Effect of Doxycycline Microencapsulation on Buccal Films: Stability, Mucoadhesion and In Vitro Drug Release
Abstract
1. Introduction
2. Results
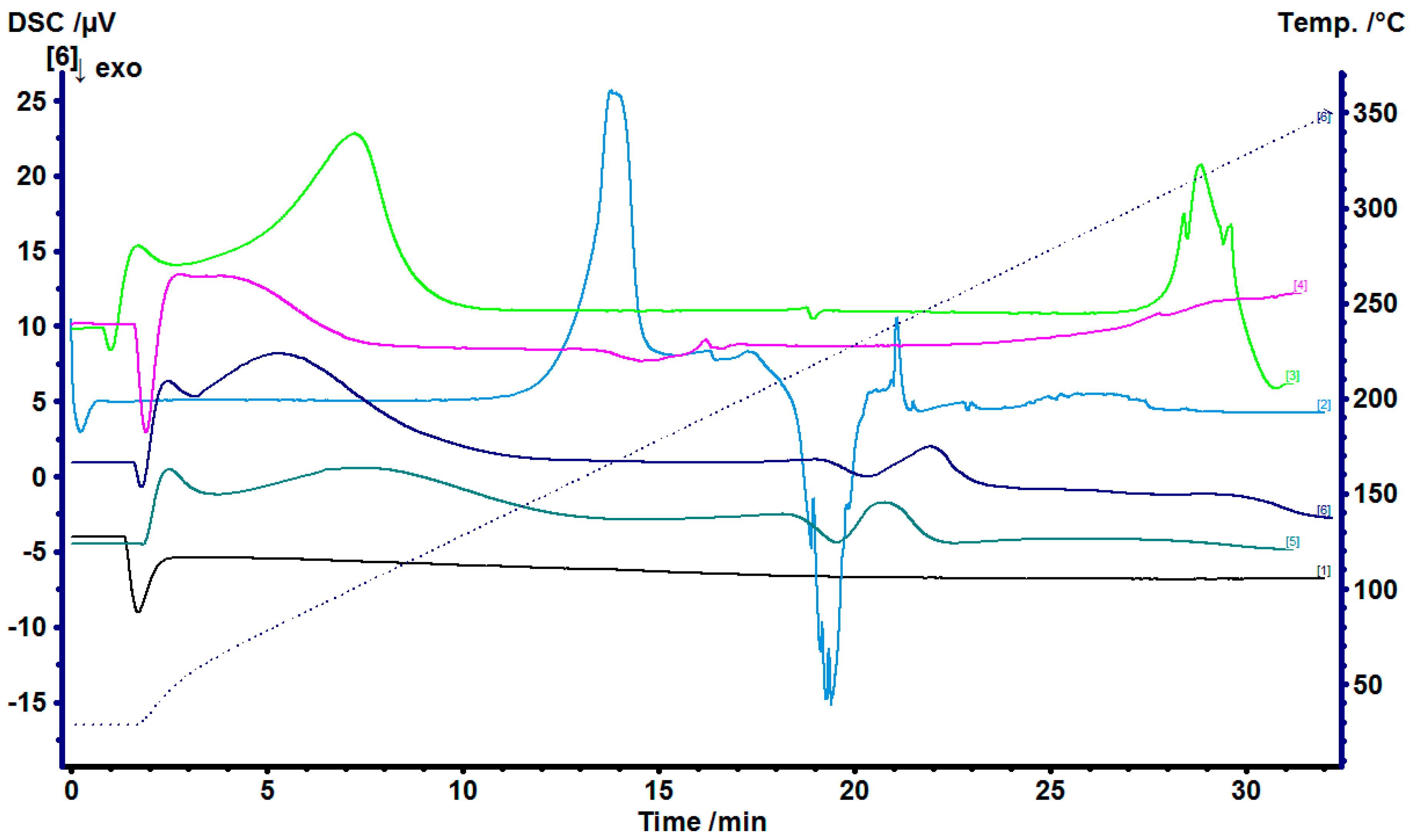
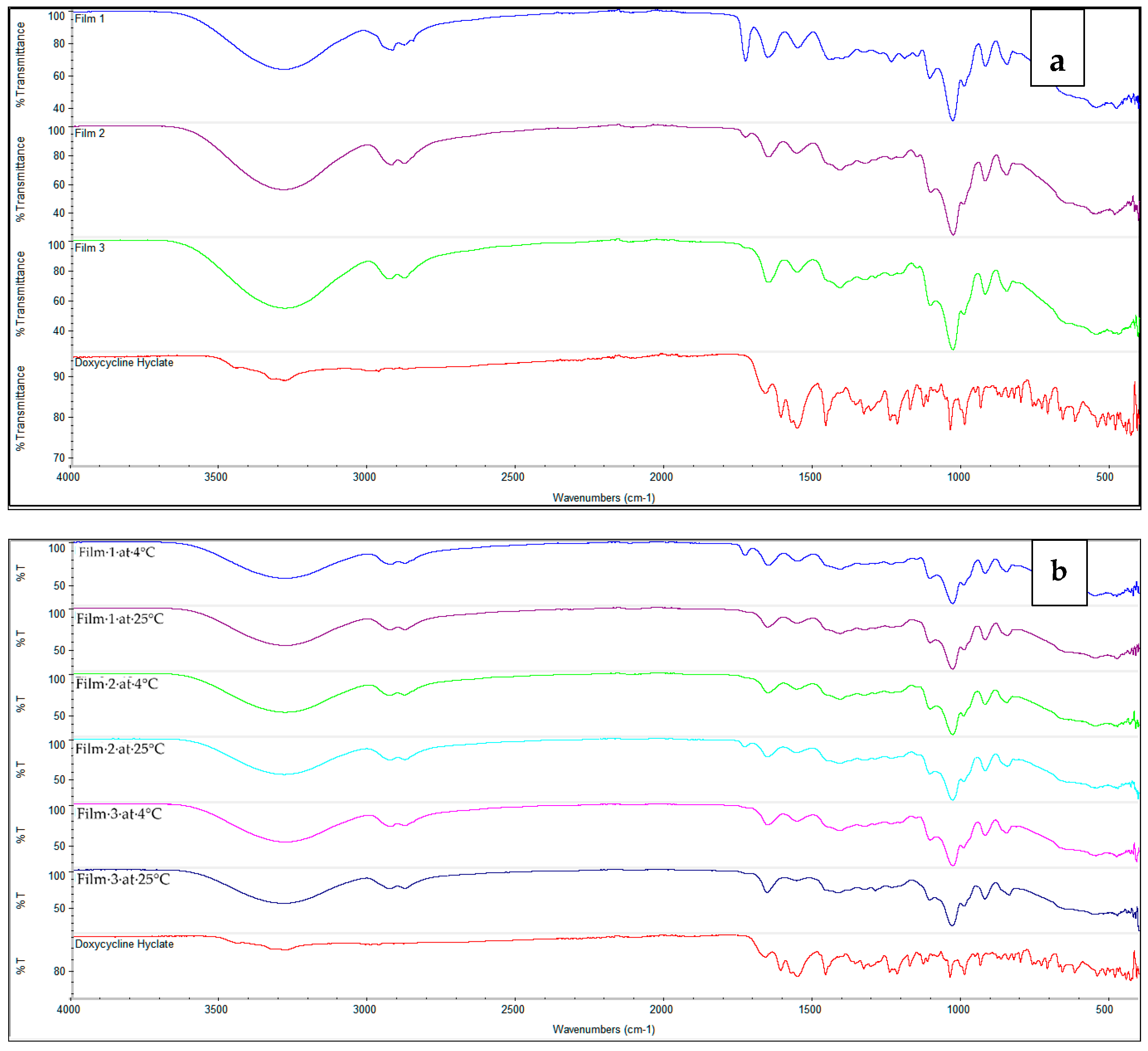
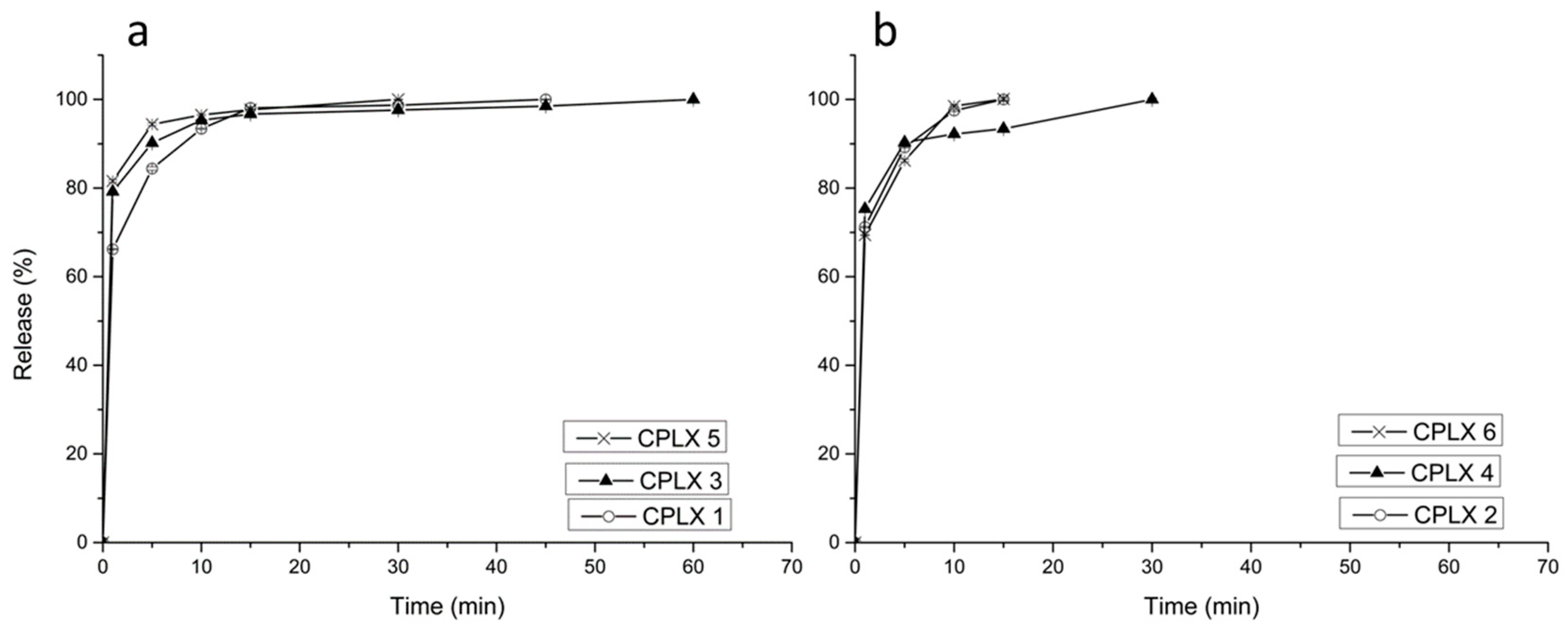
2.1. Doxycycline and β-Cyclodextrin Complexation
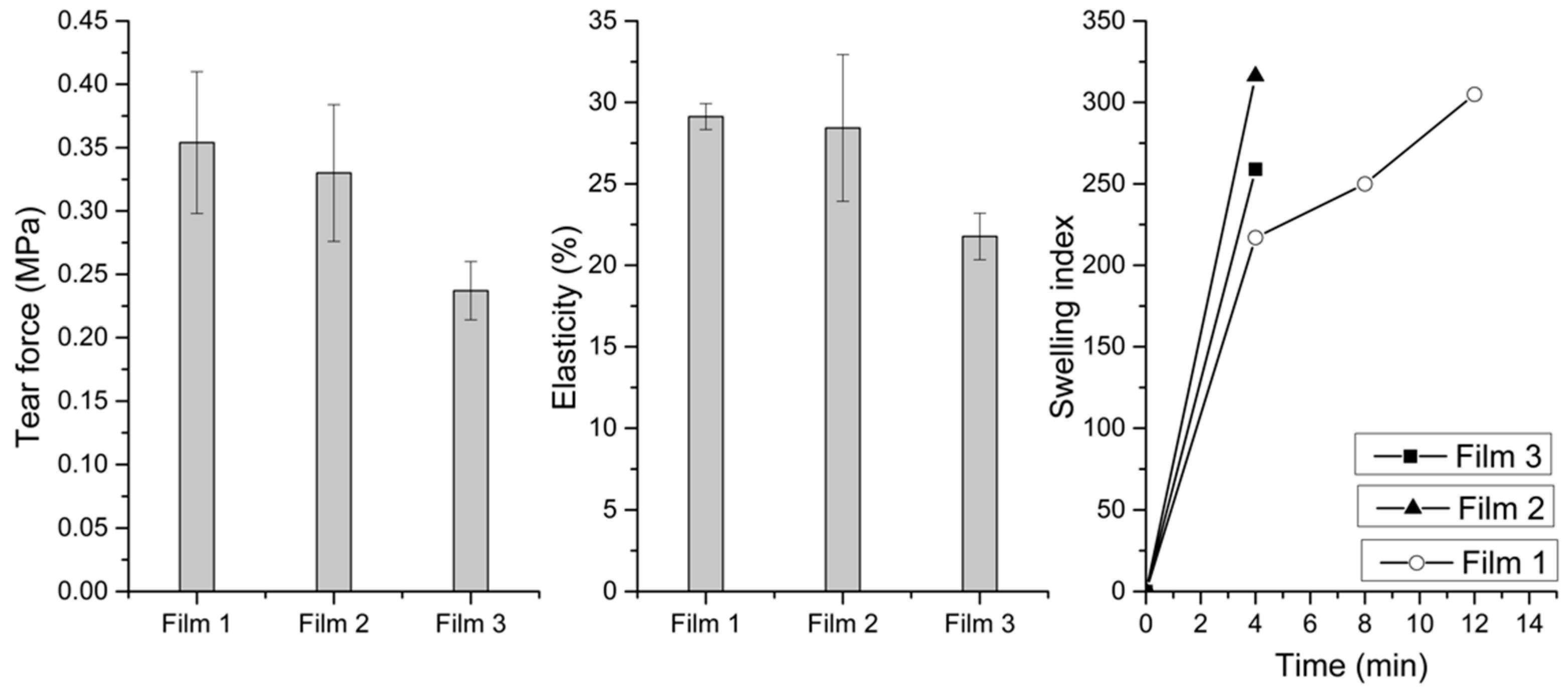
2.2. In Vitro Mucoadhesion and Properties of the Buccal Films
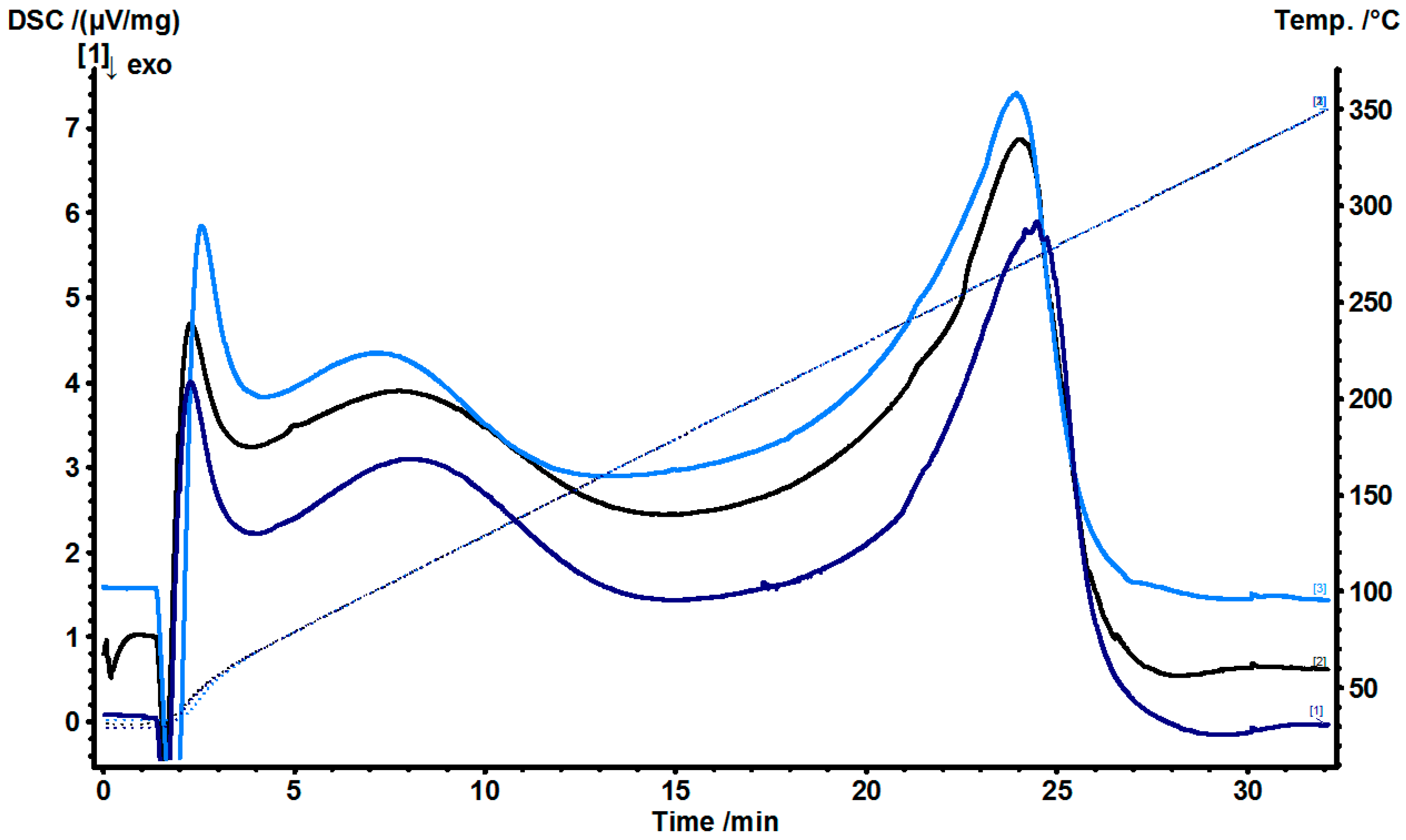
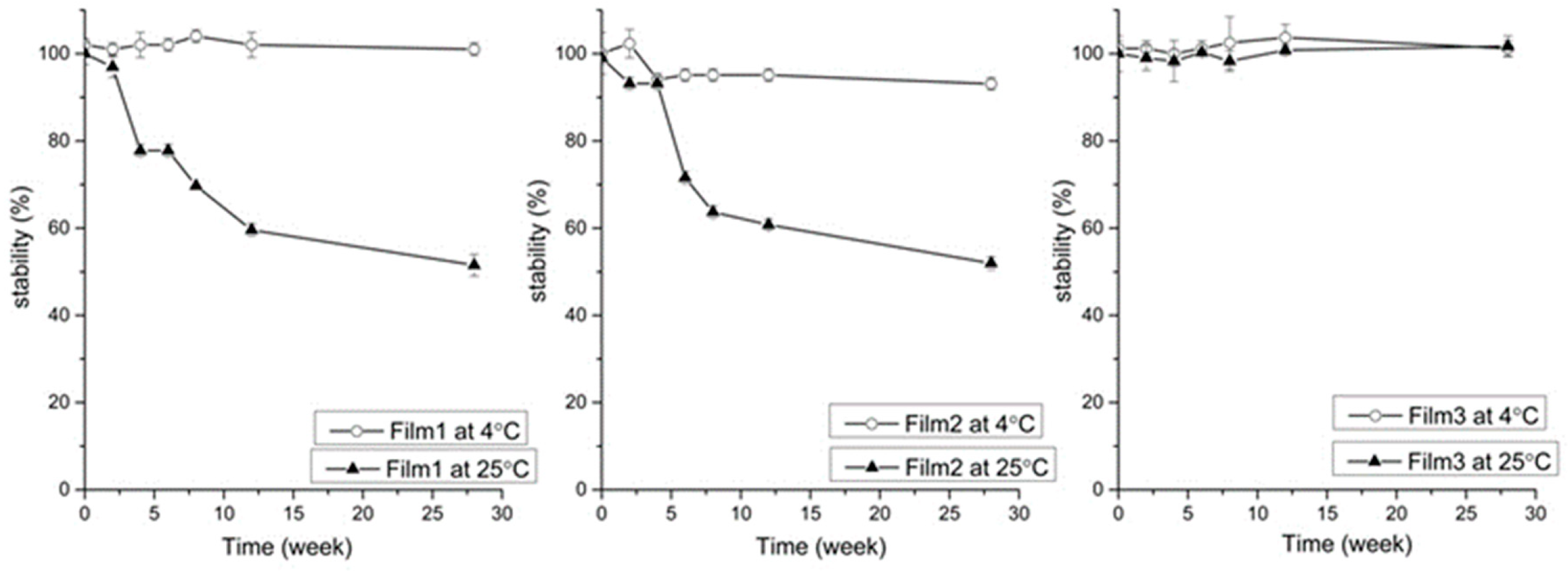
2.3. Stability Studies
2.4. Microparticle Size
