Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement
Abstract
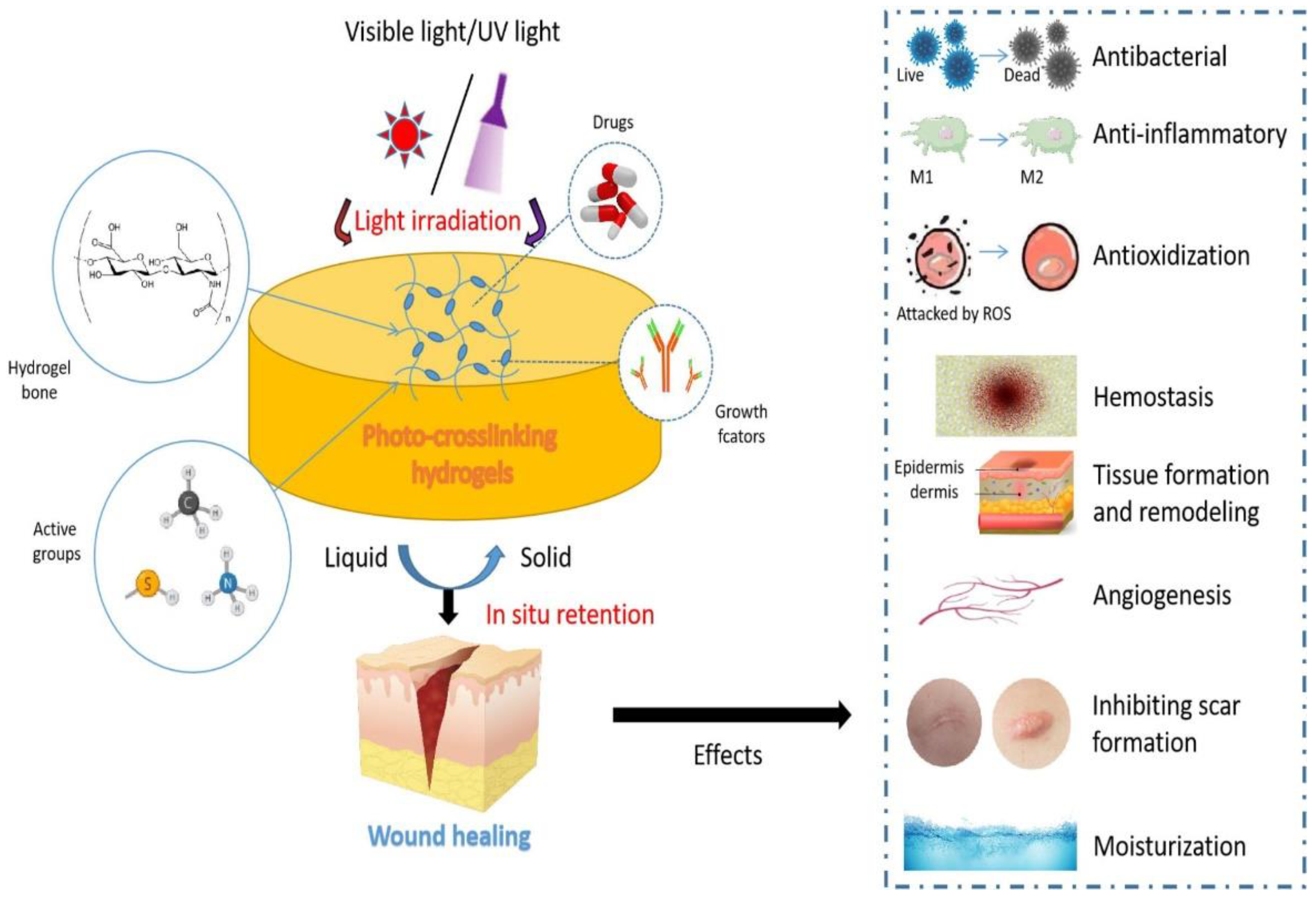
1. Introduction
2. The Design of Photo-Crosslinking Hydrogels for Wound Healing
2.1. Hyaluronic Acid
2.2. Gelatin
2.3. Natural Fibrin
2.4. Natural Chitosan Polymer
2.5. Alginate
2.6. Polyethylene Glycol (PEG)
2.7. Decellularized Extracellular Matrix (dECM)
3. Effects of Photo-Crosslinking Hydrogels in Wound Healing
3.1. Antibacteria
3.2. Anti-Inflammatory
3.3. Anti-Oxidization
3.4. Hemostasis
3.5. Tissue Formation and Remodeling
3.6. Promoting Angiogenesis
3.7. Inhibiting Scar Formation
3.8. Water Retaining Capability
4. Application of Animal Models for Wound Healing
4.1. Rat/Mouse Full-Thickness Skin Defect Model
4.2. Bleeding Model (Mouse Tail Model and Mouse Liver Bleeding Model)
4.3. Rabbit Ear Hypertrophic Scar Model
4.4. Pig Skin Defect Model
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing-aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.; Havran, W.L. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol. Rev. 2007, 215, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Keylock, K.T.; Vieira, V.J.; Wallig, M.A.; DiPietro, L.A.; Schrementi, M.; Woods, J.A. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R179–R184. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Ashworth, J.J.; Ashcroft, G.S. The hormonal regulation of cutaneous wound healing. Clin. Dermatol. 2007, 25, 56–62. [Google Scholar] [CrossRef]
- Hardman, M.J.; Ashcroft, G.S. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 2008, 9, R80. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Wilson, J.A.; Clark, J.J. Obesity: Impediment to postsurgical wound healing. Adv. Skin Wound Care 2004, 17, 426–435. [Google Scholar] [CrossRef]
- Franz, M.G.; Steed, D.L.; Robson, M.C. Optimizing healing of the acute wound by minimizing complications. Curr. Probl. Surg. 2007, 44, 691–763. [Google Scholar] [CrossRef]
- Pieringer, H.; Stuby, U.; Biesenbach, G. Patients with rheumatoid arthritis undergoing surgery: How should we deal with antirheumatic treatment? Semin. Arthritis Rheum. 2007, 36, 278–286. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Choudhry, M.A.; Chaudry, I.H. Alcohol intoxication and post-burn complications. Front. Biosci.-Landmark 2006, 11, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Siana, J.E.; Rex, S.; Gottrup, F. The effect of cigarette smoking on wound healing. Scand. J. Plast Reconstr. Surg. Hand Surg. 1989, 23, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Barbul, A. Nutrition and wound healing. Plast. Reconstr. Surg. 2006, 117, 42S–58S. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, J.; Mi, S. Photo Processing for Biomedical Hydrogels Design and Functionality: A Review. Polymers 2017, 10, 11. [Google Scholar] [CrossRef]
- Hao, R.; Cui, Z.; Zhang, X.; Tian, M.; Zhang, L.; Rao, F.; Xue, J. Rational Design and Preparation of Functional Hydrogels for Skin Wound Healing. Front. Chem. 2022, 9, 839055. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Peng, X.; He, Y.; Wei, L.; Su, W.; Wu, J.; Cui, L.; Liu, Z.; Guo, X. Photocatalytic antibacterial agent incorporated double-network hydrogel for wound healing. Colloids Surf. B Biointerfaces 2019, 180, 237–244. [Google Scholar] [CrossRef]
