Highly Flexibility, Powder Self-Healing, and Recyclable Natural Polymer Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
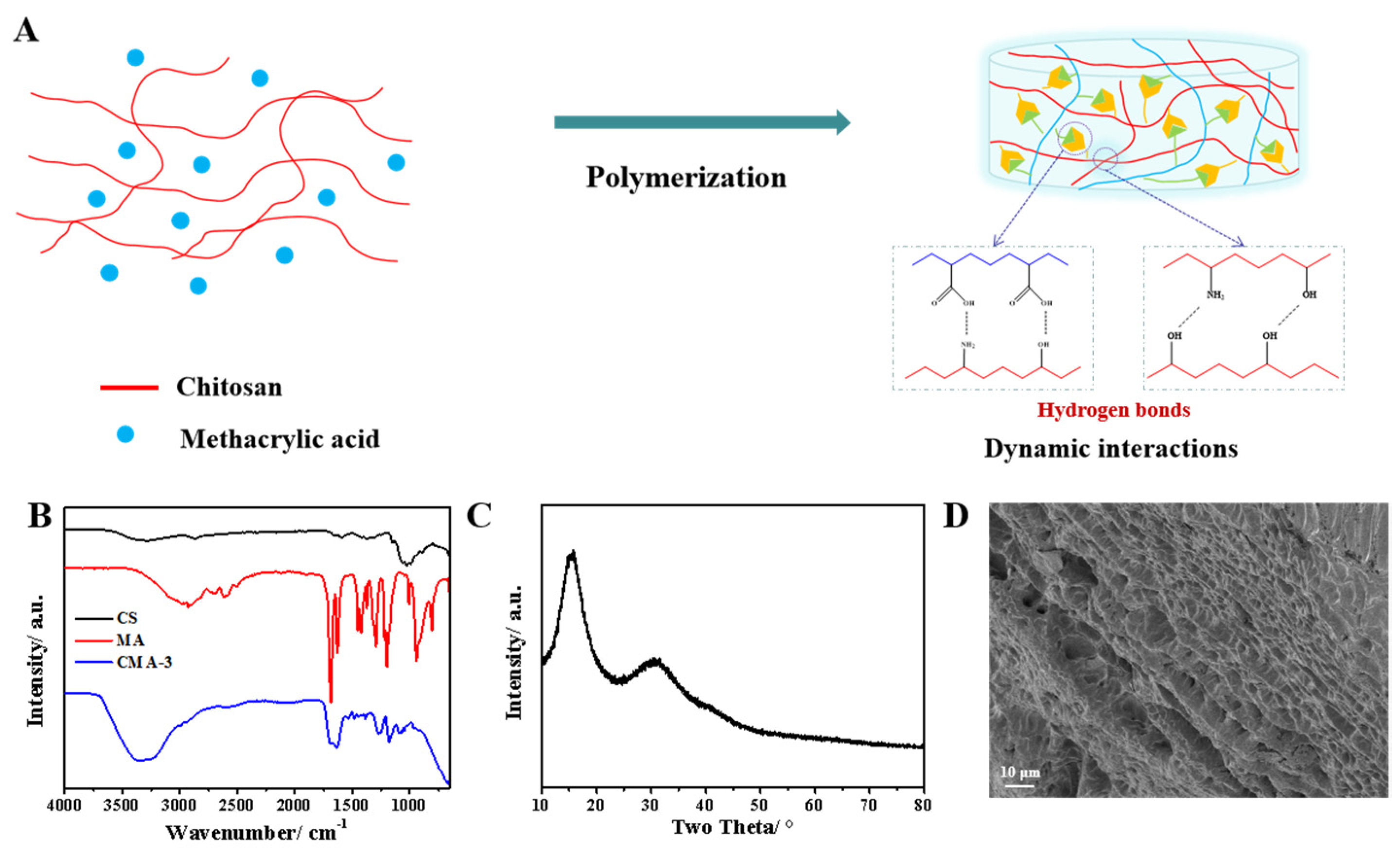
2.1. Characterization of CMA Hydrogels
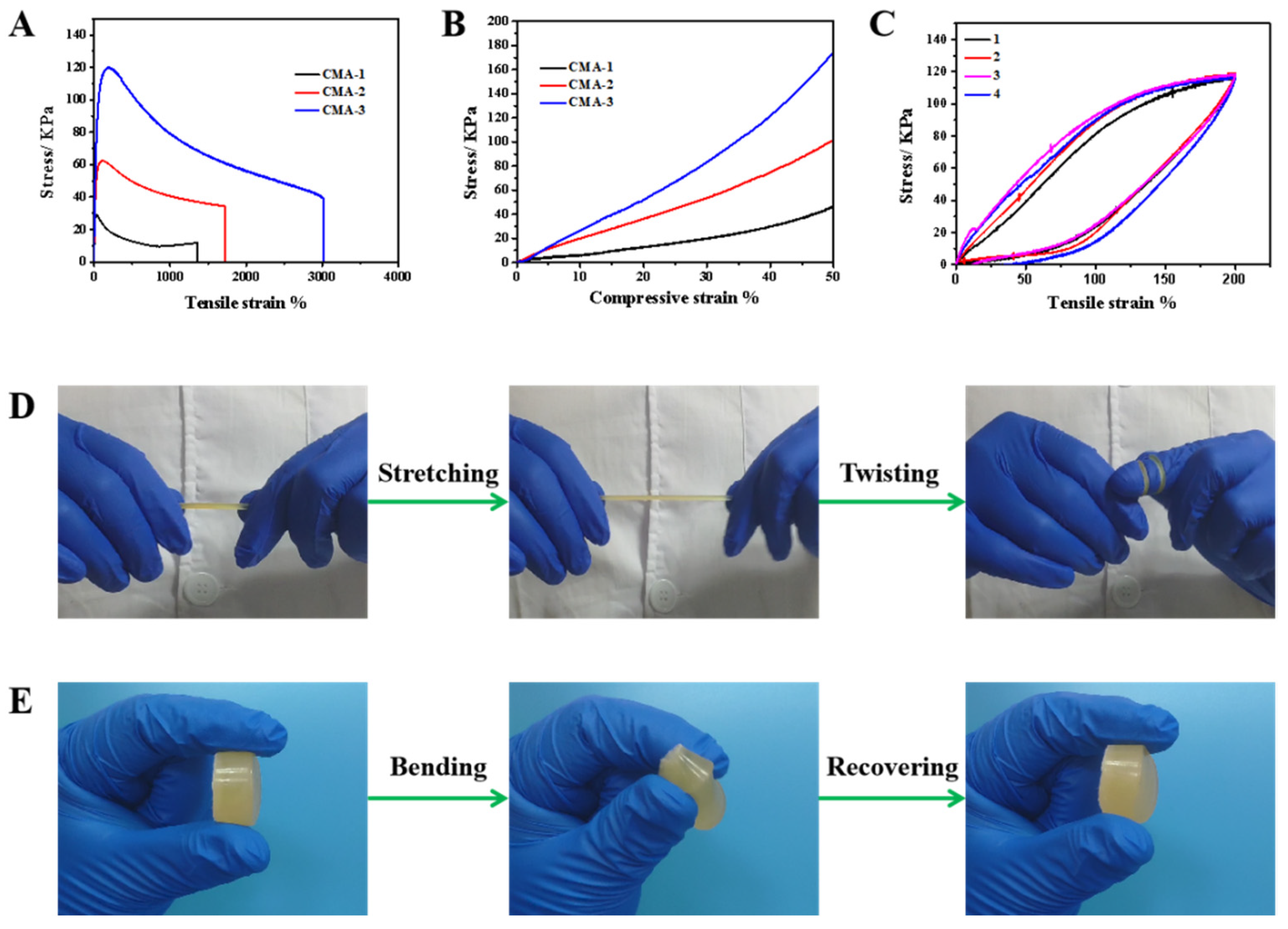
2.2. Mechanical and Water Retaining Properties of CMA Hydrogels
2.3. Self-Healing Property of CMA-3 Hydrogel
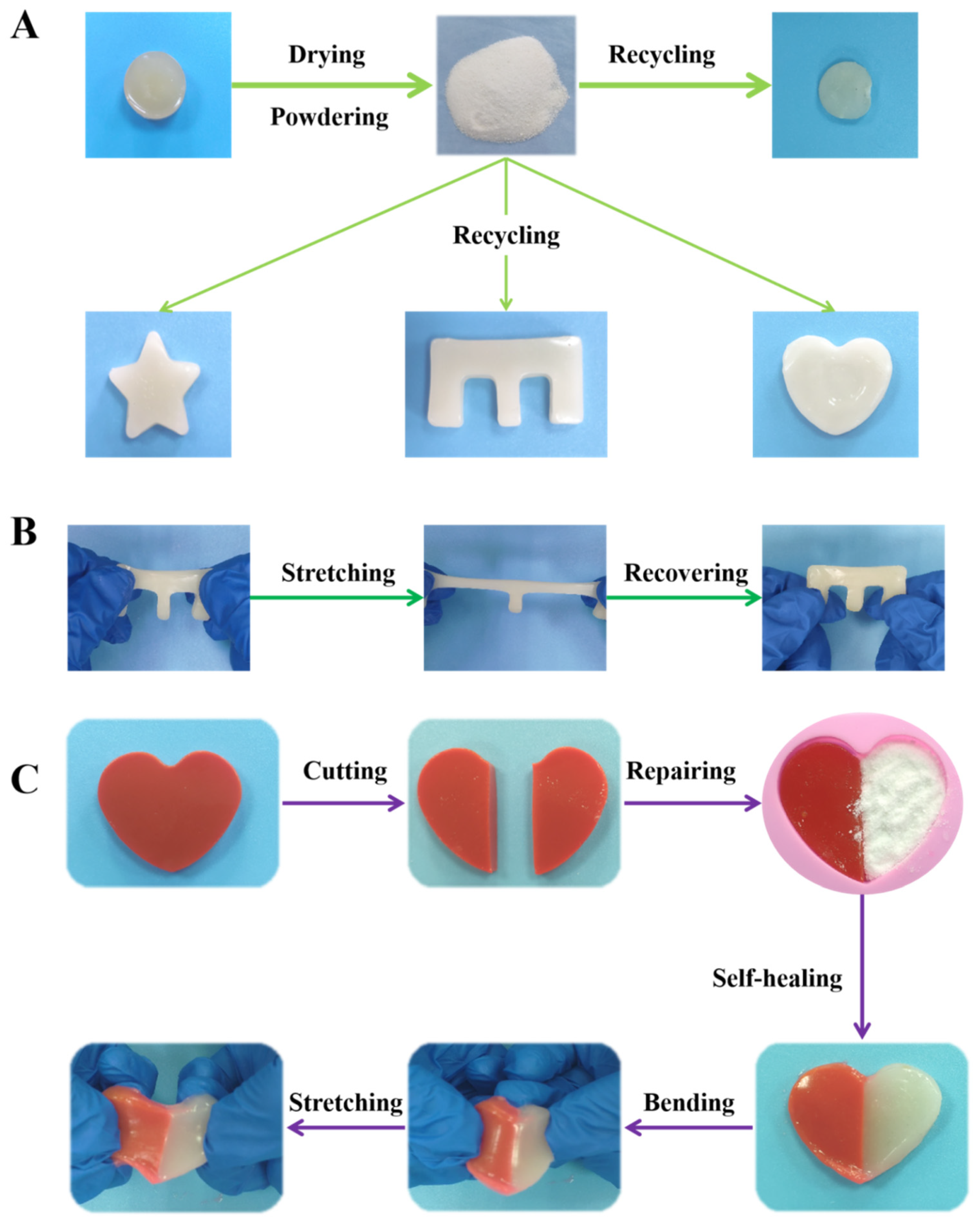
2.4. Powder Self-Healing Property of CMA-3 Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CMA Hydrogels
4.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111023. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Gu, Y.; Han, F.; Xu, X.; Huang, C.; Ma, L.; Ding, H.R.; Ma, H.J.; Li, J.Y. Preparation of sodium alginate-based super absorbent polymer by radiation grafting and crosslinking. Nucl. Tech. 2020, 43, 40–48. [Google Scholar]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.Y. Hydrogel soft robotics. Mater. Today Phy. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Schacht, E.H. Polymer chemistry and hydrogel systems. J. Phys. 2004, 3, 22–28. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Fu, X.; Wang, Y.; Yang, Z.; Pan, L.; Xu, M. Multiple Stimuli Responsive and Identifiable Zwitterionic Ionic Conductive Hydrogel for Bionic Electronic Skin. Adv. Electron. Mater. 2020, 6, 2000239. [Google Scholar] [CrossRef]
