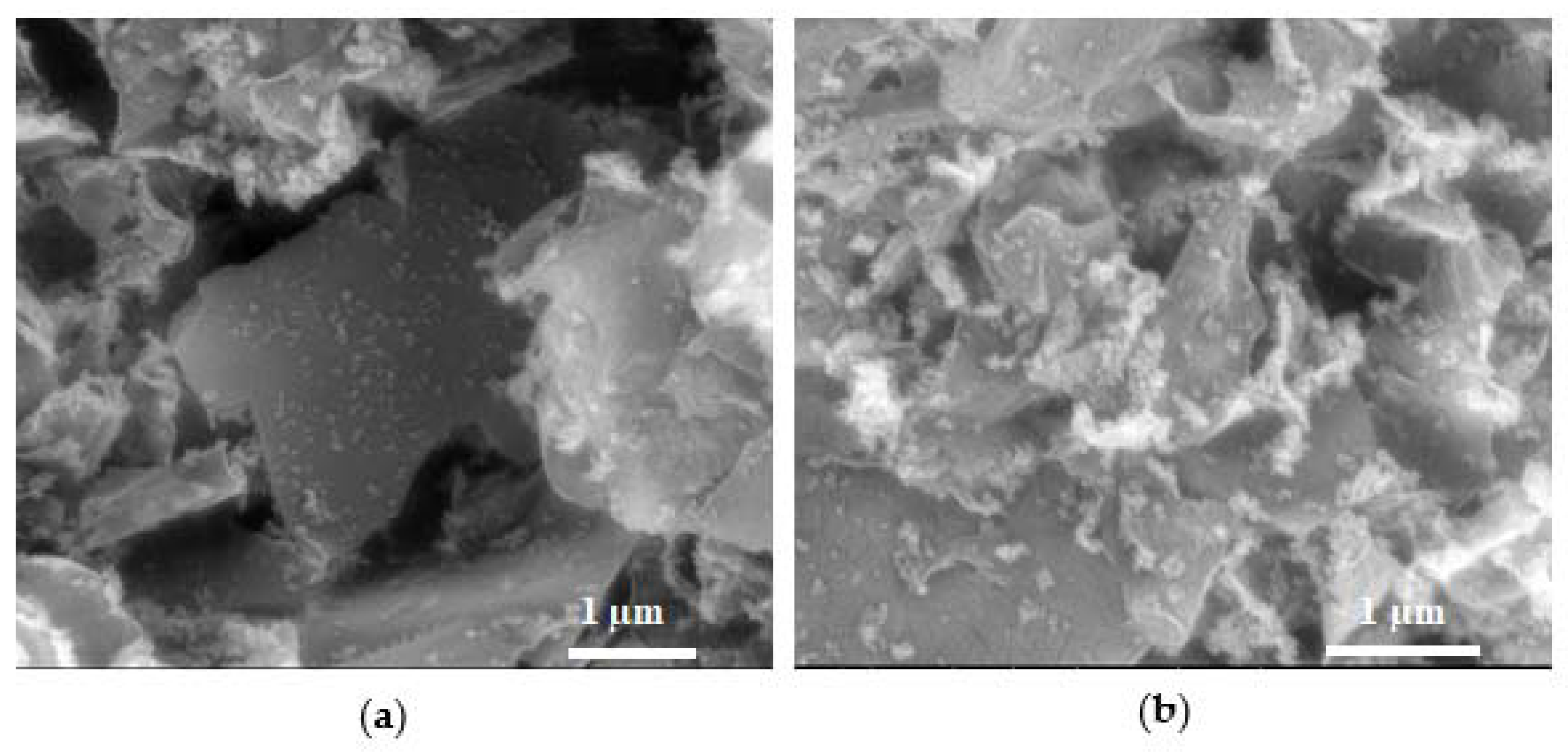
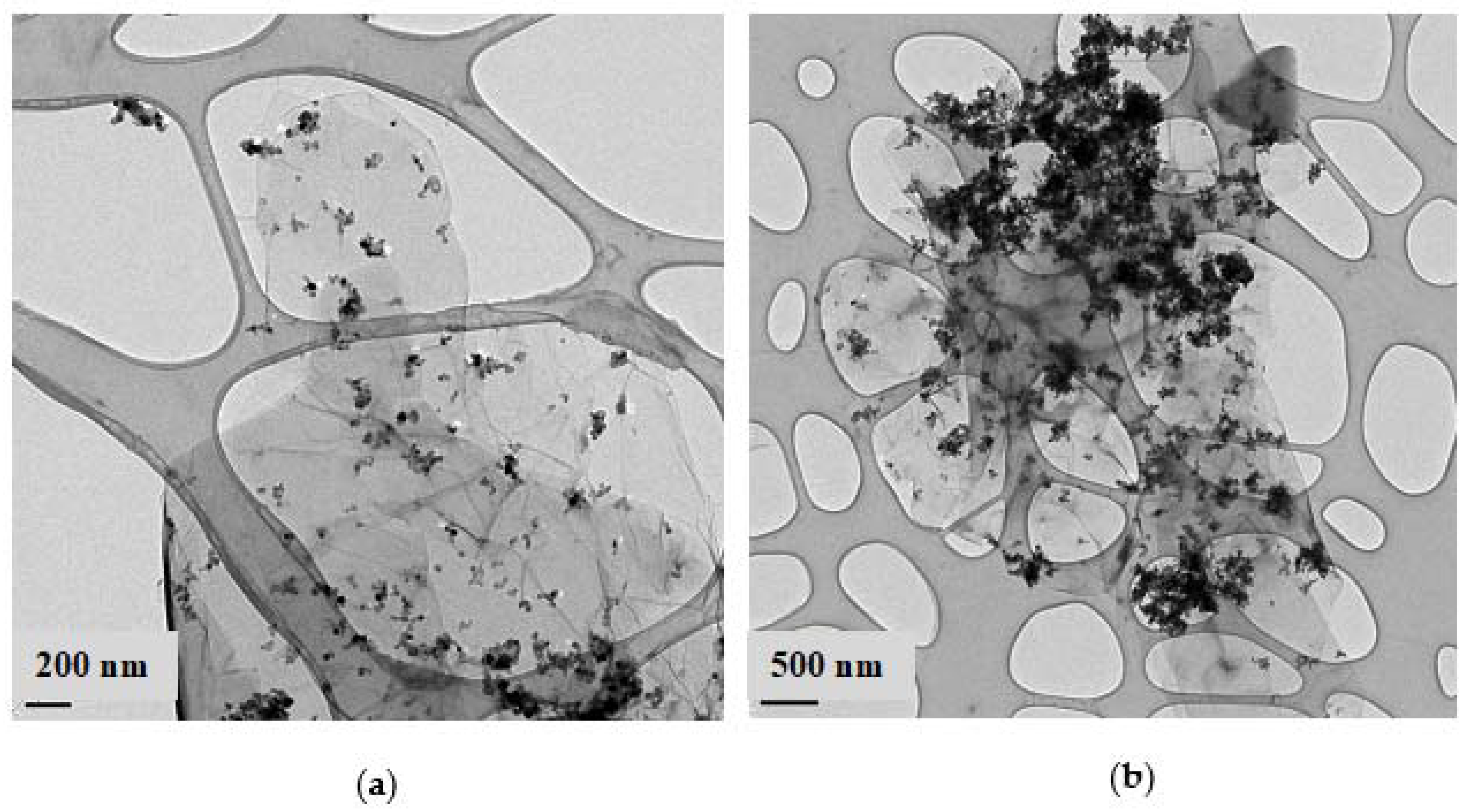
2.3. Textural Properties and Morphology
2.4. Aerogels Optical Properties
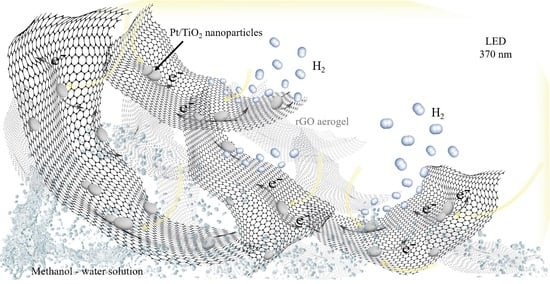
2.5. Photocatalytic Hydrogen Production
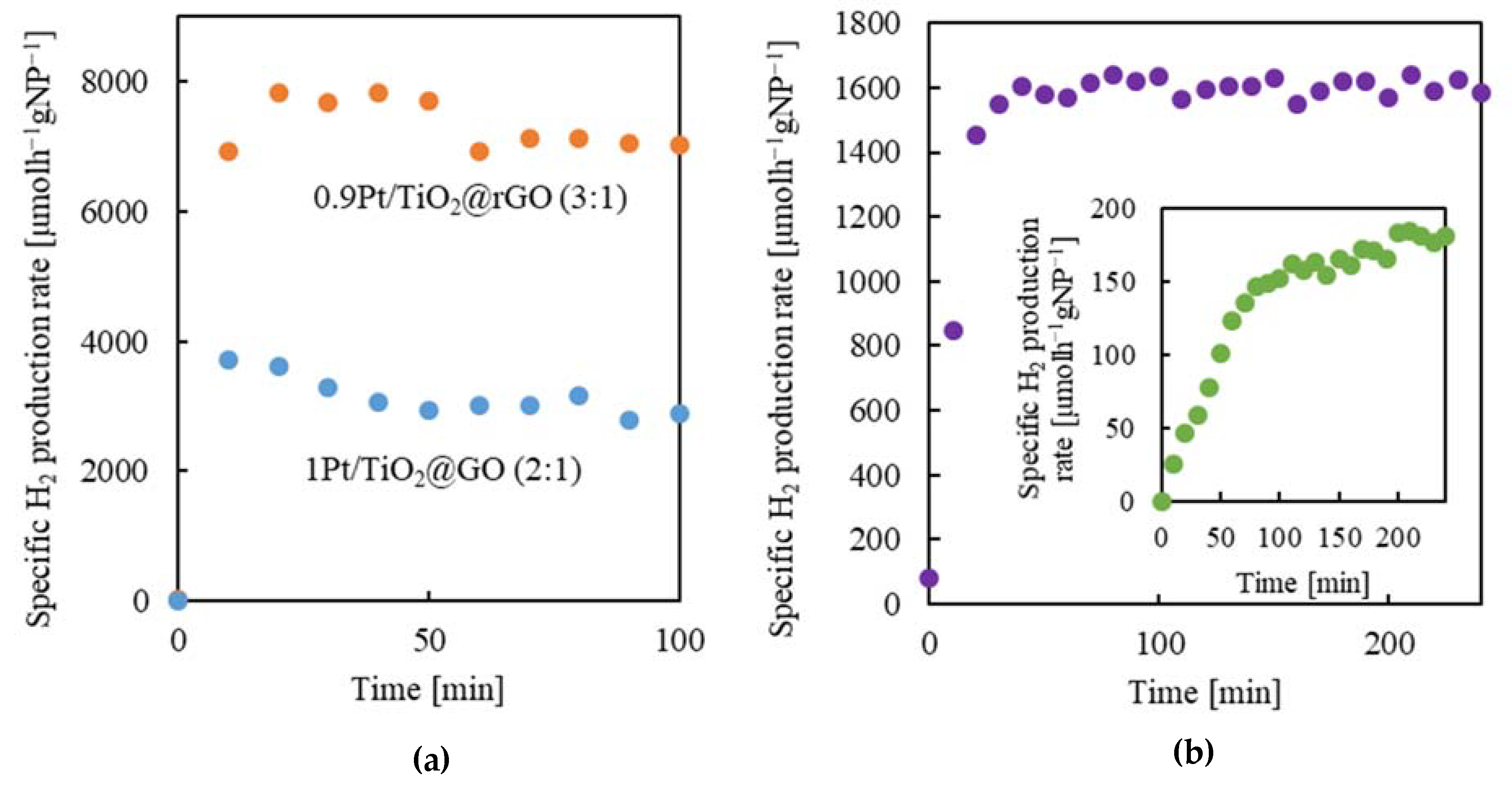
2.5.1. Aerogel Reduction Degree
2.5.2. Aerogel Architecture
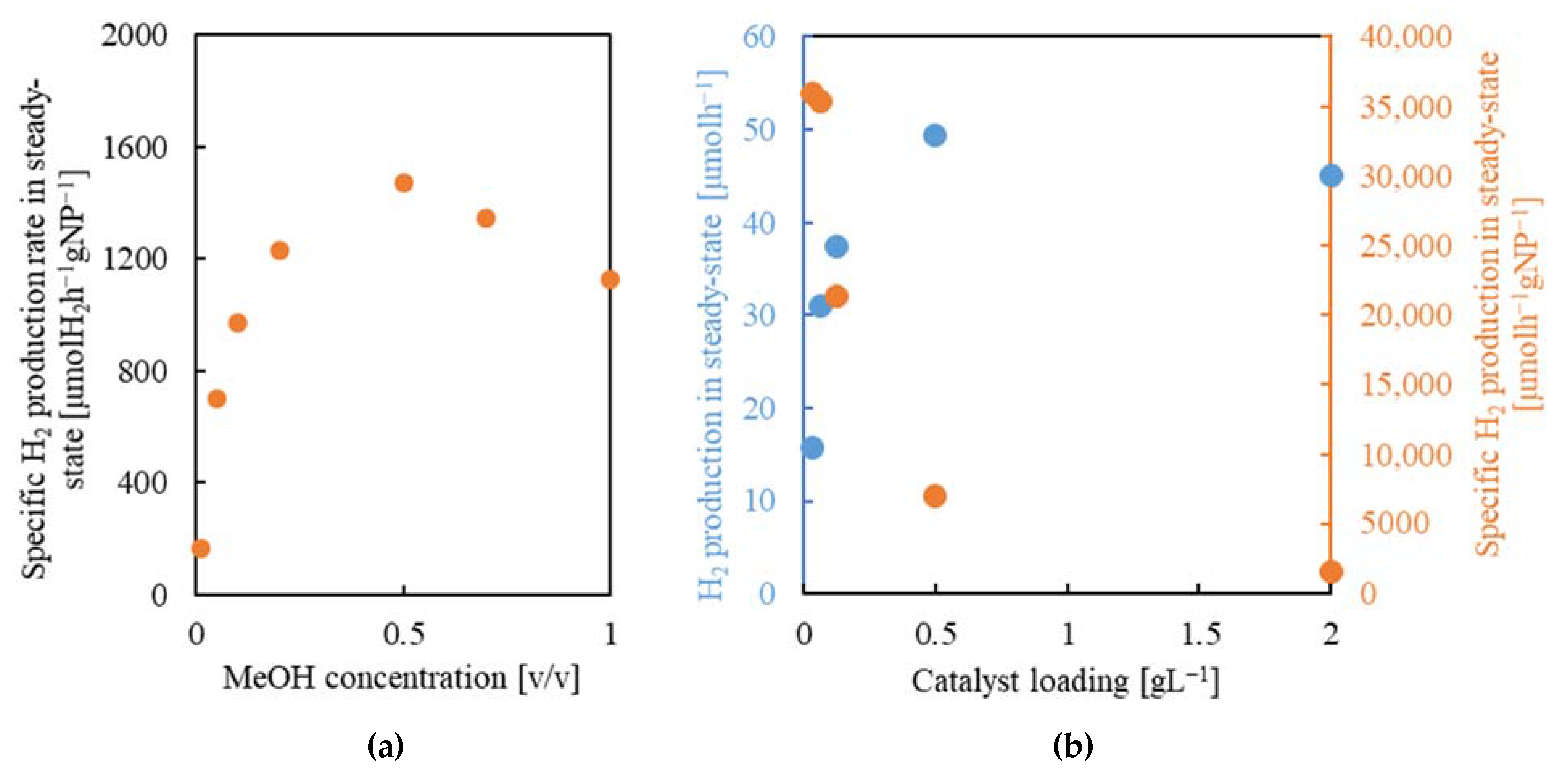
2.5.3. Methanol Concentration
