5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report
Abstract
1. Introduction
1.1. Photodynamic Therapy
1.2. Photodynamic vs. Photoinactivation Therapy
1.3. Specific Wavelength for Specific Photosensitizers
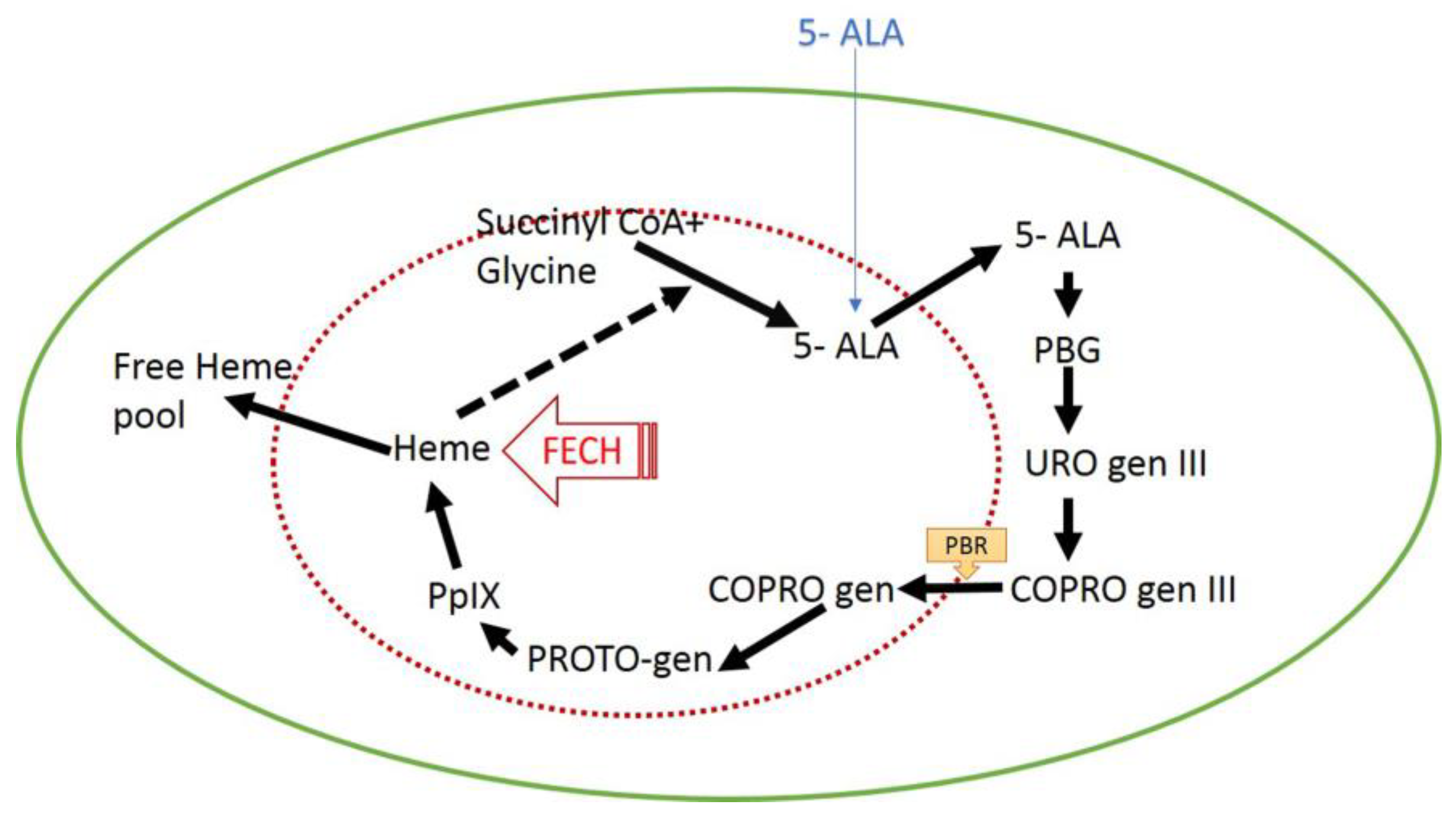
1.4. Photodynamic Therapy Based on 5-Aminolevulinic Acid and Red LED
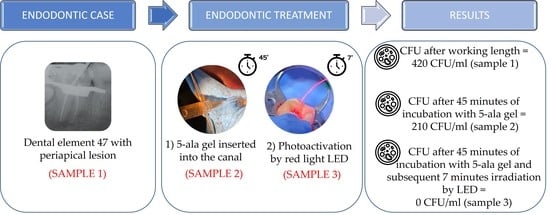
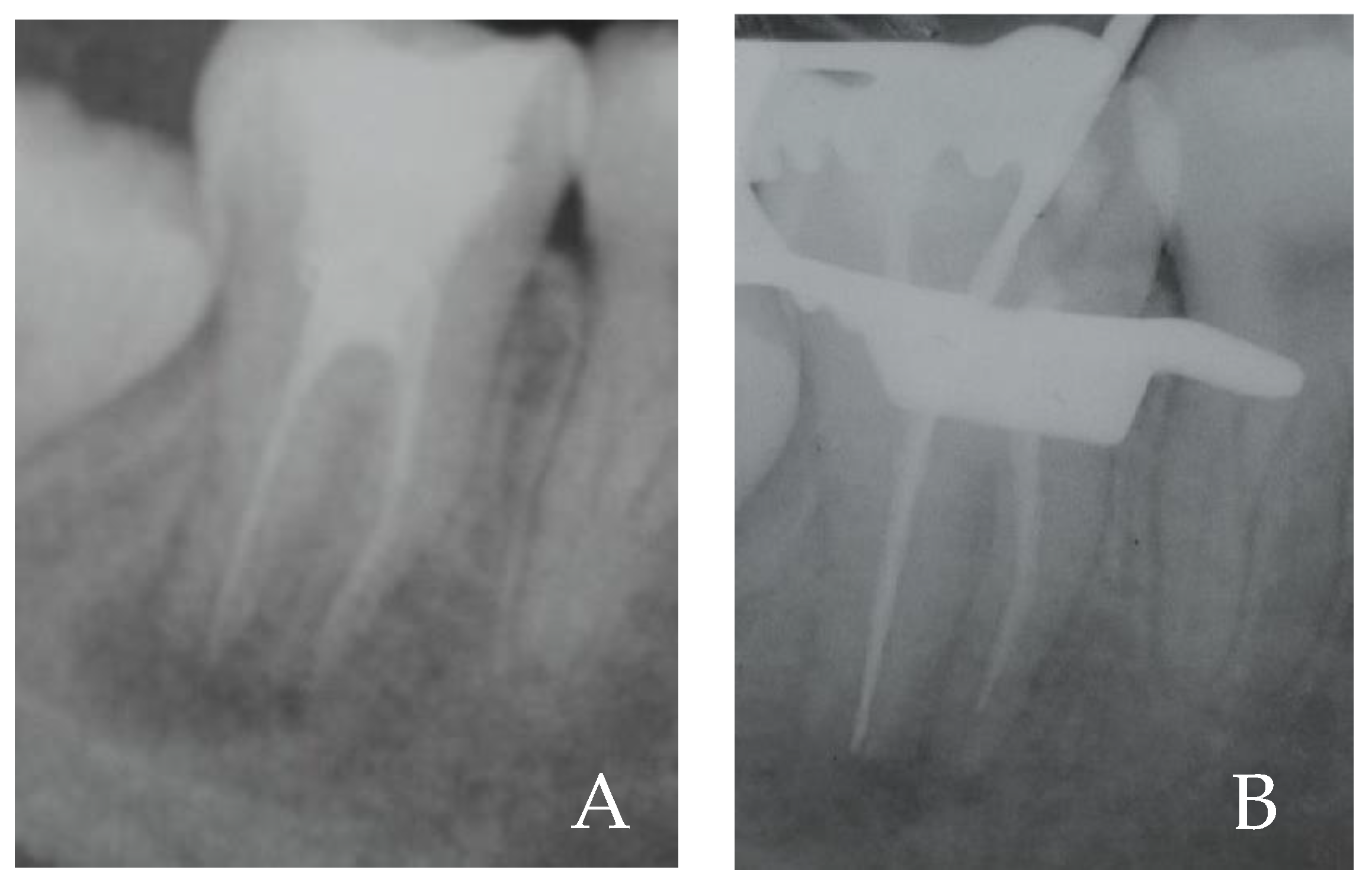
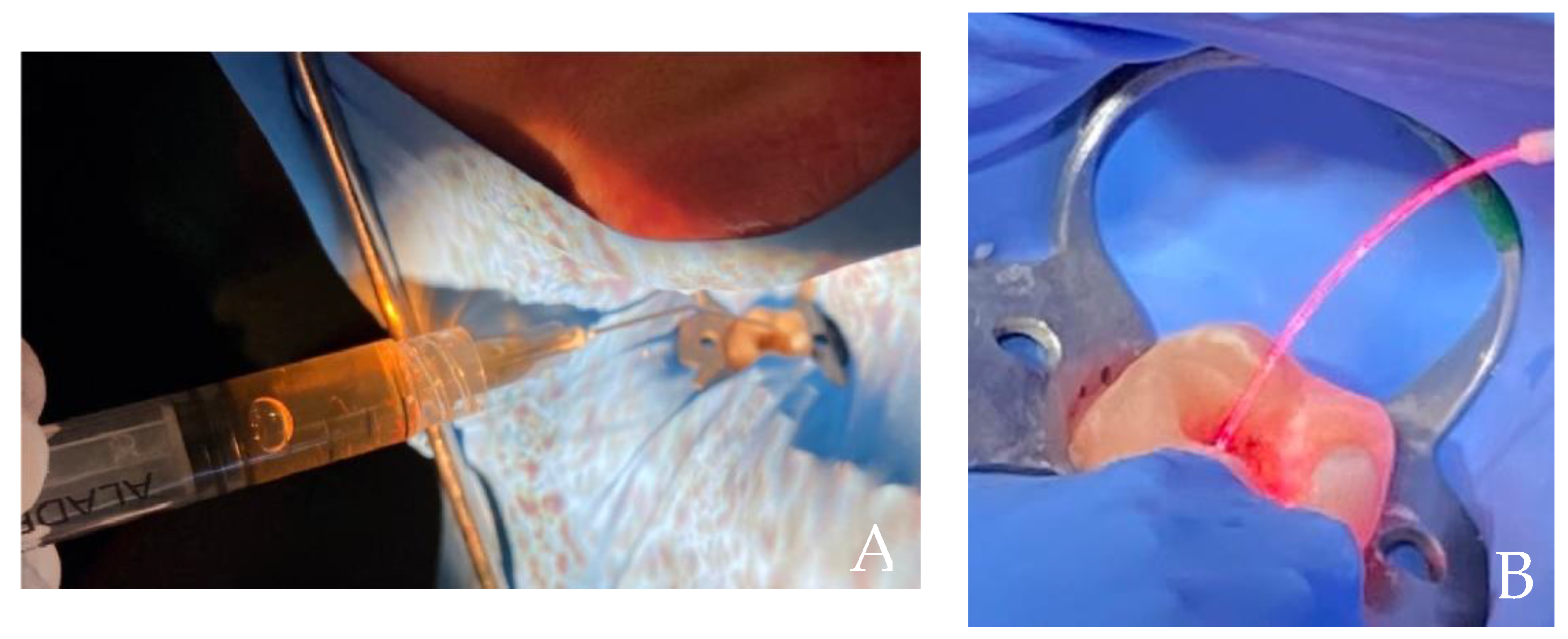
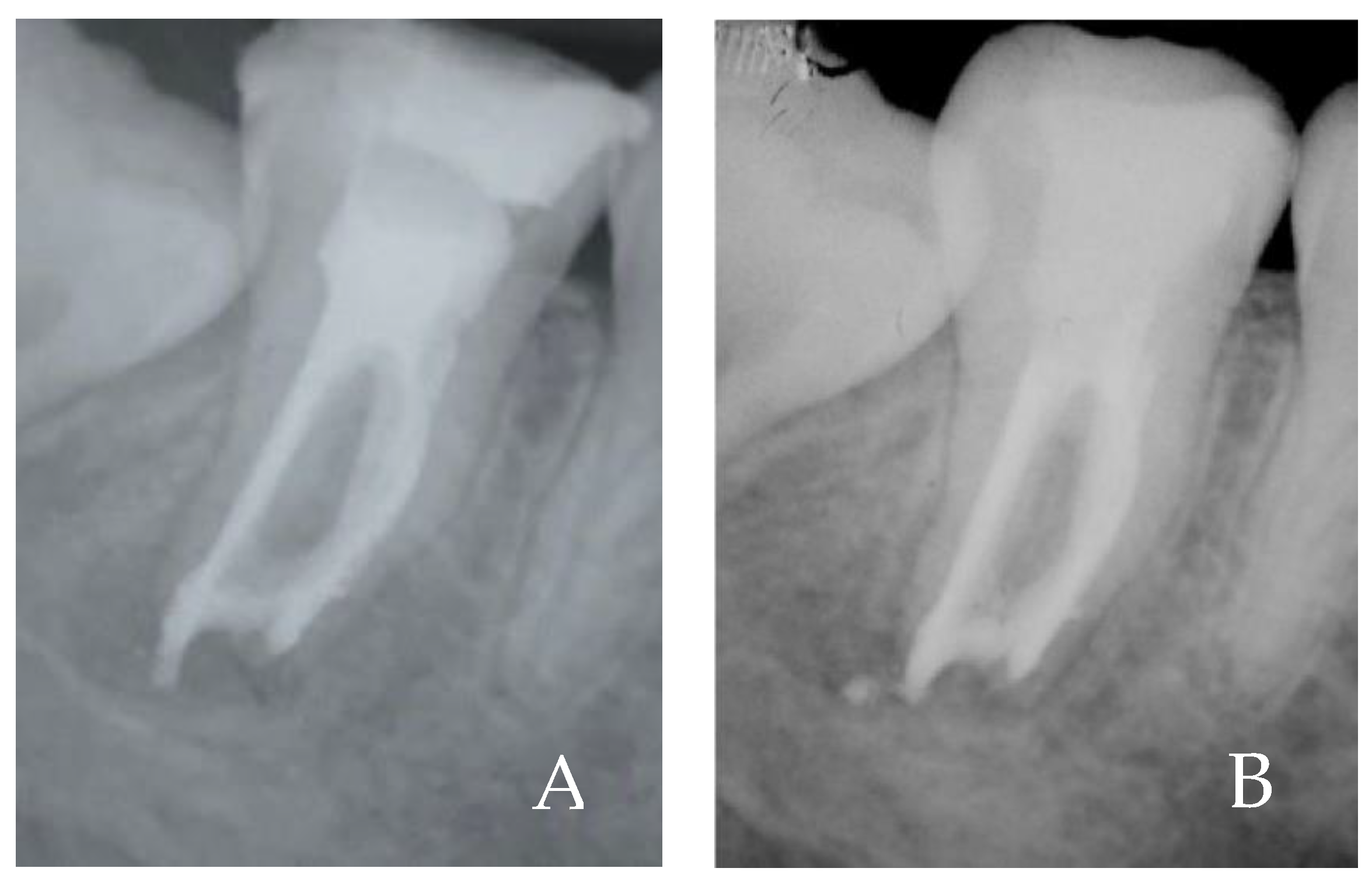
2. Case Report
3. Photodynamic Therapy in Endodontics
4. In Vitro and Ex Vivo Studies
5. In Vivo Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roguin, L.P.; Chiarante, N.; García Vior, M.C.; Marino, J. Zinc(II) Phthalocyanines as Photosensitizers for Antitumor Photodynamic Therapy. Int. J. Biochem. Cell Biol. 2019, 114, 105575. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.D.; Suggs, A.K. Introduction to Photobiology. Dermatol. Clin. 2014, 32, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic Therapy to Control Microbial Biofilms. Photodiagnosis Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of Antimicrobial Photodynamic Therapy in Periodontal and Peri-Implant Diseases. Periodontology 2009, 51, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Junqueira, J.C.; Santos, E.L.S.; Costa, A.C.B.; Jorge, A.O.C. Comparison of the Efficacy of Rose Bengal and Erythrosin in Photodynamic Therapy against Enterobacteriaceae. Lasers Med. Sci. 2010, 25, 581–586. [Google Scholar] [CrossRef]
- O’Riordan, K.; Sharlin, D.S.; Gross, J.; Chang, S.; Errabelli, D.; Akilov, O.E.; Kosaka, S.; Nau, G.J.; Hasan, T. Photoinactivation of Mycobacteria in Vitro and in a New Murine Model of Localized Mycobacterium Bovis BCG-Induced Granulomatous Infection. Antimicrob. Agents Chemother. 2006, 50, 1828–1834. [Google Scholar] [CrossRef]
- Späth, A.; Leibl, C.; Cieplik, F.; Lehner, K.; Regensburger, J.; Hiller, K.A.; Bäumler, W.; Schmalz, G.; Maisch, T. Improving Photodynamic Inactivation of Bacteria in Dentistry: Highly Effective and Fast Killing of Oral Key Pathogens with Novel Tooth-Colored Type-II Photosensitizers. J. Med. Chem. 2014, 57, 5157–5168. [Google Scholar] [CrossRef]
- Bergmans, L.; Moisiadis, P.; Huybrechts, B.; van Meerbeek, B.; Quirynen, M.; Lambrechts, P. Effect of Photo-Activated Disinfection on Endodontic Pathogens Ex Vivo. Int. Endod. J. 2008, 41, 227–239. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, M. Photodynamic Antifungal Chemotherapy. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef]
- Krüger, M.; Richter, P.; Strauch, S.M.; Nasir, A.; Burkovski, A.; Antunes, C.A.; Meißgeier, T.; Schlücker, E.; Schwab, S.; Lebert, M. What an Escherichia Coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin. Microorganisms 2019, 7, 59. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Uber Die Wirkung Fluorescirender Stoffe Auf Infusorien—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=a8c526fe-776b-4b6c-9f30-292b3ae293fd (accessed on 12 October 2022).
- Halberstaedter; Neisser, A. Zur Kenntnis Der Sensibilisierung. Dtsch. Med. Wochenschr. 1904, 30, 805–806. [Google Scholar] [CrossRef][Green Version]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-Mediated Photodynamic Therapy for the Treatment of Oral Infections-A Review. Photodiagnosis Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef]
- Soukos, N.S.; Goodson, J.M. Photodynamic Therapy in the Control of Oral Biofilms. Periodontology 2011, 55, 143–166. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L. Photodynamic Combinational Therapy in Cancer Treatment. Off. J. Balk. Union Oncol. 2018, 23, 561–567. [Google Scholar]
- Konopka, K.; Goslinski, T. Photodynamic Therapy in Dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- Sharwani, A.; Jerjes, W.; Salih, V.; MacRobert, A.J.; El-Maaytah, M.; Khalil, H.S.M.; Hopper, C. Fluorescence Spectroscopy Combined with 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence in Detecting Oral Premalignancy. J. Photochem. Photobiol. B 2006, 83, 27–33. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic Therapy for Localized Infections--State of the Art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Tasso, T.T.; Baptista, M.S. Photosensitized Oxidation of Intracellular Targets: Understanding the Mechanisms to Improve the Efficiency of Photodynamic Therapy. Methods Mol. Biol. 2022, 2451, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Queirós, C.; Garrido, P.M.; Maia Silva, J.; Filipe, P. Photodynamic Therapy in Dermatology: Beyond Current Indications. Dermatol. Ther. 2020, 33, e13997. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic Therapy for Skin Cancer: How to Enhance Drug Penetration? J. Photochem. Photobiol. B 2019, 197, 111544. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; dos Santos, F.V.; Lyon, J.P.; Maftoum-Costa, M.; Pacheco-Soares, C.; Soares Da Silva, N. Photodynamic Therapy: Porphyrins and Phthalocyanines as Photosensitizers. Aust. J. Chem. 2008, 61, 741–754. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Z.; Ren, Y.; Chen, X.; Zhang, W.; Zhu, X.; Mao, Z.; Shen, J.; Nie, S. Advances in Nanomaterials for Use in Photothermal and Photodynamic Therapeutics (Review). Mol. Med. Rep. 2019, 20, 5–15. [Google Scholar] [CrossRef]
