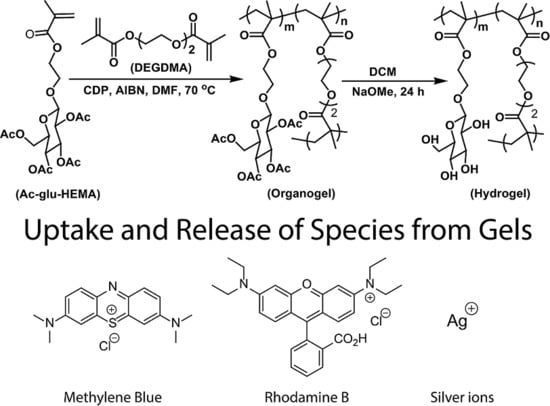
Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels
Abstract
1. Introduction
2. Results and Discussion
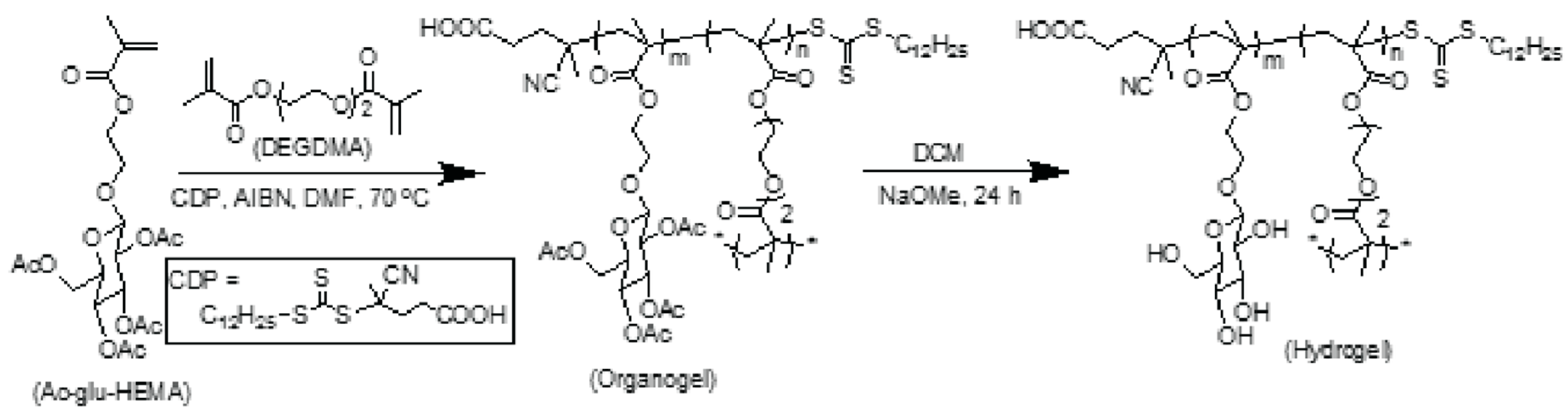
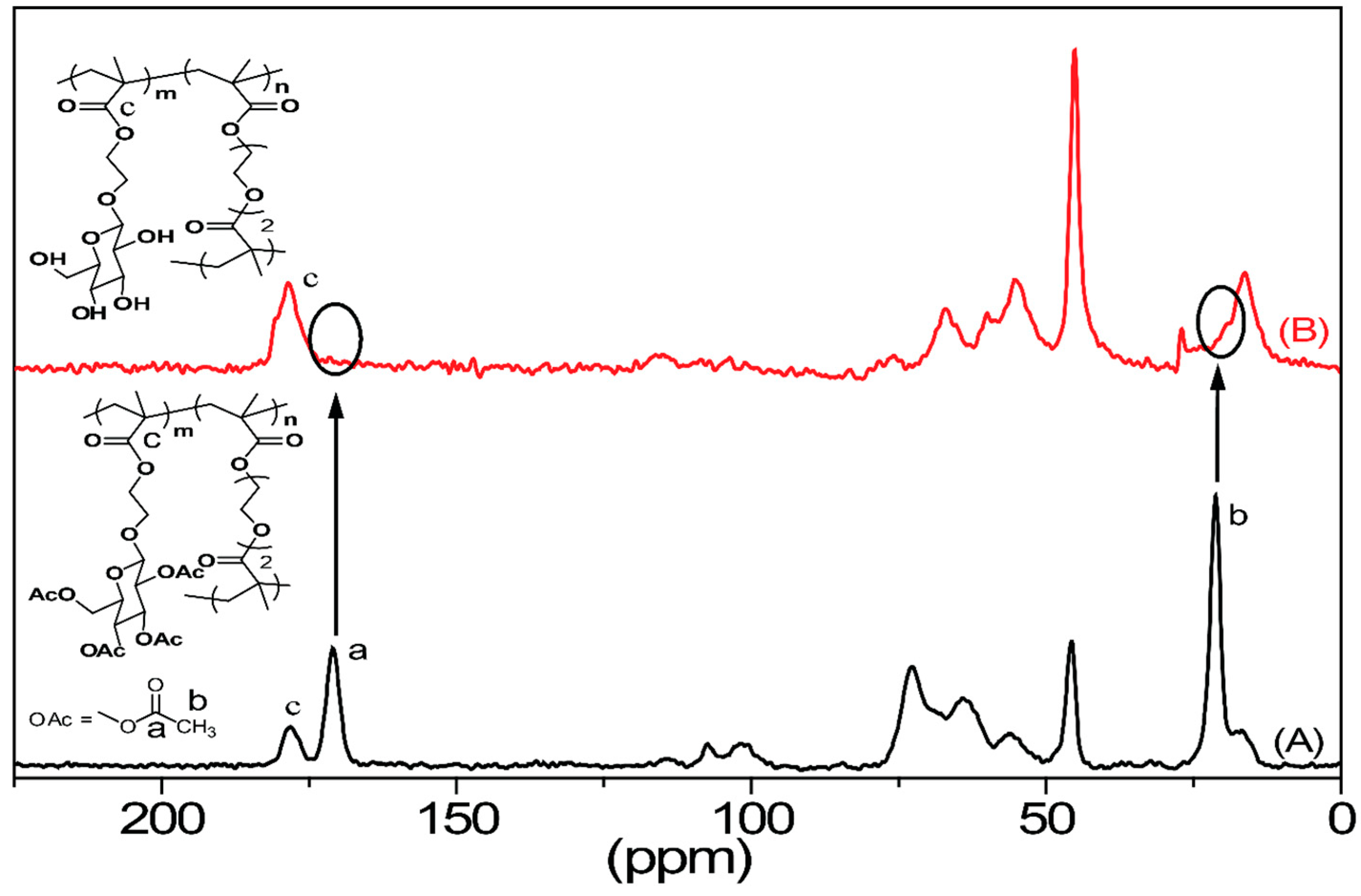
2.1. Gel Chemistry
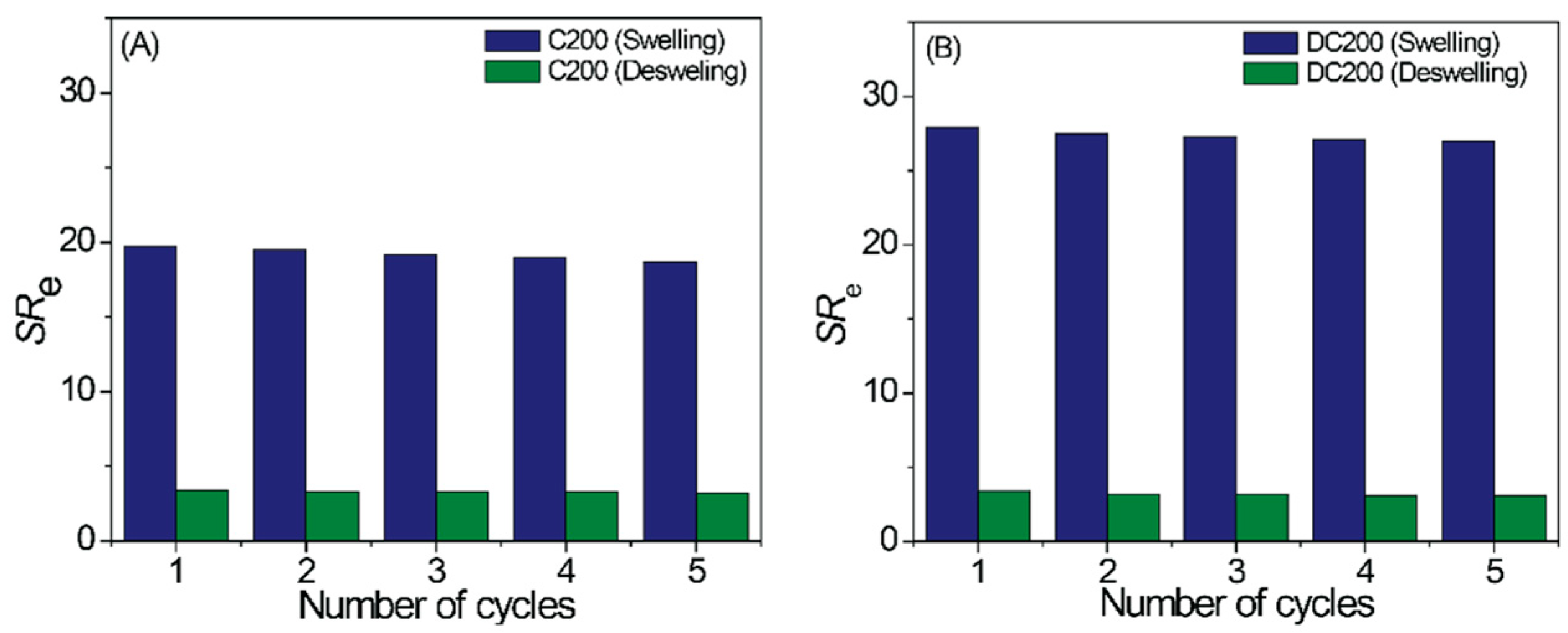
2.2. Macroscopic Properties of the Gels
2.3. Gel Swelling in Various Solvents
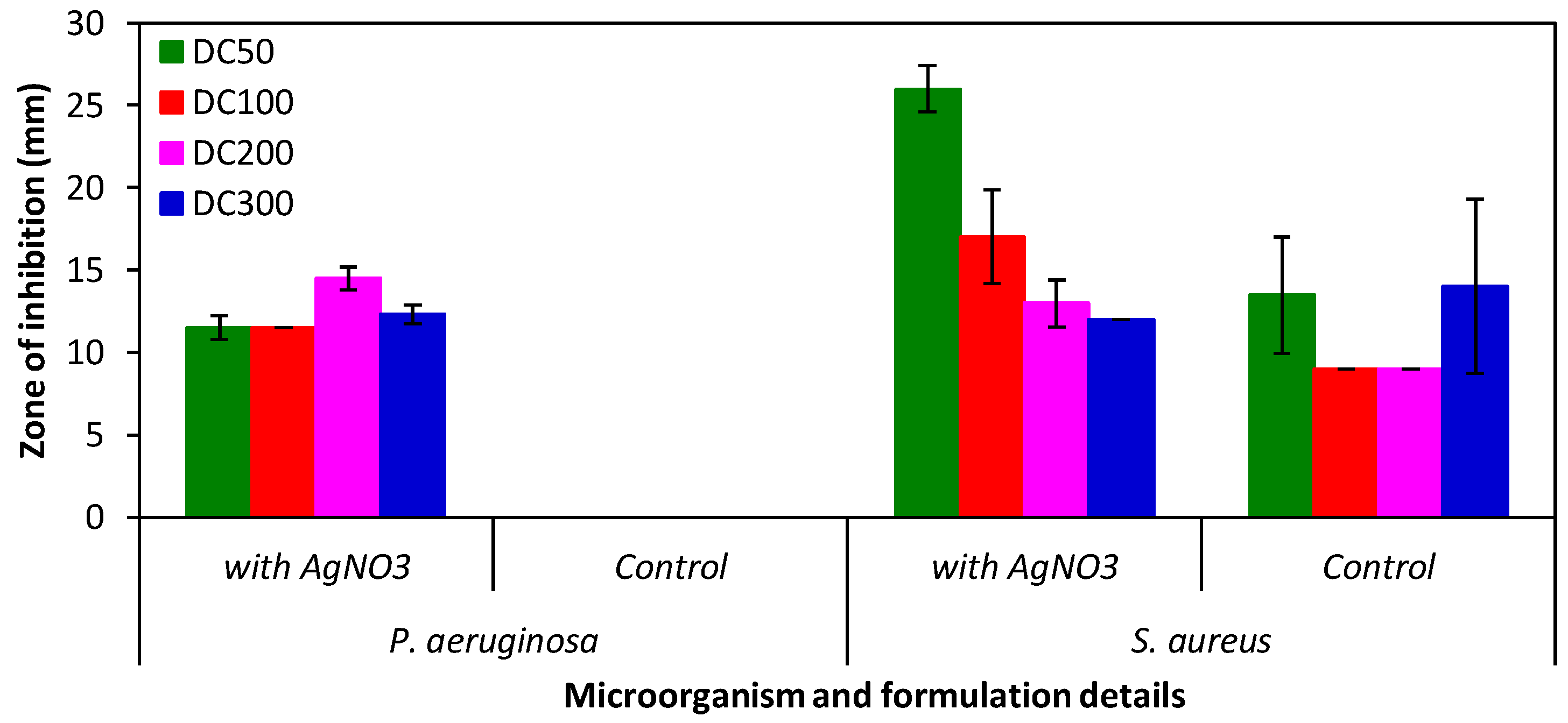
2.4. Uptake and Release of Species from Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Analytical Chemistry
4.3. Preparation of Organogels
4.4. Preparation of Hydrogels
4.5. Gel Swelling Studies
4.6. Rheology
4.7. Field Emission Scanning Electron Microscopy (FE-SEM) Analysis
4.8. Dye Uptake Study
4.9. Inductively Coupled Plasma (ICP) Analysis of Ag+ Release