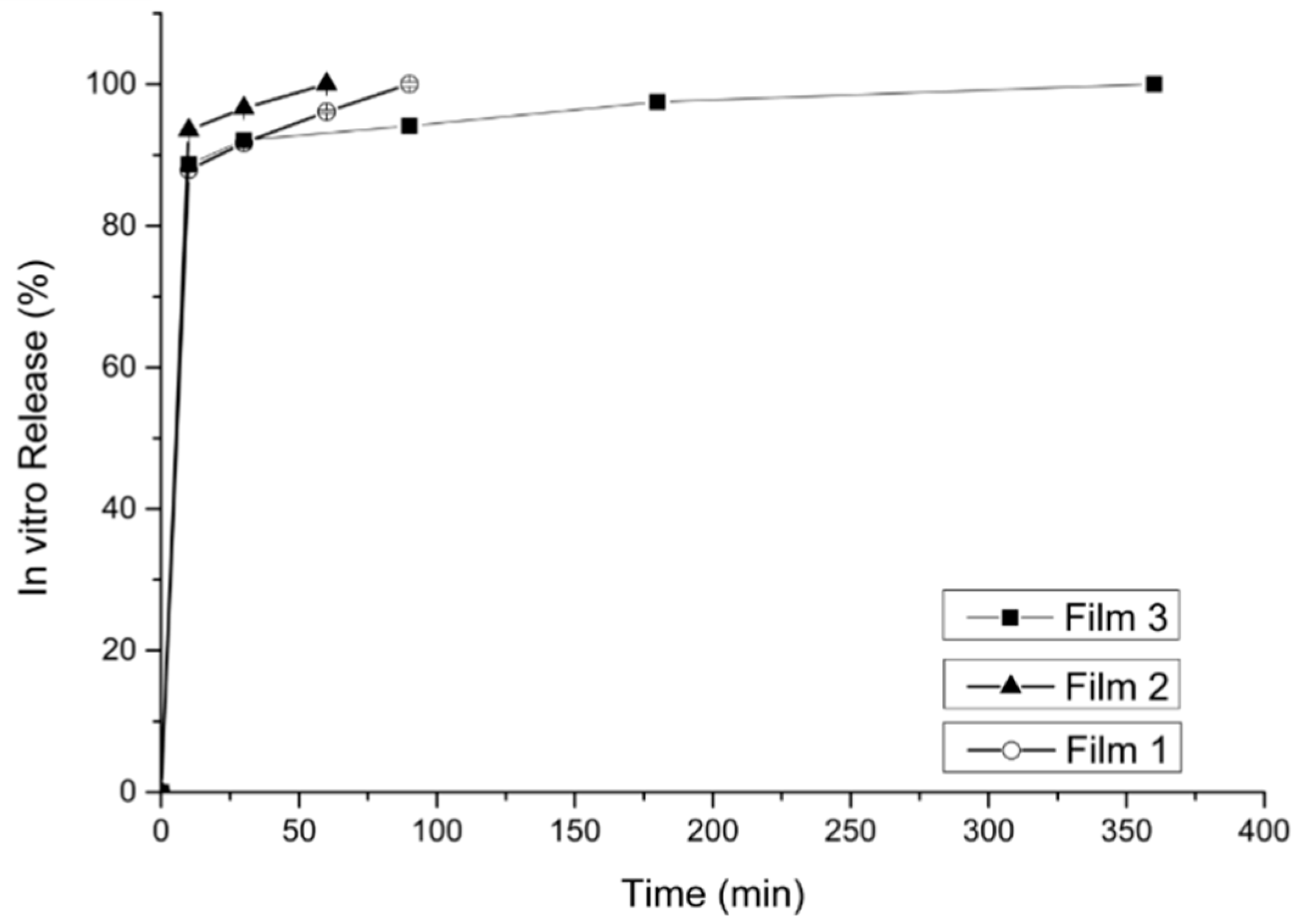
2.5. Dissolution and Release Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Doxycycline and β-Cyclodextrin Complexation
4.3. Preparation of Microparticles
4.4. Preparation of Buccal Films
4.5. Analysis
4.5.1. Fourier-Transform Infrared Spectroscopy (FTIR)
4.5.2. Differential Scanning Calorimetry (DSC)
4.6. Mucoadhesion Study of Buccal Films
4.7. Tensile Strengths and Elasticity
4.8. Swelling Study of Buccal Films
4.9. pH, Film Thickness and Stability Studies
4.10. Microparticles Dissolution Study and In Vitro Drug Release from Buccal Films
