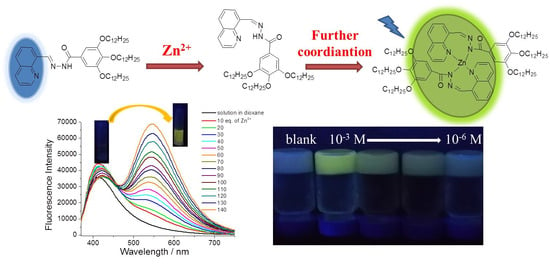
Fluorescent Quinoline-Based Supramolecular Gel for Selective and Ratiometric Sensing Zinc Ion with Multi-Modes
Abstract
:1. Introduction
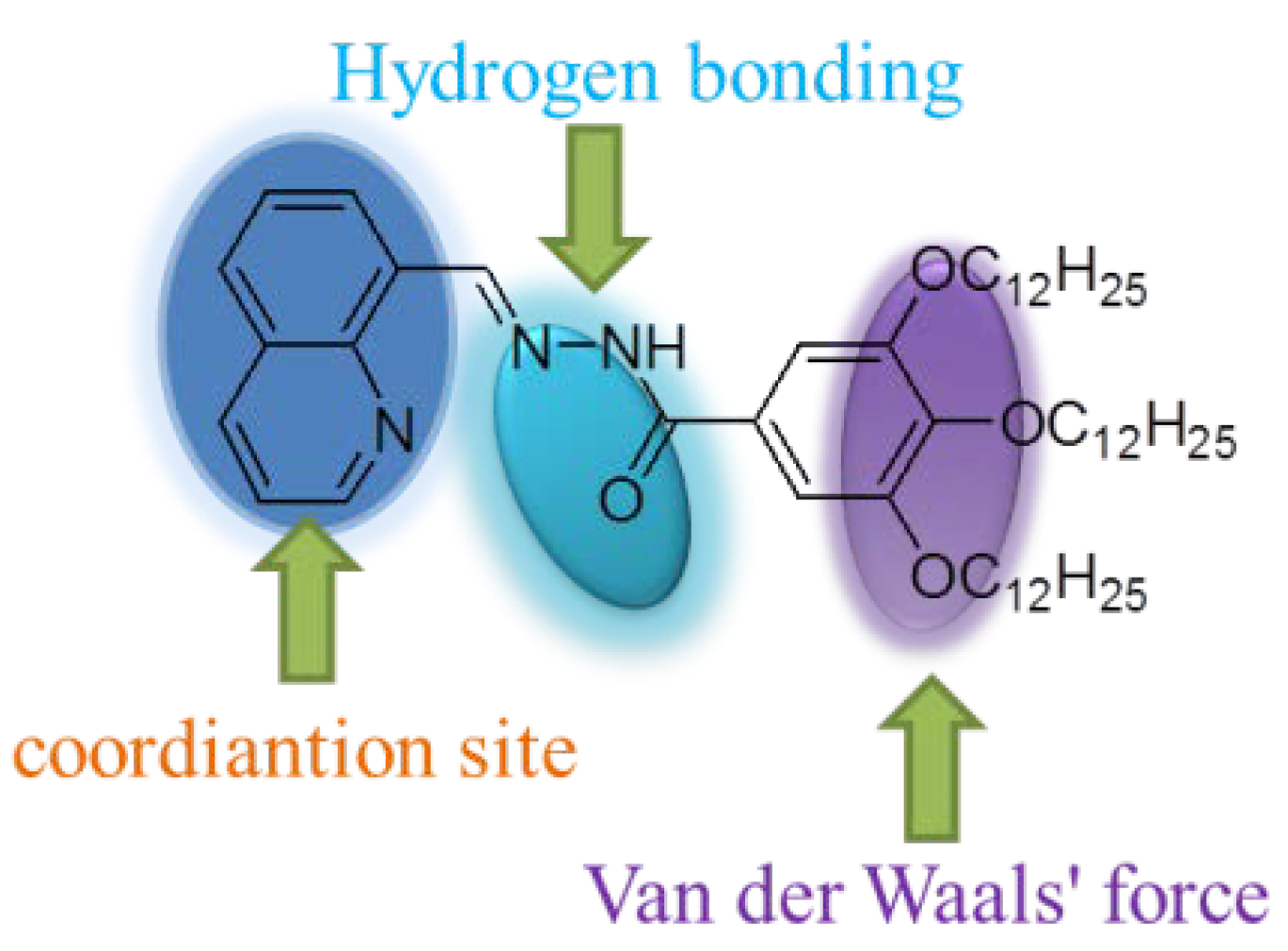
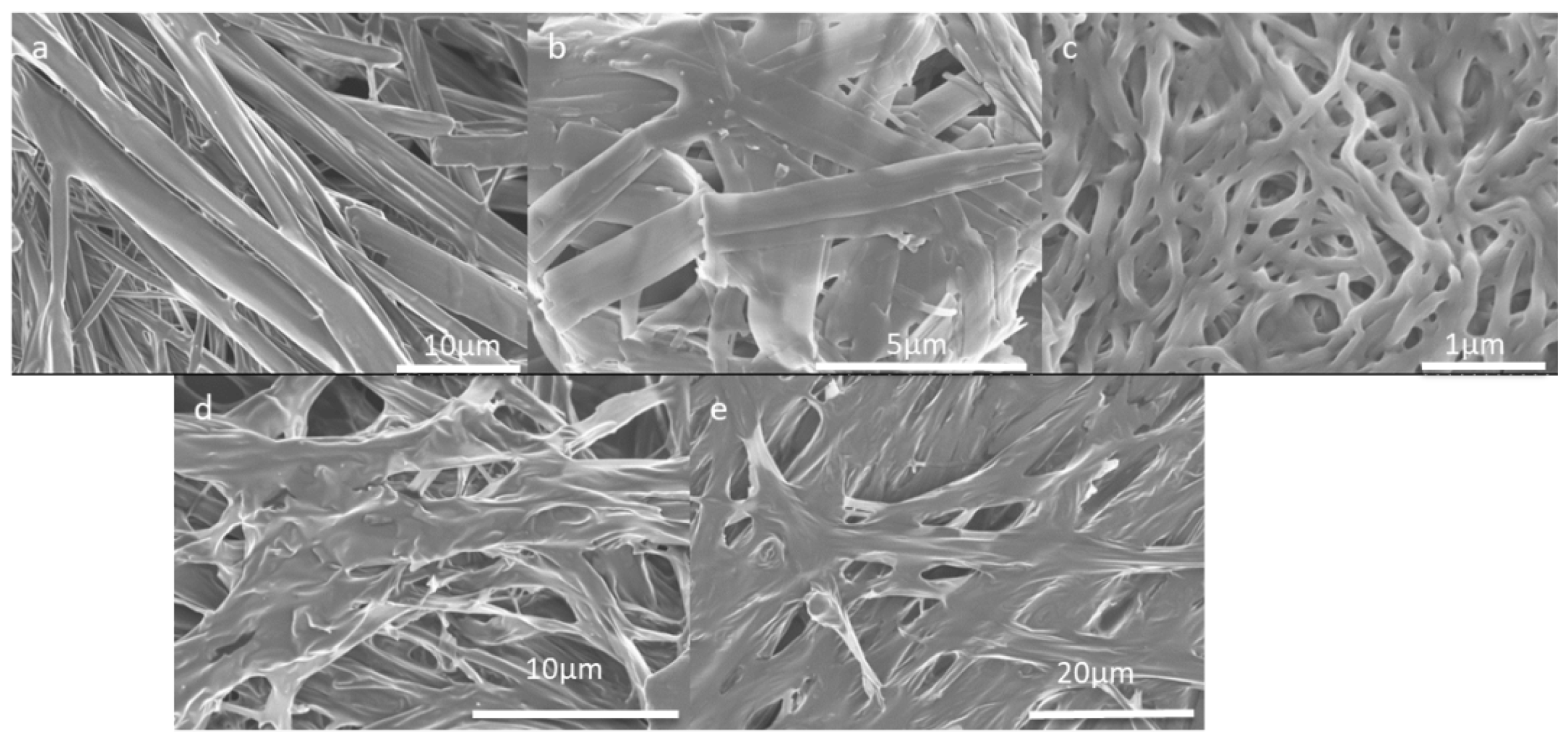
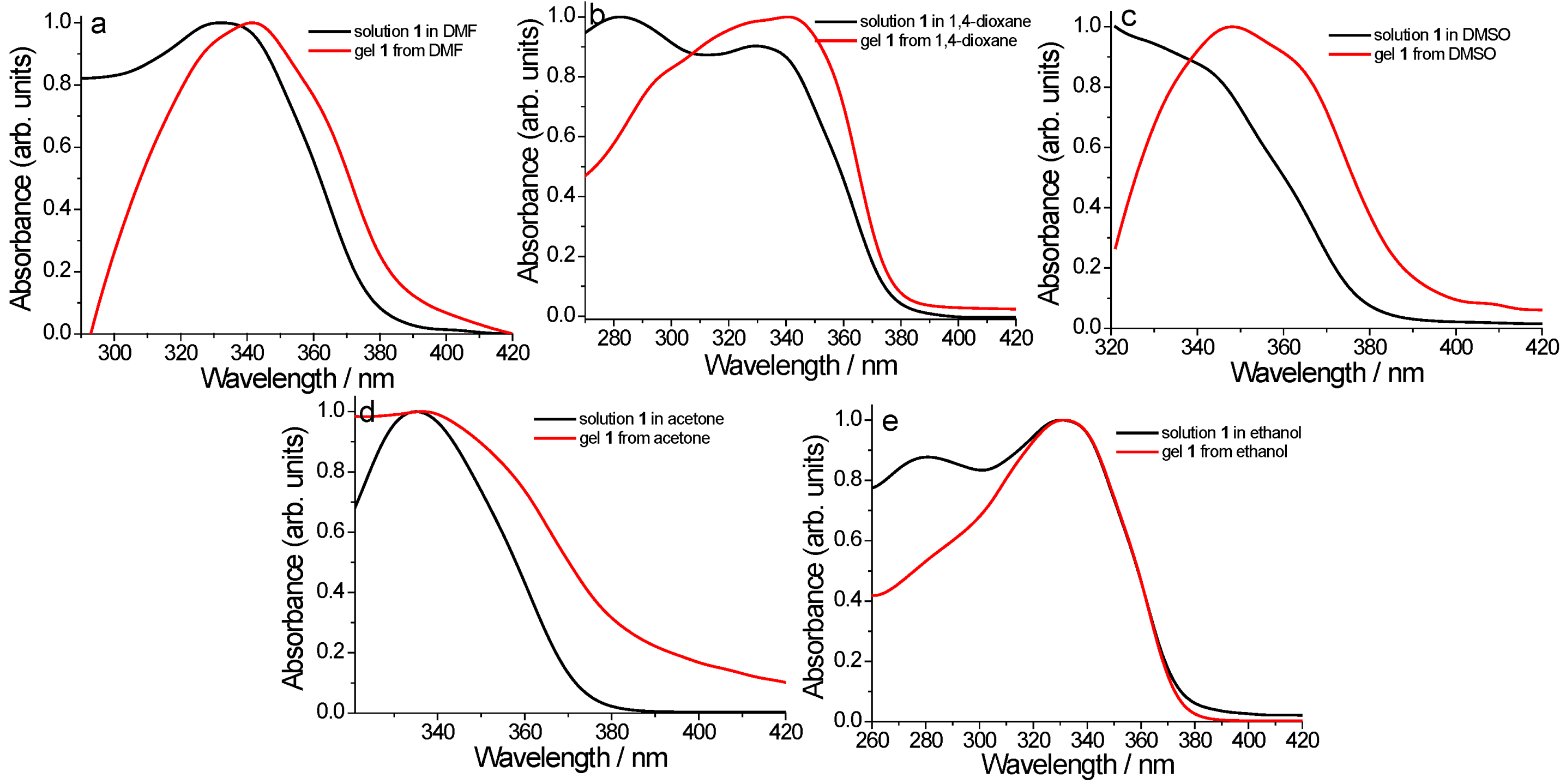
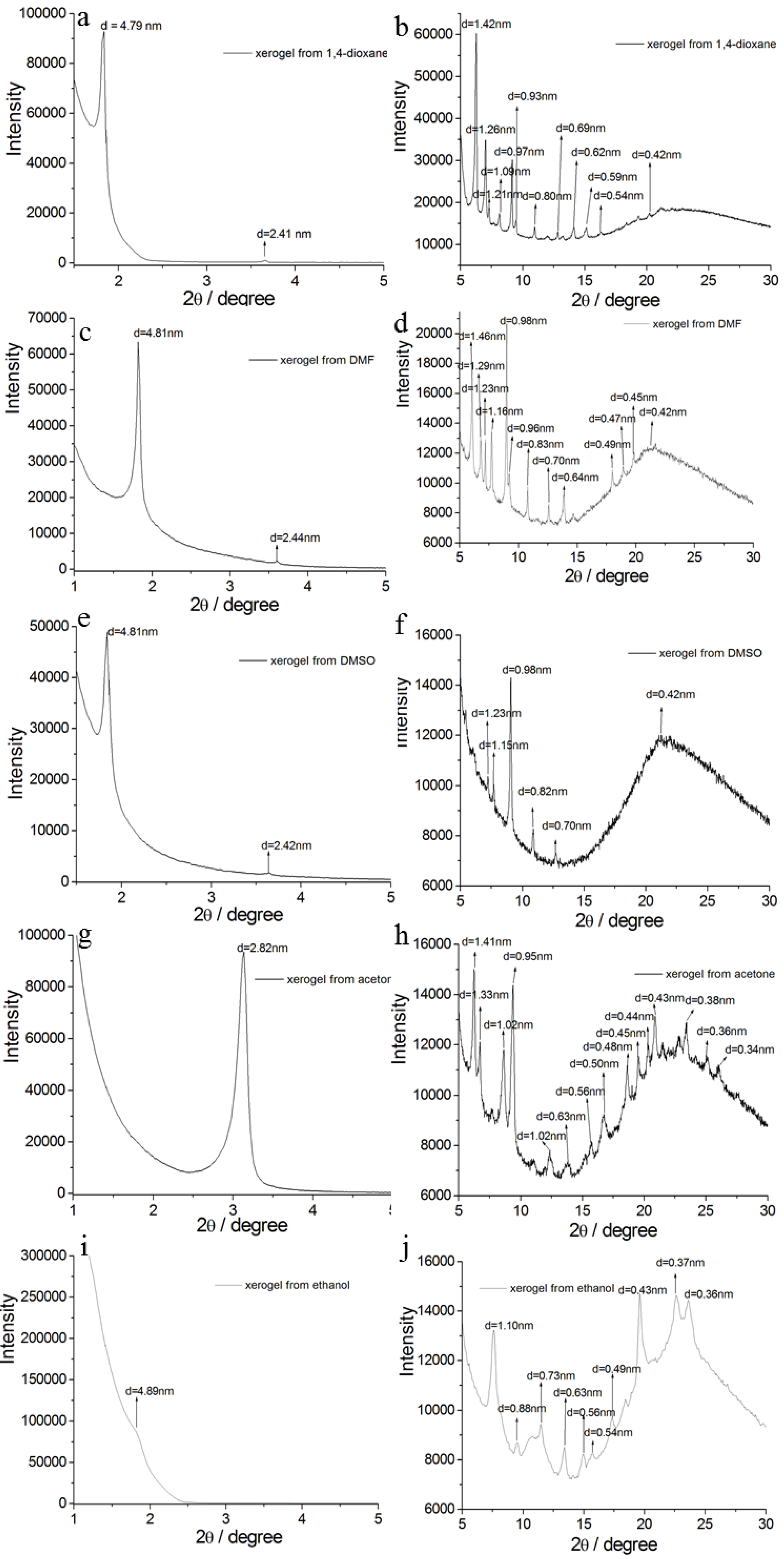
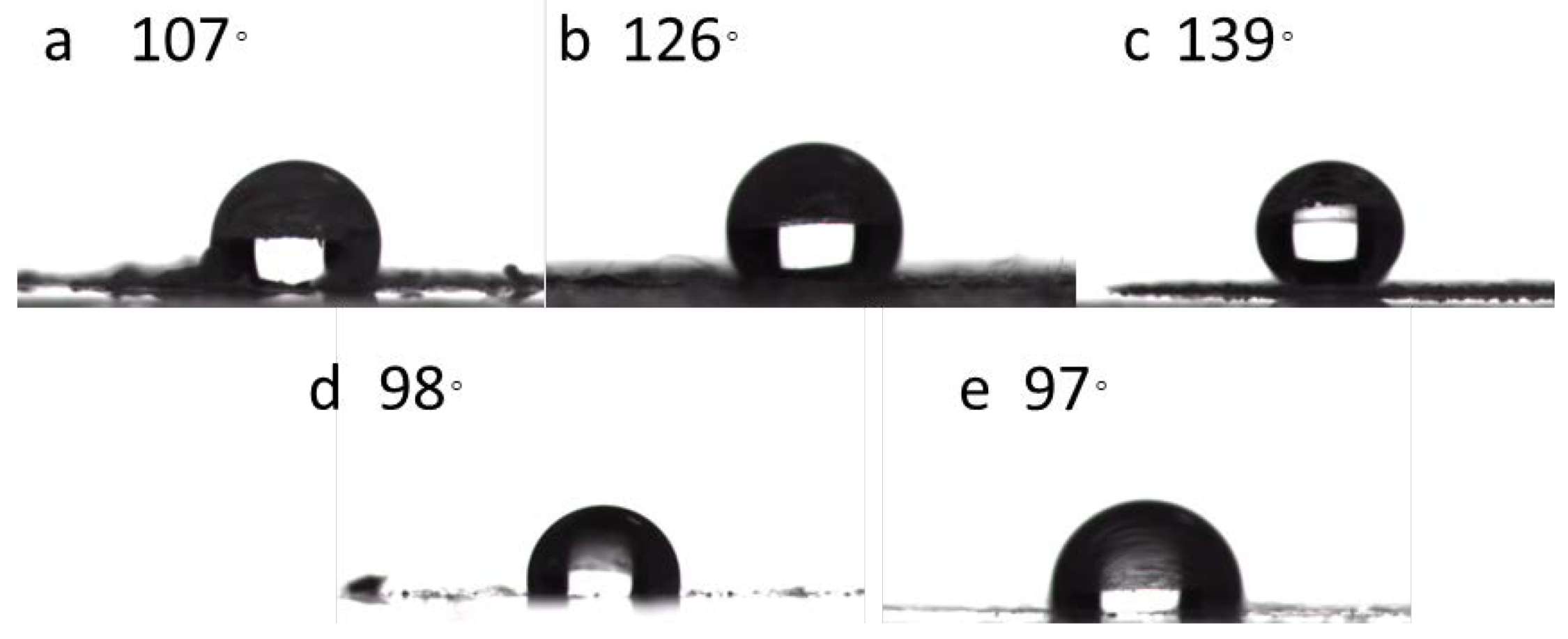
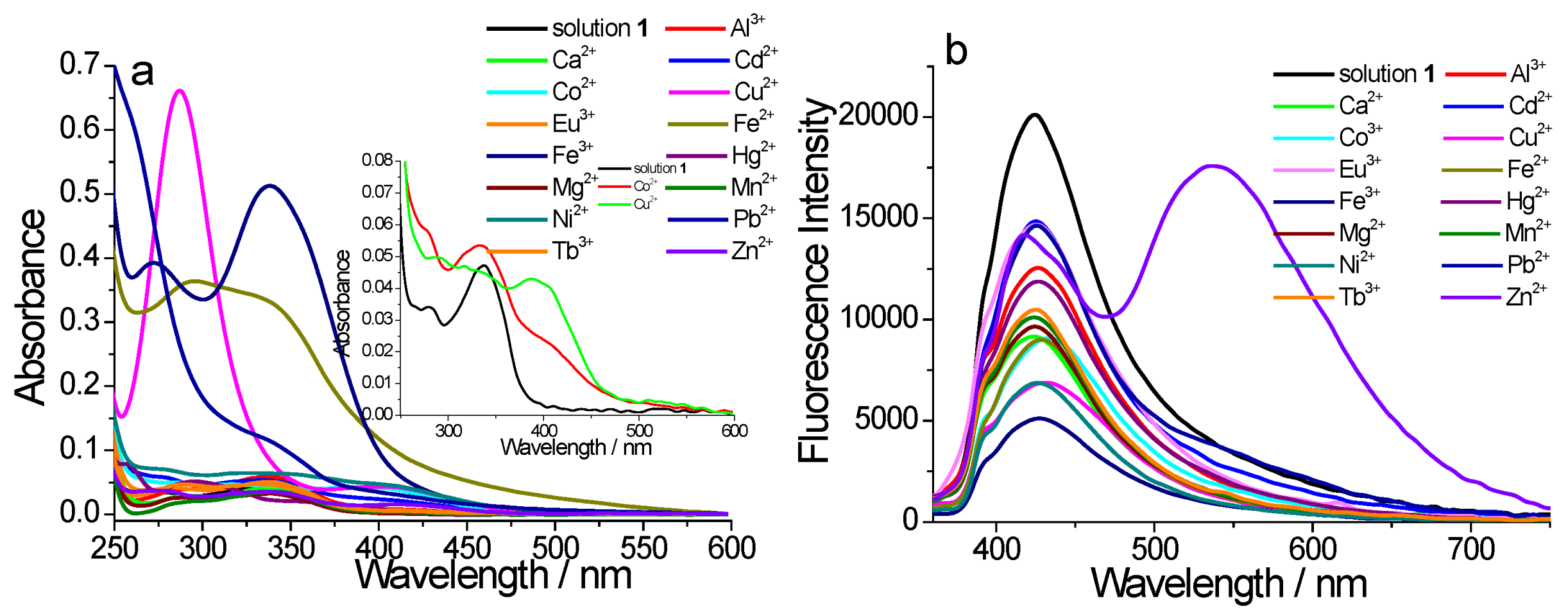
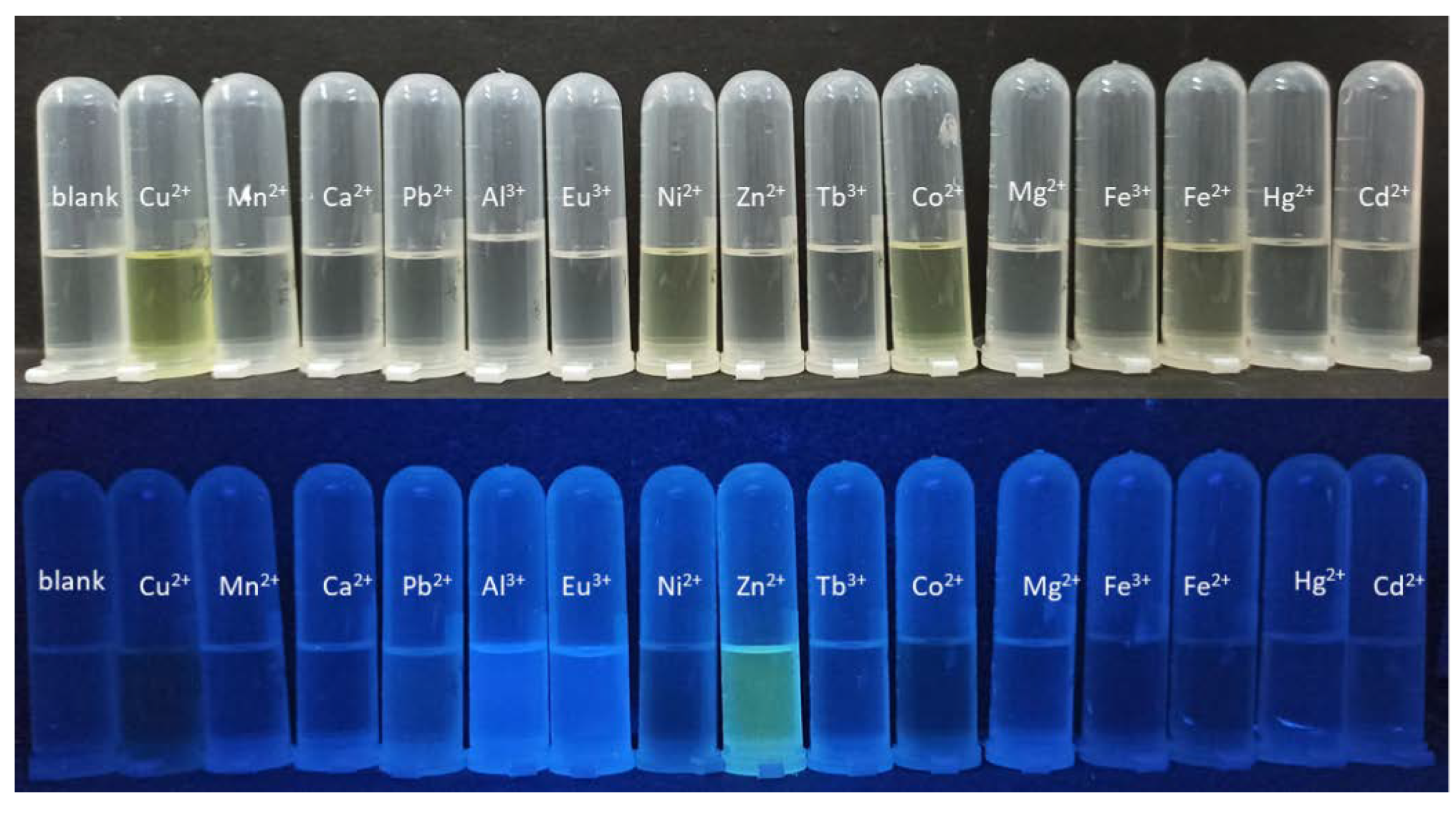
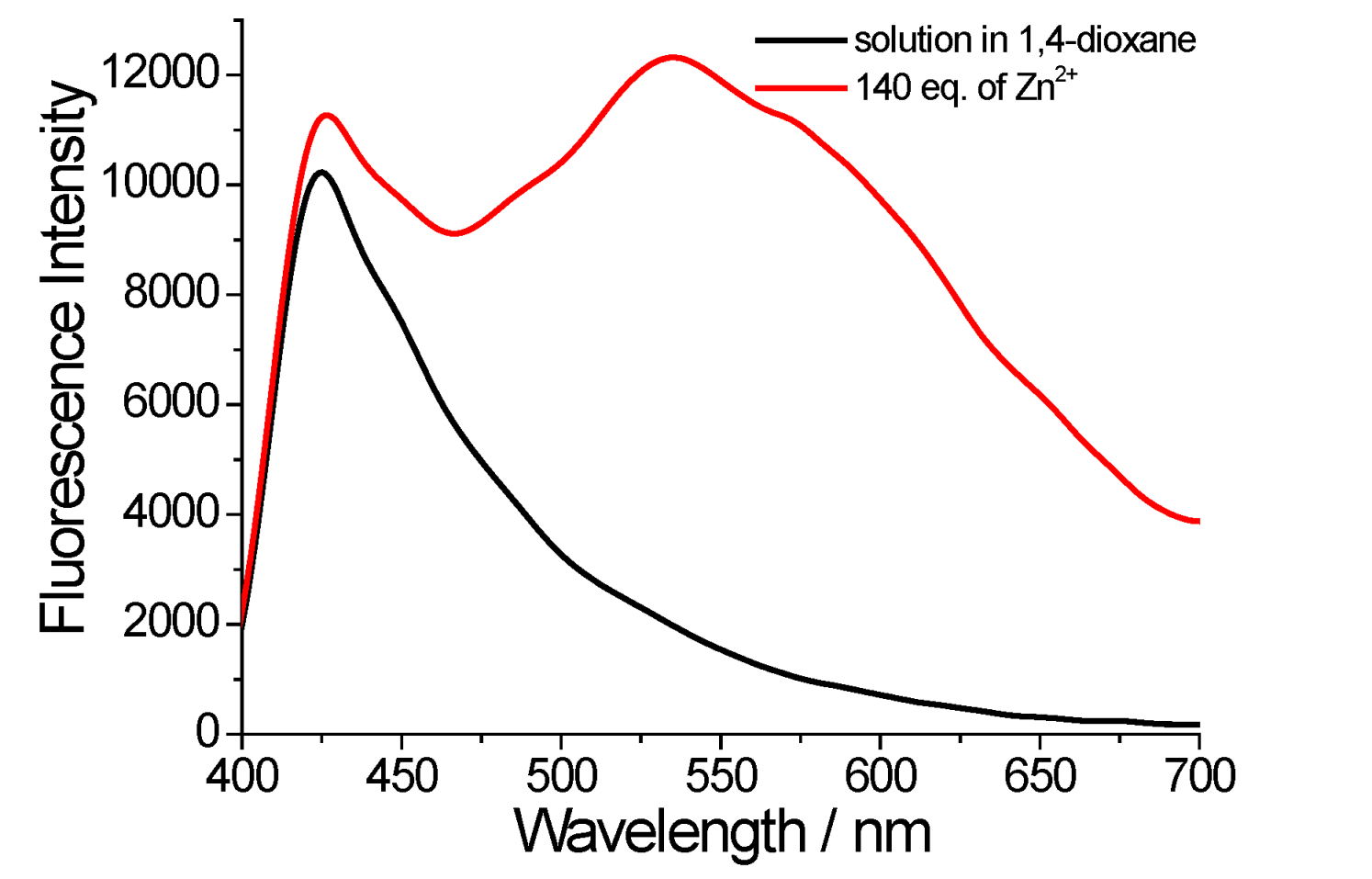
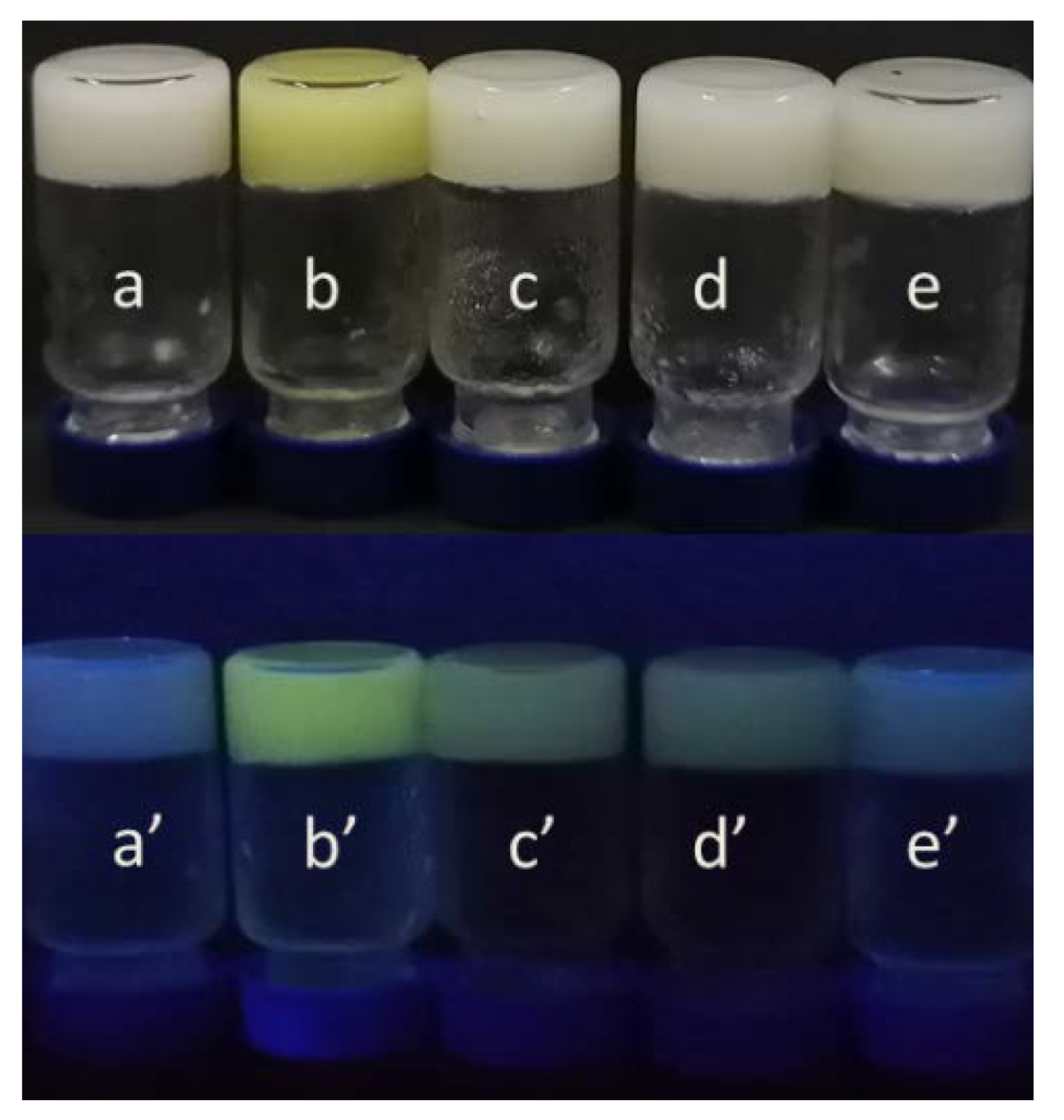
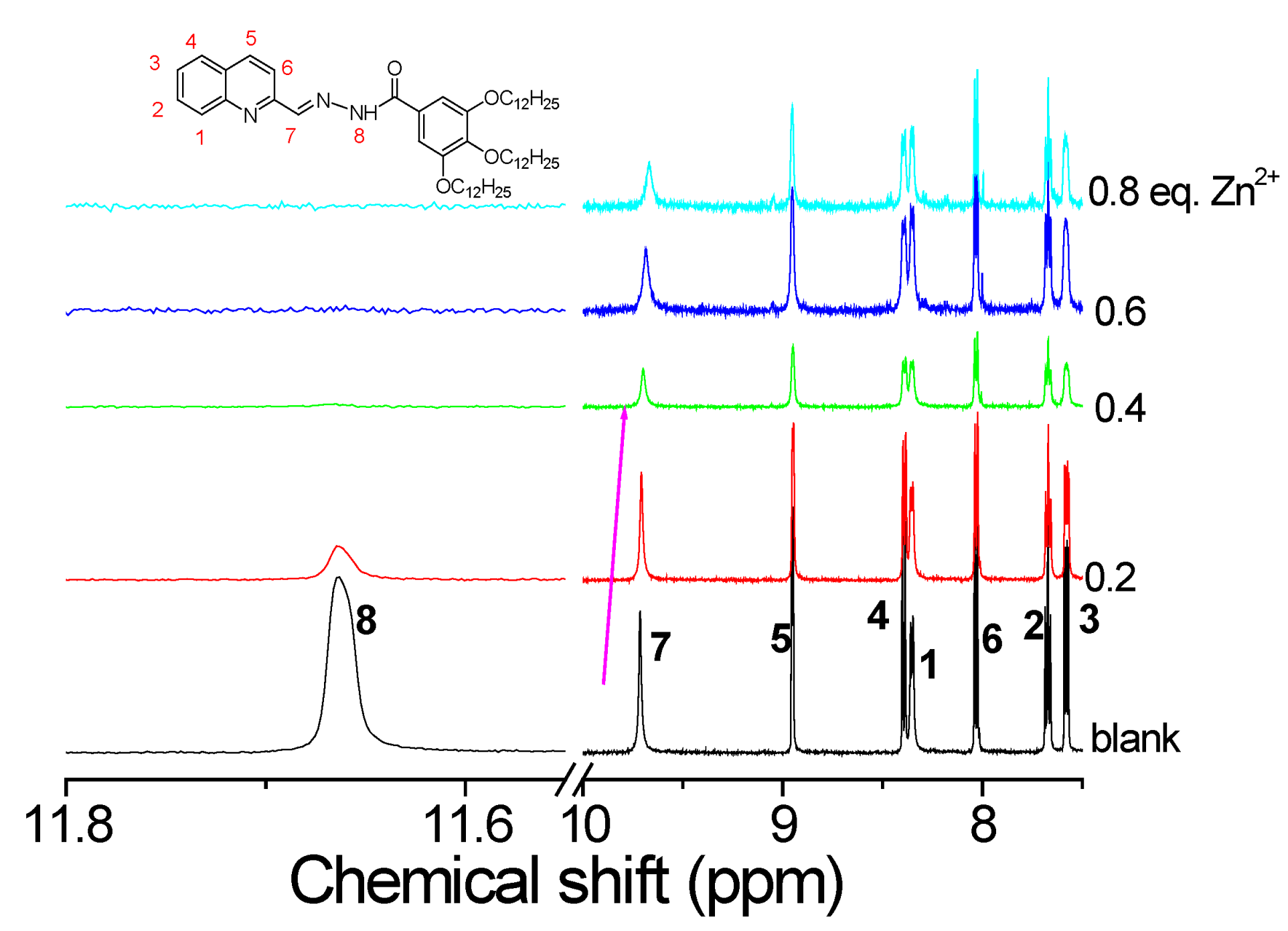
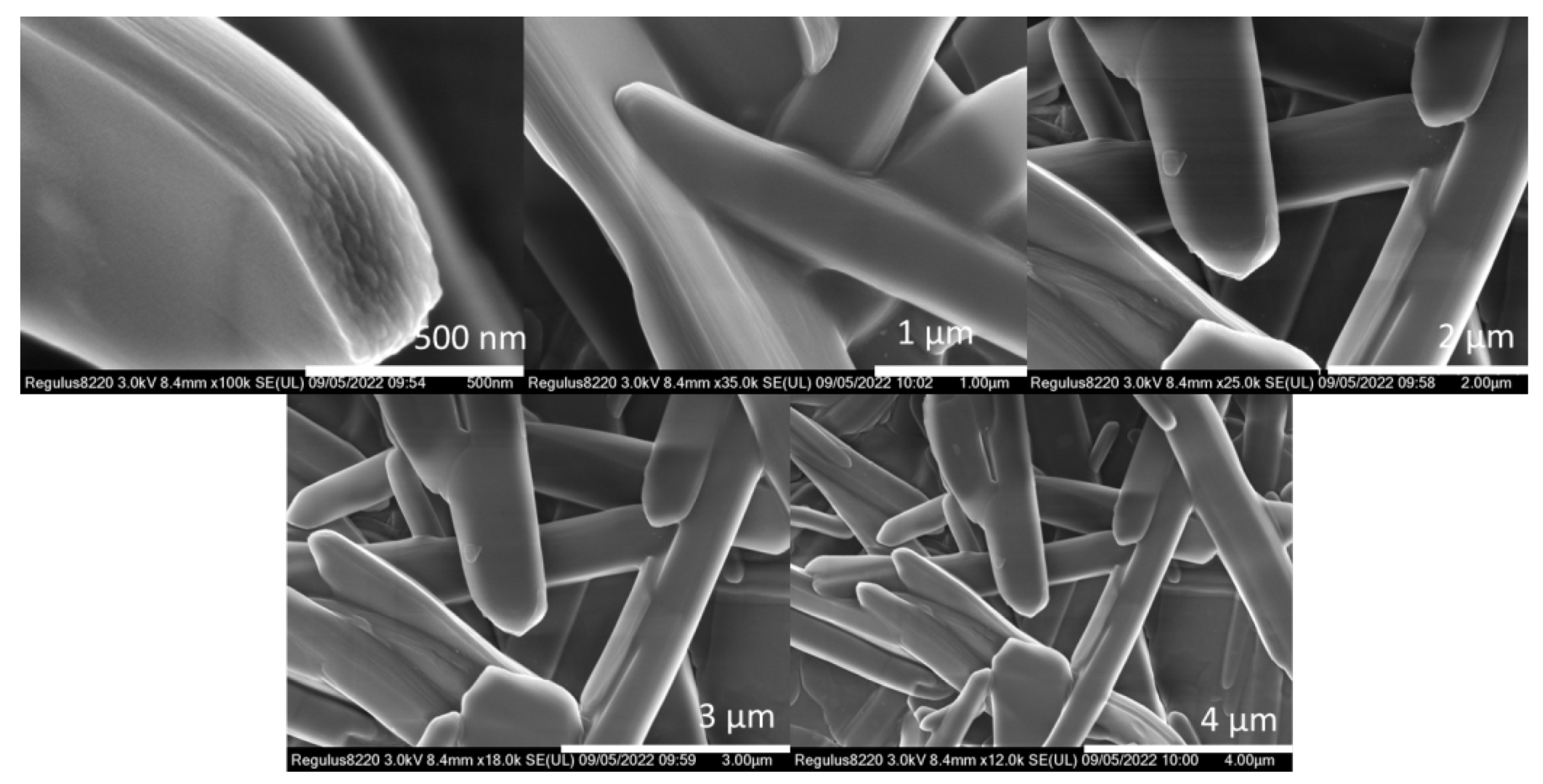
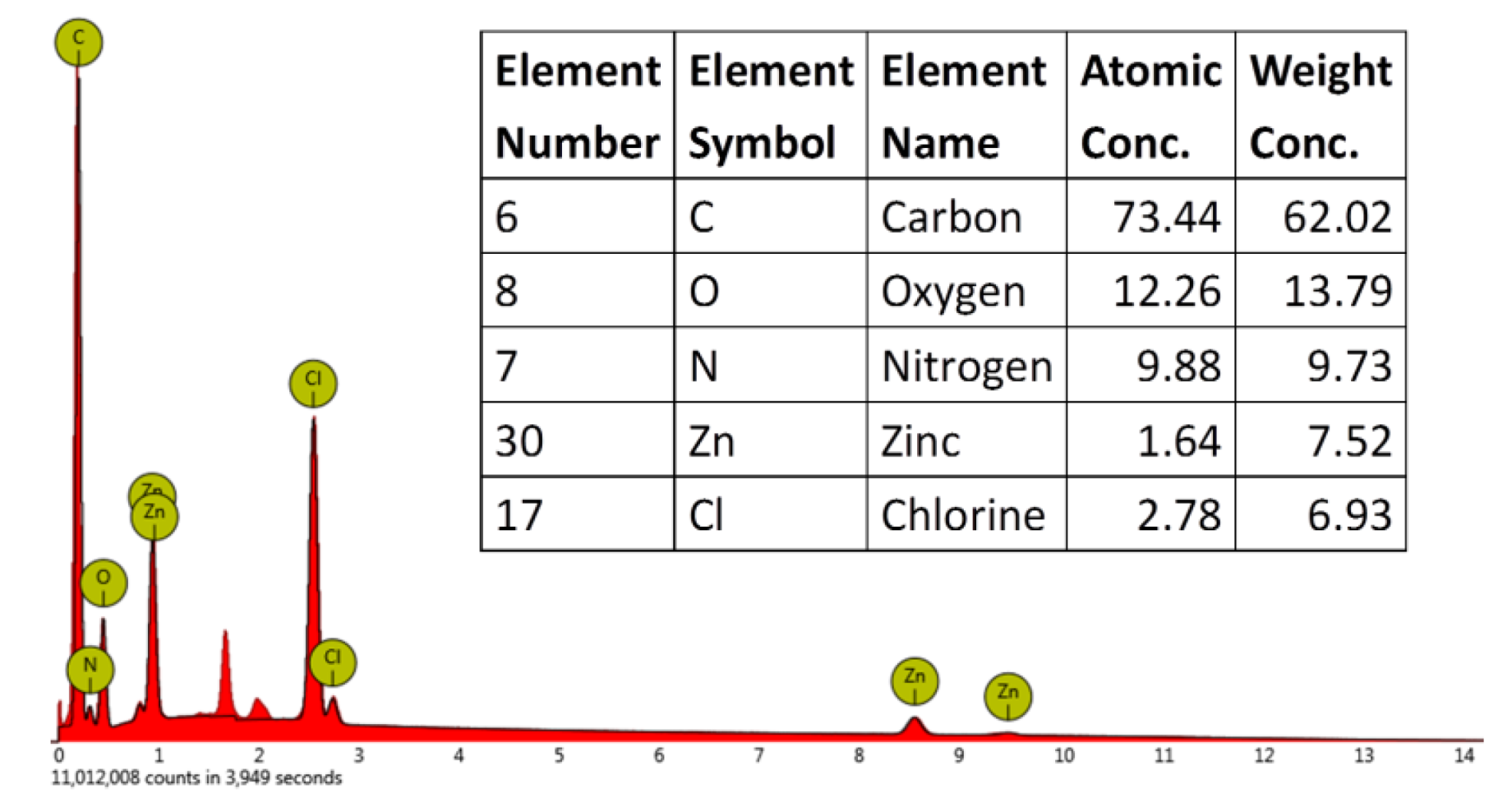
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Reagents and Solvents
4.2. Gelation Test
4.3. Techniques and Instrumentations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Q.; Cheng, Z.; Kariuki, M.; Hall, S.C.L.; Hill, S.K.; Rho, J.Y.; Perrier, S. Molecular self-assembly and supramolecular chemistry of cyclic peptides. Chem. Rev. 2021, 121, 13936–13995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.-G.; Ma, L.-L.; Li, M.; An, Y.-Y.; Han, Y.-F. Strategies for the construction of supramolecular assemblies from poly-NHC ligand precursors. Sci. China Chem. 2021, 64, 701–718. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Bai, S.; Wang, Y.-Y.; Han, Y.-F. A strategy for the construction of triply interlocked organometallic cages by rational design of poly-NHC precursors. J. Am. Chem. Soc. 2020, 142, 13614–13621. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Li, X.; Bai, S.; Wang, Y.-Y.; Han, Y.-F. Self-assembly, structural transformation, and guest-binding properties of supramolecular assemblies with triangular metal–metal bonded units. J. Am. Chem. Soc. 2020, 142, 2524–2531. [Google Scholar] [CrossRef]
- Feng, T.; Li, X.; An, Y.-Y.; Bai, S.; Sun, L.-Y.; Li, Y.; Wang, Y.-Y.; Han, Y.-F. Backbone-directed self-assembly of interlocked molecular cyclic metalla[3]catenanes. Angew. Chem. Int. Ed. 2020, 59, 13516–13520. [Google Scholar] [CrossRef]
- Newsom, J.; Payneand, K.; Krebs, M. Microgels: Modular, tunable constructs for tissue regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef]
- Yuan, Y.; Solin, N. Mechanochemical preparation and self-assembly of protein:dye hybrids for white luminescence. ACS Appl. Polym. Mater. 2021, 3, 4825–4836. [Google Scholar] [CrossRef]
