In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid–Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Hydrogel Scaffolds
2.1.1. Optical and Microscopy Observations
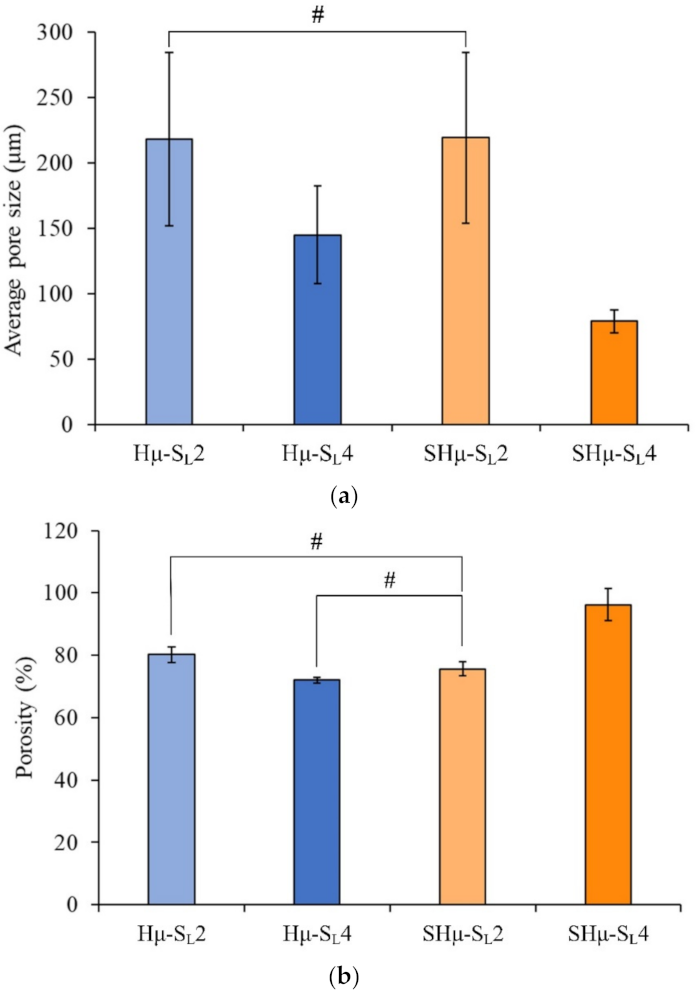
2.1.2. Pore Size and Porosity Distribution of the Prepared Hydrogel Scaffolds
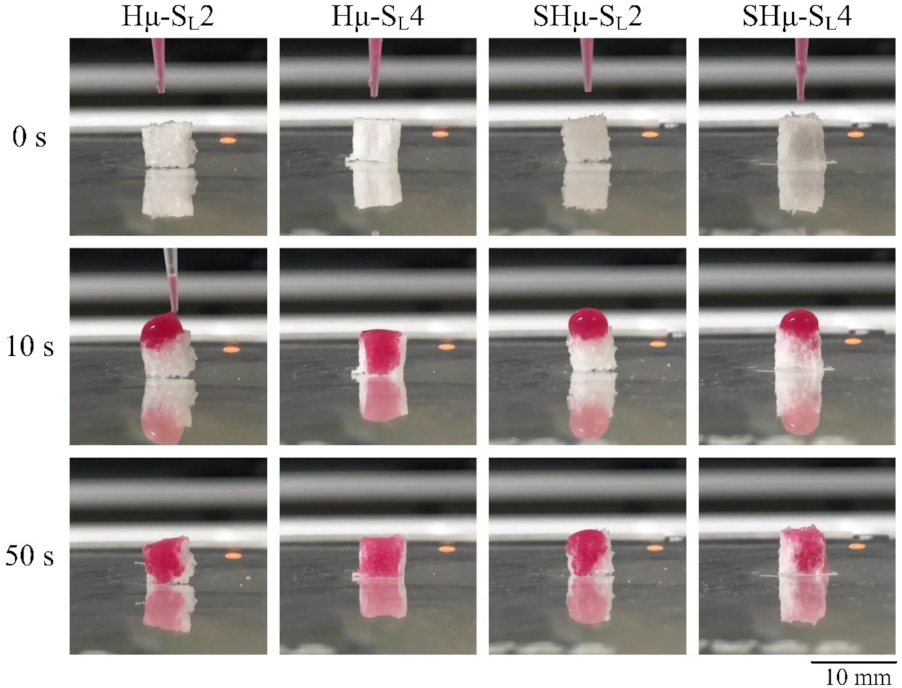
2.1.3. Perforate Pore Evaluation of the Prepared Hydrogel Scaffolds
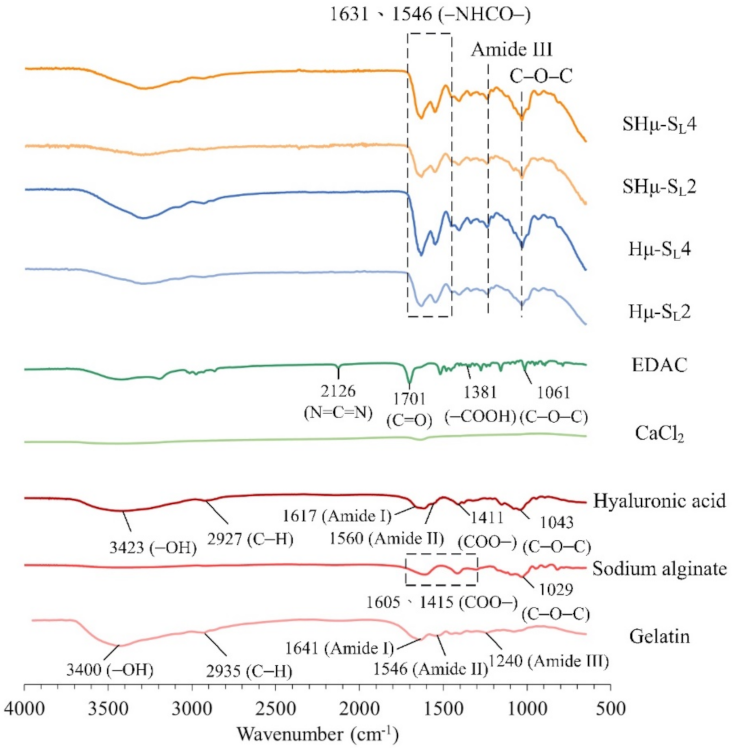
2.1.4. Functional Group Analysis of the Hydrogel Scaffolds by Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) Spectrum
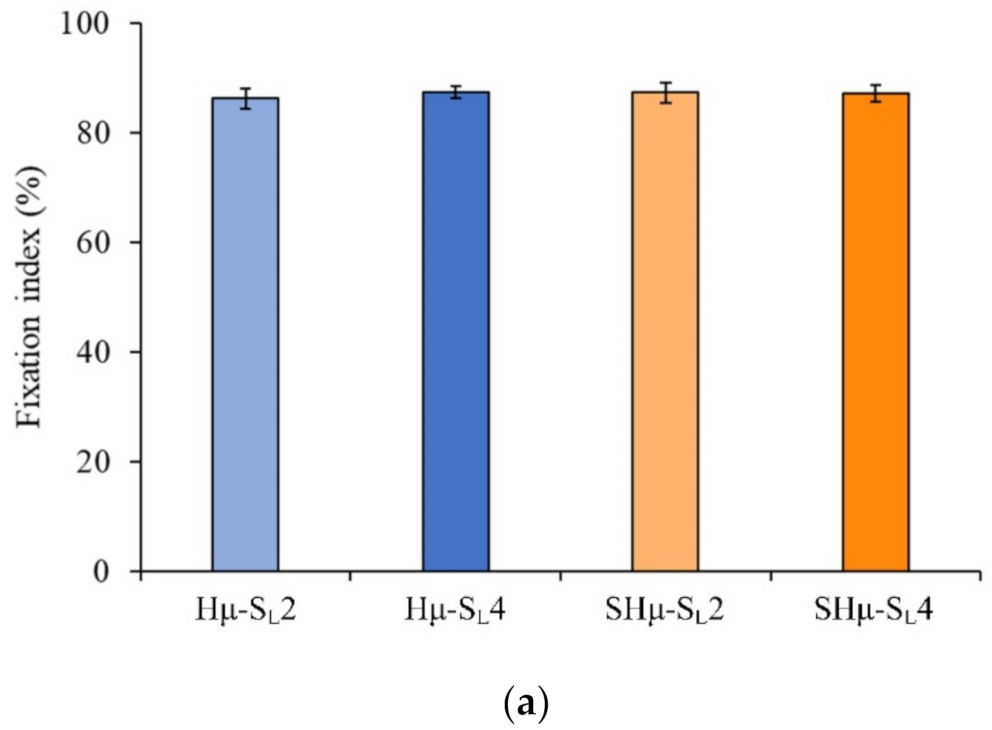
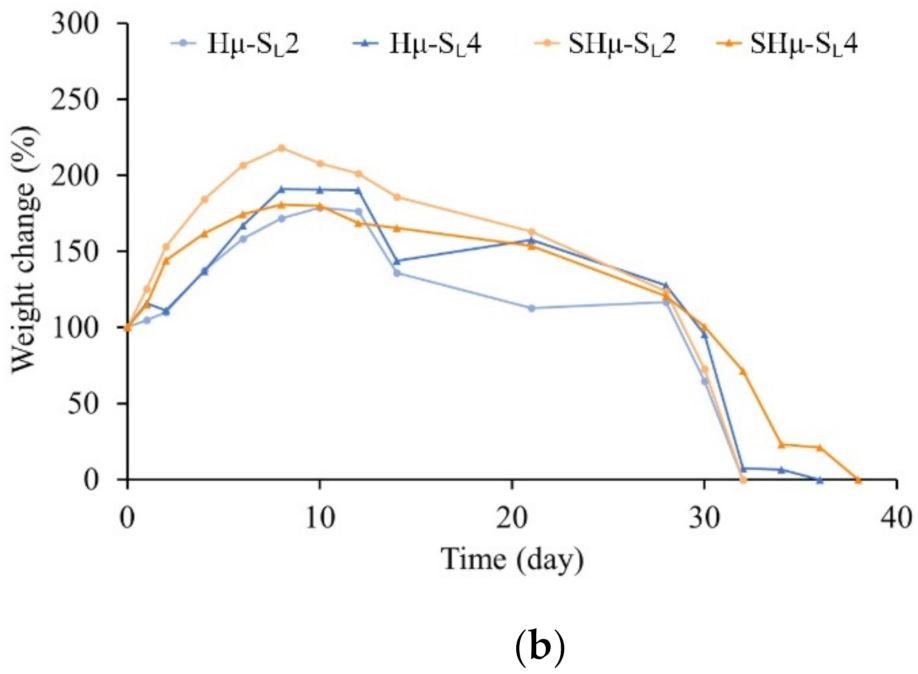
2.1.5. Fixation of Free Amine and Degradation Rate of the Hydrogel Scaffolds
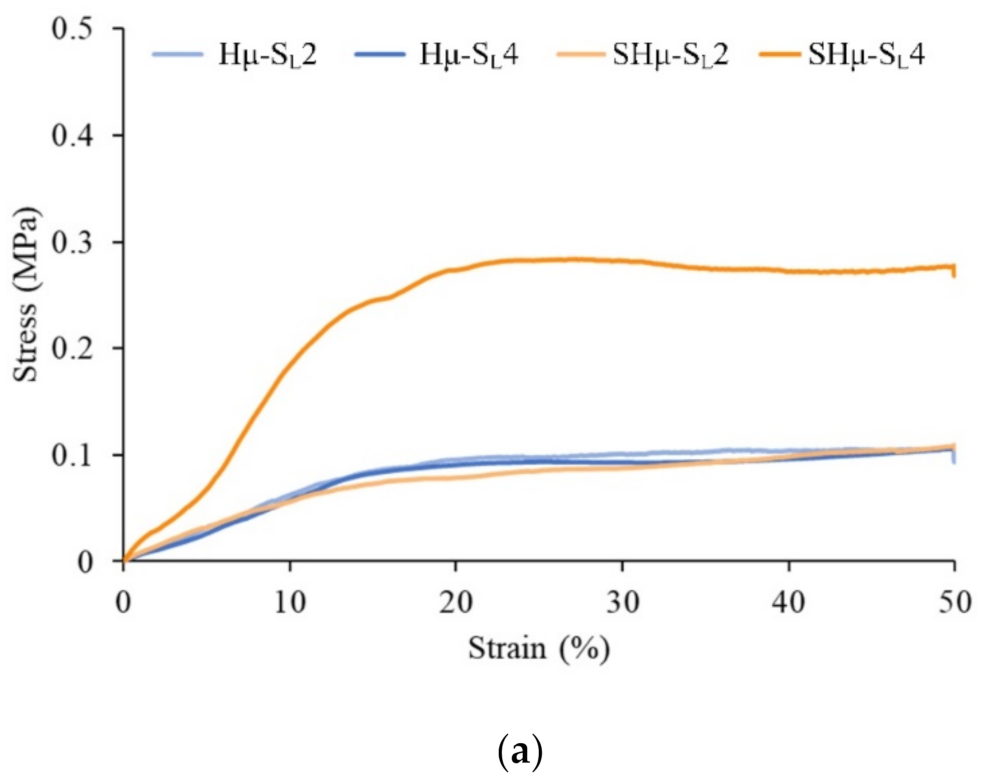
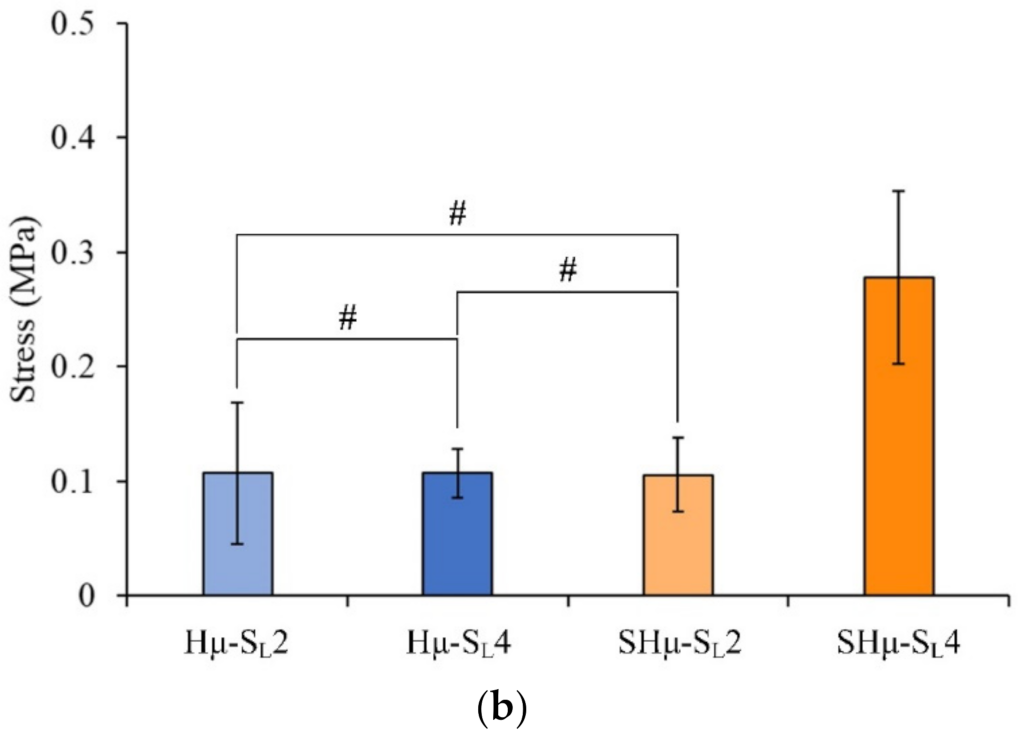
2.1.6. Strength of the Hydrogel Scaffolds
2.2. Biocompatibility and Chondrocyte Co-Culture Evaluation of the Hydrogel Scaffolds
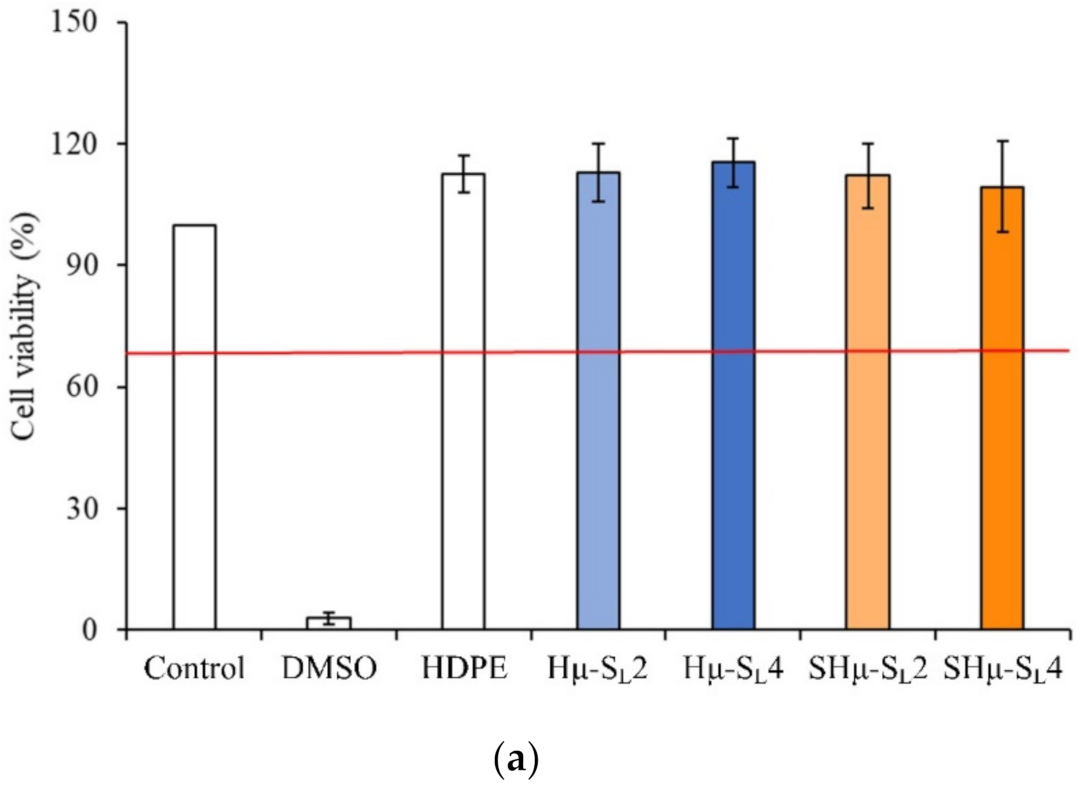
2.2.1. Cytotoxicity of the Hydrogel Scaffolds
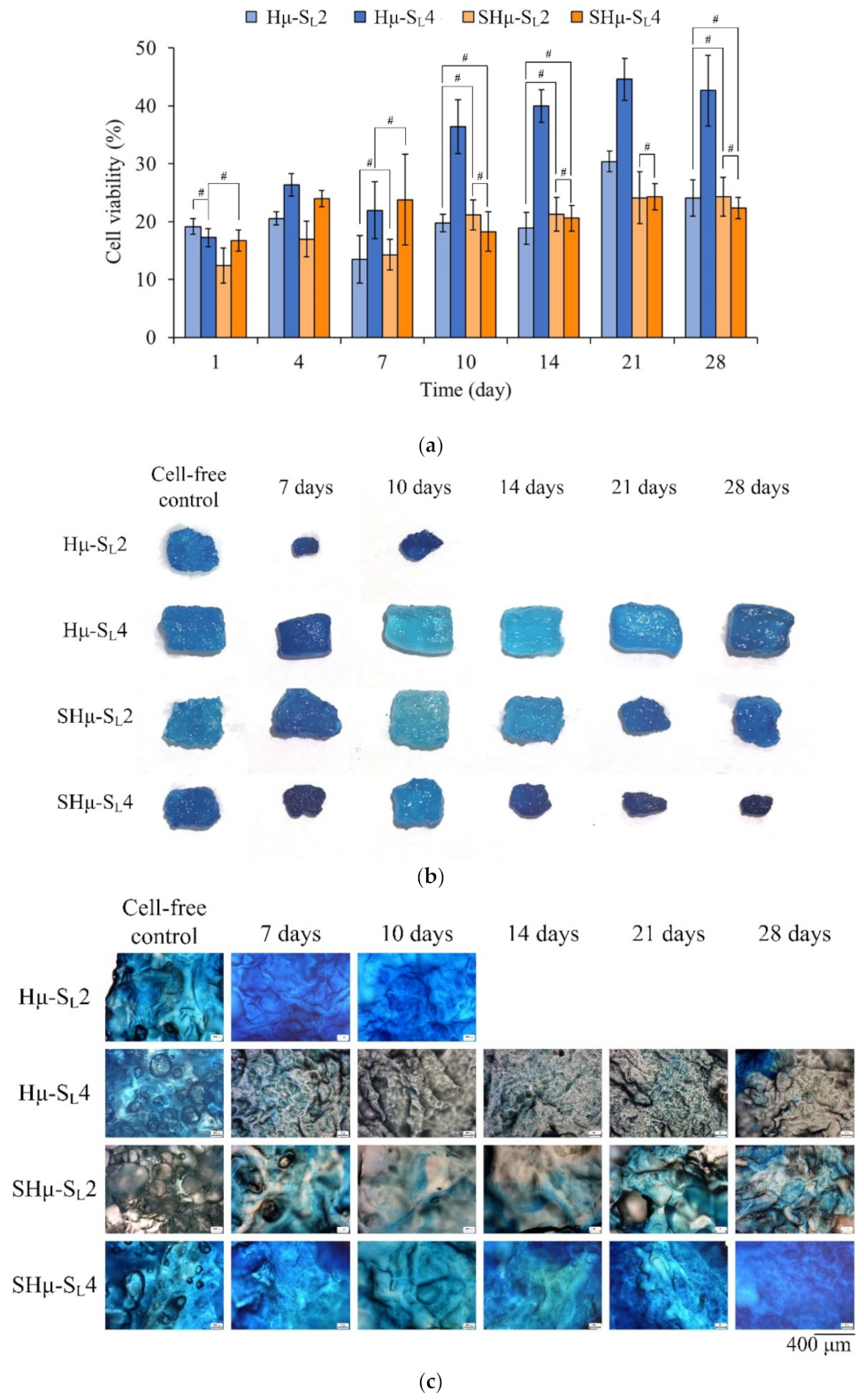
2.2.2. Chondrocyte Co-Culture Evaluation of the Hydrogel Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Raw Materials
4.2. Hydrogel Scaffold Preparation
4.3. Characterizations of the Hydrogel Scaffolds
4.3.1. Structure Analysis
4.3.2. Perforate Pore Evaluation
4.3.3. ATR-FTIR Spectrum
4.3.4. Fixation Index Evaluated by Amine Residue
4.3.5. Weight Changes of the Hydrogel Scaffolds after Immersion
4.3.6. Compressive Strength Evaluation
4.4. Biocompatibility and Chondrocyte Co-Culture Evaluation In Vitro
4.4.1. Cytotoxicity
4.4.2. Chondrocyte Co-Culture Evaluation In Vitro