- Ok, E.; Ertas, H.; Saygili, G.; Gok, T. Effect of Photo-Activated Disinfection on Bond Strength of Three Different Root Canal Sealers. Eur. J. Dent. 2014, 8, 85–89. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yılmaz, S. Photodynamic Therapy in Dentistry: A Literature Review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Petrini, M.; Spoto, G.; Scarano, A.; D’Arcangelo, C.; Tripodi, D.; di Fermo, P.; D’Ercole, S. Near-Infrared LEDS Provide Persistent and Increasing Protection against E. Faecalis. J. Photochem. Photobiol. B 2019, 197, 111527. [Google Scholar] [CrossRef]
- Petrini, M.; Trentini, P.; Tripodi, D.; Spoto, G.; D’Ercole, S. In Vitro Antimicrobial Activity of LED Irradiation on Pseudomonas Aeruginosa. J. Photochem. Photobiol. B 2017, 168, 25–29. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; Spoto, G.; Trentini, P.; Tripodi, D.; Petrini, M. In Vitro Inactivation of Enterococcus Faecalis with a Led Device. J. Photochem. Photobiol. B 2016, 160, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Spoto, G.; de Iuliis, V.; Petrini, M.; Flati, V.; di Gregorio, J.; Vitale, D.; Caruso, M.; Dadorante, V.; Ciarmoli, M.; Robuffo, I.; et al. Effect of Low Energy Light Irradiation by Light Emitting Diode on U937 Cells. J. Biol. Regul. Homeost. Agents 2017, 30, 997–1007. [Google Scholar]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.S.; de Melo, W.C.M.A.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light Based Anti-Infectives: Ultraviolet C Irradiation, Photodynamic Therapy, Blue Light, and Beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef] [PubMed]
- Maraccini, P.A.; Wenk, J.; Boehm, A.B. Photoinactivation of Eight Health-Relevant Bacterial Species: Determining the Importance of the Exogenous Indirect Mechanism. Environ. Sci. Technol. 2016, 50, 5050–5059. [Google Scholar] [CrossRef]
- Schuch, A.P.; Menck, C.F.M. The Genotoxic Effects of DNA Lesions Induced by Artificial UV-Radiation and Sunlight. J. Photochem. Photobiol. B 2010, 99, 111–116. [Google Scholar] [CrossRef]
- Yasuda, Y.; Kawamorita, T.; Yamaguchi, H.; Saito, T. Bactericidal Effect of Nd:YAG and Er:YAG Lasers in Experimentally Infected Curved Root Canals. Photomed. Laser Surg. 2010, 28 (Suppl. 2), S75. [Google Scholar] [CrossRef]
- Saydjari, Y.; Kuypers, T.; Gutknecht, N. Laser Application in Dentistry: Irradiation Effects of Nd:YAG 1064 Nm and Diode 810 Nm and 980 Nm in Infected Root Canals-A Literature Overview. Biomed. Res. Int. 2016, 2016, 8421656. [Google Scholar] [CrossRef]
- Pirnat, S.; Lukac, M.; Ihan, A. Study of the Direct Bactericidal Effect of Nd:YAG and Diode Laser Parameters Used in Endodontics on Pigmented and Nonpigmented Bacteria. Lasers Med. Sci. 2011, 26, 755–761. [Google Scholar] [CrossRef]
- Koba, K.; Kimura, Y.; Matsumoto, K.; Takeuchi, T.; Ikarugi, T.; Shimizu, T. A Histopathological Study of the Effects of Pulsed Nd:YAG Laser Irradiation on Infected Root Canals in Dogs. J. Endod. 1999, 25, 151–154. [Google Scholar] [CrossRef]
- Ng, R.; Singh, F.; Papamanou, D.A.; Song, X.; Patel, C.; Holewa, C.; Patel, N.; Klepac-Ceraj, V.; Fontana, C.R.; Kent, R.; et al. Endodontic Photodynamic Therapy Ex Vivo. J. Endod. 2011, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Nuñez, S.C.; Hamblim, M.R.; Suzuki, H.; Ribeiro, M.S. Photodynamic Therapy Associated with Conventional Endodontic Treatment in Patients with Antibiotic-Resistant Microflora: A Preliminary Report. J. Endod. 2010, 36, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Jurič, I.B.; Plečko, V.; Pandurić, D.G.; Anić, I. The Antimicrobial Effectiveness of Photodynamic Therapy Used as an Addition to the Conventional Endodontic Re-Treatment: A Clinical Study. Photodiagnosis Photodyn. Ther. 2014, 11, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Arantes-Neto, J.G.; Sellera, D.P.; Fregnani, E.R. Effects of Antimicrobial Photodynamic Therapy and Surgical Endodontic Treatment on the Bacterial Load Reduction and Periapical Lesion Healing. Three Years Follow Up. Photodiagnosis Photodyn. Ther. 2015, 12, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Godiny, M.; Azari-Marhabi, S.; Tabatabaei, F.S.; Barati, M. Comparison of the Antibacterial Effect of 810 Nm Diode Laser and Photodynamic Therapy in Reducing the Microbial Flora of Root Canal in Endodontic Retreatment in Patients With Periradicular Lesions. J. Lasers Med. Sci. 2016, 7, 99–104. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Diagnostic Accuracy of Multiplex Real-Time PCR Approaches Compared with Cultivation -Based Detection Methods: Monitoring the Endopathogenic Microbiota Pre and Post Photo-Activated Disinfection. Photodiagnosis Photodyn. Ther. 2018, 22, 140–146. [Google Scholar] [CrossRef]