- Wei, T.; Zhao, Q.; Li, Z.; Dai, X.; Niu, Y.; Yao, H.; Zhang, Y.; Qu, W.; Lin, Q. Supramolecular organogel with aggregation-induced emission for ultrasensitive detection and effective removal of Cu2+ and Hg2+ from aqueous solution. Dyes Pigments 2021, 192, 109436. [Google Scholar] [CrossRef]
- Giri, A.; Sahoo, A.; Dutta, T.; Patra, A. Cavitand and molecular cage-based porous organic polymers. ACS Omega 2020, 5, 28413–28424. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Jung, H.; Zeng, Y.; Lee, S.; Ma, K.; Swamy, K.; Zhou, X.; Kim, M.H.; Yoon, J. Effective strategy for colorimetric and fluorescence sensing of phosgene based on small organic dyes and nanofiber platforms. ACS Appl. Mater. Interf. 2016, 8, 22246–22252. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular hydrogels with properties tunable by calcium ions: A bio-inspired chemical system. ACS Appl. Bio. Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, D.; Zou, D.; Zhang, J. The photo-, electro- and photoelectro-catalytic properties and application prospects of porous coordinate polymers. J. Mater. Chem. A 2018, 6, 6130–6154. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Praveen, V.; Vedhanarayanan, B.; Mal, A.; Mishra, R.; Ajayaghosh, A. Self-assembled extended π-systems for sensing and security applications. Accounts Chem. Res. 2020, 53, 496–507. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, C.; Jian, C.; Yang, S.; Liu, M.; Huang, X.; Gao, W.; Wu, H. Salt/current-triggered stabilization of β-cyclodextrins encapsulated host-guest low-molecular-weight gels. Chin. Chem. Lett. 2020, 31, 369–372. [Google Scholar] [CrossRef]
- Zhong, Q.; Liu, B.; Yang, B.; Li, Y.; Li, J.; Yan, X. Flexible lithium metal capacitors enabled by an in situ prepared gel polymer electrolyte. Chin. Chem. Lett. 2021, 32, 3496–3500. [Google Scholar] [CrossRef]
- Gao, A.; Han, Q.; Wang, Q.; Cao, X. Synthesis and properties study of bis-pyridine derivative gel system. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2021, 34, 614–618. [Google Scholar]
- Gao, A.; Li, Y.; Cao, X. Preparation and properties of coumarin derivative gel. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2020, 33, 129–133. [Google Scholar]
- Lan, Y.; Corradini, M.; Weiss, R.; Raghavan, S.; Rogers, M. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef]