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 141. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, P.; Shamloo, A. Fabrication of a novel 3D scaffold for cartilage tissue repair: In-vitro and in-vivo study. Mater. Sci. Eng. C 2021, 128, 112285. [Google Scholar] [CrossRef] [PubMed]
- Nuernberger, S.; Cyran, N.; Albrecht, C.; Redl, H.; Vécsei, V.; Marlovits, S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011, 32, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Radossi, P.; Rajendran, S.; Dalzoppo, D.; Bortolami, M.; Bagno, A.; Grandi, F.; Gamba, P.G.; Parnigotto, P.P. Autologous chondrocytes as a novel source for neo-chondrogenesis in haemophiliacs. Cell Tissue Res. 2016, 366, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kumbar, S.G.; Nukavarapu, S.P. Biomaterial-directed cell behavior for tissue engineering. Curr. Opin. Biotechnol. 2021, 17, 100260. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Dalzoppo, D.; Lora, S.; Sartore, L.; Folin, M.; Parnigotto, P.P.; Grandi, C. Tailored PVA/ECM scaffolds for cartilage regeneration. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Vila-Parrondo, C.; García-Astrain, C.; Liz-Marzán, L.M. Colloidal systems toward 3D cell culture scaffolds. Adv. Colloid Interface Sci. 2020, 283, 102237. [Google Scholar] [CrossRef]
- Tenje, M.; Cantoni, F.; Porras Hernández, A.M.; Searle, S.S.; Johansson, S.; Barbe, L.; Antfolk, M.; Pohlit, H. A practical guide to microfabrication and patterning of hydrogels for biomimetic cell culture scaffolds. Organs-on-a-Chip 2020, 2, 100003. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, J.; Wang, B.; Li, L.; Kong, X.; Liao, J. The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109927. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. Chapter 10—The application of natural polymer–based hydrogels in tissue engineering. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–307. [Google Scholar] [CrossRef]