- Li, P.; She, W.; Luo, Y.; He, D.; Chen, J.; Ning, N.; Yu, Y.; de Beer, S.; Zhang, S. One-pot, self-catalyzed synthesis of self-adherent hydrogels for photo-thermal, antimicrobial wound treatment. J. Mater. Chem. B 2021, 9, 159–169. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Thet, N.T.; Alves, D.R.; Bean, J.E.; Booth, S.; Nzakizwanayo, J.; Young, A.E.; Jones, B.V.; Jenkins, A.T. Prototype Development of the Intelligent Hydrogel Wound Dressing and Its Efficacy in the Detection of Model Pathogenic Wound Biofilms. ACS Appl. Mater. Interfaces 2016, 8, 14909–14919. [Google Scholar] [CrossRef]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Xia, H.; Jia, L.; Zhao, J.; Zhao, D.; Yan, X.; Zhang, Y.; Tang, S.; Zhou, G.; Zhu, L.; et al. Ultrafast, tough, and adhesive hydrogel based on hybrid photocrosslinking for articular cartilage repair in water-filled arthroscopy. Sci. Adv. 2021, 7, eabg0628. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Li, Y.; Wang, Y.; Bao, C.; Chen, Y.; Lin, Q.; Zhu, L. A postoperative anti-adhesion barrier based on photoinduced imine-crosslinking hydrogel with tissue-adhesive ability. Acta Biomater. 2017, 62, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Z.; Chen, Z.; Wang, D. Application of skin traction for surgical treatment of grade IV pressure sore: A clinical report of 160 cases. Spinal Cord 2011, 49, 76–80. [Google Scholar] [CrossRef]
- Kushibiki, T.; Mayumi, Y.; Nakayama, E.; Azuma, R.; Ojima, K.; Horiguchi, A.; Ishihara, M. Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor. Sci. Rep. 2021, 11, 23094. [Google Scholar] [CrossRef]
- Wang, T.; Mu, X.; Li, H.; Wu, W.; Nie, J.; Yang, D. The photocrosslinkable tissue adhesive based on copolymeric dextran/HEMA. Carbohydr. Polym. 2013, 92, 1423–1431. [Google Scholar] [CrossRef]
- Zou, C.Y.; Lei, X.X.; Hu, J.J.; Jiang, Y.L.; Li, Q.J.; Song, Y.T.; Zhang, Q.Y.; Li-Ling, J.; Xie, H.Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2021, 8, 341–354. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, B.; Jiang, F.; Zhang, Y.; Zhou, R.; Lu, Y.; Lin, S.; Lin, Q.; Jiang, X.; Zhu, L. Promoting Oral Mucosal Wound Healing with a Hydrogel Adhesive Based on a Phototriggered S-Nitrosylation Coupling Reaction. Adv. Mater. 2021, 33, e2105667. [Google Scholar] [CrossRef]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Lee, J.; Hua, J.; Li, S.; Panchamukhi, A.; Yue, J.; Gou, X.; Xia, Z.; Zhu, L.; et al. A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nat. Commun. 2021, 12, 1670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.J. Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J. Control. Release 2020, 323, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Li, M.N.; Yu, H.P.; Ke, Q.F.; Zhang, C.Q.; Gao, Y.S.; Guo, Y.P. Gelatin methacryloyl hydrogels functionalized with endothelin-1 for angiogenesis and full-thickness wound healing. J. Mater. Chem. B 2021, 9, 4700–4709. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, C.; Jiang, Y.; Huang, J.; Liu, Y.; Nthumba, P.M.; Gu, G.; Wu, X.; Zhao, Y.; Ren, J. Engineering an adhesive based on photosensitive polymer hydrogels and silver nanoparticles for wound healing. J. Mater. Chem B 2020, 8, 5756–5764. [Google Scholar] [CrossRef]