4.10. Antimicrobial Studies—Zone of Inhibition (ZOI)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Gel | ε | ETN | α | β | π* |
|---|---|---|---|---|---|
| Hexane | 2.00 | 0.009 | 0.00 | 0.00 | −0.08 |
| Chloroform | 4.80 | 0.259 | 0.44 | 0.00 | 0.69 |
| THF | 7.58 | 0.207 | 0.00 | 0.55 | 0.55 |
| Dichloromethane | 8.93 | 0.309 | 0.30 | 0.00 | 0.73 |
| Acetone | 20.70 | 0.355 | 0.08 | 0.48 | 0.71 |
| Methanol | 32.70 | 0.762 | 0.93 | 0.62 | 0.60 |
| DMF | 36.70 | 0.386 | 0.00 | 0.76 | 0.88 |
| Acetonitrile | 37.50 | 0.460 | 0.19 | 0.31 | 0.75 |
| DMSO | 46.70 | 0.444 | 0.00 | 0.76 | 1.00 |
| Water | 78.3 | 1.100 | 1.15 | 0.15 | 1.10 |
| Gel | δt | δo | δd | δp | δh | δa |
|---|---|---|---|---|---|---|
| Hexane | 14.9 | - | 14.9 | - | - | 2.1 |
| Chloroform | 18.9 | 9.30 | 8.70 | 1.50 | 2.80 | 3.18 |
| THF | 18.6 | 9.50 | 8.20 | 2.80 | 3.90 | 4.80 |
| Dichloromethane | 20.2 | 9.90 | 8.90 | 3.10 | 3.00 | 4.31 |
| Acetone | 19.6 | 10.4 | 13.9 | - | - | - |
| Methanol | 29.7 | 10.0 | 12.7 | 6.00 | 10.90 | 17.0 |
| DMF | 24.1 | 12.7 | 16.2 | - | - | - |
| Acetonitrile | 24.7 | 16.8 | 13.3 | - | - | - |
| DMSO | 24.5 | 12.5 | 17.2 | - | - | - |
| Water | 47.9 | - | 12.9 | - | - | - |
References
- Deligkaris, K.; Tadele, T.S.; Olthuis, W.; van den Berg, A. Hydrogel-based devices for biomedical applications. Sens. Actuators B Chem. 2010, 147, 765–774. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery-A Review. J. Control. Release 2008, 125, 179. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2012, 14, 95. [Google Scholar] [CrossRef]
- Sustainable Development Goals Knowledge Platform. Available online: https://sustainabledevelopment.un.org/?menu=1300 (accessed on 11 August 2019).