- Han, L.; Huang, H.; Fu, X.; Li, J.; Yang, Z.; Liu, X.; Pan, L.; Xu, M. A flexible, high-voltage and safe zwitterionic natural polymer hydrogel electrolyte for high-energy-density zinc-ion hybrid supercapacitor. Chem. Eng. J. 2019, 392, 123733. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, M.; Wan, P. MXene hydrogel for wearable electronics. Matter 2021, 4, 2655–2658. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, D.; Wang, L.H.; Liu, W.; Chiu, A.; Shariati, K.; Liu, Q.; Wang, X.; Zhong, Z.; Webb, J.; et al. An Adhesive Hydrogel with “Load-Sharing” Effect as Tissue Bandages for Drug and Cell Delivery. Adv. Mater. 2020, 32, e2001628. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Taylor, D.L.; In Het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wu, J.; Han, L.; Yang, Z.; Jiang, Z.; Wang, R.; Huang, Z.; Xu, M. Ultrafast Self-Healing, Reusable, and Conductive Polysaccharide-Based Hydrogels for Sensitive Ionic Sensors. ACS Sustain. Chem. Eng. 2020, 8, 18506–18518. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Q.; Thundat, T.; Zeng, H. Stretchable, Injectable, and Self-Healing Conductive Hydrogel Enabled by Multiple Hydrogen Bonding toward Wearable Electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Song, M.; Yu, H.; Zhu, J.; Ouyang, Z.; Abdalkarim, S.Y.H.; Tam, K.C.; Li, Y. Constructing stimuli-free self-healing, robust and ultrasensitive biocompatible hydrogel sensors with conductive cellulose nanocrystals. Chem. Eng. J. 2020, 398, 125547. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, Y.; Tang, P.; Gan, D.; Xing, W.; Hou, Y.; Wang, K.; Xie, C.; Lu, X. Conductive, Tough, Transparent, and Self-Healing Hydrogels Based on Catechol–Metal Ion Dual Self-Catalysis. Chem. Mater. 