2.1. Solvent Properties
As alluded to above, this study focuses on the effect of varying organogel solvent (i.e., aliphatic mineral oil—MO) on gel properties. It is important to establish the foundational pure solvent properties of each MO prior to discussing gel properties. First, each MO should be midblock selective (i.e., EB selective) such that polystyrene endblocks can aggregate and subsequently vitrify into physical crosslinks. Should the MO have an affinity for polystyrene domains, these domains will be plasticized and gels will not be load-bearing materials. This attribute is typically accomplished through use of aliphatic hydrocarbon solvents. The present MOs, which include squalane (SQUAL), Hydrobrite 200 (HB 200), Hydrobrite 380 (HB 380), Hydrobrite 550 (HB 550), and Hydrobrite 1000 (HB 1000), are all advertised as primarily aliphatic hydrocarbon oils. To confirm this assertion, FTIR spectra were collected for each MO (
Figure S1). These spectra all exhibit two weak peaks at ca. 1375 and 1460 cm
−1 as well as a series of stronger peaks at ca. 2825–3000 cm
−1. The latter arise from alkane C–H stretching and the former from methyl and methylene C–H bending, respectively. Combined with the absence of peaks that would be expected for other functional groups such as aromatic rings (i.e., 1650–2000 cm
−1), these results confirm that the MOs used are primarily aliphatic hydrocarbons and should be EB selective.
The second solvent attribute of interest in this study is MO viscosity because it is hypothesized to have an effect on transport within gels. Specifically, the Stokes–Einstein Equation describes the relationship between diffusivity of a penetrant through pure solvent,
D0, and the solvent’s dynamic viscosity,
μ, as
where
kB is the Boltzmann constant,
T is temperature, and
rh is the hydrodynamic radius of the penetrant. While the chemical functionality of the MOs is uniform across the grades examined, viscosity is sensitive to the molecular weight and architecture of the compounds that comprise each MO. Thus, the viscosities may vary. Dynamic viscosities measured for each of the five MOs highlight that this factor is prevalent for these MOs (
Figure 1). In fact, there is a relatively broad range of dynamic viscosities from SQUAL up to HB 1000, which theoretically translate to a large variation of penetrant diffusion through pure solvent:
D0,SQUAL/
D0,HB 1000 ≈ 20.
2.2. Gel Preparation
In order to understand the effect of gel solvent viscosity on gel properties, organogels were fabricated using each of the MOs along with SEBS copolymer (number-average molecular weight,
Mn = 125 kDa, polystyrene fraction,
fS = 0.33 g polystyrene/g, and polydispersity
Ð = 1.01) as the gelator and either oleic acid (OA) or aerosol-OT (AOT) as the penetrant compound. Oleic acid resides in SEBS/MO gels as individual molecules (i.e., unimers), whereas aerosol-OT aggregates into reverse micelles with a radii of ca. 1.7 nm. Gels were initially formulated with 10 wt%, 20 wt%, or 30 wt% copolymer and 0.5 wt% OA or 1.0 wt% AOT (values based on previous work [
17,
18]), resulting in six series (in each series, the MO identity was varied). Note, 1.0 wt% AOT loading resulted in cloudy gels for HB 550 and HB 1000 MOs, suggesting that precipitation occurred. As a result, only SQUAL, HB 200, and HB 380 were considered when AOT was present. All gels were preswollen prior to any experimentation in a solution-matched liquid (e.g., a 10 wt% SEBS/0.5 wt% OA/89.5 wt% HB 380 gel was preswollen in a 0.5 wt% OA/99.5 wt% HB 380 solution) until equilibrium swollen mass was achieved. This process causes the concentration of SEBS to decrease due to an increase in MO, but the penetrant concentration remains fixed.
Table 1 provides a summary of the final gel formulations. In the remainder of our discussion, we refer to each of the copolymer concentration sets by their average concentrations: 6.5 wt%, 11.2 wt%, and 15.7 wt%.
2.3. Mechanical Behavior
Quasi-static, uniaxial tensile experiments were conducted for each gel batch shown in
Table 1 from
λzz = 1 to
λzz = 6, where
λzz is the stretch ratio defined by the length at a given time,
L, to that at the start of the experiment,
L0 (i.e.,
L/
L0). Two qualitative observations are apparent from engineering stress,
σeng, vs. stretch data across all 24 gel batches (
Figure 2). First, full stress–stretch profiles are closely bunched together for a fixed SEBS concentration across all MO and penetrant identities (as indicated by similar colors in
Figure 2). Second, the stress at any given stretch ratio increases with SEBS concentration implying gels become stiffer as a consequence of increasing polymer concentration.