- Chatterjee, S.; Chi-Leung Hui, P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Kabiri, K.; Azizi, A.; Zohuriaan-Mehr, M.; Marandi, G.B.; Bouhendi, H. Alcohophilic gels: Polymeric organogels composing carboxylic and sulfonic acid groups. J. Appl. Polym. Sci. 2011, 120, 3350–3356. [Google Scholar] [CrossRef]
- Basak, S.; Nanda, J.; Banerjee, A. A new aromatic amino acid based organogel for oil spill recovery. J. Mater. Chem. 2012, 22, 11658–11664. [Google Scholar] [CrossRef]
- Mondal, S.; Bairi, P.; Das, S.; Nandi, A.K. Phase selective organogel from an imine based gelator for use in oil spill recovery. J. Mater. Chem. A 2019, 7, 381–392. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Y.; Xu., Z.; Guo, Q. Hybrid high internal phase emulsion (HIPE) organogels with oil separation properties. Chem. Commun. 2014, 50, 13821–13824. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Mucoadhesive Hydrogel Nanoparticles as Smart Biomedical Drug Delivery System. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef]
- Hacker, M.C.; Nawaz, H.A. Multi-Functional Macromers for Hydrogel Design in Biomedical Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2015, 16, 27677–27706. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Parajuli, D.; Ghimire, K.N.; Biswas, B.K.; Kawakita, H.; Oshima, T.; Ohto, K. Biosorbents for Removing Hazardous Metals and Metalloids. Materials 2017, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Xiao, R.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Stokke, B.T. Polysaccharide Hydrogels. Gels 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Barbucci, R. Polysaccharide Based Hydrogels for Biomedical Applications. In Hydrogels, 1st ed.; Barbucci, R., Ed.; Springer: Milano, Italy, 2009; pp. 25–41. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carb. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT Polymerization: The Synthesis of Polymers with Defined End-Groups. Polymer 2005, 46, 8458. [Google Scholar] [CrossRef]