- Manukyan, I.A.; Risovanniy, S.I. Efficacy of The Root Canal System Disinfection by Photodynamic Therapy with A Low-Intensity Diode Laser and Photosensitizer “ELOFIT”. Int. Res. J. 2021, 5, 99–105. [Google Scholar] [CrossRef]
- Dragana, R.; Jelena, M.; Jovan, M.; Biljana, N.; Dejan, M. Antibacterial Efficiency of Adjuvant Photodynamic Therapy and High-Power Diode Laser in the Treatment of Young Permanent Teeth with Chronic Periapical Periodontitis. A Prospective Clinical Study. Photodiagnosis Photodyn. Ther. 2022, 103129. [Google Scholar] [CrossRef]
- Asnaashari, M.; Ashraf, H.; Rahmati, A.; Amini, N. A Comparison between Effect of Photodynamic Therapy by LED and Calcium Hydroxide Therapy for Root Canal Disinfection against Enterococcus Faecalis: A Randomized Controlled Trial. Photodiagnosis Photodyn. Ther. 2017, 17, 226–232. [Google Scholar] [CrossRef]
- Bharti, R.; Tikku, A.P.; Chandra, A.; Gupta, P. Antimicrobial Effectiveness of Photodynamic Therapy, 5% Sodium Hypochlorite and 2% Chlorhexidine Gluconate in Root Canal Treated Teeth: A Clinical Study. J. Adv. Oral Res. 2021, 12, 193–199. [Google Scholar] [CrossRef]
- Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Dataset of Endodontic Microorganisms Killed at 265 Nm Wavelength by an Ultraviolet C Light Emitting Diode in Root Canals of Extracted, Instrumented Teeth. Data Brief. 2021, 40, 107750. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Homayuni, H.; Paymanpour, P. The Antibacterial Effect of Additional Photodynamic Therapy in Failed Endodontically Treated Teeth: A Pilot Study. J. Lasers Med. Sci. 2016, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, Z.; Bidabadi, M.M.; Asnaashari, M.; Rahmati, A.; Tabatabaei, F.S. Comparison of the Antimicrobial Efficacy of Calcium Hydroxide and Photodynamic Therapy Against Enterococcus Faecalis and Candida Albicans in Teeth With Periapical Lesions; An In Vivo Study. J. Lasers Med. Sci. 2017, 8, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Ghorbanzadeh, R.; Parker, S.; Chiniforush, N.; Bahador, A. The Evaluation of Cultivable Microbiota Profile in Patients with Secondary Endodontic Infection before and after Photo-Activated Disinfection. Photodiagnosis Photodyn. Ther. 2017, 18, 198–203. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized Singlet Oxygen Generation and Detection: Recent Advances and Future Perspectives in Cancer Photodynamic Therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Mozayeni, M.A.; Vatandoost, F.; Asnaashari, M.; Shokri, M.; Azari-Marhabi, S.; Asnaashari, N. Comparing the Efficacy of Toluidine Blue, Methylene Blue and Curcumin in Photodynamic Therapy Against Enterococcus Faecalis. J. Lasers Med. Sci. 2020, 11, S49–S54. [Google Scholar] [CrossRef]
- Sperandio, F.; Huang, Y.-Y.; Hamblin, M. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Giordano, N.P.; Cian, M.B.; Dalebroux, Z.D. Outer Membrane Lipid Secretion and the Innate Immune Response to Gram-Negative Bacteria. Infect. Immun. 2020, 88, e00920-19. [Google Scholar] [CrossRef]
- Sculean, A.; Deppe, H.; Miron, R.; Schwarz, F.; Romanos, G.; Cosgarea, R. Effectiveness of Photodynamic Therapy in the Treatment of Periodontal and Peri-Implant Diseases. Monogr. Oral Sci. 2021, 29, 133–143. [Google Scholar] [CrossRef]
- Silva, L.A.B.; Novaes, A.B.; de Oliveira, R.R.; Nelson-Filho, P.; Santamaria, M.; Silva, R.A.B. Antimicrobial Photodynamic Therapy for the Treatment of Teeth with Apical Periodontitis: A Histopathological Evaluation. J. Endod. 2012, 38, 360–366. [Google Scholar] [CrossRef]
- Reinhard, A.; Sandborn, W.J.; Melhem, H.; Bolotine, L.; Chamaillard, M.; Peyrin-Biroulet, L. Photodynamic Therapy as a New Treatment Modality for Inflammatory and Infectious Conditions. Expert Rev. Clin. Immunol. 2015, 11, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.; Schenka, A.A.; Neto, A.A.; de Souza, C.P.; Rodriguez, H.M.H.; Ribeiro, M.C. Photodynamic Therapy in Endodontic Treatment of Deciduous Teeth. Lasers Med. Sci. 2009, 24, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.P.; Moreira, L.M.; de Moraes, P.C.G.; dos Santos, F.V.; de Resende, M.A. Photodynamic Therapy for Pathogenic Fungi. Mycoses 2011, 54, e265–e271. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Reiners, J.J. Photodynamic Therapy: Autophagy and Mitophagy, Apoptosis and Paraptosis. Autophagy 2020, 16, 2098–2101. [Google Scholar] [CrossRef] [PubMed]