- Gao, A.; Zhang, Z.; Xu, R.; Liu, Y.; Zhang, Y.; Gao, L.; Cao, X. H-bonding regulation effect of two component self-assembly system. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2015, 28, 396–399. [Google Scholar]
- Ma, X.; Zhang, Z.; Xie, H.; Ma, Y.; Liu, C.; Liu, S.; Liu, M. Emissive Intelligent supramolecular gel for highly selective sensing of al3+ and writable soft material. Chem. Commun. 2018, 54, 13674–13677. [Google Scholar] [CrossRef]
- Muir, V.G.; Burdick, J.A. Chemically modified biopolymers for the formation of biomedical hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional supramolecular polymeric networks: The marriage of covalent polymers and macrocycle-based host–guest interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Jiang, X.-M.; Gong, G.-F.; Yao, H.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. Pillararene-based aiegens: Research progress and appealing applications. Chem. Commun. 2021, 57, 284–301. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, N.; Gao, A.; Ding, Q.; Li, Y.; Chang, X. Terminal molecular isomer-effect on supramolecular self-assembly system based on naphthalimide derivative and its sensing application for mercury (II) and iron (III) ions. Langmuir 2018, 34, 7404–7415. [Google Scholar] [CrossRef]
- Cao, X.; Ding, Q.; Li, Y.; Gao, A.; Chang, X. Continuous multi-channel sensing of volatile acid and organic amine gases using a fluorescent self-assembly system. J. Mater. Chem. C 2019, 7, 133–142. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.; Huang, J.; Zhao, J.; Yan, Y. Multifunctional metallo-organic vesicles displaying aggregation-induced emission: Two-photon cell-imaging, drug delivery, and specific detection of zinc ion. ACS Appl. Nano Mater. 2018, 1, 1819–1827. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Wang, F.; Sessler, C.D.; Vogler, N.W.; Zhang, D.Y.; Loucks, W.H.; Tzounopoulos, T.; Lippard, S.J. Photoactivatable sensors for detecting mobile zinc. J. Am. Chem. Soc. 2018, 140, 2020–2023. [Google Scholar] [CrossRef]
- Webber, M.; Appel, E.; Meijer, E.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef]