- Pang, L.; Tian, P.; Cui, X.; Wu, X.; Zhao, X.; Wang, H.; Wang, D.; Pan, H. In Situ Photo-Cross-Linking Hydrogel Accelerates Diabetic Wound Healing through Restored Hypoxia-Inducible Factor 1-Alpha Pathway and Regulated Inflammation. ACS Appl. Mater. Interfaces 2021, 13, 29363–29379. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Yuan, Q.; Gu, Z.; Wu, J. Tofu-Based Hybrid Hydrogels with Antioxidant and Low Immunogenicity Activity for Enhanced Wound Healing. J. Biomed. Nanotechnol. 2019, 15, 1371–1383. [Google Scholar] [CrossRef]
- Yao, S.; Chi, J.; Wang, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Zn-MOF Encapsulated Antibacterial and Degradable Microneedles Array for Promoting Wound Healing. Adv. Healthc. Mater. 2021, 10, e2100056. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Wang, H.; Qiao, L.; Jiang, Y.; Ma, G.; Zhang, W.; Hu, Z. Photo-induced adhesive carboxymethyl chitosan-based hydrogels with antibacterial and antioxidant properties for accelerating wound healing. Carbohydr. Polym. 2022, 278, 119000. [Google Scholar] [CrossRef]
- Qi, C.; Xu, L.; Deng, Y.; Wang, G.; Wang, Z.; Wang, L. Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 2018, 6, 2859–2870. [Google Scholar] [CrossRef]
- Chandika, P.; Kim, M.S.; Khan, F.; Kim, Y.M.; Heo, S.Y.; Oh, G.W.; Kim, N.G.; Jung, W.K. Wound healing properties of triple cross-linked poly (vinyl alcohol)/methacrylate kappa-carrageenan/chitooligosaccharide hydrogel. Carbohydr. Polym. 2021, 269, 118272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chang, C.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J.; Meng, Z.; Cui, W.; Ge, Z. Photo-crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater. 2021, 125, 197–207. [Google Scholar] [CrossRef]
- Wang, L.; Yang, K.; Li, X.; Zhang, X.; Zhang, D.; Wang, L.N.; Lee, C.S. A double-crosslinked self-healing antibacterial hydrogel with enhanced mechanical performance for wound treatment. Acta Biomater. 2021, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Han, Z.; Zhang, H.; Yang, R.; Fang, J.; Wang, L.; Li, X.; Li, X. Tea polyphenol/glycerol-treated double-network hydrogel with enhanced mechanical stability and anti-drying, antioxidant and antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2022, 208, 530–543. [Google Scholar] [CrossRef]
- Mei, J.; Zhou, J.; Kong, L.; Dai, Y.; Zhang, X.; Song, W.; Zhu, C. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopaedic surgery through spatiotemporal immunomodulation. J. Nanobiotechnology 2022, 20, 232. [Google Scholar] [CrossRef]
- Popescu, I.; Turtoi, M.; Suflet, D.M.; Dinu, M.V.; Darie-Nita, R.N.; Anghelache, M.; Calin, M.; Constantin, M. Alginate/poloxamer hydrogel obtained by thiol-acrylate photopolymerization for the alleviation of the inflammatory response of human keratinocytes. Int. J. Biol. Macromol. 2021, 180, 418–431. [Google Scholar] [CrossRef]
