Peptide-Mucin Binding and Biosimilar Mucus-Permeating Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Physicochemical Properties of Peptides
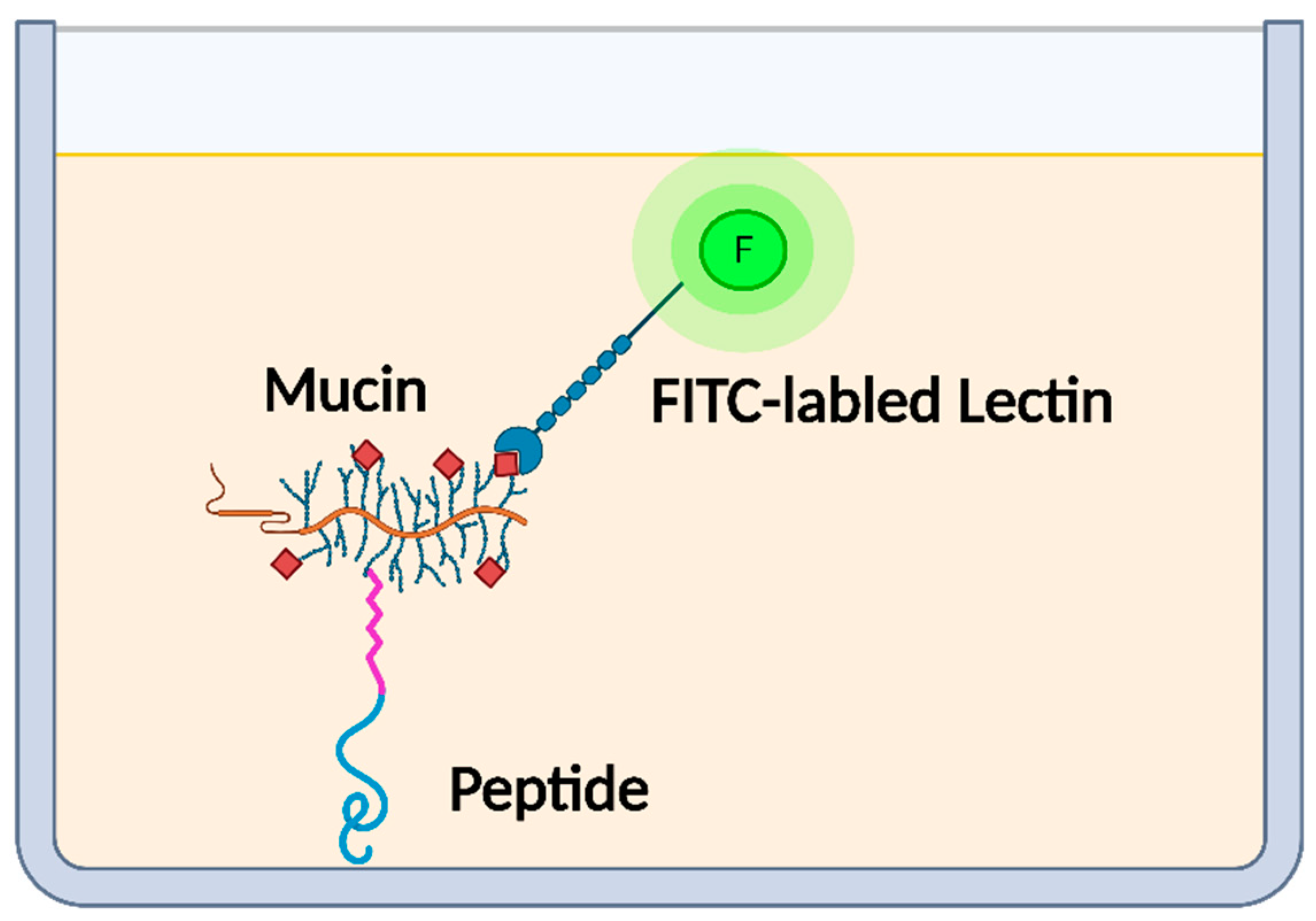
2.3. Determination of Mucin-Binding Activity of Peptides
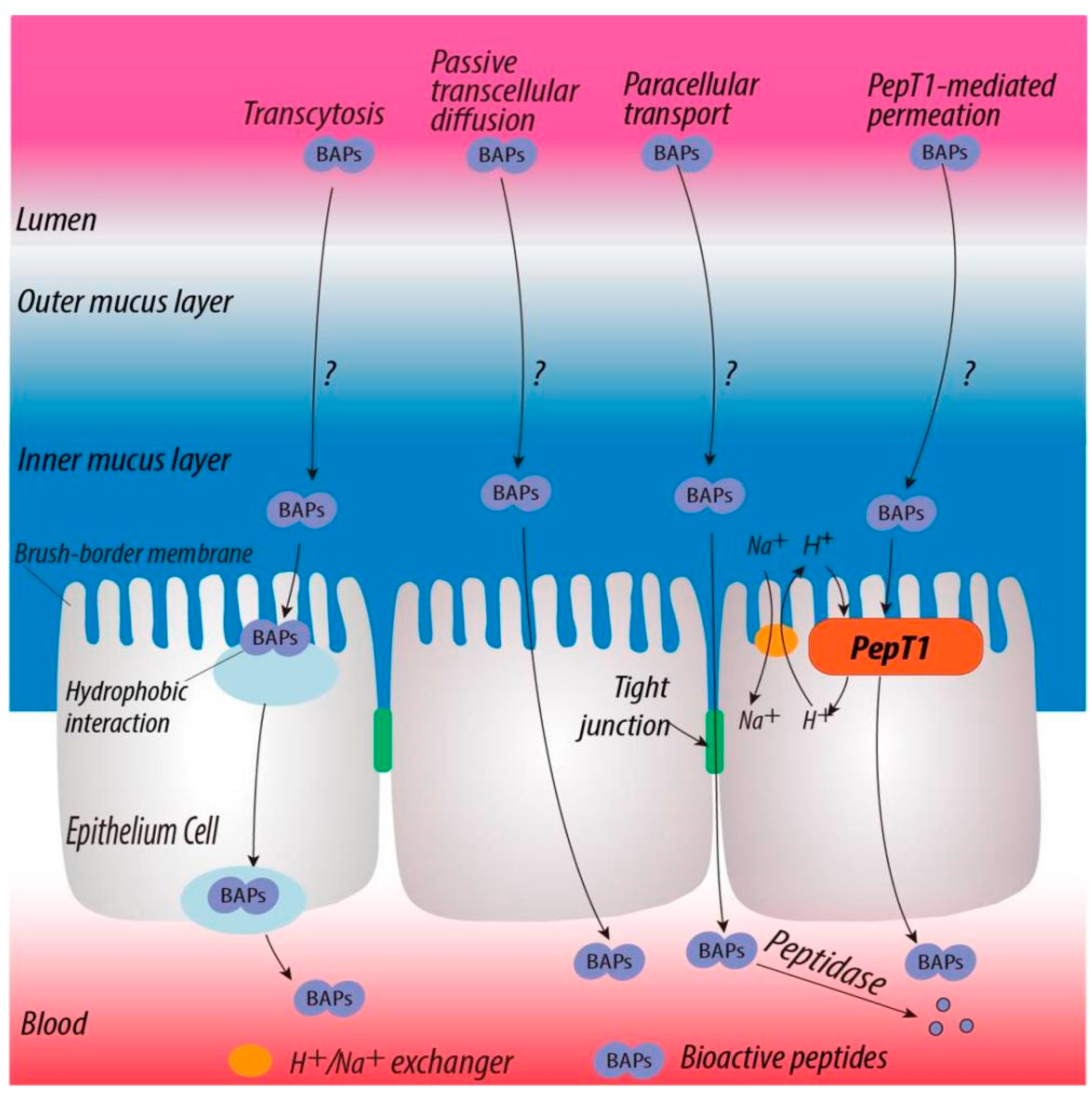
2.4. Quantification of Biosimilar Mucus-Permeating Property of Peptides
2.5. Statistical Analysis
3. Results and Discussion
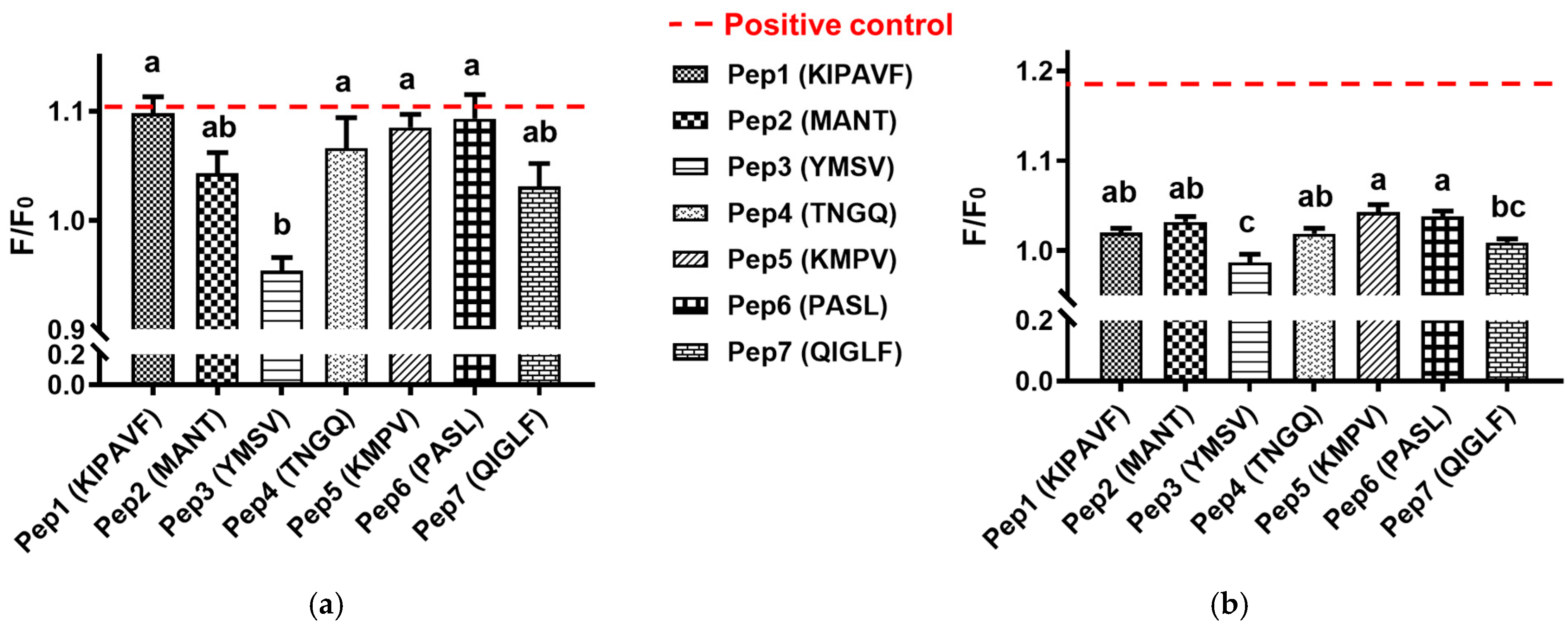
3.1. Mucin-Binding Activity of Peptides
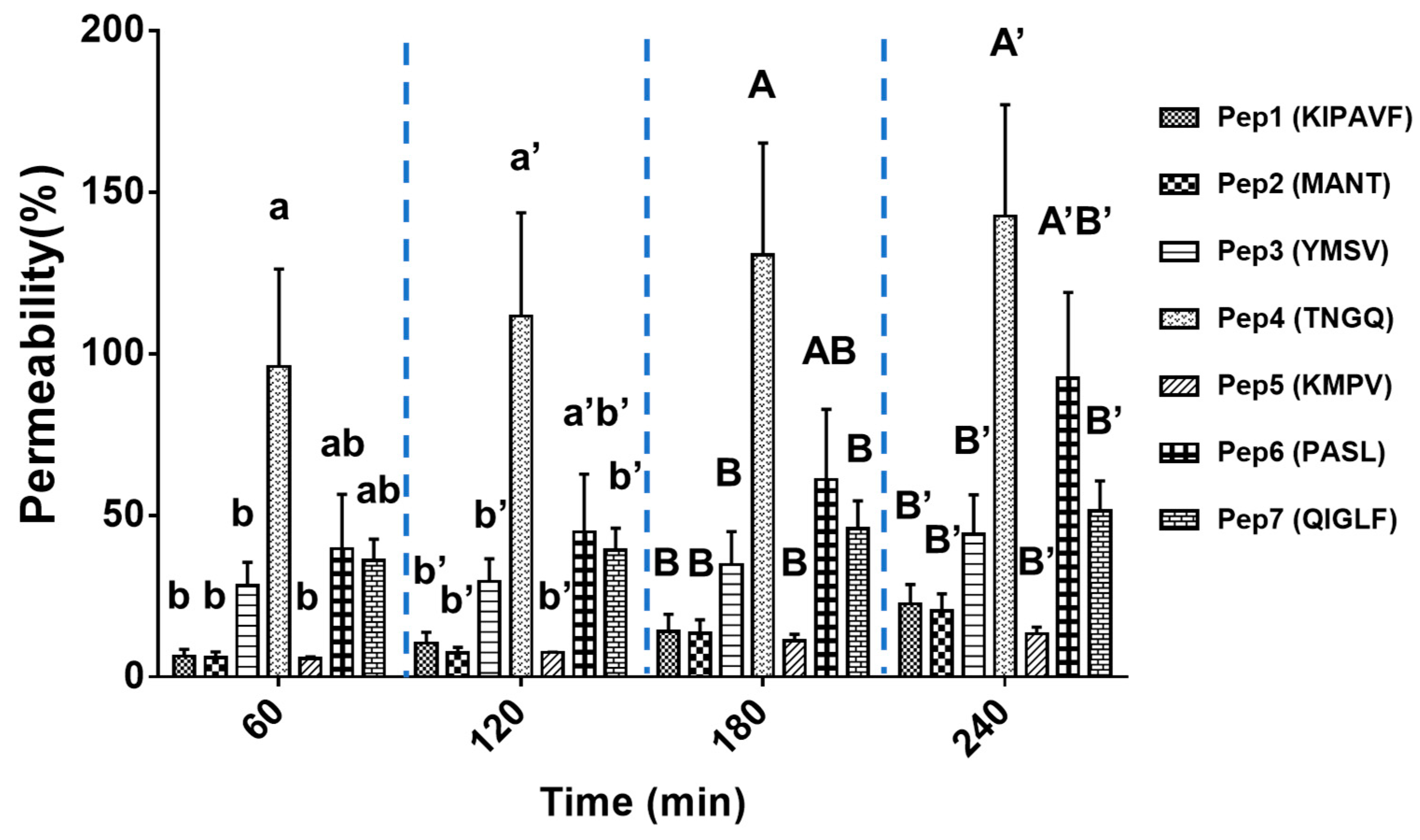
3.2. Mucus-Permeating Property of Peptides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Abioye, R.O.; Okagu, I.U.; Obeme-Nmom, J.I. Bioaccessibility of bioactive peptides: Recent advances and perspectives. Curr. Opin. Food Sci. 2021. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliver. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.; Sutton, P.; Karlsson, N.; Korolik, V.; McGuckin, M. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schömig, V.J.; Käsdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; McClements, D.J. Characterization of mucin–lipid droplet interactions: Influence on potential fate of fish oil-in-water emulsions under simulated gastrointestinal conditions. Food Hydrocolloid. 2016, 56, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Fan, H.; Yu, W.; Hong, H.; Wu, J. Transport Study of Egg-Derived Antihypertensive Peptides (LKP and IQW) Using Caco-2 and HT29 Coculture Monolayers. J. Agric. Food Chem. 2017, 65, 7406–7414. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Baldursdóttir, S.G.; Müllertz, A.; Nielsen, H.M. Property profiling of biosimilar mucus in a novel mucus-containing in vitro model for assessment of intestinal drug absorption. Eur. J. Pharm. Biopharm. 2014, 87, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Albarkah, Y.A.; Green, R.J.; Khutoryanskiy, V.V. Probing the Mucoadhesive Interactions Between Porcine Gastric Mucin and Some Water-Soluble Polymers. Macromol. Biosci. 2015, 15, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioall. Sci. 2011, 3, 89. [Google Scholar] [CrossRef]
- Menchicchi, B.; Fuenzalida, J.P.; Bobbili, K.B.; Hensel, A.; Swamy, M.J.; Goycoolea, F.M. Structure of Chitosan Determines Its Interactions with Mucin. Biomacromolecules 2014, 15, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Felgentreff, K.; Beisswenger, C.; Griese, M.; Gulder, T.; Bringmann, G.; Bals, R. The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides 2006, 27, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, Y.; Wang, Q.; Ren, T.; Gou, J.; Guo, W.; Yin, T.; He, H.; Zhang, Y.; Tang, X. Cell-penetrating