Synthesis of Oxidant Functionalised Cationic Polymer Hydrogel for Enhanced Removal of Arsenic (III)
Abstract
:1. Introduction
2. Results and Discussion
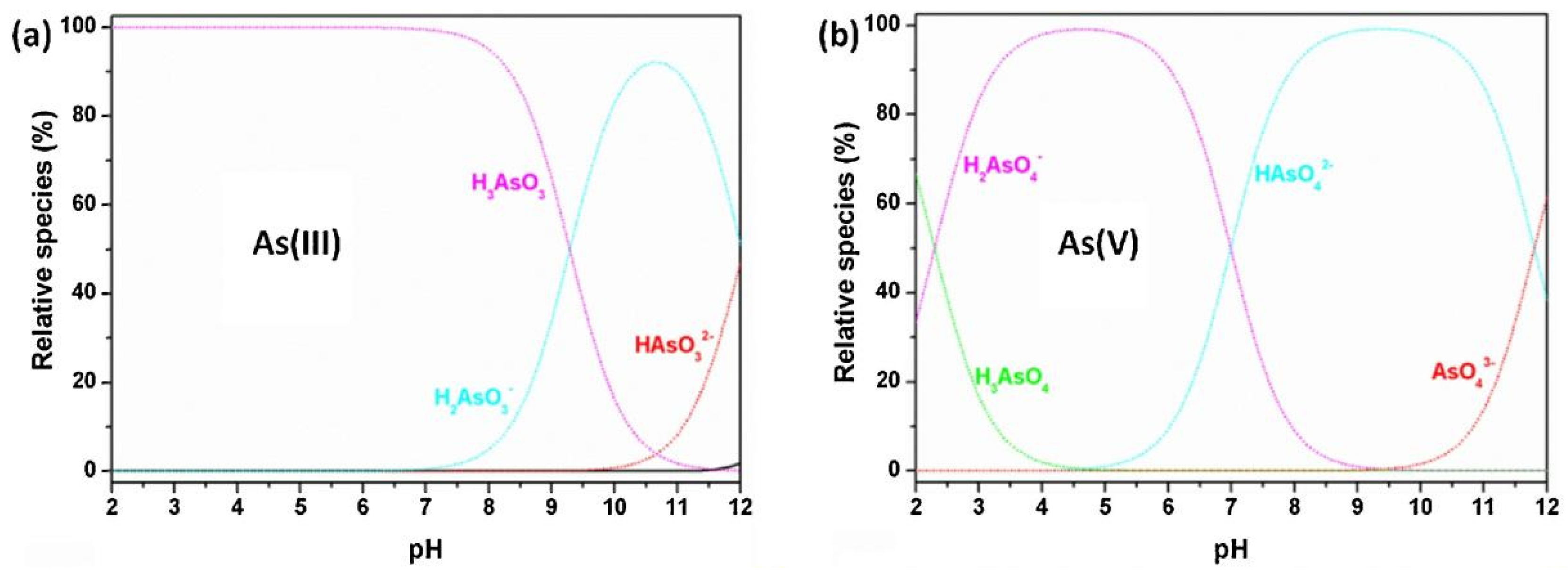
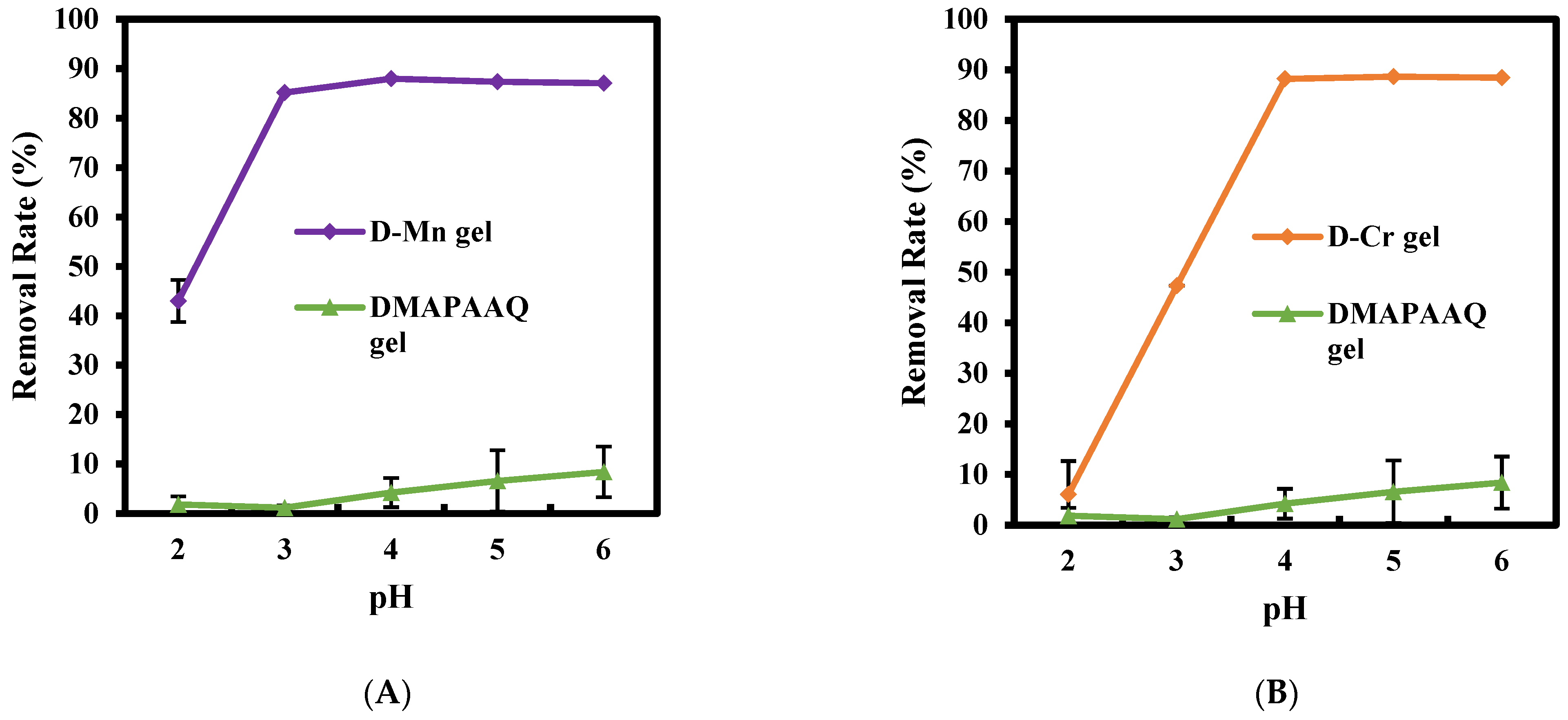
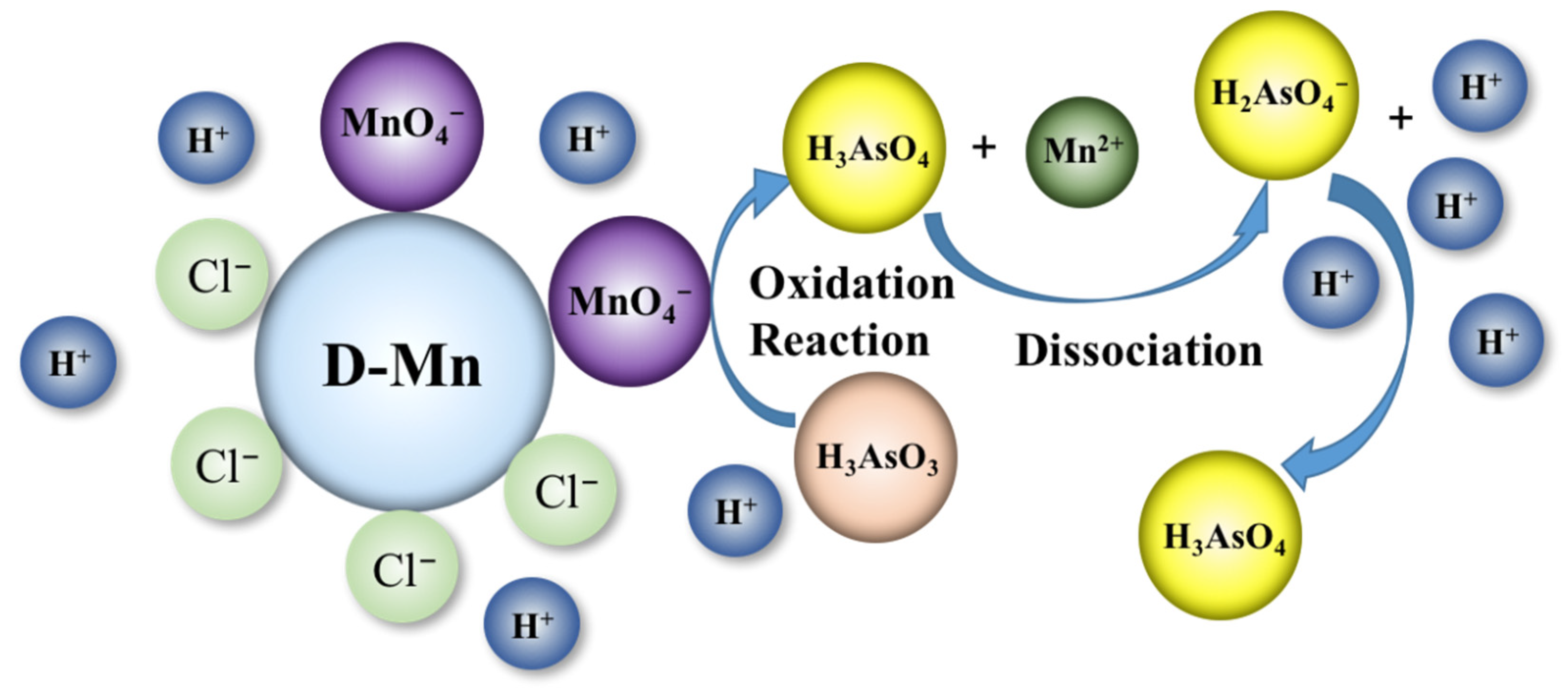
2.1. Effect of pH of the As Solution on the Gel Adsorption
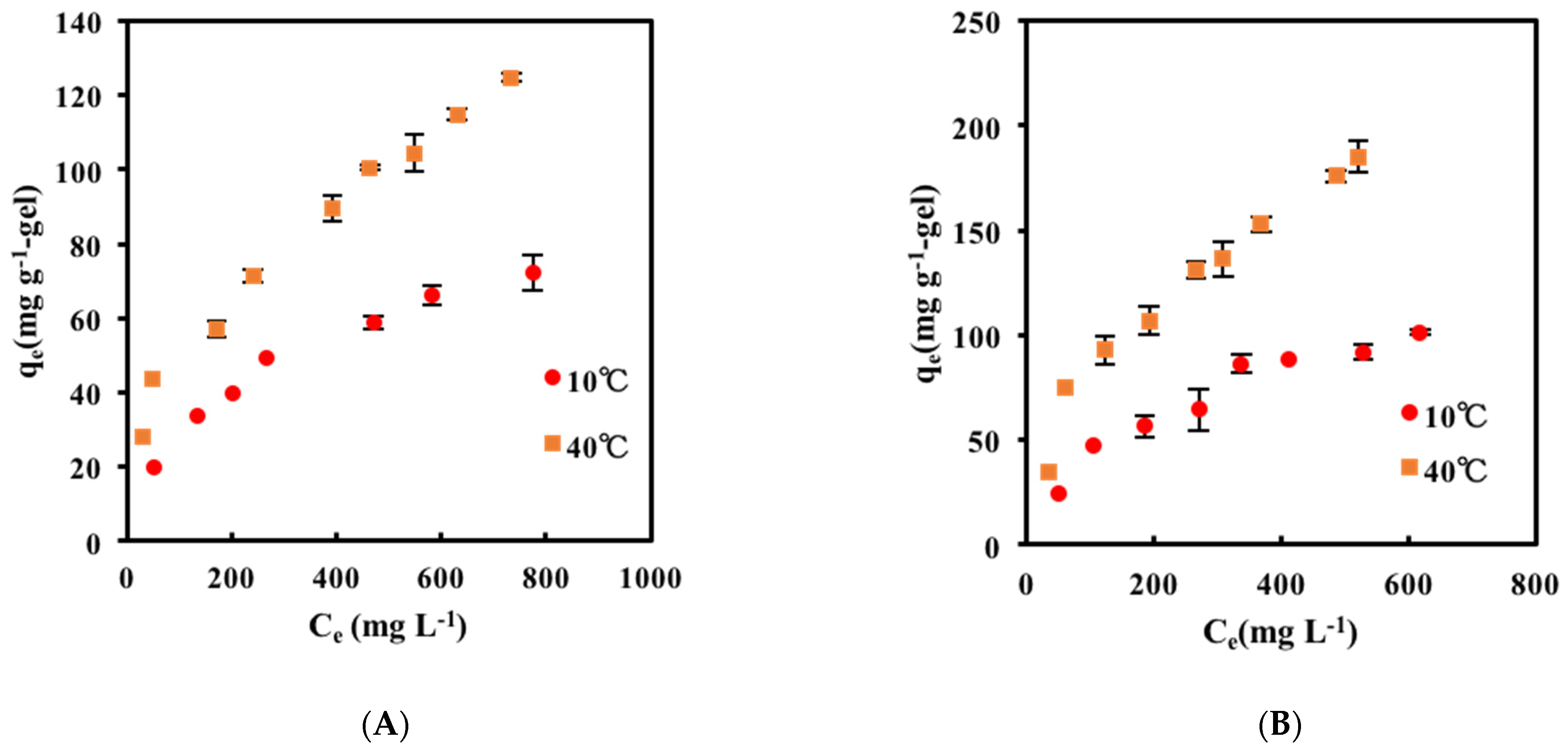
2.2. Adsorption Isotherm
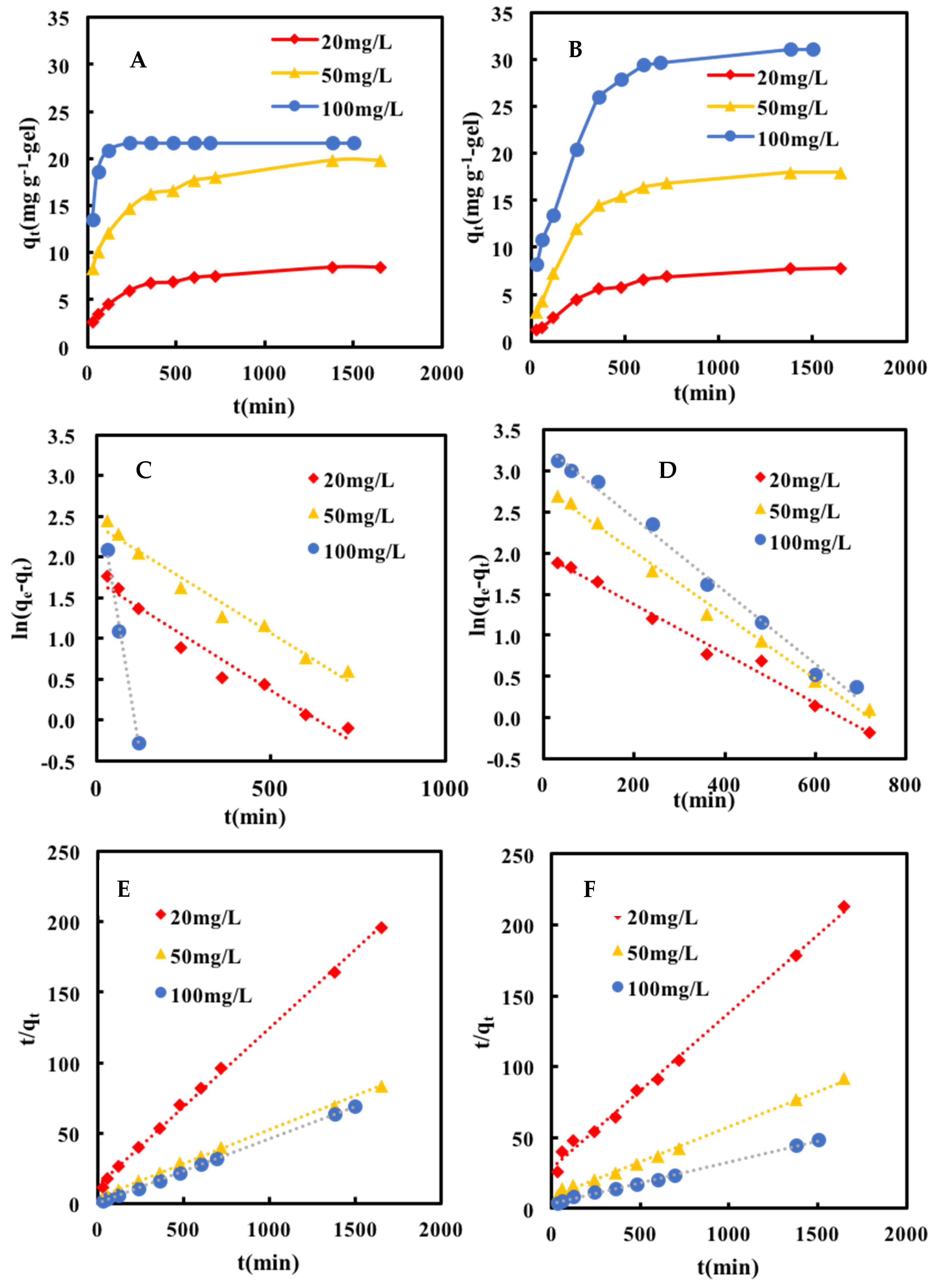