- Semsarilar, M.; Perrier, S. ‘Green’ reversible addition-fragmentation chain-transfer (RAFT) polymerization. Nat. Chem. 2010, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Maiti, B.; De, P. Carbohydrate-Conjugated Amino Acid-Based Fluorescent Block Copolymers: Their Self-Assembly, pH Responsiveness, and/or Lectin Recognition. Langmuir 2015, 31, 9422. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Min, K.; Matyjaszewski, K. Determination of Gel Point During Atom Transfer Radical Copolymerization with Cross-Linker. Macromolecules 2007, 40, 7763. [Google Scholar] [CrossRef]
- Barton, A.F.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; Routledge: New York, NY, USA, 1991. [Google Scholar] [CrossRef]
- Mancini, P.M.; Adam, C.G.; Fortunato, G.G.; Vottero, L.R. A comparison of nonspecific solvent scales. Degree of agreement of microscopic polarity values obtained by different measurement methods. ARKIVOC 2007, 16, 266–280. [Google Scholar] [CrossRef]
- Lu, J.; Brown, J.S.; Liotta, C.L.; Eckert, C.A. Polarity and hydrogen-bonding of ambient to near-critical water: Kamlet–Taft solvent parameters. Chem. Commun. 2001, 7, 665–666. [Google Scholar] [CrossRef]
- Kitak, T.; Dumičić, A.; Planinšek, O.; Šibanc, R.; Srčič, S. Determination of Solubility Parameters of Ibuprofen and Ibuprofen Lysinate. Molecules 2015, 20, 21549–21568. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K. Solvent effects on supramolecular gel-phase materials: Two-component dendritic gel. Langmuir 2004, 20, 10851–10857. [Google Scholar] [CrossRef]
- Lan, Y.; Corradini, M.G.; Weiss, R.G.; Raghavan, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef]
- Diehn, K.K.; Oh, H.; Hashemipour, R.; Weiss, R.G.; Raghavan, S.R. Insights into organogelation and its kinetics from Hansen solubility parameters. Toward a priori predictions of molecular gelation. Soft Matter 2014, 10, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, W.; Yang, B.; Li, H.; Zhou, L.; Li, S. Application of Solvent Parameters for Predicting Organogel Formation. AAPS Pharm. Sci. Tech. 2018, 19, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Hirst, A.R.; Smith, D.K. Exploring molecular recognition pathways in one- and two-component gels formed by dendritic lysine-based gelators. Soft Matter 2012, 8, 3399–3406. [Google Scholar] [CrossRef]
- Hardy, J.G.; Hirst, A.R.; Ashworth, I.; Brennan, C.; Smith, D.K. Exploring molecular recognition pathways within a family of gelators with different hydrogen bonding motifs. Tetrahedron 2007, 63, 7397–7406. [Google Scholar] [CrossRef]