- Anju, S.; Prajitha, N.; Sukanya, V.S.; Mohanan, P.V. Complicity of degradable polymers in health-care applications. Mater. Today Chem. 2020, 16, 100236. [Google Scholar] [CrossRef]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a nanocomposite scaffold of gelatin–alginate–graphene oxide for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Mase, A.; Takizawa, Y.; Shinkai, M.; Honda, H.; Hata, K.-I.; Ueda, M.; Kobayashi, T. Transglutaminase-mediated gelatin matrices incorporating cell adhesion factors as a biomaterial for tissue engineering. J. Biosci. Bioeng. 2003, 95, 196–199. [Google Scholar] [CrossRef]
- Chang, C.-H.; Liu, H.-C.; Lin, C.-C.; Chou, C.-H.; Lin, F.-H. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 2003, 24, 4853–4858. [Google Scholar] [CrossRef]
- Xia, W.; Liu, W.; Cui, L.; Liu, Y.; Zhong, W.; Liu, D.; Wu, J.; Chua, K.; Cao, Y. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dutt, D.; Kaur, P.; Singh, H.; Mishra, N.C. Microfibrous paper scaffold for tissue engineering application. J. Biomater. Sci. Polym. Ed. 2020, 31, 1091–1106. [Google Scholar] [CrossRef]
- Nikitovic, D.; Kouvidi, K.; Kavasi, R.-M.; Berdiaki, A.; Tzanakakis, G.N. Hyaluronan/Hyaladherins-a promising axis for targeted drug delivery in cancer. Curr. Drug Deliv. 2016, 13, 500–511. [Google Scholar] [CrossRef]
- Merzendorfer, H. Chitosan derivatives and grafted adjuncts with unique properties. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 95–151. [Google Scholar] [CrossRef]