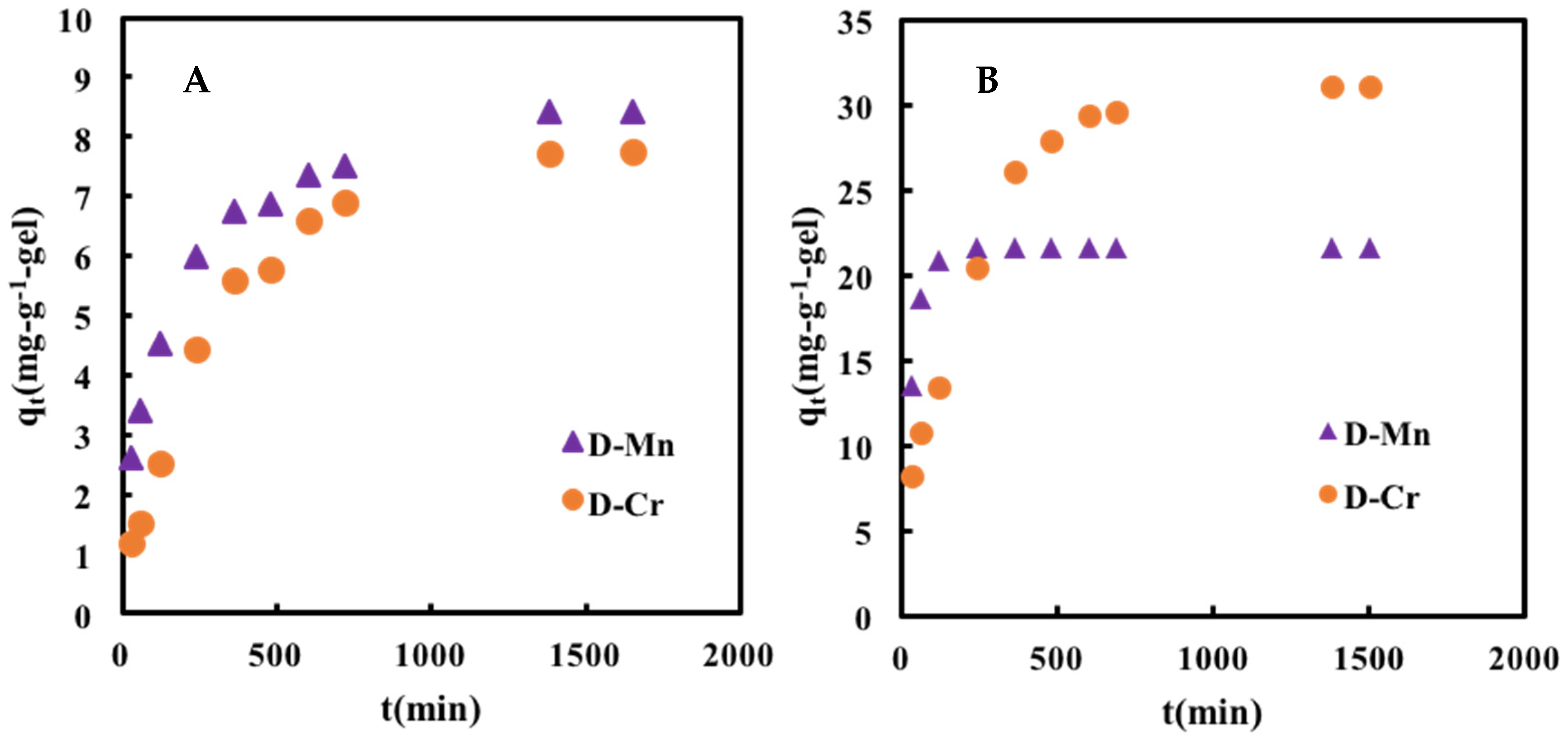
2.3. Adsorption Kinetics
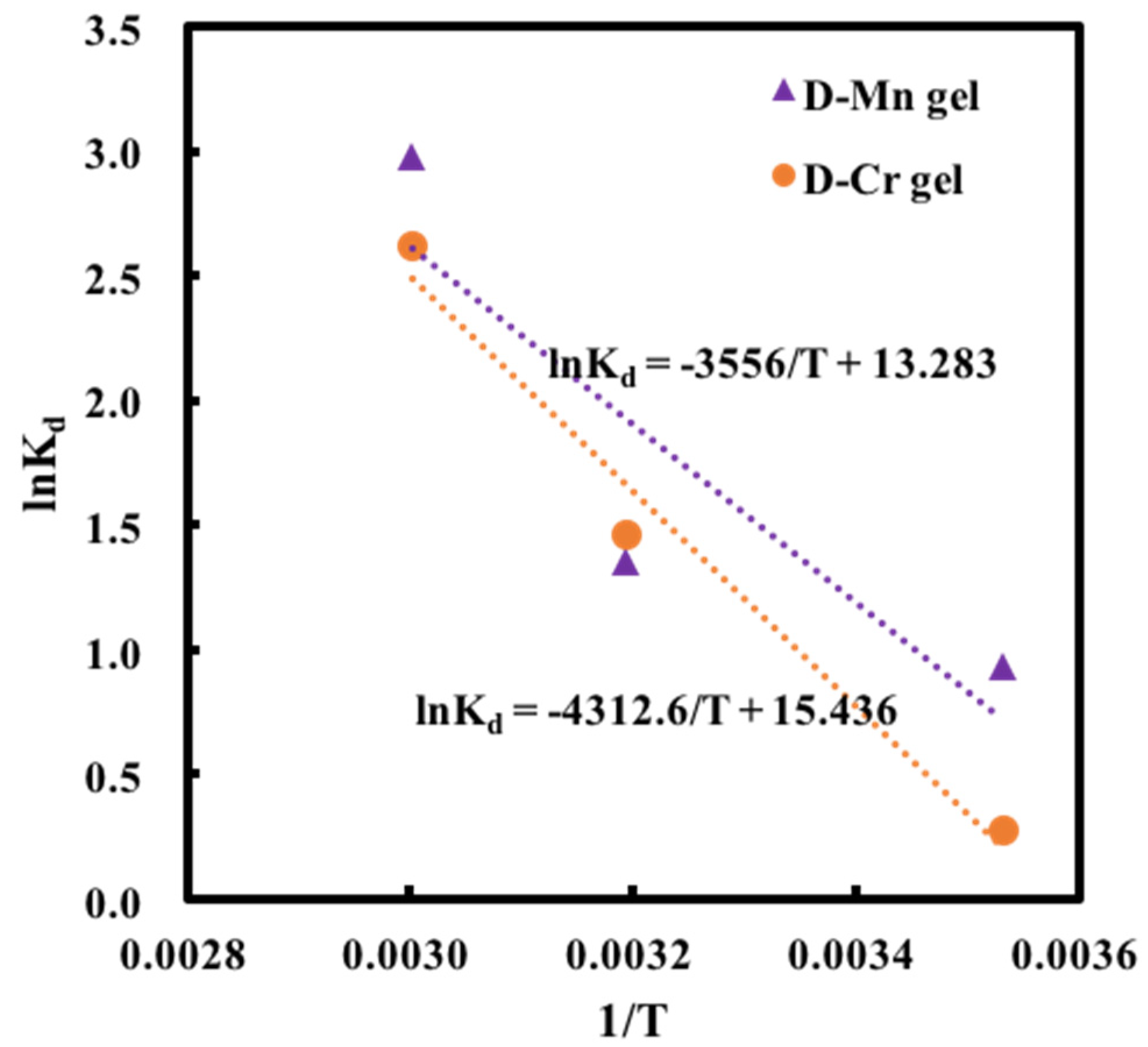
2.4. Adsorption Thermodynamics
2.5. Effect of Co-Existing Ions on the As Removal Property of the D-Mn and D-Cr Gels
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of DMAPAAQ Hydrogel
4.3. Synthesis of the D-Mn Gel and D-Cr Gel
4.4. Batch Adsorption Experiments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Shakoor, A.; Maitlo, A.A.; Chen, D.