Electrospun Fibers Derived from Peptide Coupled Amphiphilic Copolymers for Dorsal Root Ganglion (DRG) Outgrowth
Abstract
:1. Introduction
2. Results and Discussion
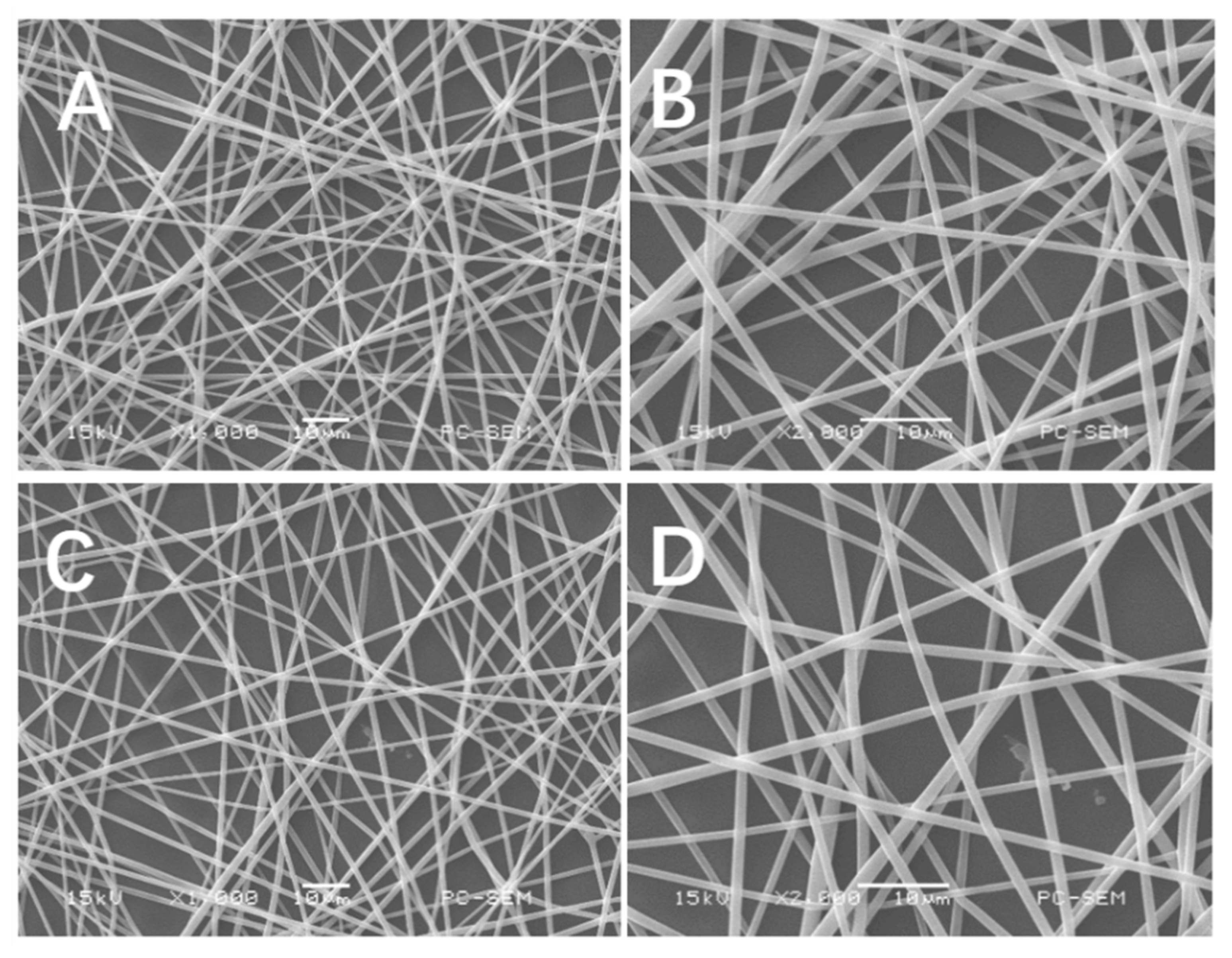
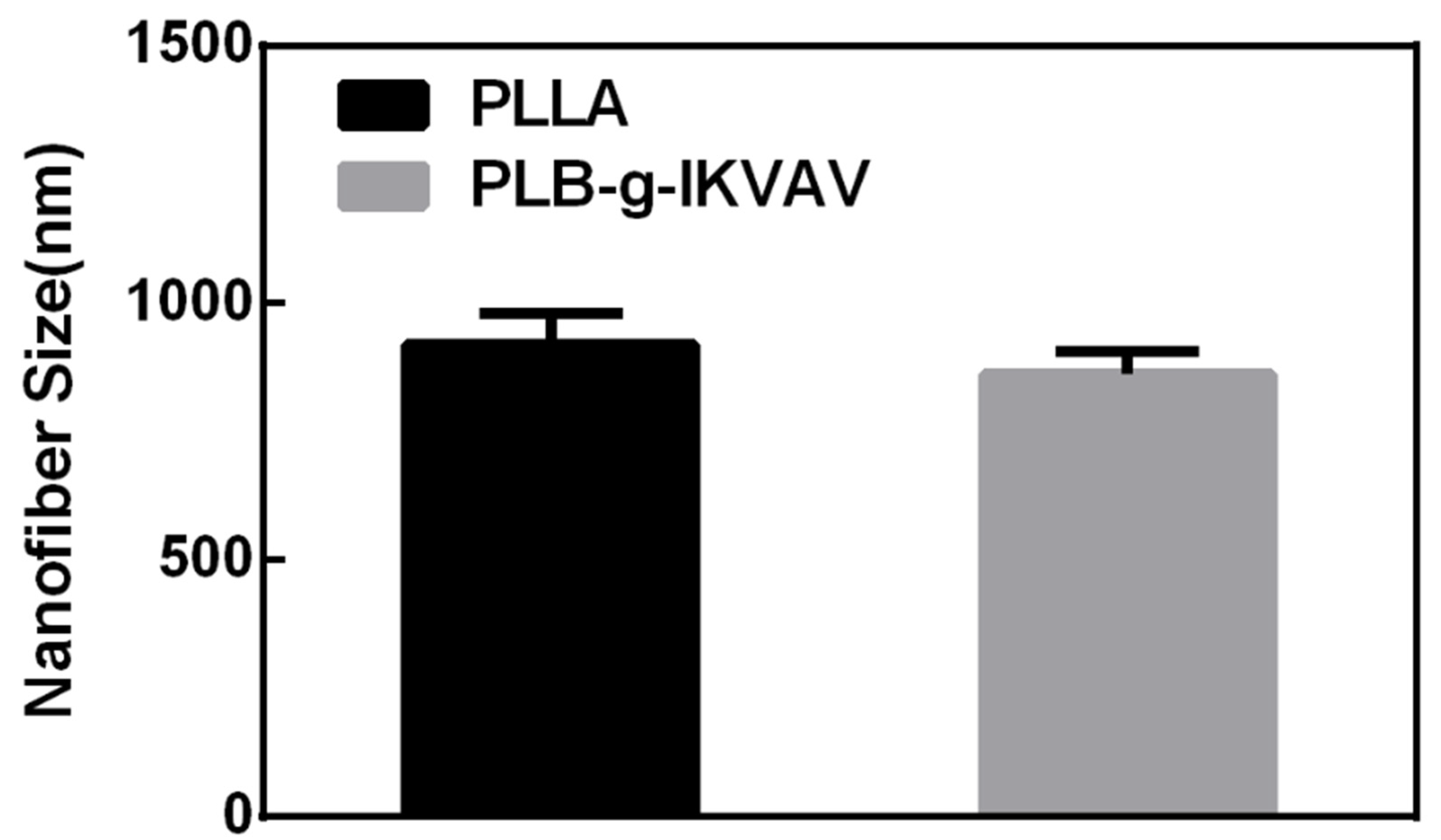
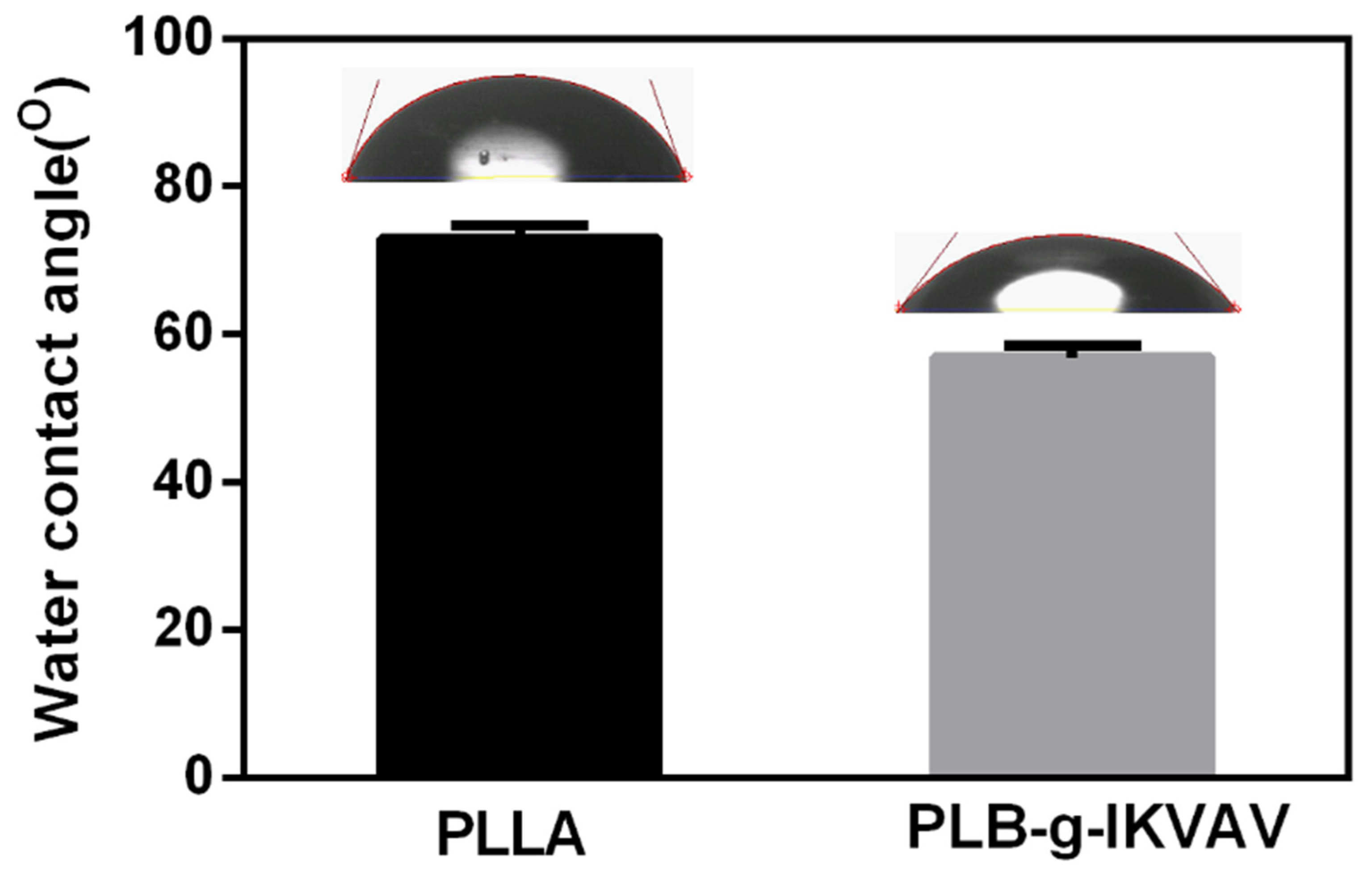
2.1. Morphology of Electrospun PLLA and PLB-g-IKVAV Fibers
2.2. DRGs Proliferation Studies on Electrospun PLLA and PLB-g-IKVAV Fiber Sheets
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Electrospinning
4.3. IKVAV Conjugation onto Electrospun Polymer Fibers
4.4. Electrospun Fiber Characterization
4.5. Surface Characterization
4.6. DRG Neurite Length
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, X.; Li, R.; Jia, W.; Liu, G.; Luo, Y.; Cheng, Z. Co-Axial Fibers with Janus-Structured Sheaths by Electrospinning Release Corn Peptides for Wound Healing. ACS. Appl. Bio. Mater. 2020, 3, 6430–6438. [Google Scholar] [CrossRef]
- Behtaj, S.; Karamali, F.; Masaeli, E.; Anissimov, Y.G.; Rybachuk, M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and pure PCL scaffolds for retinal progenitor cell cultivation. Biochem. Eng. J. 2021, 166, 107846. [Google Scholar] [CrossRef]
- Barrientos, I.J.H.; Paladino, E.; Szabó, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun collagen-based nanofibres: A sustainable material for improved antibiotic utilisation in tissue engineering applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.M.; Lee, S.H.; Lee, J.Y.; Son, J.S.; Kim, B.S.; Lee, B.; Chun, H.J.; Min, B.H.; Kim, J.H.; Kim, M.S. A