peptide together with PEG-modified mesostructured silica nanoparticles promotes mucous permeation and oral delivery of therapeutic proteins and peptides. Biomater. Sci. 2019, 7, 2934–2950. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: A review. Food Hydrocolloid. 2021, 118, 106772. [Google Scholar] [CrossRef]
| No. | Sequence | Purity (%) | MW | pI | Net Charge at pH 7.0 | Hydrophobicity Index | Boman Index |
|---|---|---|---|---|---|---|---|
| Pep1 | KIPAVF | 98.7 | 673.8 | 9.70 | +0.9977 | 1.300 | −1.36 |
| Pep2 | MANT | 98.7 | 435.5 | 6.10 | −0.0020 | −0.125 | 1.26 |
| Pep3 | YMSV | 95.5 | 498.6 | 6.10 | −0.0029 | 1.000 | −0.71 |
| Pep4 | TNGQ | 95.7 | 418.4 | 6.10 | −0.0020 | −2.025 | 3.45 |
| Pep5 | KMPV | 99.2 | 473.6 | 9.70 | +0.9977 | 0.150 | −0.21 |
| Pep6 | PASL | 99.2 | 386.4 | 6.10 | −0.0020 | 0.800 | −0.83 |
| Pep7 | QIGLF | 98.1 | 576.7 | 6.10 | −0.0020 | 1.440 | −1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Abioye, R.O.; Okagu, O.D.; Udenigwe, C.C. Peptide-Mucin Binding and Biosimilar Mucus-Permeating Properties. Gels 2022, 8, 1. https://doi.org/10.3390/gels8010001
Sun X, Abioye RO, Okagu OD, Udenigwe CC. Peptide-Mucin Binding and Biosimilar Mucus-Permeating Properties. Gels. 2022; 8(1):1. https://doi.org/10.3390/gels8010001
Chicago/Turabian StyleSun, Xiaohong, Raliat O. Abioye, Ogadimma D. Okagu, and Chibuike C. Udenigwe. 2022. "Peptide-Mucin Binding and Biosimilar Mucus-Permeating Properties" Gels 8, no. 1: 1. https://doi.org/10.3390/gels8010001
APA StyleSun, X., Abioye, R. O., Okagu, O. D., & Udenigwe, C. C. (2022). Peptide-Mucin Binding and Biosimilar Mucus-Permeating Properties. Gels, 8(1), 1. https://doi.org/10.3390/gels8010001