2.5.4. Catalyst Concentration
2.5.5. Catalyst Composition
Platinum Content
NP:rGO Ratio
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthetic Methods
4.2.1. Preparation of NPs of xPt/TiO2 Composite
4.2.2. Preparation of xPt/TiO2@rGO Composite Aerogels
4.3. Characterization
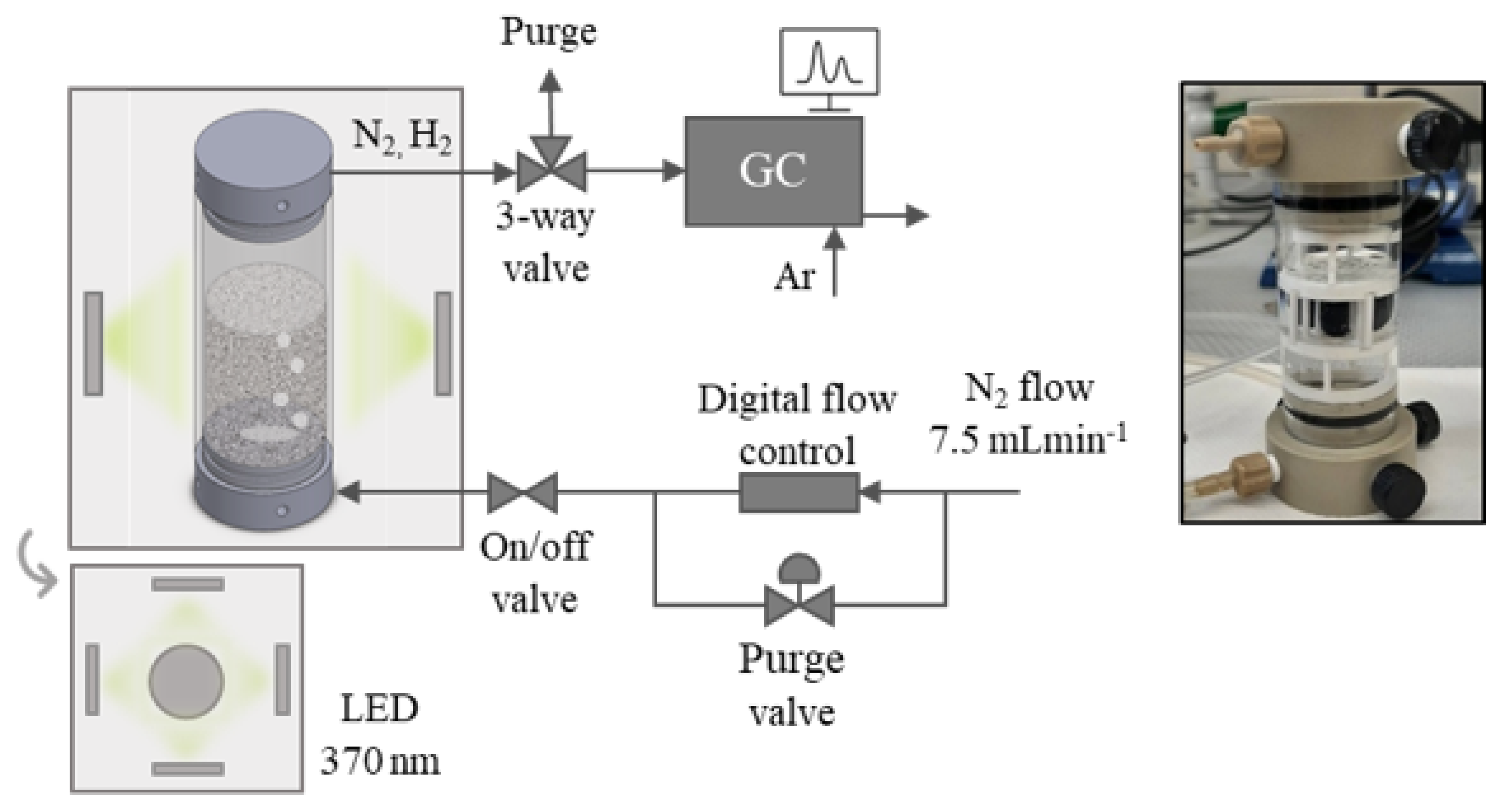
4.4. Photocatalytic H2 Production
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohd Shah, N.R.A.; Mohamad Yunus, N.N.; Wong, W.Y.; Arifin, K.; Jeffery Minggu, L. Current progress on 3D graphene-based photocatalysts: From synthesis to photocatalytic hydrogen production. Int. J. Hydrog. Energy 2021, 46, 9324–9340. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Martha, S.; Chandra Sahoo, P.; Parida, K.M. An overview on visible light responsive metal oxide based photocatalysts for hydrogen energy production. RSC Adv. 2015, 5, 61535–61553. [Google Scholar] [CrossRef]
- Kuang, P.; Sayed, M.; Fan, J.; Cheng, B.; Yu, J. 3D Graphene-Based H2-Production Photocatalyst and Electrocatalyst. Adv. Energy Mater. 2020, 10, 1–53. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a photocatalyst for water splitting—An experimental and theoretical review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Blengini, G.A.; Latunussa, C.E.L.; Eynard, U. Study on the EU’s List of Critical Raw Materials—Critical Raw Materials Factsheets; Final Report; European Comission: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Critical Raw Materials Resilience: Charting a Path Towards Greater Security and Sustainability. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0474 (accessed on 7 September 2022).