-Y. Adsorption of Arsenic (III) from Aqueous Solution by a Novel Phosphorus-Modified Biochar Obtained from Taraxacum Mongolicum Hand-Mazz: Adsorption Behavior and Mechanistic Analysis. J. Environ. Manag. 2021, 292, 112764. [Google Scholar] [CrossRef]
- Saif, S.; Adil, S.F.; Khan, M.; Hatshan, M.R.; Khan, M.; Bashir, F. Adsorption Studies of Arsenic(V) by CuO Nanoparticles Synthesized by Phyllanthus Emblica Leaf-Extract-Fueled Solution Combustion Synthesis. Sustainability 2021, 13, 2017. [Google Scholar] [CrossRef]
- Brammer, H.; Ravenscroft, P. Arsenic in Groundwater: A Threat to Sustainable Agriculture in South and South-East Asia. Environ Int. 2009, 35, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.C.S.; Rosemberg, R.S.; Bomfeti, C.A.; Monteiro, D.S.; Barbosa, F.; Oliveira, L.C.A.; Rodriguez, M.; Pereira, M.C.; Rodrigues, J.L. Arsenic Removal from Contaminated Water by Ultrafine δ-FeOOH Adsorbents. Chem. Eng. J. 2014, 237, 47–54. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Sorg, T.J.; Chen, A.S.C.; Wang, L. Arsenic Species in Drinking Water Wells in the USA with High Arsenic Concentrations. Water Res. 2014, 48, 156–169. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Home Page. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 15 January 2018).