- Boltes Cecatto, R.; Siqueira de Magalhães, L.; Fernanda Setúbal Destro Rodrigues, M.; Pavani, C.; Lino-dos-Santos-Franco, A.; Teixeira Gomes, M.; Fátima Teixeira Silva, D. Methylene Blue Mediated Antimicrobial Photodynamic Therapy in Clinical Human Studies: The State of the Art. Photodiagnosis Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef]
- Strazzi-Sahyon, H.B.; Oliveira, A.K.L.; Carvalho, A.P.; Figueiredo, R.B.; Cintra, L.T.A.; Gomes-Filho, J.E.; dos Santos, P.H.; Sivieri-Araujo, G. Influence of Photodynamic Therapy and Intracanal Medication on Martens Hardness, Elastic Modulus and Bond Strength of Glass-Fiber Posts to Endodontically Treated Root Dentin. Photodiagnosis Photodyn. Ther. 2021, 36, 102571. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Hioka, N.; Kimura, E.; Batistela, V.R.; Terada, R.S.S.; Graciano, A.X.; Baesso, M.L.; Hayacibara, M.F. Antibacterial Photodynamic Therapy for Dental Caries: Evaluation of the Photosensitizers Used and Light Source Properties. Photodiagnosis Photodyn. Ther. 2012, 9, 122–131. [Google Scholar] [CrossRef]
- Foschi, F.; Fontana, C.R.; Ruggiero, K.; Riahi, R.; Vera, A.; Doukas, A.G.; Pagonis, T.C.; Kent, R.; Stashenko, P.P.; Soukos, N.S. Photodynamic Inactivation of Enterococcus Faecalis in Dental Root Canals in Vitro. Lasers Surg. Med. 2007, 39, 782–787. [Google Scholar] [CrossRef]
- Fonseca, M.B.; Tessare, P.O.; Pallota, R.C.; Filho, H.F.; Denardin, O.V.P.; Rapoport, A.; Dedivitis, R.A.; Veronezi, J.F.; Genovese, W.J.; Ricardo, A.L.F. Photodynamic Therapy for Root Canals Infected with Enterococcus Faecalis. Photomed. Laser Surg. 2008, 26, 209–213. [Google Scholar] [CrossRef]
- Poggio, C.; Arciola, C.R.; Dagna, A.; Florindi, F.; Chiesa, M.; Saino, E.; Imbriani, M.; Visai, L. Photoactivated Disinfection (PAD) in Endodontics: An in Vitro Microbiological Evaluation. Int. J. Artif. Organs 2011, 34, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, S.; Kangarlou, A.; Shahbazi, R.; Nazari Nasab, A.; Naseri, M. Comparison of the Bactericidal Efficacy of Photodynamic Therapy, 2.5% Sodium Hypochlorite, and 2% Chlorhexidine against Enterococcous Faecalis in Root Canals; an in Vitro Study. Dent. Res. J. 2012, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- de Souza da Fonseca, A.; da Silva Sergio, L.P.; Mencalha, A.L.; de Paoli, F. Low-Power Lasers on Bacteria: Stimulation, Inhibition, or Effectless? Lasers Med. Sci. 2021, 36, 1791–1805. [Google Scholar] [CrossRef]
- Wainwright, M.; Phoenix, D.A.; Marland, J.; Wareing, D.R.A.; Bolton, F.J. A Study of Photobactericidal Activity in the Phenothiazinium Series. FEMS Immunol. Med. Microbiol. 1997, 19, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Giusti, J.S.M.; Santos-Pinto, L.; Pizzolito, A.C.; Helmerson, K.; Carvalho-Filho, E.; Kurachi, C.; Bagnato, V.S. Antimicrobial Photodynamic Action on Dentin Using a Light-Emitting Diode Light Source. Photomed. Laser Surg. 2008, 26, 281–287. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef]
- Klotz, L.O.; Pellieux, C.; Briviba, K.; Pierlot, C.; Aubry, J.M.; Sies, H. Mitogen-Activated Protein Kinase (P38-, JNK-, ERK-) Activation Pattern Induced by Extracellular and Intracellular Singlet Oxygen and UVA. Eur. J. Biochem. 1999, 260, 917–922. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. The History of PDT in Norway Part II. Recent Advances in General PDT and ALA-PDT. Photodiagnosis Photodyn. Ther. 2007, 4, 80–87. [Google Scholar] [CrossRef]
- Shi, L.; Wang, H.; Chen, K.; Yan, J.; Yu, B.; Wang, S.; Yin, R.; Nong, X.; Zou, X.; Chen, Z.; et al. Chinese Guidelines on the Clinical Application of 5-Aminolevulinic Acid-Based Photodynamic Therapy in Dermatology (2021 Edition). Photodiagnosis Photodyn. Ther. 2021, 35, 102340. [Google Scholar] [CrossRef]
- Schaechter, M.; Eisenstein, B.I. Estabelecimento de Doenças Infecciosas in: Schaechter, M.; Engleberg, N.C.; Eisenstein, B.I.; Medoff, G.Microbiologia / Mecanismo Das Doenças Infecciosas. Com. Ciências Saúde 2002, 18, 3–9. [Google Scholar]
- Reis, T.A.; Jaculi, A.E.; Ramos, K.L.V.; Souza, P.E.N.; Veiga-Souza, F.H.; Joanitti, G.A.; Azevedo, R.B.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Combination of Cyclodextrin Complexation and Iontophoresis as a Promising Strategy for the Cutaneous Delivery of Aluminum-Chloride Phthalocyanine in Photodynamic Therapy. Eur. J. Pharm. Sci. 2019, 139. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.; Nuss, A.M.; Berghoff, B.A.; Klug, G. Singlet Oxygen Stress in Microorganisms. Adv. Microb. Physiol. 2011, 58, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Bohm, G.C.; Gándara, L.; di Venosa, G.; Mamone, L.; Buzzola, F.; Casas, A. Photodynamic Inactivation Mediated by 5-Aminolevulinic Acid of Bacteria in Planktonic and Biofilm Forms. Biochem. Pharmacol. 2020, 177, 114016. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Foged, C.; Haedersdal, M.; Bik, L.; Dierickx, C.; Phillipsen, P.A.; Togsverd-Bo, K. Thermo-Mechanical Fractional Injury Enhances Skin Surface- and Epidermis- Protoporphyrin IX Fluorescence: Comparison of 5-Aminolevulinic Acid in Cream and Gel Vehicles. Lasers Surg. Med. 2021, 53, 622–629. [Google Scholar] [CrossRef]
