On the Race for More Stretchable and Tough Hydrogels
Abstract
1. Introduction
2. Stretchable and Tough Hydrogels from Synthetic Polymers
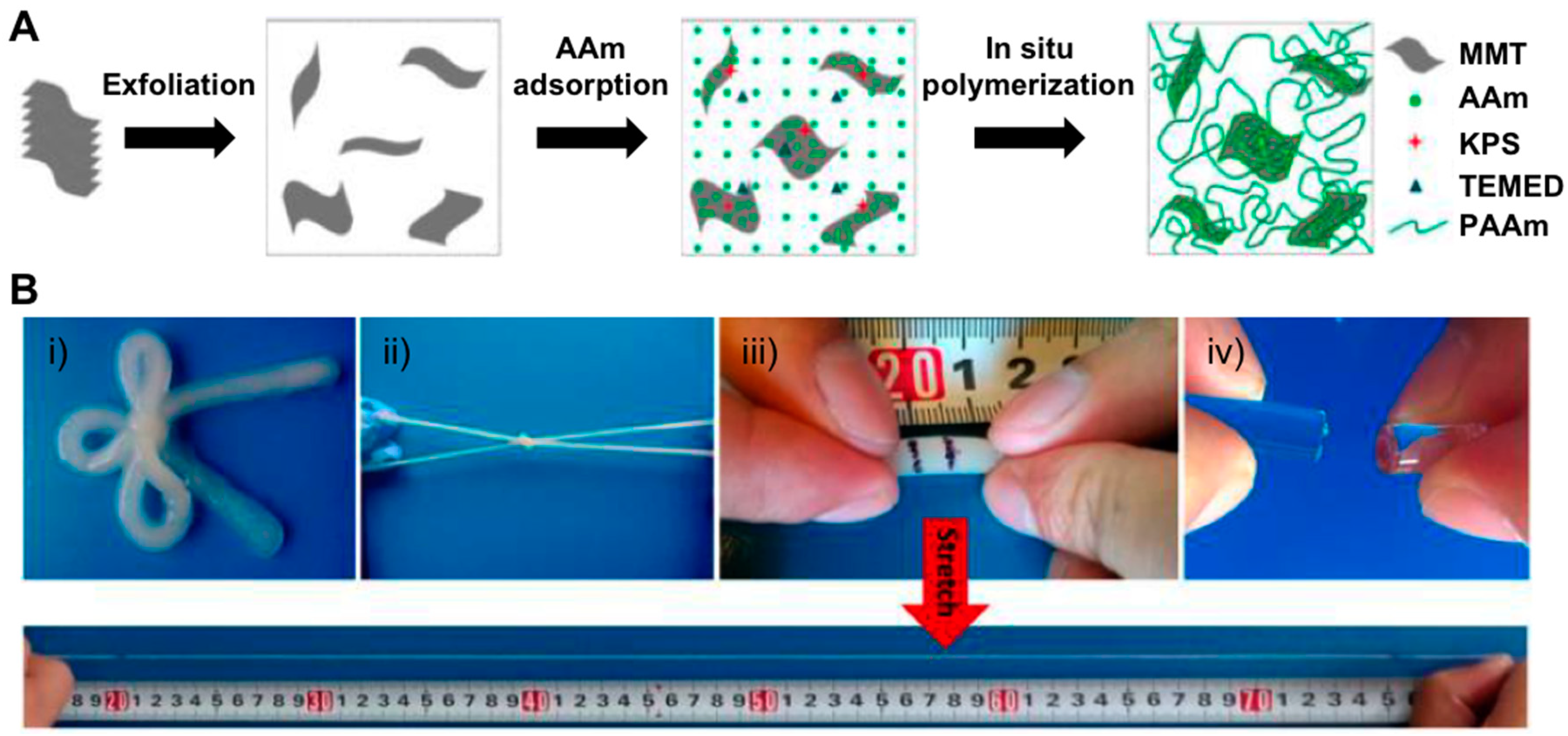
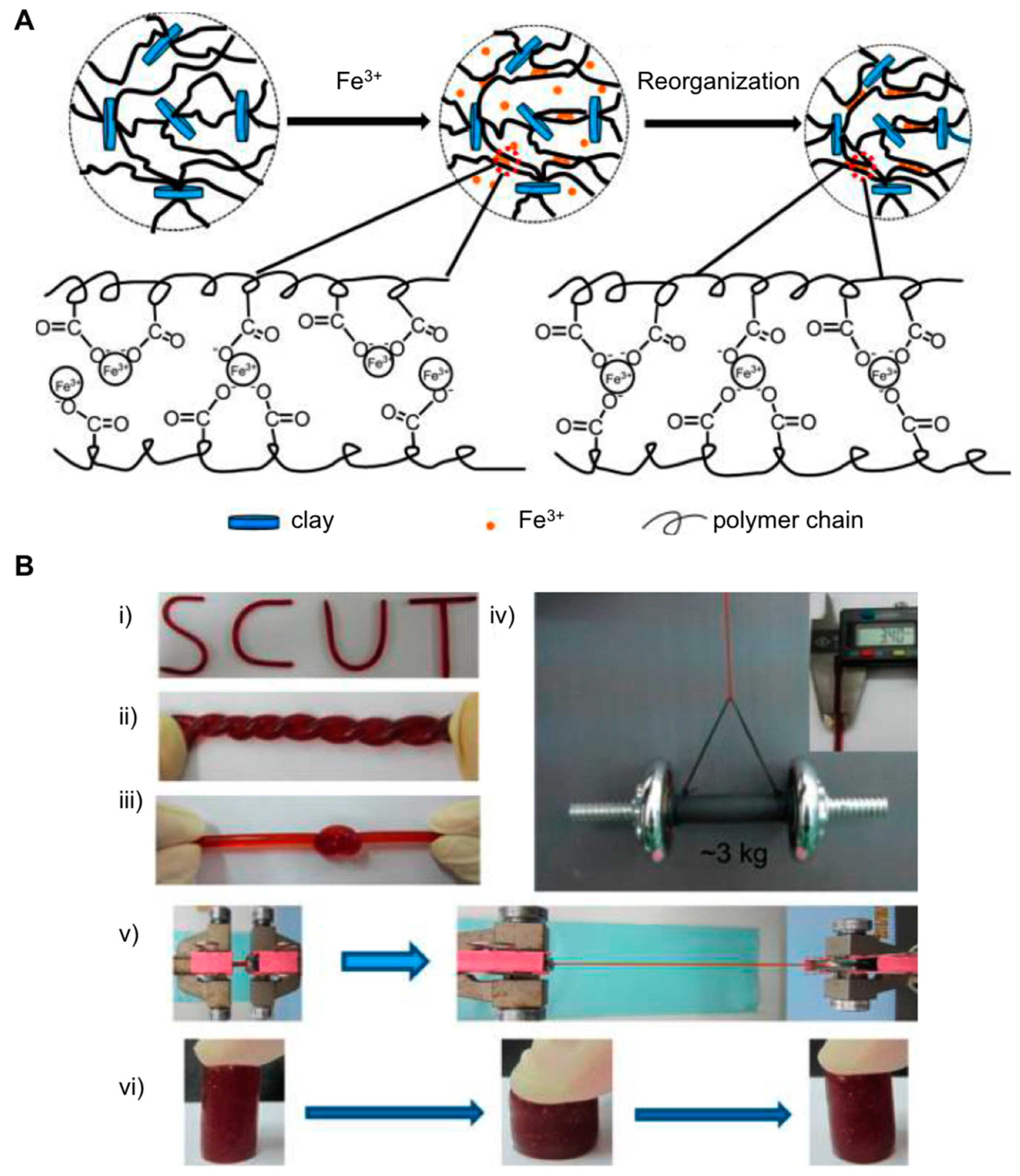