- Li, L.; Lu, C.; Wang, L.; Chen, M.; White, J.; Hao, X.; McLean, K.M.; Chen, H.; Hughes, T.C. Gelatin-Based Photocurable Hydrogels for Corneal Wound Repair. ACS Appl. Mater. Interfaces 2018, 10, 13283–13292. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, M.; Yuan, M.; Shi, X.; Song, J.; He, Y.; Mao, H.; Kong, D.; Gu, Z. Gallium(III)-Mediated Dual-Cross-Linked Alginate Hydrogels with Antibacterial Properties for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 22426–22442. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, Y.; Jin, J.; Wu, X.; Xu, K.; Dai, M.; Niu, Q.; Zheng, H.; He, X.; Shen, J. Immunoregulation in Diabetic Wound Repair with a Photoenhanced Glycyrrhizic Acid Hydrogel Scaffold. Adv. Mater. 2022, 34, e2200521. [Google Scholar] [CrossRef]
- Bian, S.; Zheng, Z.; Liu, Y.; Ruan, C.; Pan, H.; Zhao, X. A shear-thinning adhesive hydrogel reinforced by photo-initiated crosslinking as a fit-to-shape tissue sealant. J. Mater. Chem. B 2019, 7, 6488–6499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Z.; Wei, Q.; Ma, K.; Hu, W.; Huang, Q.; Su, J.; Li, H.; Zhang, C.; Fu, X. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022, 147, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, L.; Wicks, J.; Ling, C.; Zhao, X.; Yan, Y.; Qi, J.; Cui, W.; Deng, L. Quickly promoting angiogenesis by using a DFO-loaded photo-crosslinked gelatin hydrogel for diabetic skin regeneration. J. Mater. Chem. B 2016, 4, 3770–3781. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Hyun, H.; Lee, D.W.; Yang, D.H. Visible Light-Cured Glycol Chitosan Hydrogel Containing a Beta-Cyclodextrin-Curcumin Inclusion Complex Improves Wound Healing In Vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Shu, F.; Zhou, R.; Bao, B.; Xiao, S.; Li, K.; Lin, Q.; Zhu, L.; Xia, Z. In situ-formed adhesive hyaluronic acid hydrogel with prolonged amnion-derived conditioned medium release for diabetic wound repair. Carbohydr. Polym. 2022, 276, 118752. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Rao, J.; Bei, H.P.; Liu, Y.; Zhao, X. 3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair. Int. J. Bioprinting 2021, 7, 367. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018, 1059, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Thompson, R.; Palmer, J.W.; Khorazo, D. The nature of lysozyme action. Science 1934, 79, 61. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid-Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem. Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- DeBari, M.K.; King CI Altgold, T.A., 3rd; Abbott, R.D. Silk Fibroin as a Green Material. ACS Biomater. Sci. Eng. 2021, 7, 3530–3544. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Gilchrist, T.; Martin, A.M. Wound treatment with Sorbsan—An alginate fibre dressing. Biomaterials 1983, 4, 317–320. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.B.; Tang, Q.Y.; Chen, X.Y.; Tu, Y.; Sun, S.Z.; Sun, Z.L. Polyethylene glycol as a promising synthetic material for repair of spinal cord injury. Neural Regen. Res. 2017, 12, 1003–1008. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.C.; Barragan, M.; Tadros, M.H.; Gehrke, S.H.; Detamore, M.S. Approaching the compressive modulus of articular cartilage with a decellularized cartilage-based hydrogel. Acta Biomater. 2016, 38, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.K.; Han, T.T.; Marecak, D.M.; Watkins, J.F.; Amsden, B.G.; Flynn, L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 2014, 35, 1914–1923. [Google Scholar] [CrossRef]