- Han, I.S.; Han, M.H.; Kim, J.; Lew, S.; Lee, Y.J.; Horkay, F.; Magda, J.J. Constant-volume hydrogel osmometer: A new device concept for miniature biosensors. Biomacromolecules 2002, 3, 1271. [Google Scholar] [CrossRef] [PubMed]
- Aduba, D.C., Jr.; Margaretta, E.D.; Marnot, A.E.; Heifferon, K.V.; Surbey, W.R.; Chartrain, N.A.; Whittington, A.R.; Long, T.E.; Williams, C.B. Vat photopolymerization 3D printing of acid-cleavable PEG-methacrylate networks for biomaterial applications. Mater. Today Commun. 2019, 19, 204. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting Polymers, Hydrogels and Their Composites: Preparation, Properties and Bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.C.; Goh, S.S.; Liow, S.S.; Xue, K.; Loh, X.J. Molecular gel sorbent materials for environmental remediation and wastewater treatment. J. Mater. Chem. A 2019, 7, 18759–18791. [Google Scholar] [CrossRef]
- Reed, B.E.; Matsumoto, M.R.; Jensen, J.N.; Viadero, R.; Lin, W. Physicochemical processes. Water Environ. Res. 1998, 70, 449–473. [Google Scholar] [CrossRef]
- Hua, B.; Xiong, H.; Kadhom, M.; Wang, L.; Zhu, G.; Yang, J.; Cunningham, G.; Deng, B. Physico-Chemical Processes. Water Environ. Res. 2017, 89, 974. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.; Raoufi, M.; Panda, A.B.; Schönherr, H.; Pal, S. Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Int. 2014, 6, 4766–4777. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Zhang, Q.; Li, Y.; Wang, H. P25-graphene hydrogels: Room-temperature synthesis and application for removal of methylene blue from aqueous solution. J. Hazard. Mater. 2012, 205, 229. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, T.; Liu, Y.; Zhou, B.; Yang, R.; Zhu, L. Sorption behavior of methylene blue and rhodamine B mixed dyes onto chitosan graft poly (acrylic acid-co-2-acrylamide-2-methyl propane sulfonic acid) hydrogel. Adv. Polym. Technol. 2018, 37, 2568. [Google Scholar] [CrossRef]
- Bethi, B.; Manasa, V.; Srinija, K.; Sonawane, S.H. Intensification of Rhodamine-B dye removal using hydrodynamic cavitation coupled with hydrogel adsorption. Chem. Eng. Proc.-Proc. Intens. 2018, 134, 51. [Google Scholar] [CrossRef]