biodegradable, injectable, gel system based on MPEG-b-(PCL-ran-PLLA) diblock copolymers with an adjustable therapeutic window. Biomaterials 2010, 31, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; Conti, B.; Colzani, B.; Dondi, D.; Lazzaroni, S.; Modena, T.; Genta, I. Ivermectin controlled release implants based on poly-D,L-lactide and poly-ε-caprolactone. J. Drug Deliv. Sci. Tec. 2018, 46, 101–110. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Hasirci, N.; Hasirci, V. Cell behavior on the alginate-coated PLLA/PLGA scaffolds. Int. J. Biol. Macromol. 2019, 124, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Jiang, S.; Song, P.; Song, X.; Liu, Q.; Wang, L.; Chen, X. Effect of blending HA-g-PLLA on xanthohumol-loaded PLGA fiber membrane. Colloid. Surf. B Biointerfaces 2016, 146, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Smith Callahan, L.A.; Xie, S.; Barker, I.A.; Zheng, J.; Reneker, D.H.; Dove, A.P.; Becker, M.L. Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 2013, 34, 9089–9095. [Google Scholar] [CrossRef] [PubMed]
- Zahir, L.; Kida, T.; Tanaka, R.; Nakayama, Y.; Shiono, T.; Kawasaki, N.; Yamano, N.; Nakayama, A. Synthesis and properties of biodegradable thermoplastic elastomers using 2-Methyl-1,3-propanediol, succinic acid and lactide. Polym. Degrad. Stabil. 2020, 181, 109353. [Google Scholar] [CrossRef]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.H.; Awad, G.A.S. Moxifloxacin-loaded in situ synthesized Bioceramic/Poly(L-lactide-co-ε-caprolactone) composite scaffolds for treatment of osteomyelitis and orthopedic regeneration. Int. J. Pharm. 