- Visser, J.; Levett, P.A.; te Moller, N.C.; Besems, J.; Boere, K.W.; van Rijen, M.H.; de Grauw, J.C.; Dhert, W.J.; van Weeren, P.R.; Malda, J. Crosslinkable hydrogels derived from cartilage, meniscus, and tendon tissue. Tissue Eng. Part A 2015, 21, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Khan, A.U.R.; Zheng, H.; Li, X.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; Li, J.; Wu, J.; Mo, X. A photocrosslinking antibacterial decellularized matrix hydrogel with nanofiber for cutaneous wound healing. Colloids Surf. B Biointerfaces 2022, 217, 112691. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Coluccino, L.; Gottardi, R.; Ceseracciu, L.; Scaglione, S.; Goldoni, L.; Tuan, R.S. Efficacy of thermoresponsive, photocrosslinkable hydrogels derived from decellularized tendon and cartilage extracellular matrix for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e159–e170. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Ni, X.; Little, P.J.; Xu, S.; Tang, L.; Weng, J. Metformin, Macrophage Dysfunction and Atherosclerosis. Front. Immunol. 2021, 12, 682853. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, Ł.; Łabuzek, K.; Bułdak, R.J.; Kozłowski, M.; Machnik, G.; Liber, S.; Suchy, D.; Duława-Bułdak, A.; Okopień, B. Metformin affects macrophages’ phenotype and improves the activity of glutathione peroxidase, superoxide dismutase, catalase and decreases malondialdehyde concentration in a partially AMPK-independent manner in LPS-stimulated human monocytes/macrophages. Pharmacol. Rep. 2014, 66, 418–429. [Google Scholar] [CrossRef]
- Shi, X.M.; Xu, G.M.; Zhang, G.J.; Liu, J.R.; Wu, Y.M.; Gao, L.G.; Yang, Y.; Chang, Z.S.; Yao, C.W. Low-temperature Plasma Promotes Fibroblast Proliferation in Wound Healing by ROS-activated NF-κB Signaling Pathway. Curr. Med. Sci. 2018, 38, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.A.; Hee Hong, S.; Hee Lee, M.; Kwon, B.J.; Mi Seon, G.; Sung Kim, M.; Kim, D.; Chang Nam, K.; Park, J.C. Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 2019, 95, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, H.; Wu, Y. Knockdown of Dual Oxidase 1 (DUOX1) Promotes Wound Healing by Regulating Reactive Oxygen Species (ROS) by Activation of Nuclear Kactor kappa B (NF-κB) Signaling. Med. Sci. Monit. 2021, 27, e926492. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Bakker, K.; Apelqvist, J.; Lipsky, B.A.; Van Netten, J.J.; International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: Development of an evidence-based global consensus. Diabetes Metab. Res. Rev. 2016, 32, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Botusan, I.R.; Sunkari, V.G.; Savu, O.; Catrina, A.I.; Grünler, J.; Lindberg, S.; Pereira, T.; Ylä-Herttuala, S.; Poellinger, L.; Brismar, K.; et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Fox-Talbot, K.; Steenbergen, C.; Bosch-Marcé, M.; Semenza, G.L. Adenoviral transfer of HIF-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc. Natl. Acad. Sci. USA 2009, 106, 18769–18774. [Google Scholar] [CrossRef]