- Mandla, S.; Davenport Huyer, L.; Radisic, M. Review: Multimodal bioactive material approaches for wound healing. APL Bioeng. 2018, 2, 021503. [Google Scholar] [CrossRef]
- Varghese, S.; Jamora, C. Hydrogels: A versatile tool with a myriad of biomedical and research applications for the skin. Expert Rev. Dermatol. 2012, 7, 315–317. [Google Scholar] [CrossRef]
- Du, L.; Tong, L.; Jin, Y.; Jia, J.; Liu, Y.; Su, C.; Yu, S.; Li, X. A multifunctional in situ-forming hydrogel for wound healing. Wound Repair Regen. 2012, 20, 904–910. [Google Scholar] [CrossRef]
- Guthrie, H.C.; Martin, K.R.; Taylor, C.; Spear, A.M.; Whiting, R.; Macildowie, S.; Clasper, J.C.; Watts, S.A. A pre-clinical evaluation of silver, iodine and Manuka honey based dressings in a model of traumatic extremity wounds contaminated with Staphylococcus aureus. Injury 2014, 45, 1171. [Google Scholar] [CrossRef]
- Spear, A.M.; Lawton, G.; Staruch, R.M.T.; Rickard, R.F. Regenerative medicine and war: A front-line focus for UK defence. NPJ Regen. Med. 2018, 3, 13. [Google Scholar] [CrossRef]
- Low, W.L.; Martin, C.; Hill, D.J.; Kenward, M.A. Antimicrobial efficacy of silver ions in combination with tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Int. J. Antimicrob. Agents 2011, 37, 162. [Google Scholar] [CrossRef]
- Low, W.L.; Martin, C.; Hill, D.J.; Kenward, M.A. Antimicrobial efficacy of liposome-encapsulated silver ions and tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Lett. Appl. Microbiol. 2013, 57, 33. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Lavigne, R. Breaking barriers: Expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol. 2015, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.; Chen, Y.Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.L.; Papp-Wallace, K.M.; Hujer, A.M.; Domitrovic, T.N.; Hujer, K.M.; Hurless, K.N.; Tuohy, M.; Hall, G.; Bonomo, R.A. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: Resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 1020. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Mei, L.; Zhang, X.; Wang, Y.; Zhao, Y.; Li, C. Water-soluble BODIPY-conjugated glycopolymers as fluorescent probes for live cell imaging. Polym. Chem. 2013, 4, 5743. [Google Scholar] [CrossRef]