2021, 602, 120662. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mat. Sci. Eng C 2016, 58, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- Li, S.; Su, L.; Li, X.; Yang, L.; Yang, M.; Zong, H.; Zong, Q.; Tang, J.; He, H. Reconstruction of abdominal wall with scaffolds of electrospun poly (L-lactide-co caprolactone) and porcine fibrinogen: An experimental study in the canine. Mat. Sci. Eng. C 2020, 110, 110644. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Singh, R.; Garlichs, C.D.; Alexiou, C. Nano-biomaterials for cardiovascular applications: Clinical perspective. J. Control. Release 2016, 229, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, H.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; et al. Biomedicine & Pharmacotherapy Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed. Pharm. 2021, 141, 111928. [Google Scholar] [CrossRef]
- Jing, W.; Zuo, D.; Cai, Q.; Chen, G.; Wang, L.; Yang, X.; Zhong, W. Promoting neural transdifferentiation of BMSCs via applying synergetic multiple factors for nerve regeneration. Exp. Cell. Res. 2019, 375, 80–91. [Google Scholar] [CrossRef]
- Sebe, I.; Ostorhazi, E.; Fekete, A.; Kovacs, K.N.; Zelko, R.; Kovalszky, I.; Li, W.; Wade, J.D.; Szabo, D.; Otvos, L. Polyvinyl alcohol nanofiber formulation of the designer antimicrobial peptide APO sterilizes Acinetobacter baumannii-infected skin wounds in mice. Amino. Acids 2016, 48, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, G.; Noh, I. Recent trends in metal ion based hydrogel biomaterials for tissue engineering and other biomedical applications. J. Mater. Sci. Technol. 2021, 63, 35–53. [Google Scholar] [CrossRef]