Quantitative mechanical characteristics can be acquired by fitting stress–stretch profiles with an applicable model. The physically-crosslinked network structure of SEBS gels is amenable to fitting with a revised form of the slip-tube network (STN) model [
19,
20], which includes a correction for the filler effect imparted on gels’ mechanical properties by the glassy polystyrene crosslink domains
where
Gc and
Ge are the modulus contributions from the crosslinked network and the entangled EB midblocks, respectively, and
ϕPS is the volume fraction of polystyrene in the gel. The values of
ϕPS for each gel batch are calculated from known information, namely
ϕPS =
fS ×
wSEBS(
ρS/
ρg) where
fS is the fraction of polystyrene in SEBS,
wSEBS is the SEBS concentration in the gel, and
ρS and
ρg are the densities of polystyrene and the gel, respectively. As a result,
Gc and
Ge are the only fitting parameters used to describe stress–stretch data with the STN model and the fitted model represents data well up to
λzz ≈ 4 as has been noted in previous studies [
17,
19,
21]. Furthermore, the values of
Gc and
Ge determined through fitting match the observations discussed above. Penetrant and MO identity have minimal effect on
Gc and
Ge, whereas both modulus contributions increase dramatically with increasing SEBS concentration (
Figure 3). The minor fluctuations observed for
Gc and
Ge when penetrant and MO identity are varied likely arise as a result of small differences in
wSEBS across these gels. These results highlight that the properties of the solvent alone do not affect gels’ quasi-static mechanical behavior so long as the solvent-polymer interactions remain approximately the same. It also shows that the presence of penetrants in either form does not impact quasi-static mechanical response.
Deeper exploration of the modulus contribution parameters allows these results to be further understood. The affine network definition of
Gc is
where
ν is the number density of networked EB chains within gels, and is the only parameter in this expression that varies across gel systems, here. It is expected that
ν is only a function of
wSEBS because the amount of copolymer in gels directly affects the number density of EB blocks, but solvents with similar functionality should not affect this parameter. This interpretation agrees with the trends seen in
Figure 3a. Alternatively,
Ge is typically described as a power-law proportional to
wSEBS, wherein the exponent depends on the quality of the solvent for the polymer (i.e.,
Ge ∝
wSEBS2.25 for a good solvent or
Ge ∝
wSEBS2.33 for a theta solvent) [
20,
22,
23]. We expect that
Ge, like
Gc, is only dependent on
wSEBS and not
μ since the chemical functionality of the MOs is relatively unchanged. This interpretation agrees with the trends seen in
Figure 3b. There is clear experimental and theoretical dependence of
Gc and
Ge on
wSEBS; however, these relationships are not explored in detail at present because the focus of this study is on the effect of oil viscosity on gel properties and because of the considerable complexity of polymer chain conformation that exists in gels formulated at different
wSEBS followed by post-anneal swelling.
2.4. Transport Properties
In contrast to gel mechanical behavior, we hypothesize that the transport of penetrants through gels should be affected by MO viscosity. Penetrant (i.e., OA or AOT) release experiments were conducted by submersing gels into pure MO (the identity of the oil matched that of the gel under observation) and monitoring the relative amount of OA or AOT retained within each gel over time. OA and AOT concentrations were measured using a previously described FTIR-based method that takes advantage of the isolated peak position of each penetrant compound’s ester/acid carbonyl group(s) (OA = 1712 cm
−1, AOT = 1739 cm
−1) [
18]. Examples of the time evolution of FTIR spectra during release experiments are shown in
Figure S2 (OA) and
Figure S3 (AOT) and relative retained mass (i.e.,
m/
m0 where
m and
m0 are penetrant mass in a gel at time
t and time
0, respectively) corresponds directly to relative peak absorbance (
A/
A0 where
A and
A0 are carbonyl peak absorbance at time
t and time
0, respectively).