- Yeh, T.F.; Cihlář, J.; Chang, C.Y.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Solís-Fernández, P.; Bissett, M.; Ago, H. Synthesis, structure and applications of graphene-based 2D heterostructures. Chem. Soc. Rev. 2017, 46, 4572–4613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Wang, Y.; Wu, Z. 2D Graphene-TiO2 Composite and Its Photocatalytic Application in Water Pollutants. Front. Energy Res. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, S.; Xia, Y.; Ma, S.; Zhou, Q.; Li, Y. The fundamental role and mechanism of reduced graphene oxide in rGO/Pt-TiO2 nanocomposite for high-performance photocatalytic water splitting. Appl. Catal. B 2017, 207, 335–346. [Google Scholar] [CrossRef]
- Roy, S.S.; Cheruvathoor Poulose, A.; Bakandritsos, A.; Varma, R.S.; Otyepka, M. 2D graphene derivatives as heterogeneous catalysts to produce biofuels via esterification and trans-esterification reactions. Appl. Mater. Today 2021, 23, 101053. [Google Scholar] [CrossRef]
- Gusmão, R.; Veselý, M.; Sofer, Z. Recent Developments on the Single Atom Supported at 2D Materials beyond Graphene as Catalysts. ACS Catal. 2020, 10, 9634–9648. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Chen, J. Three-dimensional graphene-based composites for energy applications. Nanoscale 2015, 7, 6924–6943. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent developments of TiO2-based photocatalysis in the hydrogen evolution and photodegradation: A review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord. Chem. Rev. 2016, 312, 99–148. [Google Scholar] [CrossRef]
- Yam, K.M.; Guo, N.; Jiang, Z.; Li, S.; Zhang, C. Graphene-based heterogeneous catalysis: Role of graphene. Catalysts 2020, 10, 53. [Google Scholar] [CrossRef]
- Rivero, M.J.; Iglesias, O.; Ribao, P.; Ortiz, I. Kinetic performance of TiO2/Pt/reduced graphene oxide composites in the photocatalytic hydrogen production. Int. J. Hydrog. Energy 2019, 44, 101–109. [Google Scholar] [CrossRef]
- Mohan, P.S.; Purkait, M.K.; Chang, C.T. Experimental evaluation of Pt/TiO2/rGO as an efficient HER catalyst via artificial photosynthesis under UVB & visible irradiation. Int. J. Hydrog. Energy 2020, 45, 17174–17190. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, Q.; Zhang, X.; Peng, T. Graphite oxide-TiO2 nanocomposite and its efficient visible-light-driven photocatalytic hydrogen production. J. Alloys Compd. 2012, 516, 85–90. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Y.; Williams, T.; Wang, H.; Sun, C.; Zhang, X. Metal link: A strategy to combine graphene and titanium dioxide for enhanced hydrogen production. Int. J. Hydrog. Energy 2016, 41, 22034–22042. [Google Scholar] [CrossRef]
- Da Silva, R.O.; Heiligtag, F.J.; Karnahl, M.; Junge, H.; Niederberger, M.; Wohlrab, S. Design of multicomponent aerogels and their performance in photocatalytic hydrogen production. Catal. Today 2015, 246, 101–107. [Google Scholar] [CrossRef]
- Lin, C.C.; Wei, T.Y.; Lee, K.T.; Lu, S.Y. Titania and Pt/titania aerogels as superior mesoporous structures for photocatalytic water splitting. J. Mater. Chem. 2011, 21, 12668–12674. [Google Scholar] [CrossRef]
- Borrás, A.; Rosado, A.; Fraile, J.; López-Periago, A.M.; Giner Planas, J.; Yazdi, A.; Domingo, C. Meso/microporous MOF@graphene oxide composite aerogels prepared by generic supercritical CO2 technology. Microporous Mesoporous Mater. 2022, 335, 111825. [Google Scholar] [CrossRef]