2019, 31, 5625–5632. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Shackleford, I.C.G.; Connal, L.A. Tough, Self-Healing Hydrogels Capable of Ultrafast Shape Changing. Adv. Mater. 2019, 31, e1904956. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Tao, F.; Pan, Q. Rationally Designed Self-Healing Hydrogel Electrolyte toward a Smart and Sustainable Supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 27745–27753. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Han, L.; Fu, X.; Wang, Y.; Yang, Z.; Pan, L.; Xu, M. A Powder Self-Healable Hydrogel Electrolyte for Flexible Hybrid Supercapacitors with High Energy Density and Sustainability. Small 2021, 17, e2006807. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterials 2019, 9, 253. [Google Scholar] [CrossRef]
- Benltoufa, S.; Miled, W.; Trad, M.; Slama, R.B.; Fayala, F. Chitosan hydrogel-coated cellulosic fabric for medical end-use: Antibacterial properties, basic mechanical and comfort properties. Carbohydr. Polym. 2020, 227, 115352. [Google Scholar] [CrossRef]
- Ge, G.; Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Muscle-Inspired Self-Healing Hydrogels for Strain and Temperature Sensor. ACS Nano 2020, 14, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Lee, W.; Kim, S.; Lee, M.; Koo, J.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Ion-conductive self-healing hydrogels based on an interpenetrating polymer network for a multimodal sensor. Chem. Eng. J. 2019, 371, 452–460. [Google Scholar] [CrossRef]