- Chou, S.-F.; Luo, L.-J.; Lai, J.-Y.; Ma, D.H.-K. Role of solvent-mediated carbodiimide cross-linking in fabrication of electrospun gelatin nanofibrous membranes as ophthalmic biomaterials. Mater. Sci. Eng. C 2017, 71, 1145–1155. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, W.-C.; Chen, C.-H.; Ko, C.-L.; Liu, S.-M.; Chen, J.-C. Chemical cross-linking on gelatin-hyaluronan loaded with hinokitiol for the preparation of guided tissue regeneration hydrogel membranes with antibacterial and biocompatible properties. Mater. Sci. Eng. C 2021, 119, 111576. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Xie, S.; Shan, X.; Cai, Z. In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatin-sodium alginate/rat Schwann-cell scaffold. Mater. Sci. Eng. C 2020, 109, 110530. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surf. B 2020, 192, 111041. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-W.; Kang, J.Y.; Yoon, I.-S.; Hwang, H.-D.; Balakrishnan, P.; Cho, H.-J.; Chung, K.-D.; Kang, D.-H.; Kim, D.-D. Interpenetrating polymer network (IPN) scaffolds of sodium hyaluronate and sodium alginate for chondrocyte culture. Colloids Surf. B 2011, 88, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Lee, D.H.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef]
- Xia, L.; Luo, X.; Zhu, Y.; Zhang, X.; Luo, L. Effects of CaCl2 freeze-drying and acidic solutions on the reusability of calcium alginate beads; and degradation of phenol by immobilized Acinetobacter sp. PR1. Biochem. Eng. J. 2019, 151, 107339. [Google Scholar] [CrossRef]
- Luo, X.; Song, H.; Yang, J.; Han, B.; Feng, Y.; Leng, Y.; Chen, Z. Encapsulation of Escherichia coli strain Nissle 1917 in a chitosan―alginate matrix by combining layer-by-layer assembly with CaCl2 cross-linking for an effective treatment of inflammatory bowel diseases. Colloids Surf. B 2020, 189, 110818. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Wang, L.; Neumann, M.; Fu, T.; Li, W.; Cheng, X.; Su, B.-L. Porous and responsive hydrogels for cell therapy. Curr. Opin. Colloid Interface Sci. 2018, 38, 135–157. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Shafiq, M.; Tang, J.; Hao, J.; Xie, X.; Yuan, Z.; Xiao, X.; Liu, Y.; Mo, X. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J. Colloid Interface Sci. 2021, 603, 94–109. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Z.; Shafiq, M.; Xie, X.; Xiao, X.; Castro, R.; Rodrigues, J.; Wu, J.; Zhou, G.; Mo, X. Gas foaming of electrospun poly(L-lactide-co-caprolactone)/silk fibroin nanofiber scaffolds to promote cellular infiltration and tissue regeneration. Colloids Surf. B 2021, 201, 111637. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, X.; Tu, H.; Wan, A. The crystallization behavior of porous poly(lactic acid) prepared by modified solvent casting/particulate leaching technique for potential use of tissue engineering scaffold. Mater. Lett. 2014, 136, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Sin, D.; Miao, X.; Liu, G.; Wei, F.; Chadwick, G.; Yan, C.; Friis, T. Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater. Sci. Eng. C 2010, 30, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Coogan, K.R.; Stone, P.T.; Sempertegui, N.D.; Rao, S.S. Fabrication of micro-porous hyaluronic acid hydrogels through salt leaching. Eur. Polym. J. 2020, 135, 109870. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, X.; Yang, S.; Lu, Z.; Niu, C.; Xu, Y.; Huang, Z.; Kang, Y.J.; Feng, L. Solvent evaporation induced fabrication of porous polycaprolactone scaffold via low-temperature 3D printing for regeneration medicine researches. Polymer 2021, 217, 123436. [Google Scholar] [CrossRef]