- Sarmah, S.; Saikia, J.; Phukan, A.; Goswamee, R.L. Adsorption of As(V) from Water over a Hydroxyl-Alumina Modified Paddy Husk Ash Surface and Its Sludge Immobilization. Water Air Soil Pollut. 2019, 230, 32. [Google Scholar] [CrossRef]
- Amini, M.; Abbaspour, K.C.; Berg, M.; Winkel, L.; Hug, S.J.; Hoehn, E.; Yang, H.; Johnson, C.A. Statistical Modeling of Global Geogenic Arsenic Contamination in Groundwater. Environ. Sci. Technol. 2008, 42, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zhu, Y.; Liu, W.; Huang, H.; Li, H. Selective Removal of As(III) Using Magnetic Graphene Oxide Ion-Imprinted Polymer in Porous Media: Potential Effect of External Magnetic Field. J. Environ. Chem. Eng. 2021, 9, 105671. [Google Scholar] [CrossRef]
- Guo, L.; Ye, P.; Wang, J.; Fu, F.; Wu, Z. Three-Dimensional Fe3O4-Graphene Macroscopic Composites for Arsenic and Arsenate Removal. J. Hazard. Mater. 2015, 298, 28–35. [Google Scholar] [CrossRef]
- Yan, B.; Liang, T.; Yang, X.; Gadgil, A.J. Superior Removal of As(III) and As(V) from Water with Mn-Doped β-FeOOH Nanospindles on Carbon Foam. J. Hazard. Mater. 2021, 418, 126347. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.T.; Phong, P.T.; Minh, T.D. Synthesis of Iron Oxide Nanoparticle Functionalized Activated Carbon and Its Applications in Arsenic Adsorption. J. Anal. Methods Chem. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Kanel, S.R.; Nepal, D.; Manning, B.; Choi, H. Transport of Surface-Modified Iron Nanoparticle in Porous Media and Application to Arsenic(III) Remediation. J. Nanopart. Res. 2007, 9, 725–735. [Google Scholar] [CrossRef]
- Rahdar, S.; Taghavi, M.; Khaksefidi, R.; Ahmadi, S. Adsorption of Arsenic (V) from Aqueous Solution Using Modified Saxaul Ash: Isotherm and Thermodynamic Study. Appl. Water Sci. 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, M.; Stecher, G.; Huck, C.; Bonn, G. Preparation of Polymer Based Sorbents for Solid Phase Extraction of Polyphenolic Compounds. Open Chem. 2011, 9, 206–212. [Google Scholar] [CrossRef]
- Beaugeard, V.; Muller, J.; Graillot, A.; Ding, X.; Robin, J.-J.; Monge, S. Acidic Polymeric Sorbents for the Removal of Metallic Pollution in Water: A Review. Reactive Funct. Polym. 2020, 152, 104599. [Google Scholar] [CrossRef]
- Önnby, L.; Pakade, V.; Mattiasson, B.; Kirsebom, H. Polymer Composite Adsorbents Using Particles of Molecularly Imprinted Polymers or Aluminium Oxide Nanoparticles for Treatment of Arsenic Contaminated Waters. Water Res. 2012, 46, 4111–4120. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Shang, X.; Huang, J.-H.; Dempsey, B.A. Treating Laundry Waste Water: Cationic Polymers for Removal of Contaminants and Decreased Fouling in Microfiltration. J. Membr. Sci. 2014, 456, 167–174. [Google Scholar] [CrossRef]