- Patel, T.H.; Kimura, H.; Weiss, C.R.; Semenza, G.L.; Hofmann, L.V. Constitutively active HIF-1alpha improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovasc. Res. 2005, 68, 144–154. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Rezaie, J. Application of stem cell-derived exosomes in ischemic diseases: Opportunity and limitations. J. Transl. Med. 2021, 19, 196. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burns Trauma. 2012, 2, 18–28. [Google Scholar] [PubMed]
- Finnson, K.W.; Arany, P.R.; Philip, A. Transforming Growth Factor Beta Signaling in Cutaneous Wound Healing: Lessons Learned from Animal Studies. Adv. Wound Care 2013, 2, 225–237. [Google Scholar] [CrossRef]
- Junker, J.P.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Potthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C 2020, 110, 110619. [Google Scholar] [CrossRef] [PubMed]
- Deal, H.E.; Brown, A.C.; Daniele, M.A. Microphysiological systems for the modeling of wound healing and evaluation of pro-healing therapies. J. Mater. Chem. B 2020, 8, 7062–7075. [Google Scholar] [CrossRef]
- Perez, R.; Davis, S.C. Relevance of animal models for wound healing. Wounds 2008, 20, 3–8. [Google Scholar]
- Ansell, D.M.; Holden, K.A.; Hardman, M.J. Animal models of wound repair: Are they cutting it? Exp. Dermatol. 2012, 21, 581–585. [Google Scholar] [CrossRef]
- Razzell, W.; Wood, W.; Martin, P. Swatting flies: Modelling wound healing and inflammation in Drosophila. Dis. Model. Mech. 2011, 4, 569–574. [Google Scholar] [CrossRef]
- Henry, K.M.; Loynes, C.A.; Whyte, M.K.; Renshaw, S.A. Zebrafish as a model for the study of neutrophil biology. J. Leukoc. Biol. 2013, 94, 633–642. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011, 2011, 969618. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef]
- Gerber, P.A.; Buhren, B.A.; Schrumpf, H.; Homey, B.; Zlotnik, A.; Hevezi, P. The top skin-associated genes: A comparative analysis of human and mouse skin transcriptomes. Biol. Chem. 2014, 395, 577–591. [Google Scholar] [CrossRef]
- Kloeters, O.; Tandara, A.; Mustoe, T.A. Hypertrophic scar model in the rabbit ear: A reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007, 15, S40–S45. [Google Scholar] [CrossRef]
- Morris, D.E.; Wu, L.; Zhao, L.L.; Bolton, L.; Roth, S.I.; Ladin, D.A.; Mustoe, T.A. Acute and chronic animal models for excessive dermal scarring: Quantitative studies. Plast. Reconstr. Surg. 1997, 100, 674–681. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Bristol, D.G.; Manning, T.O.; Rogers, R.A.; Riviere, J.E. Interspecies and interregional analysis of the comparative histologic thickness and laser Doppler blood flow measurements at five cutaneous sites in nine species. J. Investig. Dermatol. 1990, 95, 582–586. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]

| Hydrogels | Design | Effects | Animal Models | Application Prospect | Ref. | |
|---|---|---|---|---|---|---|
| Hydrogel Backbones | Chemical Modification | |||||
| Poly-lactic-co-glycolic acid (PLGA) imine-crosslinking hydrogels | Hyaluronic acid (HA) | o-Nitrobenzene (NB), carbohydrazide (CDH) | TGF-β inhibitor |