- Minati, L.; Migliaresi, C.; Lunelli, L.; Viero, G.; Dalla Serra, M.; Speranza, G. Plasma assisted surface treatments of biomaterials. Biophys. Chem. 2017, 229, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.S.; Aliakbarshirazi, S.; Vijayan, V.M.; Thukkaram, M.; De Geyter, N.; Thomas, V. Nonthermal plasma processing for nanostructured biomaterials and tissue engineering scaffolds: A mini review. Curr. Opin. Biomed. Eng. 2021, 17, 100259. [Google Scholar] [CrossRef]
- Kumar, S.; Perikamana, M.; Min, Y.; Kyu, J.; Bin, Y.; Heo, Y.; Ahmad, T.; Yeon, S.; Shin, J.; Min, K.; et al. Colloids and Surfaces B: Biointerfaces Graded functionalization of biomaterial surfaces using mussel-inspired adhesive coating of polydopamine. Colloid. Surf. B Biointerfaces 2017, 159, 546–556. [Google Scholar] [CrossRef]
- Kheirkhah, M.; Fathi, M.; Reza, H.; Razavi, M. Surface & Coatings Technology Surface modification of stainless steel implants using nanostructured forsterite (Mg2SiO4) coating for biomaterial applications. Surf. Coat. Tech. 2015, 276, 580–586. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.Y.; Zarie, E.S.; Elbahri, M.; Young, T.H.; Boccaccini, A.R. Bovine Serum Albumin (BSA)/polyacrylonitrile (PAN) biohybrid nanofibers coated with a biomineralized calcium deficient hydroxyapatite (HA) shell for wound dressing. Mat. Sci. Eng. C 2020, 116, 111248. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Li, H.; Song, Y.S.; Jeong, H.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Shin, Y.K.; Park, K.C.; Kim, D.S. Laminin peptide YIGSR enhances epidermal development of skin equivalents. J. Tissue Viability 2018, 27, 117–121. [Google Scholar] [CrossRef]
- Li, Z.; Bratlie, K.M. Materials Science & Engineering C Effect of RGD functionalization and stiffness of gellan gum hydrogels on macrophage polarization and function. Mat. Sci. Eng C 2021, 128, 112303. [Google Scholar] [CrossRef]
- Jagiełło, J.; Kuśmierz, M.; Kijeńska-Gawrońska, E.; Winkowska-Struzik, M.; Święszkowski, W.; Lipińska, L. Adhesive properties of graphene oxide and its modification with RGD peptide towards L929 cells. Mater. Today Commun. 2021, 26, 102056. [Google Scholar] [CrossRef]
- Sahab Negah, S.; Oliazadeh, P.; Jahanbazi Jahan-Abad, A.; Eshaghabadi, A.; Samini, F.; Ghasemi, S.; Asghari, A.; Gorji, A. Transplantation of human meningioma stem cells loaded on a self-assembling peptide nanoscaffold containing IKVAV improves traumatic brain injury in rats. Acta Biomater. 2019, 92, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, F.A.A.; Sidney, L.E.; Kiick, K.L.; Segal, J.I.; Alexander, C.; Rose, F.R.A.J. The electrospinning of a thermo-responsive polymer with peptide conjugates for phenotype support and extracellular matrix production of therapeutically relevant mammalian cells. Biomater. Sci. 2020, 8, 2611–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silantyeva, E.A.; Nasir, W.; Carpenter, J.; Manahan, O.; Becker, M.L.; Willits, R.K. Accelerated neural differentiation of mouse embryonic stem cells on aligned GYIGSR-functionalized nanofibers. Acta Biomater. 