- Safi, S.R.; Senmoto, K.; Gotoh, T.; Iizawa, T.; Nakai, S. The Effect of γ-FeOOH on Enhancing Arsenic Adsorption from Groundwater with DMAPAAQ + FeOOH Gel Composite. Sci. Rep. 2019, 9, 11909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, T.; Zhang, Z.; Xu, L.; Zhang, C. Enhanced Adsorption of Trivalent Arsenic from Water by Functionalized Diatom Silica Shells. PLoS ONE 2015, 10, e0123395. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Rai, P.K. Ultrafast Removal of Arsenic Using Solid Solution of Aero-Gel Based Ce1-XTixO2-Y Oxide Nanoparticles. Chemosphere 2019, 217, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Lu, J.; Zhang, Y.; Cheng, G.; Huang, S.; Chen, J.; Xu, R.; Ming, Y.; Wang, Y.; Chen, R. Facile Inverse Micelle Fabrication of Magnetic Ordered Mesoporous Iron Cerium Bimetal Oxides with Excellent Performance for Arsenic Removal from Water. J. Hazard. Mater. 2020, 383, 121172. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Sahu, M.K.; Patel, R.K. Adsorption Studies of Arsenic(III) Removal from Water by Zirconium Polyacrylamide Hybrid Material (ZrPACM-43). Water Resour. Ind. 2013, 4, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, T.S.; Das, S.; Pratt, C.J.; Seal, S. One-Pot Synthesis of a Ceria–Graphene Oxide Composite for the Efficient Removal of Arsenic Species. Nanoscale 2017, 9, 3367–3374. [Google Scholar] [CrossRef]
- Li, R.; Li, Q.; Gao, S.; Shang, J.K. Exceptional Arsenic Adsorption Performance of Hydrous Cerium Oxide Nanoparticles: Part A. Adsorption Capacity and Mechanism. Chem. Eng. J. 2012, 185–186, 127–135. [Google Scholar] [CrossRef]
- Martinson, C.A.; Reddy, K.J. Adsorption of Arsenic(III) and Arsenic(V) by Cupric Oxide Nanoparticles. J. Colloid Interface Sci. 2009, 336, 406–411. [Google Scholar] [CrossRef]
- Nabi, D.; Aslam, I.; Qazi, I.A. Evaluation of the Adsorption Potential of Titanium Dioxide Nanoparticles for Arsenic Removal. J. Environ. Sci. 2009, 21, 402–408. [Google Scholar] [CrossRef]

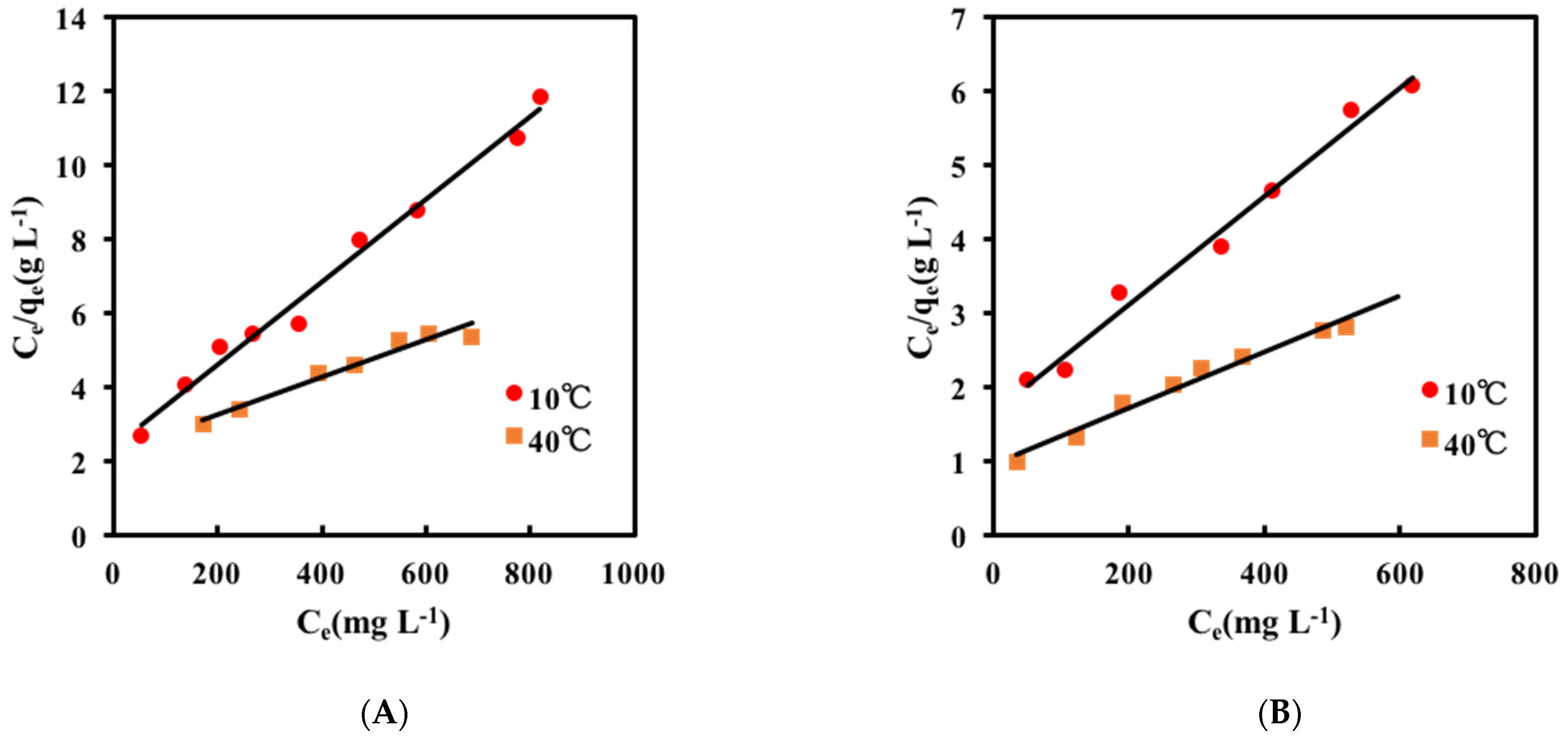


| Temperature(°C) | Qmax (mg g−1-gel) | KL (L mg−1) | R2 |
|---|---|---|---|
| 10 | 89 | 0.0005 | 0.9866 |
| 40 | 200 | 0.0002 | 0.9534 |
| Temperature(°C) | Qmax (mg g−1-gel) | KL (L mg−1) | R2 |
|---|---|---|---|
| 10 | 137 | 0.0004 | 0.9855 |
| 40 | 263 | 0.0004 | 0.9758 |
| Adsorbent | Initial As Concentration Range (mg L−1) | Adsorbent Dosage (g L−1) | Max. As(III) Adsorption Capacity (mg g−1) | References |
|---|---|---|---|---|
| D-Mn gel | 1000−10 | 2 | 200 | This work |
| D-Cr gel | 1000−10 | 2 | 263 | This work |