4.11. HPLC Method
4.12. Microscopy
4.13. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shakya, P.; Madhav, N.S.; Shakya, A.K.; Singh, K. Palatal mucosa as a route for systemic drug delivery: A review. J. Control. Release 2011, 151, 2–9. [Google Scholar] [CrossRef]
- Gandhi, R.B.; Robinson, J.R. Oral cavity as a site for bioadhesive drug delivery. Adv. Drug Deliv. Rev. 1994, 13, 43–74. [Google Scholar] [CrossRef]
- Häyrinen-Immonen, R.; Sorsa, T.; Nordström, D.; Malmström, M.; Konttinen, Y.T. Collagenase and stromelysin in recurrent aphthous ulcers (RAU). Int. J. Oral. Maxillofac. Surg. 1993, 22, 46–49. [Google Scholar] [CrossRef]
- Al-Azri, A.R.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Keefe, D.M.; Logan, R.M. Involvement of matrix metalloproteinases (MMP-3 and MMP-9) in the pathogenesis of irinotecan-induced oral mucositis. J. Oral Pathol. Med. 2014, 44, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjäderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef]
- Skulason, S.; Holbrook, W.P.; Kristmundsdottir, T. Clinical assessment of the effect of a matrix metalloproteinase inhibitor on aphthous ulcers. Acta Odontol. Scand. 