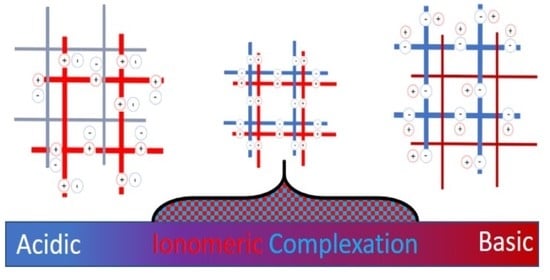
Enhanced Mechanical Properties by Ionomeric Complexation in Interpenetrating Network Hydrogels of Hydrolyzed Poly (N-vinyl Formamide) and Polyacrylamide
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
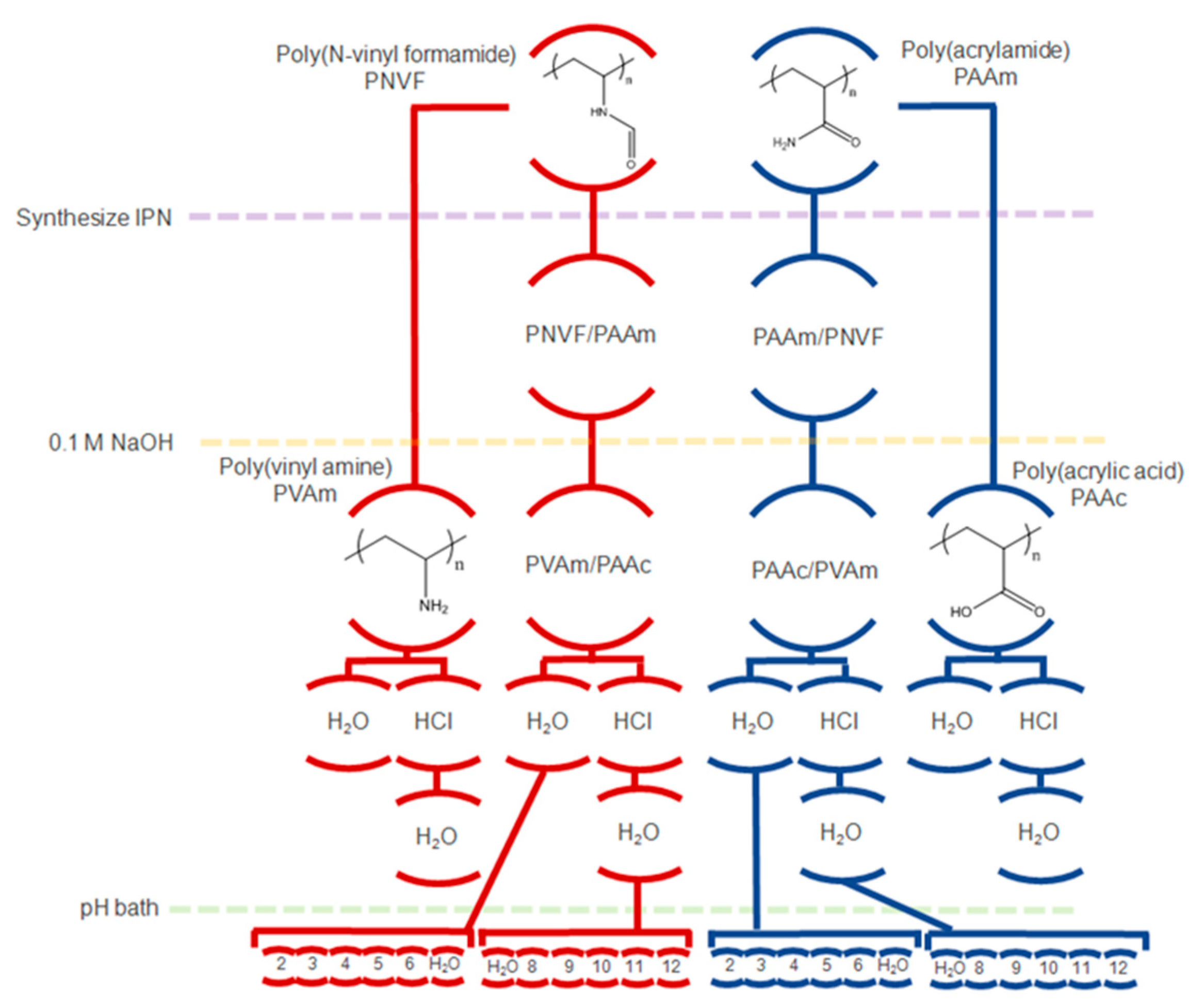
4.2. Synthesis of IPN Hydrogels
4.3. Hydrolysis of Hydrogels
4.4. Swelling and Mechanical Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Peppas, N.A. Biomaterials science: An introduction to materials in medicine. In Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Toronto, ON, Canada, 1996; p. 100. [Google Scholar]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [Green Version]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Creton, C. 50th Anniversary Perspective: Networks and Gels: Soft but Dynamic and Tough. Macromolecules 2017, 50, 8297–8316. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurokawa, T.; Furukawa, H.; Gong, J.P. Effect of the constituent networks of double-network gels on their mechanical properties and energy dissipation process. Soft Matter 2020, 16, 8618–8627. [Google Scholar] [CrossRef]
- Suekama, T.C.; Hu, J.; Kurokawa, T.; Gong, J.P.; Gehrke, S.H. Double-Network Strategy Improves Fracture Properties of Chondroitin Sulfate Networks. ACS Macro Lett. 2013, 2, 137–140. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough Physical Double-Network Hydrogels Based on Amphiphilic Triblock Copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, F.; Sun, T.L.; Nakajima, T.; King, D.; Kurokawa, T.; Zhao, Y.; Bin Ihsan, A.; Li, X.; Guo, H.; Gong, J.P. Strong and Tough Polyion-Complex Hydrogels from Oppositely Charged Polyelectrolytes: A Comparative Study with Polyampholyte Hydrogels. Macromolecules 2016, 49, 2750–2760. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Zhao, Y.; Sato, K.; Bin Ihsan, A.; Li, X.; Guo, H.; Gong, J.P. Oppositely Charged Polyelectrolytes form Tough, Self-Healing, and Rebuildable Hydrogels. Adv. Mater. 2015, 27, 2722–2727. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, C.; Tang, J.; Liu, Y.; Hua, J. Construction of a Dual Ionic Network in Natural Rubber with High Self-Healing Efficiency through Anionic Mechanism. Ind. Eng. Chem. Res. 2020, 59. [Google Scholar] [CrossRef]
- Roy, C.K.; Guo, H.L.; Sun, T.L.; Bin Ihsan, A.; Kurokawa, T.; Takahata, M.; Nonoyama, T.; Nakajima, T.; Gong, J.P. Self-Adjustable Adhesion of Polyampholyte Hydrogels. Adv. Mater. 2015, 27, 7344–7348. [Google Scholar] [CrossRef]
- Grindy, S.C.; Learsch, R.W.; Mozhdehi, D.; Cheng, J.; Barrett, D.G.; Guan, Z.; Messersmith, P.; Holten-Andersen, N. Control of hierarchical polymer mechanics with bioinspired metal-coordination dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef]
- Cristiani, T.R.; Filippidi, E.; Behrens, R.L.; Valentine, M.T.; Eisenbach, C.D. Tailoring the Toughness of Elastomers by Incorporating Ionic Cross-Linking. Macromolecules 2020, 53, 4099–4109. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-Strength, Tough, Fatigue Resistant, and Self-Healing Hydrogel Based on Dual Physically Cross-Linked Network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef]
- Fan, H.; Gong, J.P. Fabrication of Bioinspired Hydrogels: Challenges and Opportunities. Macromolecules 2020, 53, 2769–2782. [Google Scholar] [CrossRef]
