Low Molecular Weight Gelators Based on Functionalized l-Dopa Promote Organogels Formation
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
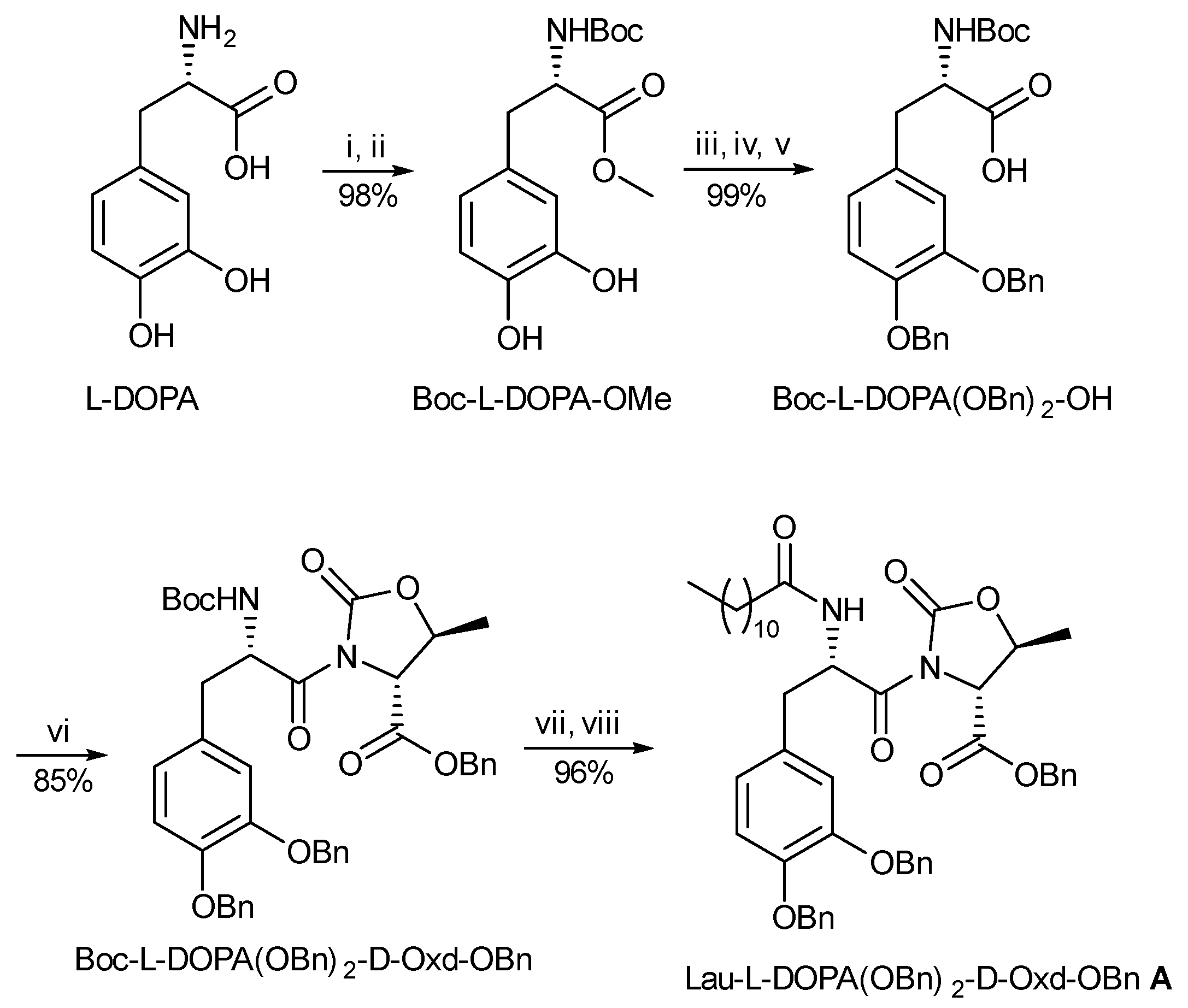
4.1. Synthesis of Lau-l-Dopa(OBn)2-d-Oxd-OBn
4.2. Conditions for the Gel Formation
4.3. Conditions for Tgel Determination
4.4. Rheology
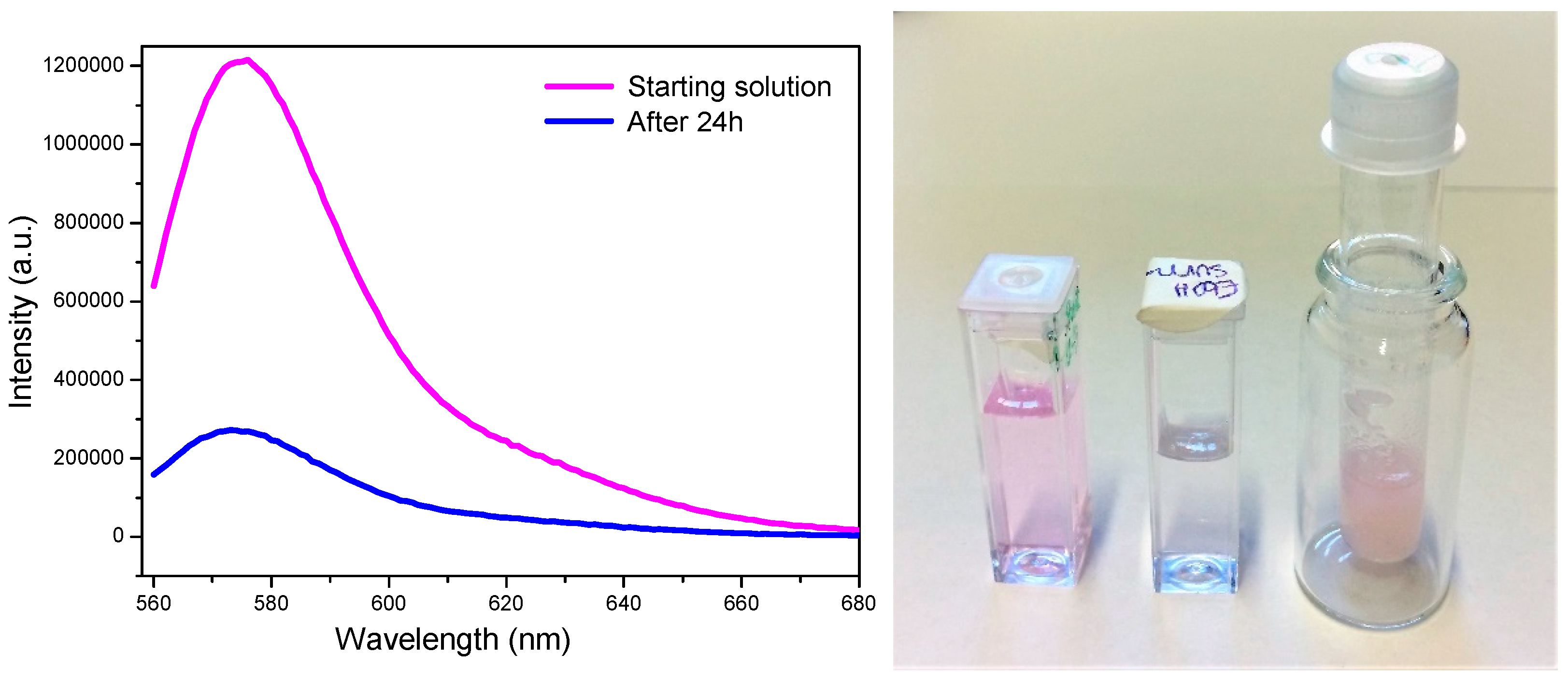
4.5. Fluorescence Spectroscopy
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–936. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Haldar, D.; Podder, D.; Roy Chowdhury, S.; Nandi, S. Tripeptide based super-organogelators: Structure and function. New J. Chem. 2019, 3743–3749. [Google Scholar]
- Pramanik, A.; Paikar, A.; Haldar, D. Sonication-induced instant fibrillation and fluorescent labeling of tripeptide fibers. RSC Adv. 2015, 5, 53886–53892. [Google Scholar] [CrossRef]
- Xiao, S.; Zou, Y.; Yu, M.; Yi, T.; Zhou, Y.; Li, F.; Huang, C. A photochromic fluorescent switch in an organogel system with non-destructive readout ability. Chem. Commun. 2007, 0, 4758–4760. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, M.; Yi, T. Low-molecular-mass gels responding to ultrasound and mechanical stress: Towards self-healing materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.J.; Dutta, D.; Stabenfeldt, S.E.; Vernon, B.L. Injectable hydrogels. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 881–903. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Maiti, B.; Bhattacharya, S. First report of charge-transfer induced heat-set hydrogel. Structural insights and remarkable properties. Nanoscale 2016, 8, 11224–11233. [Google Scholar] [CrossRef]
- Zhou, S.L.; Matsumoto, S.; Tian, H.D.; Yamane, H.; Ojida, A.; Kiyonaka, S.; Hamachi, I. pH-responsive shrinkage/swelling of a supramolecular hydrogel composed of two small amphiphilic molecules. Chem. A Eur. J. 2005, 11, 1130–1136. [Google Scholar] [CrossRef]
- Adams, D.J.; Mullen, L.M.; Berta, M.; Chen, L.; Frith, W.J. Relationship between molecular structure, gelation behaviour and gel properties of Fmoc-dipeptides. Soft Matter 2010, 6, 1971–1980. [Google Scholar] [CrossRef]
- Sutton, S.; Campbell, N.L.; Cooper, A.I.; Kirkland, M.; Frith, W.J.; Adams, D.J. Controlled release from modified amino acid hydrogels governed by molecular size or network dynamics. Langmuir 2009, 25, 10285–10291. [Google Scholar] [CrossRef] [PubMed]