- Roy, S.G.; Haldar, U.; De, P. Remarkable swelling capability of amino acid based cross-linked polymer networks in organic and aqueous medium. ACS Appl. Mater. Interfaces 2014, 6, 4233. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC standardized disc susceptibility testing method (version 8). J. Antimicrob. Chemother. 2009, 64, 454. [Google Scholar] [CrossRef]
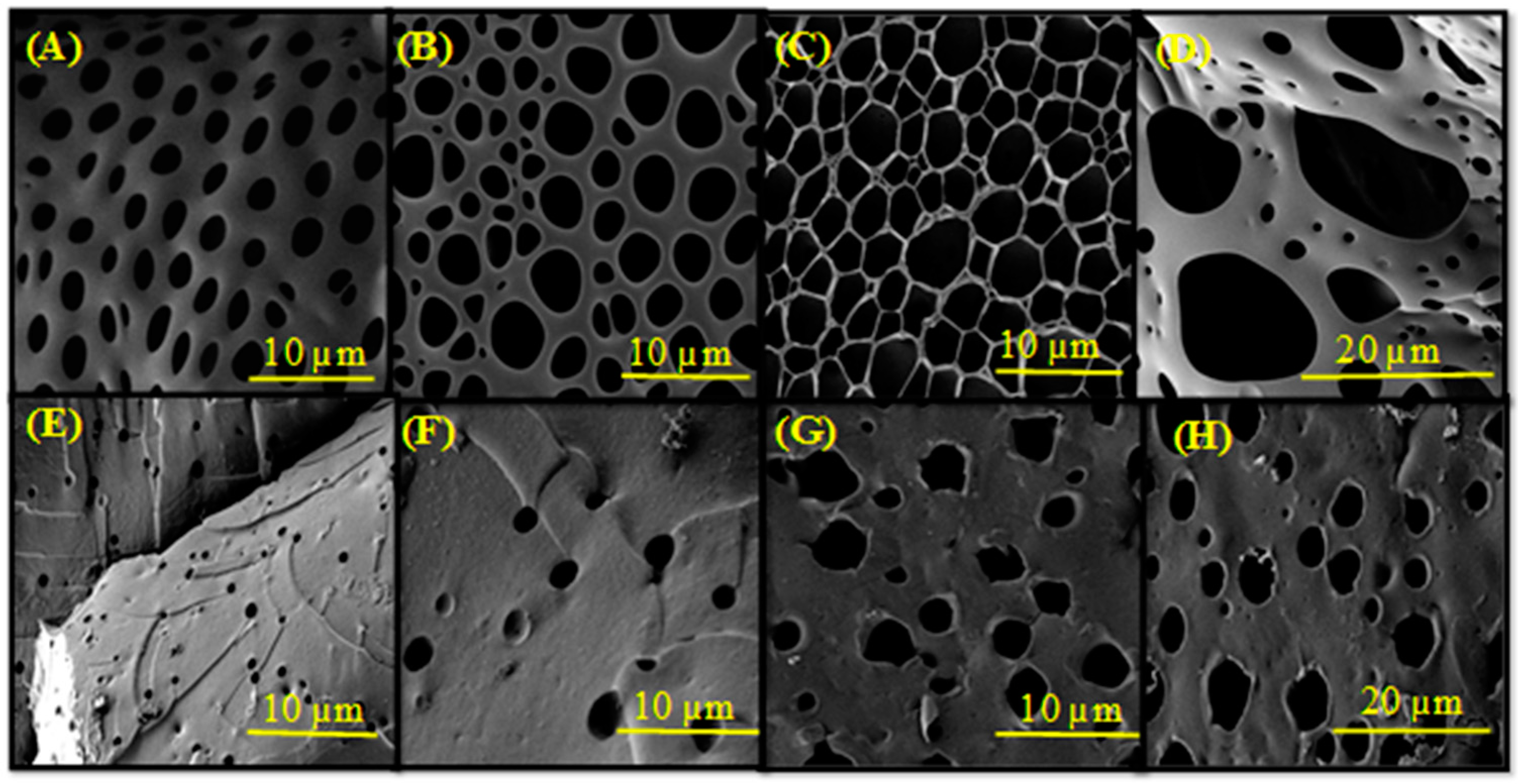

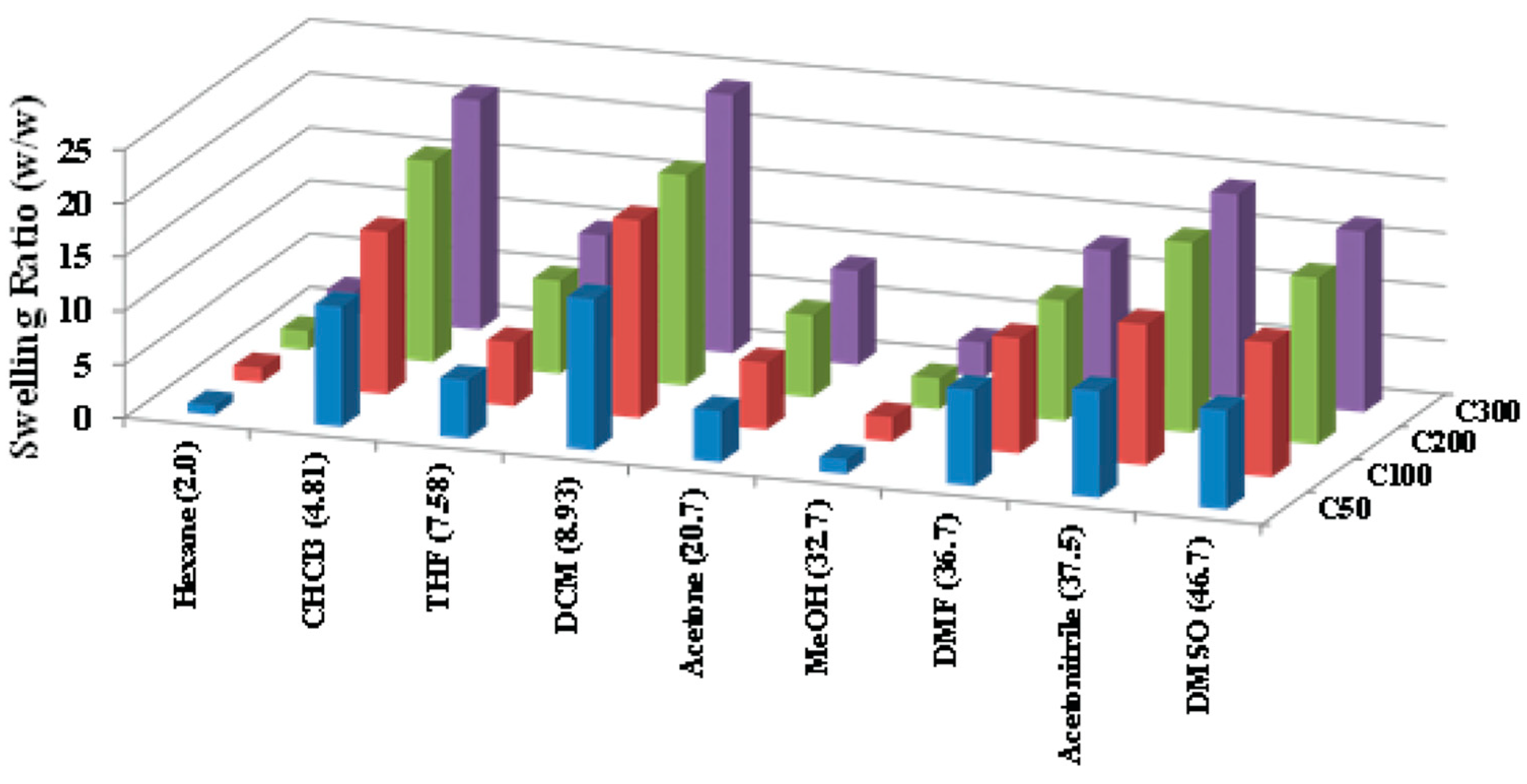
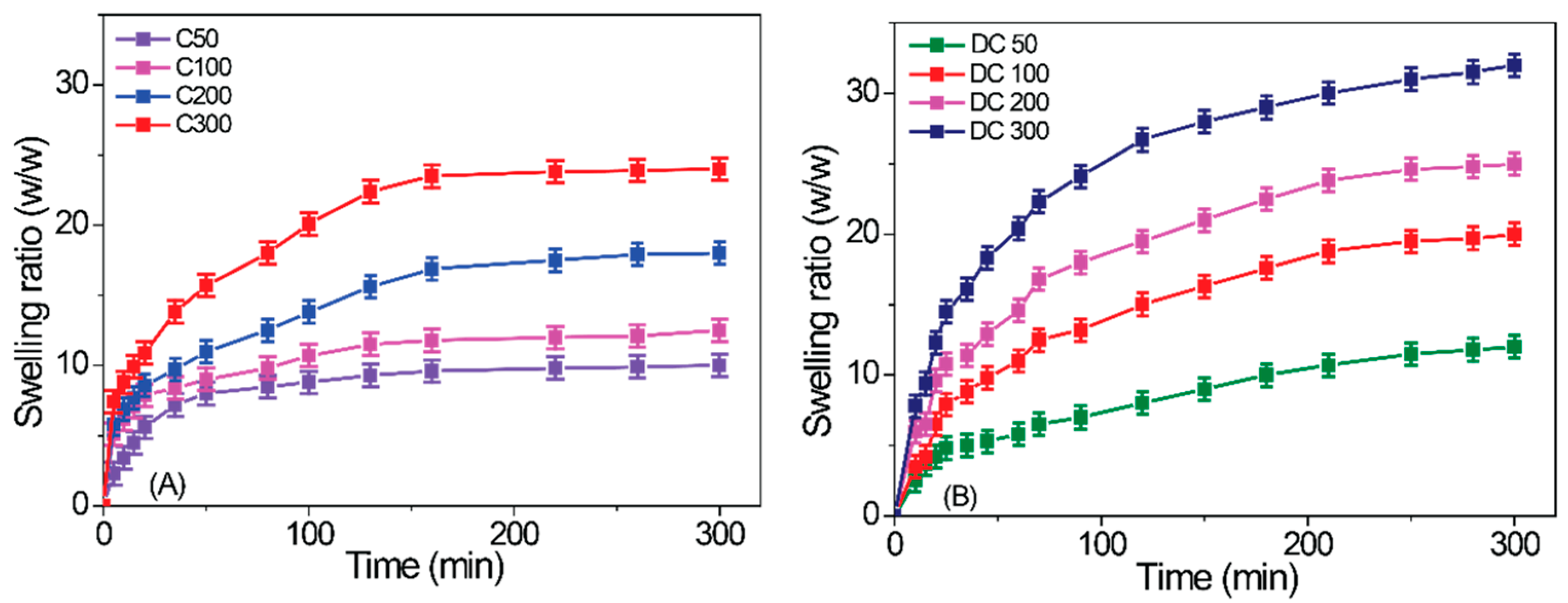

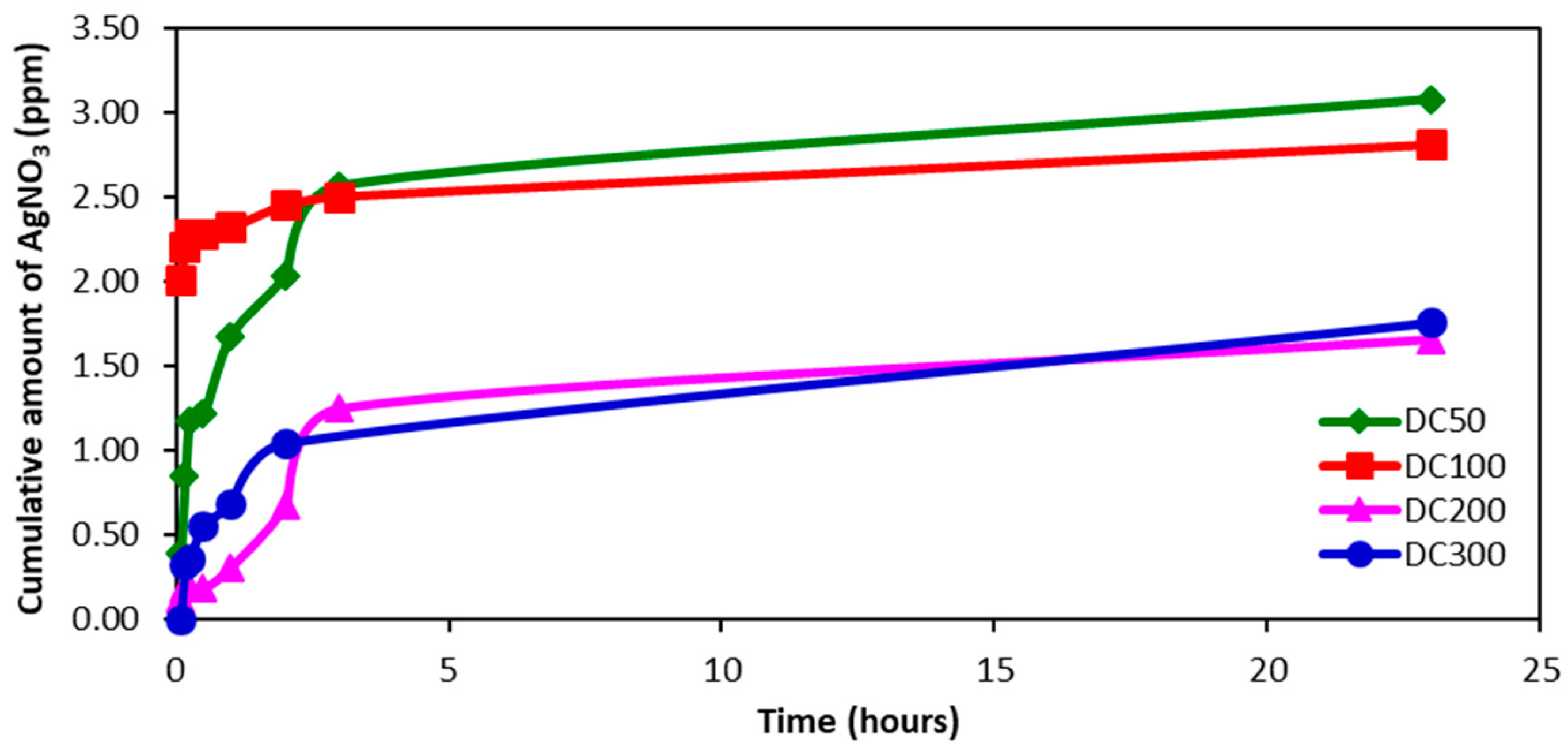
| Gel | Ac-glu-HEMA/DEGDMA/CDP/AIBN | Gelation Time (min) | Monomer Conversion after 24 h (%) |
|---|---|---|---|
| C50 | 50/2/0.5/0.15 | 90 | 77 |
| C100 | 100/2/0.5/0.15 | 105 | 75 |
| C200 | 200/2/0.5/0.15 | 135 | 80 |
| C300 | 300/2/0.5/0.15 | 148 | 79 |
| Gel | Isopropyl Palmitate | Isopropyl Myristate | Olive Oil | SSC | SSH | TTO:PVA |
|---|---|---|---|---|---|---|
| C50 | 0.12 | 0.08 | 0.15 | 0.21 | 0.25 | 1.59 |
| C100 | 0.30 | 0.18 | 0.76 | 0.42 | 0.56 | 1.09 |
| C200 | 0.39 | 1.10 | 1.67 | 1.40 | 0.87 | 1.04 |
| Hydrogel | SDW | AgNO3 Solution | Ag+ Release (ppm) |
|---|---|---|---|
| DC50 | 1.60 | 3.10 | 3.08 |
| DC100 | 2.17 | 1.44 | 2.81 |
| DC200 | 2.35 | 2.69 | 1.66 |
| DC300 | 5.28 | 3.62 | 1.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, A.; Roy, S.G.; Haldar, U.; Mahapatra, R.D.; Harper, G.R.; Low, W.L.; De, P.; Hardy, J.G. Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels. Gels 2019, 5, 43. https://doi.org/10.3390/gels5040043
Pan A, Roy SG, Haldar U, Mahapatra RD, Harper GR, Low WL, De P, Hardy JG. Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels. Gels. 2019; 5(4):43. https://doi.org/10.3390/gels5040043
Chicago/Turabian StylePan, Abhishek, Saswati G. Roy, Ujjal Haldar, Rita D. Mahapatra, Garry R. Harper, Wan Li Low, Priyadarsi De, and John G. Hardy. 2019. "Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels" Gels 5, no. 4: 43. https://doi.org/10.3390/gels5040043
APA StylePan, A., Roy, S. G., Haldar, U., Mahapatra, R. D., Harper, G. R., Low, W. L., De, P., & Hardy, J. G. (2019). Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels. Gels, 5(4), 43. https://doi.org/10.3390/gels5040043