- Ding, A.; Li, C.; Zhang, J. Topical 5-Aminolevulinic Acid Photodynamic Therapy in the Treatment of Verruca Plana: Report of 6 Cases. Photodiagnosis Photodyn. Ther. 2021, 35, 102438. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a Novel Gel Containing 5-Aminolevulinic Acid and Red LED against Bacteria Involved in Peri-Implantitis and Other Oral Infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef]
- Rossi, R.; Rispoli, L.; Lopez, M.A.; Netti, A.; Petrini, M.; Piattelli, A. Photodynamic Therapy by Mean of 5-Aminolevulinic Acid for the Management of Periodontitis and Peri-Implantitis: A Retrospective Analysis of 20 Patients. Antibiotics 2022, 11, 1267. [Google Scholar] [CrossRef]
- Yang, X.; Palasuberniam, P.; Kraus, D.; Chen, B. Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement. Int. J. Mol. Sci. 2015, 16, 25865. [Google Scholar] [CrossRef]
- Harris, F.; Pierpoint, L. Photodynamic Therapy Based on 5-Aminolevulinic Acid and Its Use as an Antimicrobial Agent. Med. Res. Rev. 2012, 32, 1292–1327. [Google Scholar] [CrossRef]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the Selectivity of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Formation. Curr. Med. Chem. Anticancer Agents 2004, 4, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Spesia, M.B.; Durantini, E.N. Evolution of Phthalocyanine Structures as Photodynamic Agents for Bacteria Inactivation. Chem. Rec. 2022, 22, e202100292. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Juzenas, P.; Moan, J. Application of 5-Aminolevulinic Acid and Its Derivatives for Photodynamic Therapy in Vitro and in Vivo. Methods Mol. Biol. 2010, 635, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Angélica Santiesteban-LÓPEZ, N.; Rosales, M.; Palou, E.; López-Malo, A. Growth Response of Escherichia Coli ATCC 35218 Adapted to Several Concentrations of Sodium Benzoate and Potassium Sorbate. J. Food Prot. 2009, 72, 2301–2307. [Google Scholar] [CrossRef]
- Aminzare, M.; Golestan, M.R.; Hashemi, M.; Amin Zare, M.; Rohani, S.M.R.; Raeisi, M.; Hosseini, S.J.; Hashemi, M. Antibacterial Effects of Monolaurin, Sorbic Acid and Potassium Sorbate on Staphylococcus aureus and Escherichia coli. J. Food Qual. Hazards Control 2014, 1, 52–55. [Google Scholar]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; Carlesi, T.; Iezzi, G.; D’arcangelo, C.; di Lodovico, S.; Piattelli, A.; D’ercole, S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus Faecalis and Human Gingival Fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 3048. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; di Piazza, S.; Chan, J.; Zotti, M.; Hanna, R.; Gheno, E.; Zekiy, A.O.; Pasquale, C.; de Angelis, N.; Amaroli, A. Newly Formulated 5% 5-Aminolevulinic Acid Photodynamic Therapy on Candida Albicans. Photodiagnosis Photodyn. Ther. 2020, 29, 101575. [Google Scholar] [CrossRef]
- Petrini, M.; Mancini, M.; Iezzi, G.; Piattelli, A.; di Campli, E.; D’ercole, S. 5-Aminolevulinic Acid and Led against Peri-Implant Disease. Dent. Cadmos 2021, 89, 358–365. [Google Scholar] [CrossRef]
- Carlesi, T.; Petrini, M.; Plotino, G.; Piattelli, A.; D’Ercole, S. Photodynamic Therapy with 5-Aminolevulinic Acid and Red LED, 660 in Vitro and in Vivo Studies. J. Endod. 2021, 7, 1–32. [Google Scholar]
- Trindade, A.C.; de Figueiredo, J.A.P.; Steier, L.; Weber, J.B.B. Photodynamic Therapy in Endodontics: A Literature Review. Photomed. Laser Surg. 2015, 33, 175–182. [Google Scholar] [CrossRef]
- Cavalli, D.; Toia, C.C.; Flores Orozco, E.I.; Khoury, R.D.; Cardoso, F.G. da R.; Alves, M.C.; Carvalho, C.A.T.; Valera, M.C. Effectiveness in the Removal of Endotoxins and Microbiological Profile in Primary Endodontic Infections Using 3 Different Instrumentation Systems: A Randomized Clinical Study. J. Endod. 2017, 43, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Cortese, T.; Grande, N.M.; Leonardi, D.P.; di Giorgio, G.; Testarelli, L.; Gambarini, G. New Technologies to Improve Root Canal Disinfection. Braz. Dent. J. 2016, 27, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bergenholtz, G. Assessment of Treatment Failure in Endodontic Therapy. J. Oral Rehabilitation 2016, 43, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Zhou, X.; Zeng, J.; Ren, Z.; Lei, L.; Kang, D.; Zhang, K.; Zou, J.; Li, Y. Inhibition of Enterococcus Faecalis Growth and Biofilm Formation by Molecule Targeting Cyclic Di-AMP Synthetase Activity. J. Endod. 2018, 44, 1381–1388.e2. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus Faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Saatchi, M.; Shokraneh, A.; Navaei, H.; Maracy, M.R.; Shojaei, H. Antibacterial Effect of Calcium Hydroxide Combined with Chlorhexidine on Enterococcus Faecalis: A Systematic Review and Meta-Analysis. J. Appl. Oral Sci. 2014, 22, 356–365. [Google Scholar] [CrossRef]