- Borrás, A.; Fraile, J.; Rosado, A.; Marbán, G.; Tobias, G.; López-Periago, A.M.; Domingo, C. Green and Solvent-Free Supercritical CO2 -Assisted Production of Superparamagnetic Graphene Oxide Aerogels: Application as a Superior Contrast Agent in MRI. ACS Sustain. Chem. Eng. 2020, 8, 4877–4888. [Google Scholar] [CrossRef]
- Rosado, A.; Borrás, A.; Fraile, J.; Navarro, J.A.R.; Suárez-García, F.; Stylianou, K.C.; López-Periago, A.M.; Giner Planas, J.; Domingo, C.; Yazdi, A. HKUST-1 Metal-Organic Framework Nanoparticle/Graphene Oxide Nanocomposite Aerogels for CO2 and CH4 Adsorption and Separation. ACS Appl. Nano Mater. 2021, 4, 12712–12725. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Veres, P.; López-Periago, A.M.; Lázár, I.; Saurina, J.; Domingo, C. Hybrid aerogel preparations as drug delivery matrices for low water-solubility drugs. Int. J. Pharm. 2015, 496, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Borrás, A.; Goncalves, G.; Marbán, G.; Sandoval, S.; Pinto, S.; Marques, P.A.A.P.; Fraile, J.; Tobais, G.; López-Periago, A.M.; Domingo, C. Preparation and Characterization of Graphene Oxide Aerogels: Exploring the Limits of Supercritical CO2 Fabrication Methods. Chem. A Eur. J. 2018, 24, 15903–15911. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Lin, Y. Further thermal reduction of reduced graphene oxide aerogel with excellent rate performance for supercapacitors. Appl. Sci. 2019, 9, 2188. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Tuning the Oxygen Content of Reduced Graphene Oxide and Effects on Its Properties. ACS Omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef]
- Litke, A.; Frei, H.; Hensen, E.J.M.; Hofmann, J.P. Interfacial charge transfer in Pt-loaded TiO2 P25 photocatalysts studied by in-situ diffuse reflectance FTIR spectroscopy of adsorbed CO. J. Photochem. Photobiol. A Chem. 2018, 370, 84–88. [Google Scholar] [CrossRef]
- Gillespie, P.N.O.; Martsinovich, N. Origin of Charge Trapping in TiO2/Reduced Graphene Oxide Photocatalytic Composites: Insights from Theory. ACS Appl. Mater. Interfaces 2019, 11, 31909–31922. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C Mater. 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, A.D.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Munk, P.; Kubelka, F. A Contribution to the Optics of Pigments. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Lan, Z.A.; Zhang, G.; Wang, X. A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting. Appl. Catal. B 2016, 192, 116–125. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, W. Photocatalytic Reactivity of Surface Platinized TiO2: Substrate Specificity and the Effect of Pt Oxidation State. J. Phys. Chem. 2005, 109, 7399–7406. [Google Scholar] [CrossRef]
- Zeng, W.; Tao, X.; Lin, S.; Lee, C.; Shi, D.; Lam, K.; Huang, B.; Wang, Q.; Zhao, Y. Defect-engineered reduced graphene oxide sheets with high electric conductivity and controlled thermal conductivity for soft and flexible wearable thermoelectric generators. Nano Energy 2018, 54, 163–174. [Google Scholar] [CrossRef]
- Wan, W.; Zhang, R.; Ma, M.; Zhou, Y. Monolithic aerogel photocatalysts: A review. J. Mater. Chem. A Mater. 2018, 6, 754–775. [Google Scholar] [CrossRef]