- Sangkert, S.; Kamolmatyakul, S.; Gelinsky, M.; Meesane, J. 3D printed scaffolds of alginate/polyvinylalcohol with silk fibroin based on mimicked extracellular matrix for bone tissue engineering in maxillofacial surgery. Mater. Today Commun. 2021, 26, 102140. [Google Scholar] [CrossRef]
- Choi, D.J.; Park, S.J.; Gu, B.K.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Effect of the pore size in a 3D bioprinted gelatin scaffold on fibroblast proliferation. J. Ind. Eng. Chem. 2018, 67, 388–395. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, G.; Junka, R.; Chang, N.; Anwar, A.; Wang, H.; Yu, X. Fabrication of polylactic acid (PLA)-based porous scaffold through the combination of traditional bio-fabrication and 3D printing technology for bone regeneration. Colloids Surf. B 2021, 197, 111420. [Google Scholar] [CrossRef]
- Agarwal, T.; Chiesa, I.; Presutti, D.; Irawan, V.; Vajanthri, K.Y.; Costantini, M.; Nakagawa, Y.; Tan, S.-A.; Makvandi, P.; Zare, E.N.; et al. Recent advances in bioprinting technologies for engineering different cartilage-based tissues. Mater. Sci. Eng. C 2021, 123, 112005. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Eviana Putri, N.R.; Wang, X.; Chen, Y.; Li, X.; Kawazoe, N.; Chen, G. Preparation of PLGA-collagen hybrid scaffolds with controlled pore structures for cartilage tissue engineering. Prog. Nat. Sci. 2020, 30, 642–650. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ramazani, S.A.A.; Mashayekhan, S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Fabrication of bioactive binary composite film based on gelatin/chitosan incorporated with cinnamon essential oil and rutin. Colloids Surf. B 2021, 204, 111830. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, P.; Cheng, Y.; Zhang, W.; Zheng, B.; Wang, Q. Nano-pearl powder/chitosan-hyaluronic acid porous composite scaffold and preliminary study of its osteogenesis mechanism. Mater. Sci. Eng. C 2020, 111, 110749. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Park, S.-N.; Park, J.-C.; Kim, H.O.; Song, M.J.; Suh, H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials 2002, 23, 1205–1212. [Google Scholar] [CrossRef]
- Sun, F.; Guo, J.; Liu, Y.; Yu, Y. Preparation, characterizations and properties of sodium alginate grafted acrylonitrile/polyethylene glycol electrospun nanofibers. Int. J. Biol. Macromol. 2019, 137, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Siqueira, N.M.; Paiva, B.; Camassola, M.; Rosenthal-Kim, E.Q.; Garcia, K.C.; dos Santos, F.P.; Soares, R.M.D. Gelatin and galactomannan-based scaffolds: Characterization and potential for tissue engineering applications. Carbohydr. Polym. 2015, 133, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Schinagl, R.M.; Gurskis, D.; Chen, A.C.; Sah, R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J. Orthop. Res. 1997, 15, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.; Zhao, H. The effect of matrix stiffness on biomechanical properties of chondrocytes. Acta Biochim. Biophys. Sin. 2016, 48, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Melo, B.A.; Jodat, Y.A.; Mehrotra, S.; Calabrese, M.A.; Kamperman, T.; Mandal, B.B.; Santana, M.H.; Alsberg, E.; Leijten, J.; Shin, S.R. 3D Printed Cartilage-Like Tissue Constructs with Spatially Controlled Mechanical Properties. Adv. Funct. Mater. 