- Zanna, N.; Merlettini, A.; Tatulli, G.; Milli, L.; Focarete, M.L.; Tomasini, C. Hydrogelation induced by Fmoc-protected peptidomimetics. Langmuir 2015, 31, 12240–12250. [Google Scholar] [CrossRef]
- Zanna, N.; Focaroli, S.; Merlettini, A.; Gentilucci, L.; Teti, G.; Falconi, M.; Tomasini, C. Thixotropic Peptide-Based Physical Hydrogels Applied to Three-Dimensional Cell Culture. ACS Omega 2017, 2, 2374–2381. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Loh, X.J. Natural rheological modifiers for personal care. Polym. Adv. Technol. 2016, 27, 1664–1679. [Google Scholar] [CrossRef]
- Chakraborty, P.; Gazit, E. Amino Acid Based Self-assembled Nanostructures: Complex Structures from Remarkably Simple Building Blocks. Chem. Nano. Mat. 2018, 4, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Zanna, N.; Merlettini, A.; Tomasini, C. Self-healing hydrogels triggered by amino acids. Org. Chem. Front. 2016, 3, 1699–1704. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef]
- Uzan, S.; Barış, D.; Çolak, M.; Aydın, H.; Hoşgören, H. Organogels as novel carriers for dermal and topical drug delivery vehicles. Tetrahedron 2016, 72, 7517–7525. [Google Scholar] [CrossRef]
- Van Esch, J.H. We can design molecular gelators, but do we understand them? Langmuir 2009, 25, 8392–8394. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C.; Sommerdijk, N.A.J.M.; Ajayaghosh, A.; Meskers, S.C.J.; Schenning, A.P.H.J.; Silva, C.; Friend, R.H.; Aida, T. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef]
- Bera, S.; Haldar, D. A rechargeable self-healing safety fuel gel. J. Mater. Chem. A 2016, 4, 6933–6939. [Google Scholar] [CrossRef]
- Vibhute, A.M.; Muvvala, V.; Sureshan, K.M. A Sugar-Based Gelator for Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. 2016, 55, 7782–7785. [Google Scholar] [CrossRef]
- Li, J.; Huo, Y.; Zeng, H. Combinatorial identification of a highly soluble phase-selective organogelator with high gelling capacity for crude oil gelation. J. Mater. Chem. A 2018, 6, 10196–10200. [Google Scholar] [CrossRef]
- Ren, C.; Chen, F.; Zhou, F.; Shen, J.; Su, H.; Zeng, H. Low-Cost Phase-Selective Organogelators for Rapid Gelation of Crude Oils at Room Temperature. Langmuir 2016, 32, 13510–13516. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Mohanty, S.; Wu, Y.; Chakraborty, N.; Mohanty, P.; Ghosh, G. Impact of alginate concentration on the viability, cryostorage, and angiogenic activity of encapsulated fibroblasts. Mater. Sci. Eng. C 2016, 65, 269–277. [Google Scholar] [CrossRef]