- Cui, K.; Ye, Y.N.; Sun, T.L.; Yu, C.; Li, X.; Kurokawa, T.; Gong, J.P. Phase Separation Behavior in Tough and Self-Healing Polyampholyte Hydrogels. Macromolecules 2020, 53, 5116–5126. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Bin Ihsan, A.; Akasaki, T.; Sato, K.; Haque, A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Barroso, N.; Guaresti, O.; Álvarez, L.P.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L. Self-healable hyaluronic acid/chitosan polyelectrolyte complex hydrogels and multilayers. Eur. Polym. J. 2019, 120, 109268. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nat. Cell Biol. 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Nisato, G.; Munch, J.P.; Candau, S.J. Swelling, Structure, and Elasticity of Polyampholyte Hydrogels†. Langmuir 1999, 15, 4236–4244. [Google Scholar] [CrossRef]
- Bin Ihsan, A.; Sun, T.L.; Kurokawa, T.; Karobi, S.N.; Nakajima, T.; Nonoyama, T.; Roy, C.K.; Luo, F.; Gong, J.P. Self-Healing Behaviors of Tough Polyampholyte Hydrogels. Macromolecules 2016, 49, 4245–4252. [Google Scholar] [CrossRef] [Green Version]
- Shchipunov, Y.; Sarin, S.; Kim, I.; Ha, C.-S. Hydrogels formed through regulated self-organization of gradually charging chitosan in solution of xanthan. Green Chem. 2010, 12, 1187–1195. [Google Scholar] [CrossRef]
- Morikawa, K.; Masubuchi, Y.; Shchipunov, Y.; Zinchenko, A. DNA-Chitosan Hydrogels: Formation, Properties, and Functionalization with Catalytic Nanoparticles. ACS Appl. Bio Mater. 2021, 4, 1823–1832. [Google Scholar] [CrossRef]