2009, 67, 25–29. [Google Scholar] [CrossRef]
- Skúlason, S.; Holbrook, W.P.; Thormar, H.; Gunnarsson, G.B.; Kristmundsdottir, T. A study of the clinical activity of a gel combining monocaprin and doxycycline: A novel treatment for herpes labialis. J. Oral Pathol. Med. 2011, 41, 61–67. [Google Scholar] [CrossRef]
- Vijayabala, G.S.; Kalappanavar, A.N.; Annigeri, R.G.; Sudarshan, R.; Shettar, S.S. Single application of topical doxycycline hyclate in the management of recurrent aphthous stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 440–446. [Google Scholar] [CrossRef]
- Skúlason, S.; Ingólfsson, E.; Kristmundsdóttir, T. Development of a simple HPLC method for separation of doxycycline and its degradation products. J. Pharm. Biomed. Anal. 2003, 33, 667–672. [Google Scholar] [CrossRef]
- Power, D.F.; Fieldson, G.; Chang, Y. Tetracycline Stabilizing Formulations. World Intellectual Property Organization (PCT). Patent No. WO2010033800A2, 25 March 2010. [Google Scholar]
- Loftsson, T. Chapter 6–Drug Degradation in Solid State. In Drug Stability for Pharmaceutical Scientists; Loftsson, T., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 115–120. [Google Scholar]
- Hassan, E.E.; Gallo, J.M. A Simple Rheological Method for the in Vitro Assessment of Mucin-Polymer Bioadhesive Bond Strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A. Mucoadhesive systems in oral drug delivery. Drug Discov. Today Technol. 2005, 2, 83–87. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- FDA. GRAS Notice Inventory. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ (accessed on 27 November 2019).