- Meng, Z.; Mirica, K.A. Covalent organic frameworks as multifunctional materials for chemical detection. Chem. Soc. Rev. 2021, 50, 13498–13558. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.C.; Goh, S.S.; Loh, X.J. Bottom-up engineering of responsive hydrogel materials for molecular detection and biosensing. ACS Mater. Lett. 2020, 2, 918–950. [Google Scholar] [CrossRef]
- Xiong, H.; He, L.; Zhang, Y.; Wang, J.; Yang, Z. A ratiometric fluorescent probe for the detection of hypochlorous acid in living cells and zebra fish with a long wavelength emission. Chin. Chem. Lett. 2019, 30, 1075–1077. [Google Scholar] [CrossRef]
- Xu, Q.-Y.; Tan, Z.; Liao, X.-W.; Wang, C. Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers. Chin. Chem. Lett. 2022, 33, 22–32. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.; Wang, Y.; Zhang, P.; Yu, P.; Wang, M.; Zhen, Y.; Dong, H.; Hu, W. A new fluorescent quinoline derivative toward the acid-responsivity in both solution and solid states. Chin. Chem. Lett. 2020, 31, 2909–2912. [Google Scholar] [CrossRef]
- Hou, J.-T.; Wang, B.; Zou, Y.; Fan, P.; Chang, X.; Cao, X.; Wang, S.; Yu, F. Molecular fluorescent probes for imaging and evaluation of hypochlorite fluctuations during diagnosis and therapy of osteoarthritis in cells and in a mouse model. ACS Sensors 2020, 5, 1949–1958. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Xie, S.; Mei, L.; Meng, R.; Chen, L.; Zhao, J. Double-oxalate-bridging tetralanthanide containing divacant lindqvist isopolytungstates with an energy transfer mechanism and luminous color adjustablility through Eu3+/Tb3+ codoping. Inorg. Chem. 2020, 59, 648–660. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Pang, J.; Liu, Y.; Li, P.; Chen, L.; Zhao, J. Two penta-REIII encapsulated tetravacant dawson selenotungstates and nanoscale derivatives and their luminescence properties. Inorg. Chem. 2019, 58, 7078–7090. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, C.; Dong, X.-Y.; Zang, S.-Q.; Mak, T.C.W. Shell engineering to achieve modification and assembly of atomically-precise silver clusters. Chem. Soc. Rev. 2021, 50, 2297–2319. [Google Scholar] [CrossRef]
- Yu, C.; Hu, F.; Song, J.; Zhang, J.; Liu, S.; Wang, B.; Meng, H.; Liu, L.; Ma, L. Ultrathin two-dimensional metal-organic framework nanosheets decorated with tetra-pyridyl calix[4]arene: Design, synthesis and application in pesticide detection. Sens. Actuators B-Chem. 2020, 310, 127819. [Google Scholar] [CrossRef]