- Suekama, T.C.; Aziz, V.; Mohammadi, Z.; Berkland, C.; Gehrke, S.H. Synthesis and characterization of poly(N-vinyl formamide) hydrogels-A potential alternative to polyacrylamide hydrogels. J. Polym. Sci. Part A Polym. Chem. 2012, 51, 435–445. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, S.; Hrymak, A.N. Acidic and basic hydrolysis of poly(N-vinylformamide). J. Appl. Polym. Sci. 2002, 86, 3412–3419. [Google Scholar] [CrossRef]
- Chen, Y.M.; Sun, P.J. pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers 2019, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Dogu, S.; Kilic, M.; Okay, O. Collapse of acrylamide-based polyampholyte hydrogels in water. J. Appl. Polym. Sci. 2009, 113, 1375–1382. [Google Scholar] [CrossRef]
- Kudryavtsev, Y.V.; Litmanovich, A.D.; Platé, N.A. On the Kinetics of Polyacrylamide Alkaline Hydrolysis. Macromolecules 1998, 31, 4642–4644. [Google Scholar] [CrossRef]
- Ricka, J.; Tanaka, T. Swelling of ionic gels: Quantitative performance of the Donnan theory. Macromolecules 1984, 17, 2916–2921. [Google Scholar] [CrossRef]
- Nakajima, T.; Hoshino, K.-I.; Guo, H.; Kurokawa, T.; Gong, J. Experimental Verification of the Balance between Elastic Pressure and Ionic Osmotic Pressure of Highly Swollen Charged Gels. Gels 2021, 7, 39. [Google Scholar] [CrossRef]
- Gong, J.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Erman, B.; Mark, J.E. Rubber-like elasticity. Annu. Rev. Phys. Chem. 1989, 40, 351–374. [Google Scholar] [CrossRef]
- Yang, H.; Ghiassinejad, S.; Van Ruymbeke, E.; Fustin, C.-A. Tunable Interpenetrating Polymer Network Hydrogels Based on Dynamic Covalent Bonds and Metal–Ligand Bonds. Macromolecules 2020, 53, 6956–6967. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Sudre, G.; Tran, Y.; Creton, C.; Hourdet, D. pH/Temperature control of interpolymer complexation between poly(acrylic acid) and weak polybases in aqueous solutions. Polymer 2012, 53, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Johl, R.; Pacelli, S.; Gehrke, S.; Paul, A. Fabricating Tough Interpenetrating Network Cryogels with DNA as the Primary Network for Biomedical Applications. ACS Macro Lett. 2020, 9, 1230–1236. [Google Scholar] [CrossRef]
- Han, W.H.; Horka, F.; McKenna, G.B. Mechanical and Swelling Behaviors of Rubber: A Comparison of Some Molecular Models with Experiment. Math. Mech. Solids 1999, 4, 139–167. [Google Scholar] [CrossRef]
- Gehrke, S.H.; Cussler, E.L. Mass-transfer in ph-sensitive hydrogels. Chem. Eng. Sci. 1989, 44, 559–566. [Google Scholar] [CrossRef]
- Suekama, T.C.; Hu, J.; Kurokawa, T.; Gong, J.P.; Gehrke, S.H. Tuning Mechanical Properties of Chondroitin Sulfate-Based Double-Network Hydrogels. Macromol. Symp. 2013, 329, 9–18. [Google Scholar] [CrossRef]
- Calvert, P. Hydrogels for Soft Machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Patras, G.; Qiao, G.G.; Solomon, D. Controlled Formation of Microheterogeneous Polymer Networks: Influence of Monomer Reactivity on Gel Structure. Macromolecules 2001, 34, 6396–6401. [Google Scholar] [CrossRef]
- Suekama, T.C.; Khanlari, A.; Gehrke, S.H. Tuning Mechanical Properties of Chondroitin Sulfate-Based Hydrogels Using the Double-Network Strategy. MRS Proc. 2014, 1622, 79–84. [Google Scholar] [CrossRef]
- Nagase, K.; Sakaguchi, K. Alkaline hydrolysis of polyacrylamide. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 2475–2482. [Google Scholar] [CrossRef]