- Bolourchian, N.; Bahjat, M. Design and in vitro evaluation of Eudragit-based extended release diltiazem microspheres for once and twice daily administrations: The effect of coating on drug release behavior. Turk. J. Pharm. Sci. 2018, 16, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, V.G.R.; Holbrook, W.P.; Gizurarson, S.; Kristmundsdottir, T. Long-term Stabilization of Aqueous Doxycycline Formulations, in Mucoadhesive Hydrogels for Treatment of Oral Mucosal Conditions. Curr. Drug Discov. Technol. 2020, 17, 376–386. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.; He, Z.; Wang, Z.; Zhang, M.; Wan, Q. Molecular modeling-based inclusion mechanism and stability studies of doxycycline and hydroxypropyl-beta-cyclodextrin complex for ophthalmic delivery. AAPS PharmSciTech 2013, 14, 10–18. [Google Scholar] [CrossRef][Green Version]
- Akazawa, Y.; Yagyu, M.; Nagoyashi; Nozawa, S.; Yasui, S. Aqueous Doxycycline Compositions. United States Patent No. US3846548A, 5 November 1974. [Google Scholar]
- Kogawa, A.C.; Zoppi, A.; Quevedo, M.A.; Salgado, H.R.N.; Longhi, M.R. Increasing Doxycycline Hyclate Photostability by Complexation with β-Cyclodextrin. AAPS PharmSciTech 2014, 15, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Biswas, S.; Ghosh, T. Preparation and Evaluation of Silymarin β-cyclodextrin Molecular Inclusion Complexes. J. Young Pharm. 2011, 3, 205–210. [Google Scholar] [CrossRef]
- Batista, P.; Castro, P.; Madureira, A.R.; Sarmento, B.; Pintado, M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals 2019, 12, 32. [Google Scholar] [CrossRef]
- Skulason, S.; Asgeirsdottir, M.S.; Magnusson, J.P.; Kristmundsdottir, T. Evaluation of polymeric films for buccal drug delivery. Die Pharm. 2009, 64, 197–201. [Google Scholar]
- Bahri-Najafi, R.; Tavakoli, N.; Senemar, M.; Peikanpour, M. Preparation and pharmaceutical evaluation of glibenclamide slow release mucoadhesive buccal film. Res. Pharm. Sci. 2015, 9, 213–223. [Google Scholar]
- Rana, P.; Murthy, R.S.R. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: A potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013, 20, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Peh, K.K.; Wong, C.F. Polymeric films as vehicle for buccal delivery: Swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 2000, 2, 53–61. [Google Scholar]
- Muzib, Y.I.; Kumari, K.S. Mucoadhesive buccal films of glibenclamide: Development and evaluation. Int. J. Pharm. Investig. 2011, 1, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hazra, M.; Mandal, D.D.; Mandal, T.; Bhuniya, S.; Ghosh, M. Designing polymeric microparticulate drug delivery system for hydrophobic drug quercetin. Saudi Pharm. J. 2015, 23, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia Europea. Doxycycline Hyclate, Monograph, 8th ed.; Council of Europe: Strasbourg, France, 2014. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patlolla, V.G.R.; Popovic, N.; Peter Holbrook, W.; Kristmundsdottir, T.; Gizurarson, S. Effect of Doxycycline Microencapsulation on Buccal Films: Stability, Mucoadhesion and In Vitro Drug Release. Gels 2021, 7, 51. https://doi.org/10.3390/gels7020051
Patlolla VGR, Popovic N, Peter Holbrook W, Kristmundsdottir T, Gizurarson S. Effect of Doxycycline Microencapsulation on Buccal Films: Stability, Mucoadhesion and In Vitro Drug Release. Gels. 2021; 7(2):51. https://doi.org/10.3390/gels7020051
Chicago/Turabian StylePatlolla, Venu Gopal Reddy, Nikolina Popovic, William Peter Holbrook, Thordis Kristmundsdottir, and Sveinbjörn Gizurarson. 2021. "Effect of Doxycycline Microencapsulation on Buccal Films: Stability, Mucoadhesion and In Vitro Drug Release" Gels 7, no. 2: 51. https://doi.org/10.3390/gels7020051
APA StylePatlolla, V. G. R., Popovic, N., Peter Holbrook, W., Kristmundsdottir, T., & Gizurarson, S. (2021). Effect of Doxycycline Microencapsulation on Buccal Films: Stability, Mucoadhesion and In Vitro Drug Release. Gels, 7(2), 51. https://doi.org/10.3390/gels7020051