2018, 75, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.C.; Kang, M.S.; Park, R.; Chae, S.Y.; Han, D.W.; Hong, S.W. Differential cellular interactions and responses to ultrathin micropatterned graphene oxide arrays with or without ordered in turn RGD peptide films. Appl. Surf. Sci. 2021, 561, 150115. [Google Scholar] [CrossRef]
- Zou, Z.; Zheng, Q.; Wu, Y.; Song, Y.; Wu, B. Growth of rat dorsal root ganglion neurons on a novel self-assembling scaffold containing IKVAV sequence. Mat. Sci. Eng. C 2009, 29, 2099–2103. [Google Scholar] [CrossRef]
- Lee, J.; Tae, G.; Kim, Y.H.; Park, I.S.; Kim, S.H.; Kim, S.H. The effect of gelatin incorporation into electrospun poly(l-lactide-co-ε-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials 2008, 29, 1872–1879. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Monavari, M.; Koenen, B.; Boccaccini, A.R. Biomimetic biohybrid nanofibers containing bovine serum albumin as a bioactive moiety for wound dressing. Mat. Sci. Eng C 2021, 123, 111965. [Google Scholar] [CrossRef]
- Ghane-Motlagh, B.; Javanbakht, T.; Shoghi, F.; Wilkinson, K.J.; Martel, R.; Sawan, M. Physicochemical properties of peptide-coated microelectrode arrays and their in vitro effects on neuroblast cells. Mat. Sci. Eng. C 2016, 68, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Qiang, N.; Tang, S.; Liao, X.; Liang, H.; Xie, F.; Zhu, J.X. Synthesis of Functional Polyester Based on Polylactic Acid and Its Effect on PC12 Cells after Coupling with Small Peptides. Int. J. Polym. Sci. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
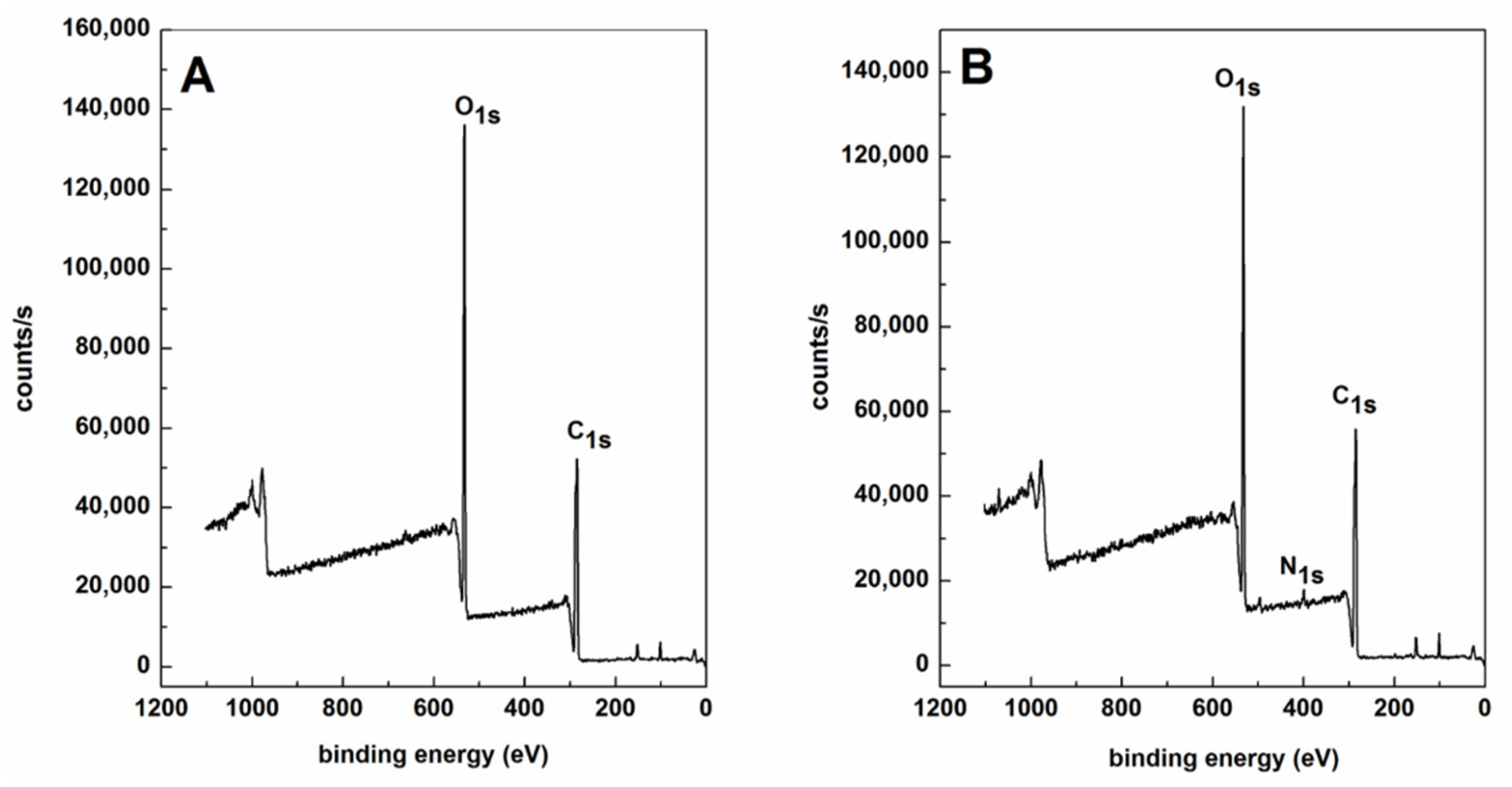


| Samples | C Atomic Concentration(%) | N Atomic Concentration(%) | O Atomic Concentration(%) |
|---|---|---|---|
| PLLA | 57.22 | 0 | 40.73 |
| PCB-IKVAV | 57.11 | 1.83 | 37.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, N.; Lin, W.; Zhou, X.; Liu, Z.; Lu, M.; Qiu, S.; Tang, S.; Zhu, J. Electrospun Fibers Derived from Peptide Coupled Amphiphilic Copolymers for Dorsal Root Ganglion (DRG) Outgrowth. Gels 2021, 7, 196. https://doi.org/10.3390/gels7040196
Qiang N, Lin W, Zhou X, Liu Z, Lu M, Qiu S, Tang S, Zhu J. Electrospun Fibers Derived from Peptide Coupled Amphiphilic Copolymers for Dorsal Root Ganglion (DRG) Outgrowth. Gels. 2021; 7(4):196. https://doi.org/10.3390/gels7040196
Chicago/Turabian StyleQiang, Na, Wensheng Lin, Xingwu Zhou, Zhu Liu, Ming Lu, Si Qiu, Shuo Tang, and Jixiang Zhu. 2021. "Electrospun Fibers Derived from Peptide Coupled Amphiphilic Copolymers for Dorsal Root Ganglion (DRG) Outgrowth" Gels 7, no. 4: 196. https://doi.org/10.3390/gels7040196
APA StyleQiang, N., Lin, W., Zhou, X., Liu, Z., Lu, M., Qiu, S., Tang, S., & Zhu, J. (2021). Electrospun Fibers Derived from Peptide Coupled Amphiphilic Copolymers for Dorsal Root Ganglion (DRG) Outgrowth. Gels, 7(4), 196. https://doi.org/10.3390/gels7040196