2019, 29, 1906330. [Google Scholar] [CrossRef]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M.; Shastri, V.P. Optimization strategies on the structural modeling of gelatin/chitosan scaffolds to mimic human meniscus tissue. Mater. Sci. Eng. C 2013, 33, 4777–4785. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Sung, H.-W.; Chang, Y.; Chiu, Y.-T.; Hsu, H.-L.; Shih, C.-C.; Lu, J.-H.; Yang, P.-C. Evaluation of an epoxy-fixed biological patch with ionically bound heparin as a pericardial substitute. Biomaterials 1996, 17, 1693–1701. [Google Scholar] [CrossRef]



| Leaching Sucrose Particle Size (μm) | Leaching Weight (g) | Designated Groups |
|---|---|---|
| 88–177 | 2 | Hμ-SL2 |
| 88–177 | 4 | Hμ-SL4 |
| 44–74 | 2 | SHμ-SL2 |
| 44–74 | 4 | SHμ-SL4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haung, S.-M.; Lin, Y.-T.; Liu, S.-M.; Chen, J.-C.; Chen, W.-C. In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid–Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration. Gels 2021, 7, 165. https://doi.org/10.3390/gels7040165
Haung S-M, Lin Y-T, Liu S-M, Chen J-C, Chen W-C. In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid–Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration. Gels. 2021; 7(4):165. https://doi.org/10.3390/gels7040165
Chicago/Turabian StyleHaung, Ssu-Meng, Yu-Ting Lin, Shih-Ming Liu, Jian-Chih Chen, and Wen-Cheng Chen. 2021. "In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid–Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration" Gels 7, no. 4: 165. https://doi.org/10.3390/gels7040165
APA StyleHaung, S.-M., Lin, Y.-T., Liu, S.-M., Chen, J.-C., & Chen, W.-C. (2021). In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid–Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration. Gels, 7(4), 165. https://doi.org/10.3390/gels7040165