- Guzman, F.; Chuang, S.S.C.; Yang, C. Role of methanol sacrificing reagent in the photocatalytic evolution of hydrogen. Ind. Eng. Chem. Res. 2013, 52, 61–65. [Google Scholar] [CrossRef]
- Nomikos, G.N.; Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Kinetic and mechanistic study of the photocatalytic reforming of methanol over Pt/TiO2 catalyst. Appl. Catal. B 2014, 146, 249–257. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Chen, W.T.; Dong, Y.; Yadav, P.; Aughterson, R.D.; Sun-Waterhouse, D.; Waterhouse, G.I.N. Effect of alcohol sacrificial agent on the performance of Cu/TiO2 photocatalysts for UV-driven hydrogen production. Appl. Catal. A Gen. 2020, 602, 117703. [Google Scholar] [CrossRef]
- Chen, W.T.; Chan, A.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H.; Waterhouse, G.I.N. Performance comparison of Ni/TiO2 and Au/TiO2 photocatalysts for H2 production in different alcohol-water mixtures. J. Catal. 2018, 367, 27–42. [Google Scholar] [CrossRef]
- Pendolino, F.; Capurso, G.; Maddalena, A.; lo Russo, S. The structural change of graphene oxide in a methanol dispersion. RSC Adv. 2014, 4, 32914–32917. [Google Scholar] [CrossRef]
- Curcó, J.; Giménez, D.; Addardak, A.; Cervera-March, S.; Esplugas, S. Effects of radiation absorption and catalyst concentration on the photocatalytic degradation of pollutants. Catal. Today 2002, 76, 177–188. [Google Scholar] [CrossRef]
- Jiang, X.; Fu, X.; Zhang, L.; Meng, S.; Chen, S. Photocatalytic reforming of glycerol for H2 evolution on Pt/TiO2: Fundamental understanding the effect of co-catalyst Pt and the Pt deposition route. J. Mater. Chem. A Mater. 2015, 3, 2271–2282. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, The European Green Deal. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 7 September 2022).
- Lu, Y.; Ma, B.; Yang, Y.; Huang, E.; Ge, Z.; Zhang, T.; Zhang, S.; Li, L.; Guan, N.; Ma, Y.; et al. High activity of hot electrons from bulk 3D graphene materials for efficient photocatalytic hydrogen production. Nano Res. 2017, 10, 1662–1672. [Google Scholar] [CrossRef]
- Naffati, N.; Sampaio, M.J.; Da Silva, E.S.; Nsib, M.F.; Arfaoui, Y.; Houas, A.; Faria, J.L.; Silva, C.G. Carbon-nanotube/TiO2 materials synthesized by a one-pot oxidation/hydrothermal route for the photocatalytic production of hydrogen from biomass derivatives. Mater. Sci. Semicond. Process. 2020, 115, 105098. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubovics, M.; Silva, C.G.; López-Periago, A.M.; Faria, J.L.; Domingo, C. Photocatalytic Hydrogen Production Using Porous 3D Graphene-Based Aerogels Supporting Pt/TiO2 Nanoparticles. Gels 2022, 8, 719. https://doi.org/10.3390/gels8110719
Kubovics M, Silva CG, López-Periago AM, Faria JL, Domingo C. Photocatalytic Hydrogen Production Using Porous 3D Graphene-Based Aerogels Supporting Pt/TiO2 Nanoparticles. Gels. 2022; 8(11):719. https://doi.org/10.3390/gels8110719
Chicago/Turabian StyleKubovics, Márta, Cláudia G. Silva, Ana M. López-Periago, Joaquim L. Faria, and Concepción Domingo. 2022. "Photocatalytic Hydrogen Production Using Porous 3D Graphene-Based Aerogels Supporting Pt/TiO2 Nanoparticles" Gels 8, no. 11: 719. https://doi.org/10.3390/gels8110719
APA StyleKubovics, M., Silva, C. G., López-Periago, A. M., Faria, J. L., & Domingo, C. (2022). Photocatalytic Hydrogen Production Using Porous 3D Graphene-Based Aerogels Supporting Pt/TiO2 Nanoparticles. Gels, 8(11), 719. https://doi.org/10.3390/gels8110719