| Tissue adhesive, scar inhibitor | [45] |
| Photoresponse supramolecular hyaluronic acid hydrogels | HA | Azobenzenes (Azo), β-cyclodextrin (CD) | EGF delivery (improving granulation tissue formation, high growth factor levels, and angiogenesis) | Rat full-thickness skin defect model | New smart dressings | [46] |
| Photo-crosslinking GelMA-ET-1 hydrogels | Gelatin (Gel) | methacryloyl (MA) | ET-1 delivery (accelerating new blood vessel formation, collagen deposition, and re-epithelialization) | Rat full-thickness skin defect model | Wound dressings | [47] |
| Methacrylated hyaluronan–polyacrylamide (MHA–PAAm) hydrogels | HA | MA | Antibacterial and hemostatic activities Tissue formation (enhancing wound granulation tissue formation, vascular tissue formation, and collagen formation, as well as alleviating inflammation) |
| Skin adhesive | [48] |
| SF-MA-BS hydrogels | Silk fibroin (SF) | MA | Antibacterial Angiogenesis by restoring the HIF-1α pathway Transformation of macrophages to M2 type | Rat streptozotocin-induced diabetic wound repair model | Diabetic wound dressings | [49] |
| Tofu-based hybrid GelMA hydrogels | Gel | MA | Antioxidant Transformation of macrophages to M2 type | Rat full-thickness skin defect model | Antioxidant | [50] |
| Cyclic o-nitrobenzyl-modified hyaluronic acid (HA-CNB) hydrogels | HA | Cyclic o-nitrobenzyl (CNB) | Transformation of macrophages to M2 type | Rat and pig mucosa defect model | Oral mucosal adhesive | [51] |
| Zn-MOF encapsulated methacrylated hyaluronic acid (MeHA) hydrogels | HA | MA | Antibacterial tissue formation (promoting angiogenesis, deposition of collagen, and reduced inflammation) | Rat full-thickness skin defect model | Microneedles (MNs) | [51] |
| HB-QCMCS/PEG hydrogels | Polyethylene glycol (PEG) | NB | Antibacterial Antioxidant | Rat full-thickness skin defect model | Tissue adhesive | [52] |
| Photo-crosslinked gelatin hydrogels | Gel | MA | b-FGF delivery (improving granulation tissue formation) | Rat diabetic wound repair model | Irregular wound healing | [37] |
| Methacrylated sericin (SerMA) hydrogels | Sericin (Ser) | MA | Antibacterial Inhibiting inflammation Promoting angiogenesis TGF-β inhibitor | Mouse full-thickness skin injury model | Skin wound dressing | [53] |
| H (Non-P), H (P), and H (P + T) multifunctional bioadhesive hydrogels | Carboxymethyl chitosan (CMCS) | MA | Antibacterial Antioxidant Promoting angiogenesis |
| First-aid hemostasis, wound dressing | [39] |
| Triple crosslinked poly (vinyl alcohol)/methacrylate kappa-carrageenan/chitooligosaccharide hydrogels | Kappa-carrageenan (κ-Ca) | MA | Antibacterial Antioxidant | Mouse full-thickness skin injury model | Wound dressing | [54] |
| GelMA-dHAMMA composite hydrogels | Gelatin | MA | Promoting angiogenesis Tissue formation (deposition of collagen) | Rabbit skin defect model | Skin substitute | [55] |
| PQB2 hydrogels (a double-crosslinked self-healing antibacterial hydrogel) | Quaternized methacryloyl chitosan (QMC) | Methacrylic anhydride | Antibacterial inhibiting inflammation | Mouse infected full-thickness skin defect model | Dressing for promoting infectious wound healing | [56] |