- Yao, S.-L.; Liu, S.-J.; Tian, X.-M.; Zheng, T.-F.; Cao, C.; Niu, C.-Y.; Chen, Y.-Q.; Chen, J.-L.; Huang, H.; Wen, H.-R. A ZnII-based metal–organic framework with a rare tcj Topology as a turn-on fluorescent sensor for acetylacetone. Inorg. Chem. 2019, 58, 3578–3581. [Google Scholar] [CrossRef]
- Qin, Z.-S.; Dong, W.-W.; Zhao, J.; Wu, Y.-P.; Zhang, Q.-C.; Li, D.-S. A water-stable Tb(III)-based metal–organic gel (MOG) for detection of antibiotics and explosives. Inorg. Chem. Front. 2018, 5, 120–126. [Google Scholar] [CrossRef]
- Ni, A.-Y.; Mu, Y.; Pan, J.; Han, S.-D.; Shang, M.-M.; Wang, G.-M. An organic–inorganic hybrid zinc phosphite framework with room temperature phosphorescence. Chem. Commun. 2018, 54, 3712–3714. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Cao, X.; Gao, A.; Hou, J.-T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. Metal oxide catalyst assisted SnO2 thin film based SO2 gas sensor. Sens. Actuators B Chem. 2016, 224, 282. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. A comparative study of RGO-SnO2 and MWCNT-SnO2 nanocomposites based SO2 gas sensors. Sens. Actuators B Chem. 2017, 248, 980. [Google Scholar] [CrossRef]
- Echizen, M.; Motoyama, S.; Tatsuta, T.; Tsuji, O.; Katiyar, R.S.; Kumar, A.; Scott, J.F. Comparison of the electrical properties of ZnO thin films on different substrates by pulsed laser deposition. Integr. Ferroelectr. 2012, 133, 9. [Google Scholar] [CrossRef]
- Zhu, Z.; Chung, C.-L.; Kumar, U.; Chung, M.-H.; Wu, C.-H.; Wu, R.-J. Facial construction of Pd/BiPO4 toward efficient photocatalytic oxidation of formaldehyde under UV light irradiation. J. Photochem. Photobio. A Chem. 2022, 427, 113840. [Google Scholar] [CrossRef]
- Kumar, U.; Yang, Y.-H.; Deng, Z.-Y.; Lee, M.-W.; Huang, W.-M.; Wu, C.-H. In situ growth of ternary metal sulfide based quantum dots to detect dual gas at extremely low levels with theoretical investigations. Sens. Actuators B Chem. 2022, 353, 131192. [Google Scholar] [CrossRef]
- Singh, M.; Yadav, B.C.; Ranjan, A.; Sonker, R.K.; Kaur, M. Detection of liquefied petroleum gas below lowest explosion limit (LEL) using nanostructured hexagonal strontium ferrite thin film. Sens. Actuators B Chem. 2017, 249, 96. [Google Scholar] [CrossRef]