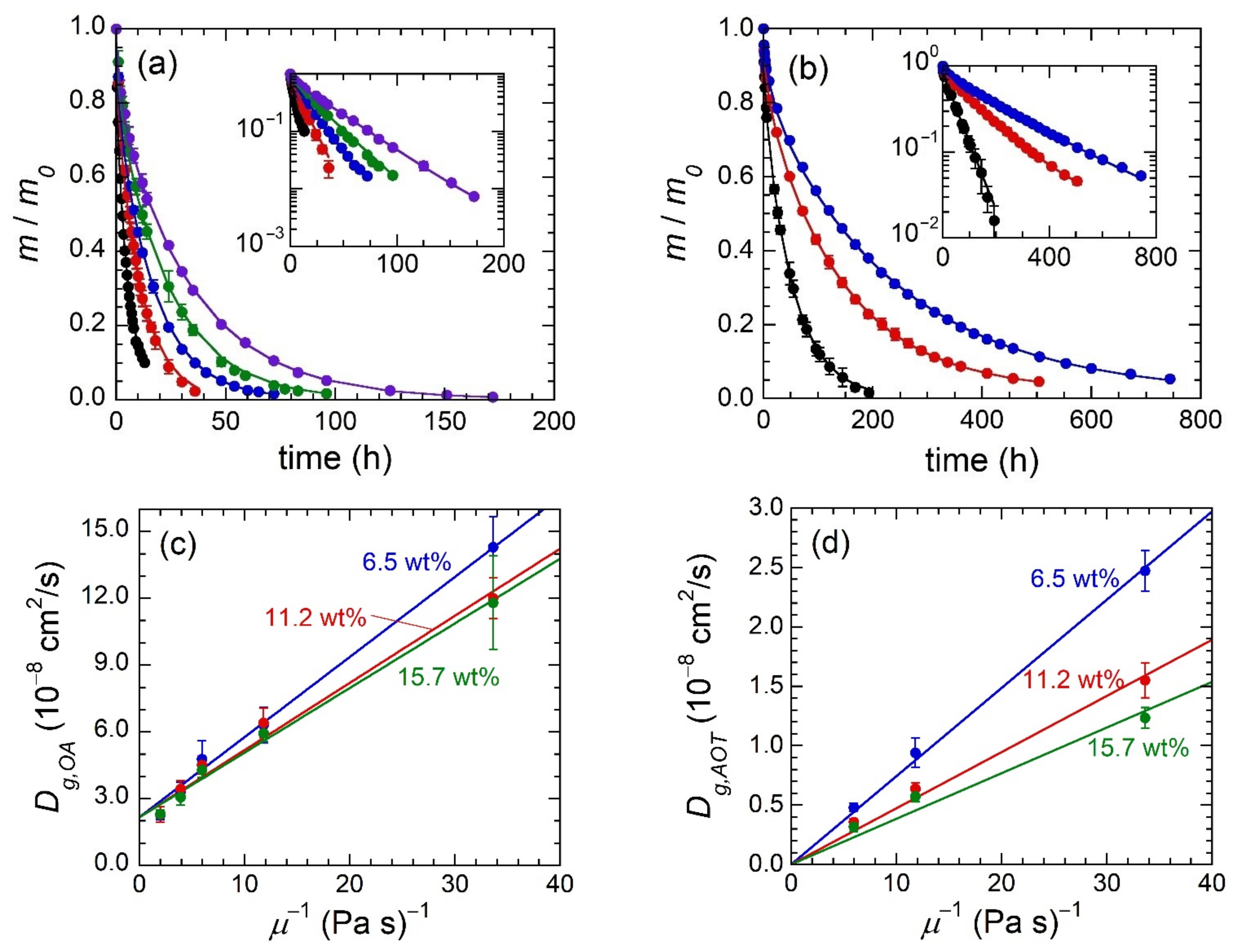
Examination of OA and AOT retained mass profiles (
Figure 4a,b) clearly shows that increasing MO viscosity leads to slower release of the penetrant compound. In gels containing OA, the time required for 90% penetrant release ranges from ≈10 h for the lowest viscosity MO (SQUAL) to ≈72 h for the highest viscosity MO (HB 1000). Similarly, gels that contain AOT range in the time required for 90% penetrant release from ≈120 h (SQUAL) to ≈600 h (HB 380). Each OA, or AOT, retained mass profile can be modeled to quantify penetrant diffusivity through gels—
Dg,i, where
i is either OA or AOT. Fick’s Second Law of Diffusion for a disk is
where
Ci is the concentration of penetrant in the gel,
r,
θ, and
z are radial, angular, and axial coordinates, and
t is time. This expression can be simplified by assuming that diffusion occurs predominantly in the axial direction of gels due the absence of a concentration gradient in the
θ direction and their geometry (radius ≈ 10 × thickness), which eliminates the first and second terms in the square brackets. The simplified form of Equation (4) can be solved by applying one initial condition (the penetrant concentration is uniform in gels at
t = 0) and two boundary conditions (the penetrant concentration gradient at the center of gels is zero and the liquid oil bath provides an effective concentration sink). Subsequent integration of this solution over the full gel thickness yields
where
L is half the gel thickness. Modeling of the retained mass profiles in
Figure 4a,b using Equation (5) only requires fitting of
Dg,i since gel thickness is known. The resultant model fits represent retained mass profiles very well as seen most clearly by retained mass versus time semi-log plots (
Figure 4a,b insets).