- Dastidar, P. Designing Supramolecular Gelators: Challenges, Frustrations, and Hopes. Gels 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Corradini, M.G.; Weiss, R.G.; Raghavan, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Tomasini, C. Synthesis of oligomers of trans-(4S,5R)-4-carboxybenzyl 5-methyl oxazolidin-2-one: An approach to new foldamers. J. Org. Chem. 2001, 66, 727–732. [Google Scholar] [CrossRef]
- Tomasini, C.; Trigari, V.; Lucarini, S.; Bernardi, F.; Garavelli, M.; Peggion, C.; Formaggio, F.; Toniolo, C. Pseudopeptide foldamers—The homo-oligomers of benzyl (4S,5R)-5-methyl-2-oxo-1,3-oxazolidine-4-carboxylate. Eur. J. Org. Chem. 2003, 4, 259–267. [Google Scholar] [CrossRef]
- Angelici, G.; Castellucci, N.; Falini, G.; Huster, D.; Monari, M.; Tomasini, C. Pseudopeptides designed to form supramolecular helixes: The role of the stereogenic centers. Cryst. Growth Des. 2010, 10, 923–929. [Google Scholar] [CrossRef]
- Angelici, G.; Falini, G.; Hofmann, H.J.; Huster, D.; Monari, M.; Tomasini, C. Nanofibers from oxazolidi-2-one containing hybrid foldamers: What is the right molecular size? Chem.—A Eur. J. 2009, 15, 8037–8048. [Google Scholar] [CrossRef]
- Angelici, G.; Castellucci, N.; Contaldi, S.; Falini, G.; Hofmann, H.J.; Monari, M.; Tomasini, C. A network of small molecules connected by cross-linked NH bonds. Cryst. Growth Des. 2010, 10, 244–251. [Google Scholar] [CrossRef]
- Castellucci, N.; Sartor, G.; Calonghi, N.; Parolin, C.; Falini, G.; Tomasini, C. A peptidic hydrogel that may behave as a “trojan Horse”. Beilstein J. Org. Chem. 2013, 9, 417–424. [Google Scholar] [CrossRef]
- Castellucci, N.; Angelici, G.; Falini, G.; Monari, M.; Tomasini, C. L-Phe-D-Oxd: A privileged scaffold for the formation of supramolecular materials. Eur. J. Org. Chem. 2011, 3082–3088. [Google Scholar] [CrossRef]
- Castellucci, N.; Falini, G.; Angelici, G.; Tomasini, C. Formation of gels in the presence of metal ions. Amino Acids 2011, 41, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.Z.; Mears, L.L.E.; Cattoz, B.N.; Griffiths, P.C.; Schweins, R.; Adams, D.J. Linking micellar structures to hydrogelation for salt-triggered dipeptide gelators. Soft Matter 2016, 12, 3612–3621. [Google Scholar] [CrossRef]