| Fe(III)/La(III)-chitosan | 1−0.05 | - | 109 | [19] |
| ZrPACM-43 | 100−10 | 13 | 41.48 | [25] |
| Ceria-GO composite | 200–0.01 | 0.5 | 185 | [26] |
| Hydrous Cerium Oxide | 100–1 | 0.5 | 170 | [27] |
| CuO nanoparticles | 400−200 | 0.08 | 39 | [28] |
| TiO2 nanoparticles | 90−5 | 1 | 31.35 | [29] |
| Fe3O4-graphene composite | 1–0.1 | 2 | 0.313 | [11] |
| Initial Concentration | Pseudo-First-Order Equation | Pseudo-Second-Order Equation | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | |
| 20 mg L−1 | 0.0027 | 5.45 | 0.96586 | 0.00097 | 8.98 | 0.99830 |
| 50 mg L−1 | 0.0027 | 10.91 | 0.97547 | 0.00056 | 20.75 | 0.99822 |
| 100 mg L−1 | − | − | − | 0.00680 | 21.79 | 0.9999 |
| Initial Concentration | Pseudo-First-Order Equation | Pseudo-Second-Order Equation | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | |
| 20 mg L−1 | 0.0030 | 7.23 | 0.98977 | 0.00043 | 9.08 | 0.99546 |
| 50 mg L−1 | 0.0039 | 16.27 | 0.99419 | 0.00029 | 20.20 | 0.99681 |
| 100 mg L−1 | 0.0044 | 27.46 | 0.99267 | 0.00023 | 34.01 | 0.99683 |
| T(K) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K−1) | |
|---|---|---|---|---|
| 283.15 | 0.93258781 | −1.5815 | 29.565 | 0.110 |
| 313.15 | 1.35287955 | −4.8815 | 29.565 | 0.110 |
| 333.15 | 2.97647589 | −7.0815 | 29.565 | 0.110 |
| T(K) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K−1) | |
|---|---|---|---|---|
| 283.15 | 0.2777234 | −0.3872 | 35.856 | 0.128 |
| 313.15 | 1.46240344 | −4.2272 | 35.856 | 0.128 |
| 333.15 | 2.61940407 | −6.7872 | 35.856 | 0.128 |
| Component | Function | Molecular Weight (g mol−1) | Concentration (mol m−3) | Mass (g) |
|---|---|---|---|---|
| DMAPAAQ | Monomer | 206.71 | 1000 | 4.1342 |
| MBAA | Linker | 154.17 | 50 | 0.1156 |
| TEMED | Accelerator | 116.21 | 20 | 0.0349 |
| APS | Initiator | 228.19 | 20 | 0.0685 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Gotoh, T.; Nakai, S. Synthesis of Oxidant Functionalised Cationic Polymer Hydrogel for Enhanced Removal of Arsenic (III). Gels 2021, 7, 197. https://doi.org/10.3390/gels7040197
Song Y, Gotoh T, Nakai S. Synthesis of Oxidant Functionalised Cationic Polymer Hydrogel for Enhanced Removal of Arsenic (III). Gels. 2021; 7(4):197. https://doi.org/10.3390/gels7040197
Chicago/Turabian StyleSong, Yu, Takehiko Gotoh, and Satoshi Nakai. 2021. "Synthesis of Oxidant Functionalised Cationic Polymer Hydrogel for Enhanced Removal of Arsenic (III)" Gels 7, no. 4: 197. https://doi.org/10.3390/gels7040197
APA StyleSong, Y., Gotoh, T., & Nakai, S. (2021). Synthesis of Oxidant Functionalised Cationic Polymer Hydrogel for Enhanced Removal of Arsenic (III). Gels, 7(4), 197. https://doi.org/10.3390/gels7040197