- Gautam, C.; Tiwary, C.S.; Machado, L.D.; Jose, S.; Ozden, S.; Biradar, S.; Galvao, D.S.; Sonker, R.K.; Yadav, B.C.; Vajtai, R.; et al. Synthesis and porous h-BN 3D architectures for effective humidity and gas sensors. RSC Adv. 2016, 6, 87888. [Google Scholar] [CrossRef]
- Kumar, R.; Kulriya, P.K.; Mishra, M.; Singh, F.; Gupta, G.; Kumar, M. Highly selective and reversible NO2 gas sensor using vertically aligned MoS2 flake networks. Nanotechnology 2018, 29, 464001. [Google Scholar] [CrossRef]
- Sahare, P.D.; Kumar, S.; Kumar, S.; Singh, F. n-ZnO/p-Si heterojunction nanodiodes based sensor for monitoring UV radiation. Sens. Actuators A Phys. 2018, 279, 351. [Google Scholar] [CrossRef]
- Borkar, H.; Gaikwad, V.M.; Dutta, S.; Tomar, M.; Gupta, V.; Kumar, A. Lead-free laminated structures for eco-friendly energy harvesters and magnetoelectric sensors. J. Phys. Chem. Solids 2022, 160, 110306. [Google Scholar] [CrossRef]
- Lin, R.-B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Gong, G.-F.; Fan, Y.-Q.; Zhou, Q.; Zhang, Q.-P.; Yao, H.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. A novel aie-based supramolecular polymer gel serves as an ultrasensitive detection and efficient separation material for multiple heavy metal ions. Soft Matter 2019, 15, 6878–6884. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, T.-T.; Zhu, X.; Wei, T.-B.; Li, H.; Zhang, Y.-M. Rationally introduce multi-competitive binding interactions in supramolecular gels: A simple and efficient approach to develop multi-analyte sensor array. Chem. Sci. 2016, 7, 5341–5346. [Google Scholar] [CrossRef]
- Shen, Z.; Jiang, Y.; Wang, T.; Liu, M. Symmetry breaking in the supramolecular gels of an achiral gelator exclusively driven by π–π stacking. J. Am. Chem. Soc. 2015, 137, 16109–16115. [Google Scholar] [CrossRef]
- Yang, X.; Lu, R.; Zhou, H.; Xue, P.; Wang, F.; Chen, P.; Zhao, Y. Aggregation-induced blue shift of fluorescence emission due to suppression of TICT in a phenothiazine-based organogel. J. Colloid Interface Sci. 2009, 339, 527–532. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Zhang, Y.; Ren, J.; Yu, X.; Cao, X. Switchable supramolecular configurations of Al3+/LysTPY coordination polymers in a hydrogel network controlled by ultrasound and heat. ACS Appl. Mater. Interface 2021, 13, 40079–40087. [Google Scholar] [CrossRef]