- Oppermann, W. Swelling Behavior and Elastic Properties of Ionic Hydrogels. ACS Symp. Ser. 1992, 480, 159–170. [Google Scholar] [CrossRef]

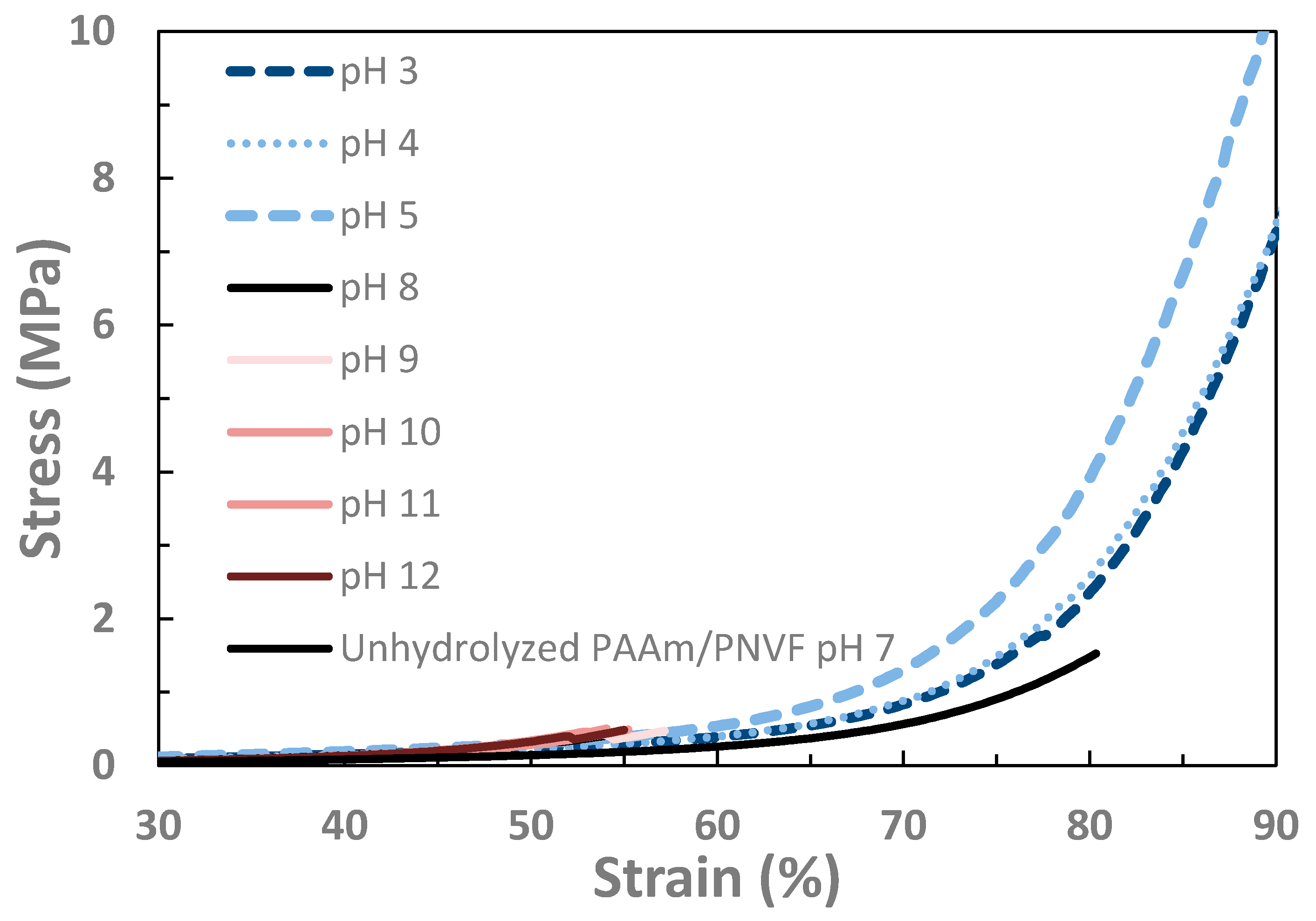





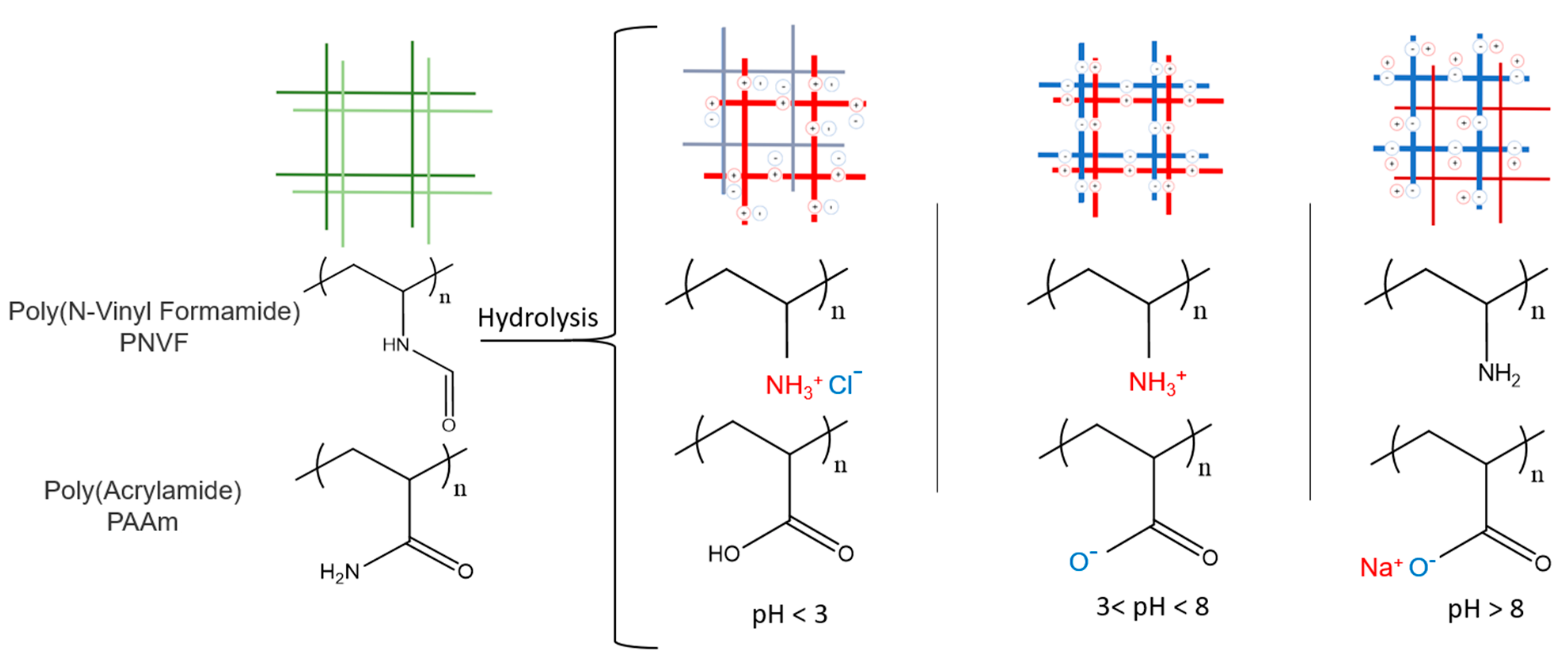
| pH | Failure Stress (MPa) | E (kPa) | G (kPa) | E/G | Q (g/g) | ρx (mol/m3) |
|---|---|---|---|---|---|---|
| 2 | 1.00 ± 0.76 | 140 ± 12 | 45 ± 3 | 3.15 ± 0.13 | 21.0 ± 0.8 | 0.0064 ± 0.0002 |
| 3 | 13.72 ± 3.30 | 190 ± 17 | 66 ± 4 | 2.86 ± 0.78 | 3.4 ± 0.6 | 0.0330 ± 0.0037 |
| 4 | 13.44 ± 1.50 | 210 ± 9 | 69 ± 2 | 3.97 ± 0.24 | 2.5 ± 0.1 | 0.0410 ± 0.0008 |
| 5 | 15.74 ± 1.50 | 240 ± 25 | 80 ± 9 | 3.01 ± 0.03 | 2.9 ± 0.2 | 0.0430 ± 0.0041 |
| 6 | 13.82 ± 0.89 | 210 ± 24 | 85 ± 8 | 2.50 ± 0.08 | 2.7 ± 0.1 | 0.0480 ± 0.0041 |
| 8 | 0.47 ± 0.46 | 260 ± 30 | 56 ± 2 | 4.63 ± 0.05 | 30.0 ± 0.5 | 0.0063 ± 0.0003 |
| 9 | 0.40 ± 0.11 | 170 ± 6 | 45 ± 4 | 3.68 ± 0.05 | 30.0 ± 0.4 | 0.0052 ± 0.0004 |
| 10 | 0.62 ± 0.14 | 140 ± 14 | 56 ± 13 | 2.49 ± 0.13 | 29.0 ± 0.1 | 0.0065 ± 0.0012 |
| 11 | 0.46 ± 0.98 | 160 ± 11 | 52 ± 5 | 3.05 ± 0.10 | 30.0 ± 2.2 | 0.0059 ± 0.0001 |
| 12 | 0.61 ± 0.42 | 170 ± 6 | 55 ± 1 | 3.08 ± 0.03 | 26.0 ± 1.7 | 0.0068 ± 0.0004 |
| pH | Failure Stress (MPa) | E (kPa) | G (kPa) | E/G | Q (g/g) | ρx (mol/m3) |
|---|---|---|---|---|---|---|
| 2 | 0.54 ± 0.43 | 87 ± 18 | 45 ± 10 | 1.95 ± 0.83 | 14.0 ± 0.9 | 0.0090 ± 0.0021 |
| 3 | 13.44 ± 1.50 | 130 ± 52 | 52 ± 6 | 2.56 ± 0.78 | 2.5 ± 0.1 | 0.0340 ± 0.0040 |
| 4 | 14.88 ± 0.58 | 210 ± 24 | 71 ± 8 | 2.98 ± 0.02 | 2.6 ± 0.2 | 0.0450 ± 0.0058 |
| 5 | 13.81 ± 1.50 | 210 ± 24 | 72 ± 4 | 2.95 ± 0.16 | 2.5 ± 0.1 | 0.0460 ± 0.0026 |