| CSG-PEG/DMA/Zn hydrogels | Chitosan (CS) | Methacrylate anhydride | Antibacteria Antioxidant Hemostasis inhibiting inflammation |
| Drug-resistant bacteria infected skin wound dressing | [41] |
| NGLG20/TG hydrogels | Gel | MA | Water-retaining capability Antibacteria Antioxidant Tissue formation (deposition of collagen) | Rat full-thickness skin defect model | Wound dressing | [57] |
| M@M–Ag–Sil-MA hydrogels | Silk fibroin | MA | Antibacterial Transformation of macrophages to M2 type Promoting angiogenesis | Rat diabetic wound repair model | Engineered nanodressing | [58] |
| Degradable alginate/poloxamer hydrogels | Alginate | Acrylate | Inhibiting inflammation | —— | Wound dressing | [59] |
| Thiol–acrylate hydrogels | Gel | Acrylate anhydride, cysteamine | Tissue formation (exhibiting epithelial wound coverage) | Focal corneal injury rabbit model | Corneal substitutes | [60] |
| Gallium (III)-mediated dual-crosslinked alginate hydrogels | Alginate | Acrylate | Antibacterial TGF-β inhibitor Tissue formation (promoting angiogenesis, deposition of collagen, and reduced inflammation) Water retaining capability | Mouse bacteria-infected wound repair model | Wound dressing | [61] |
| SF/GA/Zn hybrid hydrogels | Silk fibroin | MA | Inhibiting inflammation transformation of macrophages to M2 type | Rat diabetic wound repair model | Diabetic wound dressing | [62] |
| MCS/PEGDF/PEGDA/DMA hydrogels (CF gel for short) | Maleic anhydride modified chitosan (MCS) | MA | —— | Rat full-thickness skin defect model | Tissue sealant | [63] |
| GelMA/Gel-VH-EVs hydrogels | Gel | MA | Promoting angiogenesis tissue formation (deposition of collagen) | Mouse diabetic wound repair model | Diabetic wound dressing | [64] |
| DFO–Gelma hydrogels | Gel | MA | Promoting angiogenesis Tissue formation (granulation tissue remodeling and epithelial crawling) | Rat diabetic wound repair model | Diabetic wound dressing | [65] |
| Curcumin complexed β-CD loaded glycol chitosan hydrogels | Glycol chitosan (GC) | Glycidyl methacrylate (GM) | Promoting angiogenesis Inhibiting inflammation Tissue formation (granulation tissue remodeling and epithelial crawling) | Mouse full-thickness skin defect model | Wet dressing | [66] |
| HA-MA-NB (HNM) hydrogels | HA | MA | Transformation of macrophages to M2 type Promoting angiogenesis | Rat diabetic wound repair model | Wound dressing | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Peng, Y.; Zhang, S.; Zhang, Y.; Min, P. Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement. Gels 2022, 8, 609. https://doi.org/10.3390/gels8100609
Ma H, Peng Y, Zhang S, Zhang Y, Min P. Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement. Gels. 2022; 8(10):609. https://doi.org/10.3390/gels8100609
Chicago/Turabian StyleMa, Hao, Yuan Peng, Shunuo Zhang, Yixin Zhang, and Peiru Min. 2022. "Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement" Gels 8, no. 10: 609. https://doi.org/10.3390/gels8100609
APA StyleMa, H., Peng, Y., Zhang, S., Zhang, Y., & Min, P. (2022). Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement. Gels, 8(10), 609. https://doi.org/10.3390/gels8100609