- Streck, L.; Caroni, A.L.P.F.; Fonseca, J.L.C. Temperature and composition effects on the morphology of o/w dispersions based on poly(oxyethylene 20) sorbitan monolaurate and sorbitan monolaurate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 720–728. [Google Scholar] [CrossRef]
- Galindo-Alvarez, J.; Sadtler, V.; Marchal, P.; Perrin, P.; Tribet, C.; Marie, E.; Durand, A. Nanoemulsions with enhanced temperature stability using thermo-sensitive association of nonionic surfactant and amphiphilic polyelectrolytes. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 115–121. [Google Scholar] [CrossRef]
- Shah, D.O.; Ranch, K.M.; Desai, A.R.; Choksi, H.H.; Vyas, B.A.; Patil, R.J.; Maulvi, F.A. Effect of surfactant chain length on drug release kinetics from microemulsion-laden contact lenses. Int. J. Pharm. 2017, 524, 193–204. [Google Scholar]
- Zanna, N.; Iaculli, D.; Tomasini, C. The effect of L-DOPA hydroxyl groups on the formation of supramolecular hydrogels. Org. Biomol. Chem. 2017, 15, 5797–5804. [Google Scholar] [CrossRef]
- Latxague, L.; Gaubert, A.; Maleville, D.; Baillet, J.; Ramin, M.A.; Barthélémy, P. Carbamate based bolaamphiphile as Low Molecular Weight Hydrogelators. Gels 2016, 2, 25. [Google Scholar] [CrossRef]
- Smallwood, I.M. Handbook of organic solvents properties. Oppor. Emerg. Mark. 2015, 1–326. [Google Scholar]
- Gong, Z.; Yang, Y.; Ren, Q.; Chen, X.; Shao, Z. Injectable thixotropic hydrogel comprising regenerated silk fibroin and hydroxypropylcellulose. Soft Matter 2012, 8, 2875. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, S.; Wang, S.; Chen, X.; Shao, Z. Thixotropic silk nanofibril-based hydrogel with extracellular matrix-like structure. Biomater. Sci. 2014, 2, 1338–1342. [Google Scholar] [CrossRef]
- Pek, Y.S.; Wan, A.C.A.; Ying, J.Y. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 2010, 31, 385–391. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Wen, Y.; Liu, K.; Chen, L.; Mao, Y.; Yang, S.; Yi, T. (-)-Menthol based thixotropic hydrogel and its application as a universal antibacterial carrier. Soft Matter 2014, 10, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Nanda, J.; Banerjee, A. Multi-stimuli responsive self-healing metallo-hydrogels: Tuning of the gel recovery property. Chem. Commun. 2014, 50, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Karan, C.K.; Bhattacharjee, M. Self-Healing and Moldable Metallogels as the Recyclable Materials for Selective Dye Adsorption and Separation. ACS Appl. Mater. Interfaces 2016, 8, 5526–5535. [Google Scholar] [CrossRef]