- Zancan, R.F.; Calefi, P.H.S.; Borges, M.M.B.; Lopes, M.R.M.; de Andrade, F.B.; Vivan, R.R.; Duarte, M.A.H. Antimicrobial Activity of Intracanal Medications against Both Enterococcus Faecalis and Candida Albicans Biofilm. Microsc. Res. Tech. 2019, 82, 494–500. [Google Scholar] [CrossRef]
- Soares, J.A.; Soares, S.M.C.S.; de Jesus Tavarez, R.R.; de Castro Rizzi, C.; Vaz Rodrigues, S.C.G.; Maia Filho, E.M.; Brito-Júnior, M.; Pereira, R.D.; Magalhães, P.P.; de Macêdo Farias, L. Exploring Different Photodynamic Therapy Parameters to Optimize Elimination of Enterococcus Faecalis in Planktonic Form. Photodiagnosis Photodyn. Ther. 2018, 22, 127–131. [Google Scholar] [CrossRef]
- Nagayoshi, M.; Nishihara, T.; Nakashima, K.; Iwaki, S.; Chen, K.-K.; Terashita, M.; Kitamura, C. Bactericidal Effects of Diode Laser Irradiation on Enterococcus Faecalis Using Periapical Lesion Defect Model. ISRN Dent. 2011, 2011, 870364. [Google Scholar] [CrossRef]
- Garcez, A.S.; Nuñez, S.C.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial Effects of Photodynamic Therapy on Patients with Necrotic Pulps and Periapical Lesion. J. Endod. 2008, 34, 138–142. [Google Scholar] [CrossRef]
- Zorita-García, M.; Alonso-Ezpeleta, L.Ó.; Cobo, M.; del Campo, R.; Rico-Romano, C.; Mena-Álvarez, J.; Zubizarreta-Macho, Á. Photodynamic Therapy in Endodontic Root Canal Treatment Significantly Increases Bacterial Clearance, Preventing Apical Periodontitis. Quintessence Int. 2019, 50, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N. Optimising Single-Visit Disinfection with Supplementary Approaches: A Quest for Predictability. Aust. Endod. J. 2011, 37, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rabello, D.G.D.; Corazza, B.J.M.; Ferreira, L.L.; Santamaria, M.P.; Gomes, A.P.M.; Martinho, F.C. Does Supplemental Photodynamic Therapy Optimize the Disinfection of Bacteria and Endotoxins in One-Visit and Two-Visit Root Canal Therapy? A Randomized Clinical Trial. Photodiagnosis Photodyn. Ther. 2017, 19, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tennert, C.; Drews, A.M.; Walther, V.; Altenburger, M.J.; Karygianni, L.; Wrbas, K.T.; Hellwig, E.; Al-Ahmad, A. Ultrasonic Activation and Chemical Modification of Photosensitizers Enhances the Effects of Photodynamic Therapy against Enterococcus Faecalis Root-Canal Isolates. Photodiagnosis Photodyn. Ther. 2015, 12, 244–251. [Google Scholar] [CrossRef]
- Fimple, J.L.; Fontana, C.R.; Foschi, F.; Ruggiero, K.; Song, X.; Pagonis, T.C.; Tanner, A.C.R.; Kent, R.; Doukas, A.G.; Stashenko, P.P.; et al. Photodynamic Treatment of Endodontic Polymicrobial Infection in Vitro. J. Endod. 2008, 34, 728–734. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic Therapy in Endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- Nunes, G.P.; Delbem, A.C.B.; Gomes, J.M.L.; Lemos, C.A.A.; Pellizzer, E.P. Postoperative Pain in Endodontic Retreatment of One Visit versus Multiple Visits: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Oral Investig. 2021, 25, 455–468. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Awan, K.H.; Javed, F. Bactericidal Efficacy of Photodynamic Therapy against Enterococcus Faecalis in Infected Root Canals: A Systematic Literature Review. Photodiagnosis Photodyn. Ther. 2013, 10, 632–643. [Google Scholar] [CrossRef]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-Based Endodontic Antimicrobial Photodynamic Therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef]
- Kishen, A.; Upadya, M.; Tegos, G.P.; Hamblin, M.R. Efflux Pump Inhibitor Potentiates Antimicrobial Photodynamic Inactivation of Enterococcus Faecalis Biofilm. Photochem. Photobiol. 2010, 86, 1343–1349. [Google Scholar] [CrossRef]