- Cao, X.; Meng, L.; Li, Z.; Mao, Y.; Lan, H.; Chen, L.; Fan, Y.; Yi, T. Large red-shifted fluorescent emission via intermolecular π–π stacking in 4-ethynyl-1,8-naphthalimide-based supramolecular assemblies. Langmuir 2014, 30, 11753–11760. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, K.; Niu, L.; Li, Y.; Song, J. Effects of salt on the gelation mechanism of a d-sorbitol-based hydrogelator. J. Phys. Chem. B 2013, 117, 5989–5995. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Y.; Zhang, X.; Gao, A.; Xu, R.; Yu, Y.; Hei, X. Surface wettability and emission behavior tuned via solvent in a supramolecular self-assembly system based on a naphthalene diimides derivative. Appl. Surf. Sci. 2020, 501, 144256. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, X.; Ren, T.; Yuan, L. Molecular engineering of ultra-sensitive fluorescent probe with large stokes shift for imaging of basal HOCl in tumor cells and tissues. Chin. Chem. Lett. 2020, 31, 2980–2984. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.-C.; Wang, Y.; Guo, D.-S.; Gao, J.; Yang, Z. Fast naked-eye detection of zinc ions by molecular assembly- assisted polymerization of diacetylene. Nanoscale 2018, 10, 18829. [Google Scholar] [CrossRef]
- Jia, M.-Y.; Wang, Y.; Liu, Y.; Niu, L.-Y.; Feng, L. BODIPY-based self-assembled nanoparticles as fluorescence turn-on sensor for the selective detection of zinc in human hair. Biosens. Bioelectron. 2016, 85, 515. [Google Scholar] [CrossRef]
- Velmurugan, K.; Raman, A.; Don, D.; Tang, L.; Easwaramoorthi, S.; Nandhakumar, R. Quinoline benzimidazole-conjugate for the highly selective detection of Zn(ii) by dual colorimetric and fluorescent turn-on responses. RSC Adv. 2015, 5, 44463. [Google Scholar] [CrossRef]
- Hu, J.-H.; Sun, Y.; Qi, J.; Li, Q.; Wei, T.-B. A new unsymmetrical azine derivative based on coumarin group as dual-modal sensor for CN− and fluorescent “OFF–ON” for Zn2+. Spectrochim. Acta Part A 2017, 175, 125. [Google Scholar] [CrossRef]
- Qu, W.-J.; Guan, J.; Wei, T.-B.; Yan, G.-T.; Lin, Q.; Zhang, Y.-M. A turn–on fluorescent sensor for relay recognition of two ions: From F– selective sensor to highly Zn2+ selective sensor by tuning electronic effects. RSC Adv. 2016, 6, 35804. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, P.; Wei, T.-B.; Lin, Q.; Yao, H.; Zhang, Y.-M. A highly selective PET-based chemosensor for instant detecting of Zn2+. RSC Adv. 2014, 4, 35797. [Google Scholar] [CrossRef]