- Roy, S.; Baral, A.; Banerjee, A. An amino-acid-based self-healing hydrogel: Modulation of the self-healing properties by incorporating carbon-based nanomaterials. Chem. Eur. J. 2013, 19, 14950–14957. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Lei, J.; Kim, J.H. Dye adsorption characteristics of alginate/polyaspartate hydrogels. J. Ind. Eng. Chem. 2008, 14, 726–731. [Google Scholar] [CrossRef]
- Naskar, J.; Palui, G.; Banerjee, A. Tetrapeptide-based hydrogels: For encapsulation and slow release of an anticancer drug at physiological ph. J. Phys. Chem. B 2009, 113, 11787–11792. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, X.; Hong, C.; Zhu, F.; Yao, Y.; Xue, Z. Oxidation Degradation of Rhodamine B in Aqueous by UV/S2O8 2− Treatment System. Int. J. Photoenergy 2012, 2012, 1–5. [Google Scholar]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9781420015195. [Google Scholar]
- Genovese, D.; Bonacchi, S.; Juris, R.; Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Prevention of Self-Quenching in Fluorescent Silica Nanoparticles by Efficient Energy Transfer. Angew. Chem. Int. Ed. 2013, 52, 5965–5968. [Google Scholar] [CrossRef]



| Solvent | Solvent Polarity 1 | Solvent b.p. (°C) | Organogel m.p. (°C) | G′ (KPa) | G″ (KPa) |
|---|---|---|---|---|---|
| Toluene | 9.9 | 110.6 | 40–41 | 10 | 1.5 |
| Ethyl acetate | 23 | 77 | 40–43 | 19 | 2.9 |
| Acetonitrile | 46 | 81.6 | 42–45 | 56 | 12.2 |
| Ethanol | 65.4 | 78.5 | 60–65 | 82 | 19 |
| Methanol | 76.2 | 64.6 | 25–28 | 2.3 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuri, D.; Zanna, N.; Tomasini, C. Low Molecular Weight Gelators Based on Functionalized l-Dopa Promote Organogels Formation. Gels 2019, 5, 27. https://doi.org/10.3390/gels5020027
Giuri D, Zanna N, Tomasini C. Low Molecular Weight Gelators Based on Functionalized l-Dopa Promote Organogels Formation. Gels. 2019; 5(2):27. https://doi.org/10.3390/gels5020027
Chicago/Turabian StyleGiuri, Demetra, Nicola Zanna, and Claudia Tomasini. 2019. "Low Molecular Weight Gelators Based on Functionalized l-Dopa Promote Organogels Formation" Gels 5, no. 2: 27. https://doi.org/10.3390/gels5020027
APA StyleGiuri, D., Zanna, N., & Tomasini, C. (2019). Low Molecular Weight Gelators Based on Functionalized l-Dopa Promote Organogels Formation. Gels, 5(2), 27. https://doi.org/10.3390/gels5020027