| 6 | 13.86 ± 1.70 | 260 ± 30 | 78 ± 10 | 3.29 ± 0.03 | 2.8 ± 0.3 | 0.0460 ± 0.0027 |
| 8 | 0.18 ± 0.38 | 230 ± 31 | 60 ± 4 | 3.82 ± 0.09 | 45.0 ± 1.1 | 0.0057 ± 0.0005 |
| 9 | 0.23 ± 0.31 | 180 ± 10 | 65 ± 4 | 2.85 ± 0.03 | 43.0 ± 0.9 | 0.0062 ± 0.0003 |
| 10 | 0.24 ± 0.12 | 200 ± 10 | 53 ± 9 | 3.75 ± 0.02 | 42.0 ± 0.8 | 0.0065 ± 0.0012 |
| 11 | 0.22 ± 0.83 | 130 ± 30 | 43 ± 9 | 2.97 ± 0.15 | 42.0 ± 0.6 | 0.0043 ± 0.0011 |
| 12 | 0.14 ± 0.54 | 140 ± 11 | 46 ± 4 | 3.08 ± 0.06 | 36.0 ± 0.7 | 0.0050 ± 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalet, J.M.; Suekama, T.C.; Jeong, J.; Gehrke, S.H. Enhanced Mechanical Properties by Ionomeric Complexation in Interpenetrating Network Hydrogels of Hydrolyzed Poly (N-vinyl Formamide) and Polyacrylamide. Gels 2021, 7, 80. https://doi.org/10.3390/gels7030080
Scalet JM, Suekama TC, Jeong J, Gehrke SH. Enhanced Mechanical Properties by Ionomeric Complexation in Interpenetrating Network Hydrogels of Hydrolyzed Poly (N-vinyl Formamide) and Polyacrylamide. Gels. 2021; 7(3):80. https://doi.org/10.3390/gels7030080
Chicago/Turabian StyleScalet, Joseph M., Tiffany C. Suekama, Jeayoung Jeong, and Stevin H. Gehrke. 2021. "Enhanced Mechanical Properties by Ionomeric Complexation in Interpenetrating Network Hydrogels of Hydrolyzed Poly (N-vinyl Formamide) and Polyacrylamide" Gels 7, no. 3: 80. https://doi.org/10.3390/gels7030080
APA StyleScalet, J. M., Suekama, T. C., Jeong, J., & Gehrke, S. H. (2021). Enhanced Mechanical Properties by Ionomeric Complexation in Interpenetrating Network Hydrogels of Hydrolyzed Poly (N-vinyl Formamide) and Polyacrylamide. Gels, 7(3), 80. https://doi.org/10.3390/gels7030080