- Xue, P.; Ding, J.; Jin, M.; Lu, R. Rapid gel-to-sol transition triggered by a photoacid generator under low-power light. J. Mater. Chem. C 2017, 5, 5299–5303. [Google Scholar] [CrossRef]
- Qin, P.; Wu, Z.; Li, P.; Niu, D.; Liu, M.; Yin, M. Triple-modulated chiral inversion of co-assembly system based on alanine amphiphile and cyanostilbene derivative. ACS Appl. Mater. Interfaces 2021, 13, 18047–18055. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian (Revision A.02); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]





| Solvents | 1 | Solvents | 1 |
|---|---|---|---|
| hexane | P | 1,4-dioxane | G(8.33) |
| toluene | S | ethyl acetate | P |
| methanol | P | petroleum ether | P |
| ethanol | G(8.33) | DMSO | G(12.5) |
| acetonitrile | P | DMF | (8.33) |
| acetone | G(8.33) | THF | S |
| Compound | HOMO (eV) | LUMO (eV) |
|---|---|---|
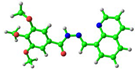 simplified 1 (trans-) | 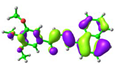 −5.64 eV | 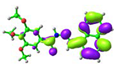 −1.68 eV |
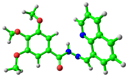 simplified 1 (cis-) | 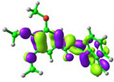 −5.75 eV | 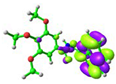 −1.57 eV |
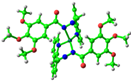 simplified 1 complex |  −5.01 eV | 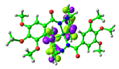 −2.30 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Wang, Q.; Gao, A.; Ge, X.; Wan, R.; Cao, X. Fluorescent Quinoline-Based Supramolecular Gel for Selective and Ratiometric Sensing Zinc Ion with Multi-Modes. Gels 2022, 8, 605. https://doi.org/10.3390/gels8100605
Han Q, Wang Q, Gao A, Ge X, Wan R, Cao X. Fluorescent Quinoline-Based Supramolecular Gel for Selective and Ratiometric Sensing Zinc Ion with Multi-Modes. Gels. 2022; 8(10):605. https://doi.org/10.3390/gels8100605
Chicago/Turabian StyleHan, Qingqing, Qingqing Wang, Aiping Gao, Xuefei Ge, Rong Wan, and Xinhua Cao. 2022. "Fluorescent Quinoline-Based Supramolecular Gel for Selective and Ratiometric Sensing Zinc Ion with Multi-Modes" Gels 8, no. 10: 605. https://doi.org/10.3390/gels8100605
APA StyleHan, Q., Wang, Q., Gao, A., Ge, X., Wan, R., & Cao, X. (2022). Fluorescent Quinoline-Based Supramolecular Gel for Selective and Ratiometric Sensing Zinc Ion with Multi-Modes. Gels, 8(10), 605. https://doi.org/10.3390/gels8100605