- Rios, A.; He, J.; Glickman, G.N.; Spears, R.; Schneiderman, E.D.; Honeyman, A.L. Evaluation of Photodynamic Therapy Using a Light-Emitting Diode Lamp against Enterococcus Faecalis in Extracted Human Teeth. J. Endod. 2011, 37, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Raoofian, R.; Ghorbanzadeh, R.; Bahador, A. An Experimental Study for Rapid Detection and Quantification of Endodontic Microbiota Following Photo-Activated Disinfection via New Multiplex Real-Time PCR Assay. Photodiagnosis Photodyn. Ther. 2018, 21, 344–350. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Chiniforush, N.; Shahabi, S.; Ghorbanzadeh, R.; Bahador, A. Sub-Lethal Doses of Photodynamic Therapy Affect Biofilm Formation Ability and Metabolic Activity of Enterococcus Faecalis. Photodiagnosis Photodyn. Ther. 2016, 15, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Chiniforush, N.; Pourhajibagher, M.; Shahabi, S.; Kosarieh, E.; Bahador, A. Can Antimicrobial Photodynamic Therapy (APDT) Enhance the Endodontic Treatment? J. Lasers Med. Sci. 2016, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Borba, A.S.M.; da Silva Pereira, S.M.; Borba, M.C.M.; Paschoal, M.A.B.; de Jesus Tavarez, R.R.; de Castro Rizzi, C.; Ferreira, M.C.; Maia Filho, E.M. Photodynamic Therapy with High-Power LED Mediated by Erythrosine Eliminates Enterococcus Faecalis in Planktonic Forms. Photodiagnosis Photodyn. Ther. 2017, 19, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus Faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-Activated Nanoparticles: An In Vitro Study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The Effect of Indocyanine Green Loaded on a Novel Nano-Graphene Oxide for High Performance of Photodynamic Therapy against Enterococcus Faecalis. Photodiagnosis Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef]
- Beltes, C.; Sakkas, H.; Economides, N.; Papadopoulou, C. Antimicrobial Photodynamic Therapy Using Indocyanine Green and Near-Infrared Diode Laser in Reducing Entrerococcus Faecalis. Photodiagnosis Photodyn. Ther. 2017, 17, 5–8. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; Aguiar, C.M.; Câmara, A.C.; de Albuquerque, M.M.; Correia, A.C.R. de B.; Soares, M.F. de L.R. The Efficacy of Photodynamic Therapy and Sodium Hypochlorite in Root Canal Disinfection by a Single-File Instrumentation Technique. Photodiagnosis Photodyn. Ther. 2015, 12, 436–443. [Google Scholar] [CrossRef]
- Schlafer, S.; Vaeth, M.; Hørsted-Bindslev, P.; Frandsen, E.V.G. Endodontic Photoactivated Disinfection Using a Conventional Light Source: An in Vitro and Ex Vivo Study. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 634–641. [Google Scholar] [CrossRef]
- Darmani, H.; Tawalbeh, K.H.; Al-Hiyasat, A.S.; Al-Akhras, M.A. Comparison of the Photosensitivity of Biofilms of Different Genera of Cariogenic Bacteria in Tooth Slices. Pol. J. Microbiol. 2018, 67, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.; Cheng, J.L.; Lim, T.W.; Teo, E.G.; Wong, J.; George, S.; Kishen, A. Light Activated Disinfection: An Alternative Endodontic Disinfection Strategy. Aust. Dent. J. 2009, 54, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guan, S.; Lu, H.; Zhao, C.; Chen, X.; Li, N.; Bai, Q.; Tian, Y.; Yu, Q. Evaluation of the Bactericidal Effect of Nd:YAG, Er:YAG, Er,Cr:YSGG Laser Radiation, and Antimicrobial Photodynamic Therapy (APDT) in Experimentally Infected Root Canals. Lasers Surg. Med. 2012, 44, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Silva Garcez, A.; Núñez, S.C.; Lage-Marques, J.L.; Jorge, A.O.C.; Ribeiro, M.S. Efficiency of NaOCl and Laser-Assisted Photosensitization on the Reduction of Enterococcus Faecalis in Vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e93–e98. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Brito, P.R.R.; Machado de Oliveira, J.C.; Alves, F.R.F.; Moreira, E.J.L.; Sampaio-Filho, H.R.; Rôças, I.N.; Siqueira, J.F. Photodynamic Therapy with Two Different Photosensitizers as a Supplement to Instrumentation/Irrigation Procedures in Promoting Intracanal Reduction of Enterococcus Faecalis. J. Endod. 2010, 36, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.G.; Santos, E.B.; Souto, R.M.; Gusman, H.; Colombo, A.P.V. Ex Vivo Antimicrobial Efficacy of the EndoVac System plus Photodynamic Therapy Associated with Calcium Hydroxide against Intracanal Enterococcus Faecalis. Int. Endod. J. 2013, 46, 499–505. [Google Scholar] [CrossRef]
- da Silva, C.C.; Chaves Júnior, S.P.; Pereira, G.L.D.; da C. Fontes, K.B.F.; Antunes, L.A.A.; Póvoa, H.C.C.; Antunes, L.S.; Iorio, N.L.P.P. Antimicrobial Photodynamic Therapy Associated with Conventional Endodontic Treatment: A Clinical and Molecular Microbiological Study. Photochem. Photobiol. 2018, 94, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Bonsor, S.J.; Nichol, R.; Reid, T.M.S.; Pearson, G.J. An Alternative Regimen for Root Canal Disinfection. Br. Dent. J. 2006, 201, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, C.B.; Bussadori, S.K.; Prates, R.A.; da Mota, A.C.C.; Tempestini Horliana, A.C.R.; Fernandes, K.P.S.; Motta, L.J. Photodynamic Therapy for Endodontic Treatment of Primary Teeth: A Randomized Controlled Clinical Trial. Photodiagnosis Photodyn. Ther. 2020, 30, 101732. [Google Scholar] [CrossRef] [PubMed]