Diffusivity values extracted through fitting of the data in
Figure 4a,b can now be used to directly compare the rate of penetrant transport between gels comprised of different MOs and SEBS concentrations (
Figure 4c,d). First, it can be seen that MO viscosity has a strong influence on the diffusivity of both penetrants and their trend qualitatively matches the Stokes–Einstein Equation, which states that
Dg,i ∝
μ−1. Second,
wSEBS has a small effect on OA diffusivity and a moderate effect on AOT diffusivity, but in both cases the impact of
wSEBS is considerably smaller than
μ.
We set out to further understand the relationship between diffusivity and formulation parameters by theoretically describing our data with an established model for solute diffusion through a polymeric gel medium. Specifically, we elect to use the hydrodynamic description of solute diffusion as it aligns most directly with the Stokes–Einstein Equation. The hydrodynamic description of solute diffusion treats the penetrant as a hard sphere moving at a constant velocity through the gel medium. The hard spheres’ motion is hindered by frictional drag within the gel stemming from the properties of the solvent and the concentration and properties of the polymer chains. The hydrodynamic model derived for penetrant diffusion through homogenous gels [
24,
25] takes the form
where
kc is a polymer/solvent-specific constant,
ϕp is the polymer volume fraction, and the penetrant hydrodynamic radii are
rh,OA ≈ 5 Å (unimers) and
rh,AOT ≈ 17 Å (reverse micelles). The term in square brackets of Equation (6) reflects frictional drag at infinite polymer dilution (i.e.,
ϕp = 0) and can, therefore, be thought of as the solvent contribution to diffusivity. Alternatively, the exponential term of Equation (6) arises from the frictional drag associated with polymer chains within gels.
It is important to reframe the definition of
ϕp in Equation (6) in the context of the current systems before proceeding. Because penetrant molecules are excluded from the crosslink domains of the present organogels, their diffusion only occurs in the MO-rich continuous phase. Therefore,
ϕp is more specifically defined as the volume fraction of EB midblocks in the EB/MO continuous phase. This new definition yields
where
VEB and
VEB/MO are the volumes of EB midblocks and of the EB/MO continuous phase in gels, respectively, and
ρEB and
ρEB/MO are the densities of the EB midblocks (0.878 g/cm
3) and of the EB/MO continuous phase (≈0.861 g/cm
3), respectively. For reference, approximate values of
ϕp for 6.5 wt% SEBS, 11.2 wt% SEBS, and 15.7 wt% SEBS gels are 4.4 vol%, 7.6 vol%, and 10.9 vol%, respectively.
With Equation (6) and corresponding considerations in place, we now revisit the experimental data in
Figure 4c,d. If the relationship between
Dg,i and
μ is accurately described by Equation (6), the trends of
Dg,i versus
μ−1 should be linear with a
y-intercept of zero. In all six cases (three SEBS concentrations and two penetrant identities), linear fits describe data well and in the case of AOT diffusion the fit can be forced through a
y-intercept of 0 cm
2/s while maintaining a good fit to the data (
Figure 4d). OA diffusion, on the other hand, is clearly not amenable to fitting with a
y-intercept of zero. However, all three series roughly converge to a common
y-intercept of ca. 2.2 × 10
−8 cm
2/s. (This point will be addressed further below). The slope of each individual linear fit, which has a fixed
rh,i and
ϕp, should correspond to [
kBT/(6π
rh,i)]exp(−
rh,i kc ϕp3/4), wherein only
kc is unknown, but should remain constant across all data series. Minimizing the squared difference between experimental and theoretical slopes (
Table 2) results in
kc = 0.39 Å
−1, which is of appropriate magnitude based on previous studies [
25].
The collection of evidence in the preceding paragraph mostly describes the experimental penetrant transport findings. The only point that does not agree with the hydrodynamic theory is the non-zero
y-intercept of the three gel series that contain OA as the penetrant. The non-zero
y-intercept suggests that diffusion of OA molecules will occur in gels comprised of MO with infinite viscosity. In contrast, the AOT diffusivity values match expectation; the diffusivity approaches zero as viscosity approaches infinity. One possible explanation is that a fraction of OA diffusion occurs through hopping of OA molecules amongst the free volume voids between MO and EB molecules (referred to as free volume theory) [
25,
26]. Even when the solvent viscosity approaches infinity and the fraction of OA diffusion occurring based on the hydrodynamic description becomes negligible, the OA molecules diffuse via the free volume sites. On the other hand, the AOT reverse micelles are too large to hop among free volume voids and are fully described by the hydrodynamic diffusion model.