- Chang, S.; Wang, B.; Liu, Y.; Li, Z.; Hu, X.; Zhang, X.; Zhang, H. Radiation-assistant preparation of highly conductive, transparent and self-healing hydrogels with triple-network structure. Polymer 2020, 188, 122156. [Google Scholar] [CrossRef]
- Ji, F.; Li, J.; Zhang, G.; Lan, W.; Sun, R.; Wong, C.P. Alkaline monomer for mechanical enhanced and self-healing hydrogels based on dynamic borate ester bonds. Polymer 2019, 184, 121882. [Google Scholar] [CrossRef]
- Ma, A.; Wang, G.; Yang, Z.; Bai, L.; Chen, H.; Wang, W.; Yang, H.; Wei, D.; Yang, L. Fabrication of Janus graphene oxide hybrid nanosheets by Pickering emulsion template for self-healing nanocomposite hydrogels. Chem. Eng. J. 2020, 385, 123962. [Google Scholar] [CrossRef]
- Bai, L.; Jiang, X.; Sun, Z.; Pei, Z.; Ma, A.; Wang, W.; Chen, H.; Yang, H.; Yang, L.; Wei, D. Self-healing nanocomposite hydrogels based on modified cellulose nanocrystals by surface-initiated photoinduced electron transfer ATRP. Cellulose 2019, 26, 5305–5319. [Google Scholar] [CrossRef]
- Pang, J.; Wang, L.; Xu, Y.; Wu, M.; Wang, M.; Liu, Y.; Yu, S.; Li, L. Skin-inspired cellulose conductive hydrogels with integrated self-healing, strain, and thermal sensitive performance. Carbohydr. Polym. 2020, 240, 116360. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; In Het Panhuis, M. Hydrogel properties and applications. J. Mater. Chem. B 2019, 7, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Han, L.; Li, J.; Fu, X.; Wang, Y.; Yang, Z.; Xu, X.; Pan, L.; Xu, M. Super-stretchable, elastic and recoverable ionic conductive hydrogel for wireless wearable, stretchable sensor. J. Mater. Chem. A 2020, 8, 10291–10300. [Google Scholar] [CrossRef]
- Zhang, X.N.; Wang, Y.J.; Sun, S.; Hou, L.; Wu, P.; Wu, Z.L.; Zheng, Q. A Tough and Stiff Hydrogel with Tunable Water Content and Mechanical Properties Based on the Synergistic Effect of Hydrogen Bonding and Hydrophobic Interaction. Macromolecules 2018, 51, 8136–8146. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, H.; Hao, W.; Liu, H.; Liu, Y.; Fu, X.; Huang, H.; Ge, M.; Qian, Y. Highly Flexibility, Powder Self-Healing, and Recyclable Natural Polymer Hydrogels. Gels 2022, 8, 89. https://doi.org/10.3390/gels8020089
Miao H, Hao W, Liu H, Liu Y, Fu X, Huang H, Ge M, Qian Y. Highly Flexibility, Powder Self-Healing, and Recyclable Natural Polymer Hydrogels. Gels. 2022; 8(2):89. https://doi.org/10.3390/gels8020089
Chicago/Turabian StyleMiao, Haiyue, Weiju Hao, Hongtao Liu, Yiyang Liu, Xiaobin Fu, Hailong Huang, Min Ge, and Yuan Qian. 2022. "Highly Flexibility, Powder Self-Healing, and Recyclable Natural Polymer Hydrogels" Gels 8, no. 2: 89. https://doi.org/10.3390/gels8020089
APA StyleMiao, H., Hao, W., Liu, H., Liu, Y., Fu, X., Huang, H., Ge, M., & Qian, Y. (2022). Highly Flexibility, Powder Self-Healing, and Recyclable Natural Polymer Hydrogels. Gels, 8(2), 89. https://doi.org/10.3390/gels8020089