- Kosarieh, E.; Khavas, S.S.; Rahimi, A.; Chiniforush, N.; Gutknecht, N. The Comparison of Penetration Depth of Two Different Photosensitizers in Root Canals with and without Smear Layer: An in Vitro Study. Photodiagnosis Photodyn. Ther. 2016, 13, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.D.S.; Mello, I.; Albergaria, S.J.; Habitante, S.M.; Lage-Marques, J.L.; Raldi, D.P. Effect of Chemical Substances in Removing Methylene Blue after Photodynamic Therapy in Root Canal Treatment. Photomed. Laser Surg. 2011, 29, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Moreo, G.; Palmieri, A.; della Vella, F.; Petruzzi, M.; Botticelli, D.; Carinci, F. Photodynamic Therapy Using 5-Aminolevulinic Acid (Ala) for the Treatment of Chronic Periodontitis: A Prospective Case Series. Appl. Sci. 2022, 12, 3102. [Google Scholar] [CrossRef]
- Abdelkarim-Elafifi, H.; Parada-Avendaño, I.; Arnabat-Dominguez, J. Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review. Antibiotics 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Prazmo, E.J.; Kwaśny, M.; Łapiński, M.; Mielczarek, A. Photodynamic Therapy As a Promising Method Used in the Treatment of Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the Outcome of Various Laser Therapy Applications in Root Canal Disinfection: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef] [PubMed]



| Light Source | Wavelength (nm) | Irradiation Time (s/m) | Authors/Year |
|---|---|---|---|
| Diode laser | 660 | 240 s | Garcez et al., 2010 [43] |
| Helbo laser | 660 | 60 s | Juric et al., 2014 [44] |
| Diode laser | 660 | 360 s | Garcez et al., 2015 [45] |
| Diode laser | 810 | 40 s | Asnaashari et al., 2016 [46] |
| Diode laser | 665 | 240 s | Asnaashari et al., 2016 [53] |
| LED | 880 | 5, 10 and 20 min | D’Ercole et al., 2016 [33] |
| Diode laser | 810 | 10 s | Ahangari et al., 2017 [54] |
| LED | 630 | 60 s | Asnaashari et al., 2017 [50] |
| Diode laser | 635 | 30 s | Pourhajibagher et al., 2017 [55] |
| LED | 880 | 5 min | Petrini et al., 2019 [31] |
| LED | 628 | 60 s | Bharti et al., 2021 [51] |
| Diode laser | 662 | 40 s | Manukyan & Risovanniy, 2021 [48] |
| Diode laser | 940 | 60 s | Dragana et al., 2022 [49] |
| LED | 265 and 280 | 30, 60 and 90 s | Morio et al., 2022 [52] |
| In Vivo Test | CFU/mL |
|---|---|
| 1 sample (After working length) | 420 |
| 2 sample (After ALAD 45 min) | 210 |
| 3 sample (After ALAD/LED 7 min) | 0 |
| Garced et al., 2008 [111] | Polyethyleneimine Conjugate and Chloro-e6 Activated by Laser Fiber, Increased the Effectiveness of the Traditional Chemo Mechanical Therapy |
| Pourhajibagher and Bahador, 2018 [47] | PDT with toluidine blue was effective in decreasing bacterial load in endodontic infections on necrotic teeth not endodontically treated |
| Bharti et al., 2021 [51] | PDT with toluidine blue activated by LED light was efficacy in teeth with periapical lesions |
| Garcia-Zorita et al., 2019 [112] | PDT induced a reduction in bacterial load |
| Okamoto et al., 2020 [140] | CMP combined with antimicrobial photoactivation was effective, but not statistically significant efficacy alone conventional endodontic treatment |
| Silva et al., 2012 [62] | PDT may be a promising adjunct therapy to cleaning and shaping procedures in teeth affected by apical periodontitis treated in a single session |
| Lauritano et al., 2022 [143] | Sites treated with the combination of SRP and ALAD gel showed a significantly reduced total bacterial loading compared to the SRP treated sites |
| Rossi R et al., 2022 [89] | ALAD-PDT was a support for the conventional treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ercole, S.; Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Tripodi, D.; Iezzi, G.; Piattelli, A.; Petrini, M. 5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report. Gels 2022, 8, 697. https://doi.org/10.3390/gels8110697
D’Ercole S, Carlesi T, Dotta TC, Pierfelice TV, D’Amico E, Tripodi D, Iezzi G, Piattelli A, Petrini M. 5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report. Gels. 2022; 8(11):697. https://doi.org/10.3390/gels8110697
Chicago/Turabian StyleD’Ercole, Simonetta, Teocrito Carlesi, Tatiane Cristina Dotta, Tania Vanessa Pierfelice, Emira D’Amico, Domenico Tripodi, Giovanna Iezzi, Adriano Piattelli, and Morena Petrini. 2022. "5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report" Gels 8, no. 11: 697. https://doi.org/10.3390/gels8110697
APA StyleD’Ercole, S., Carlesi, T., Dotta, T. C., Pierfelice, T. V., D’Amico, E., Tripodi, D., Iezzi, G., Piattelli, A., & Petrini, M. (2022). 5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report. Gels, 8(11), 697. https://doi.org/10.3390/gels8110697