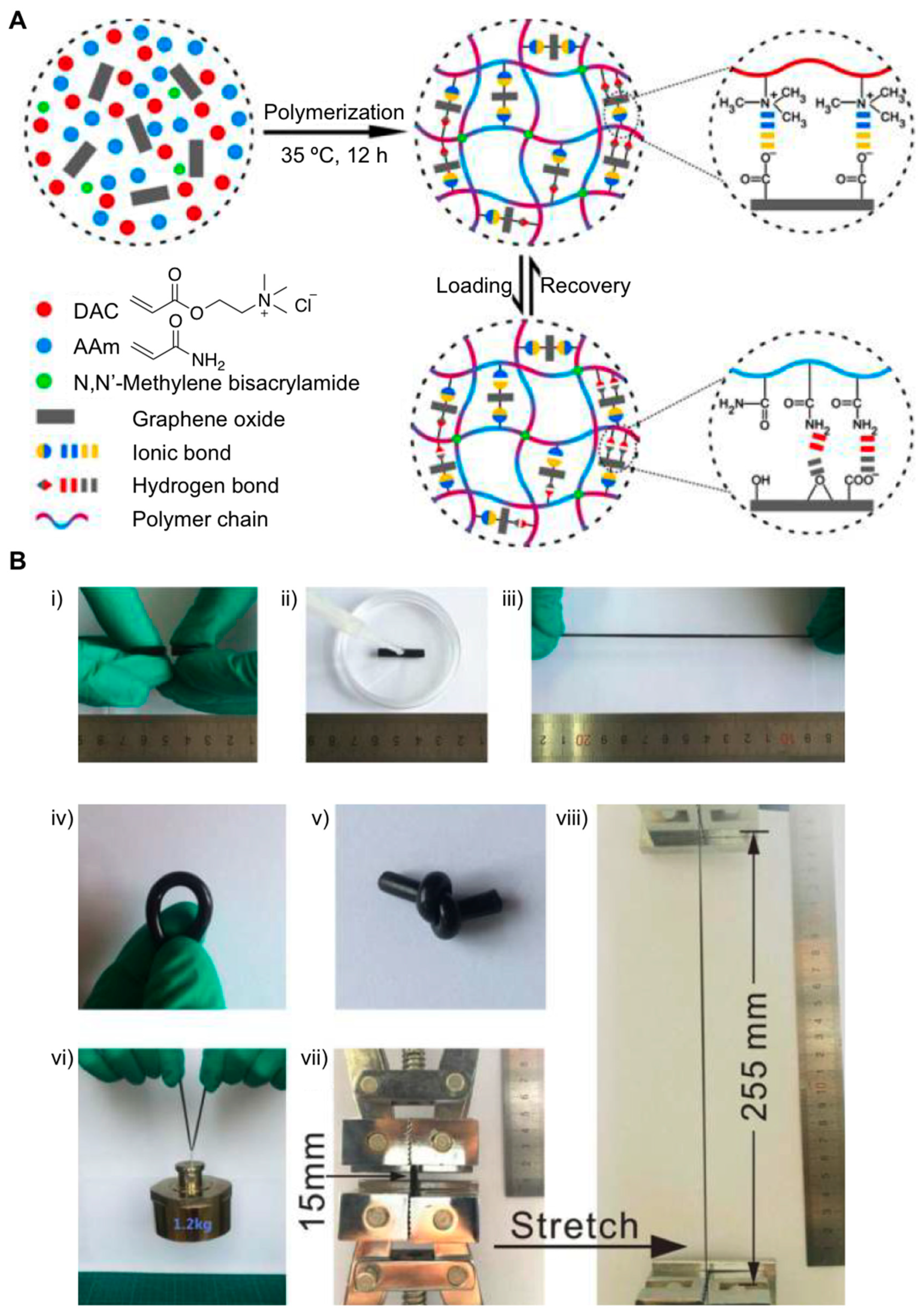
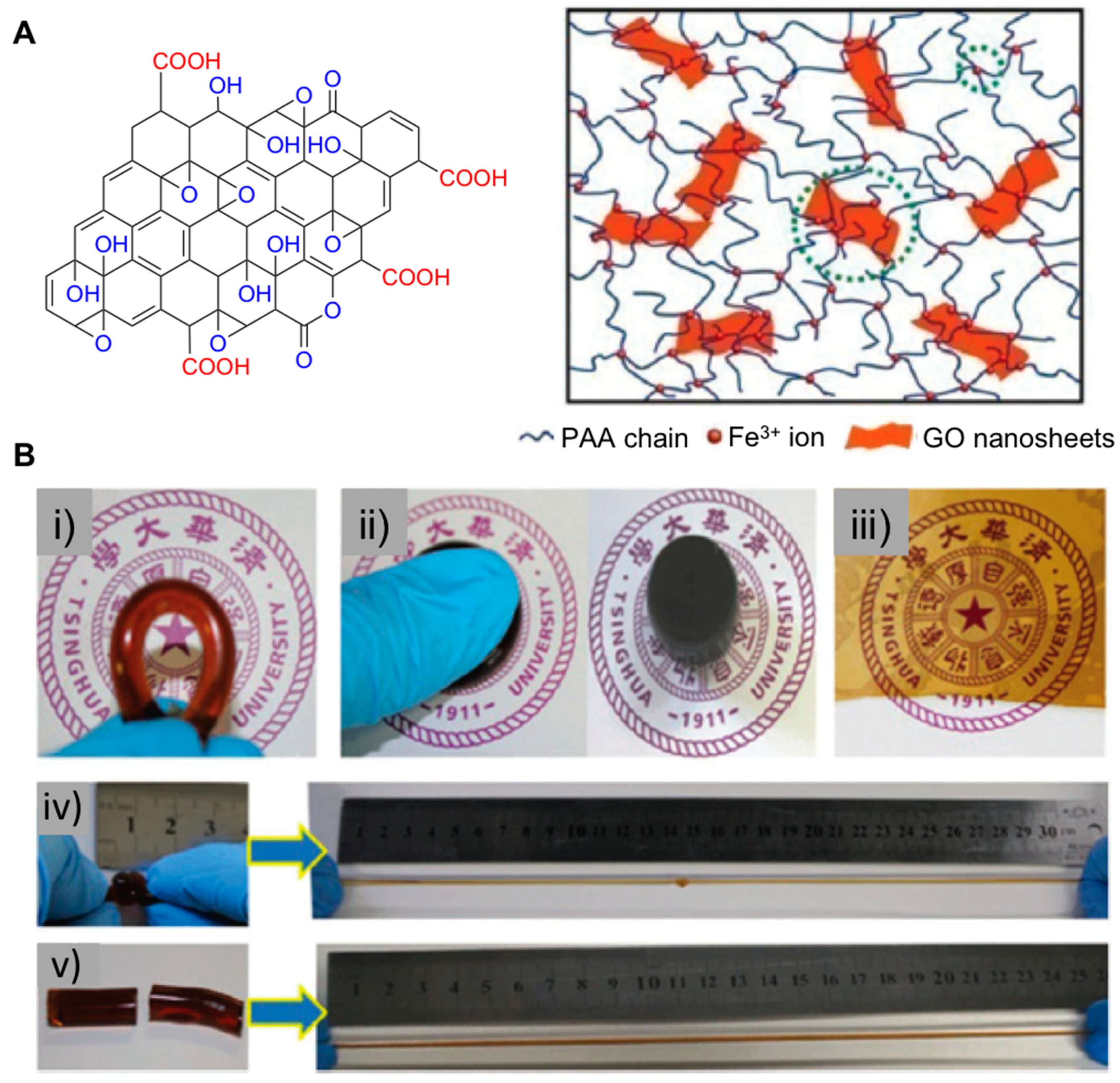
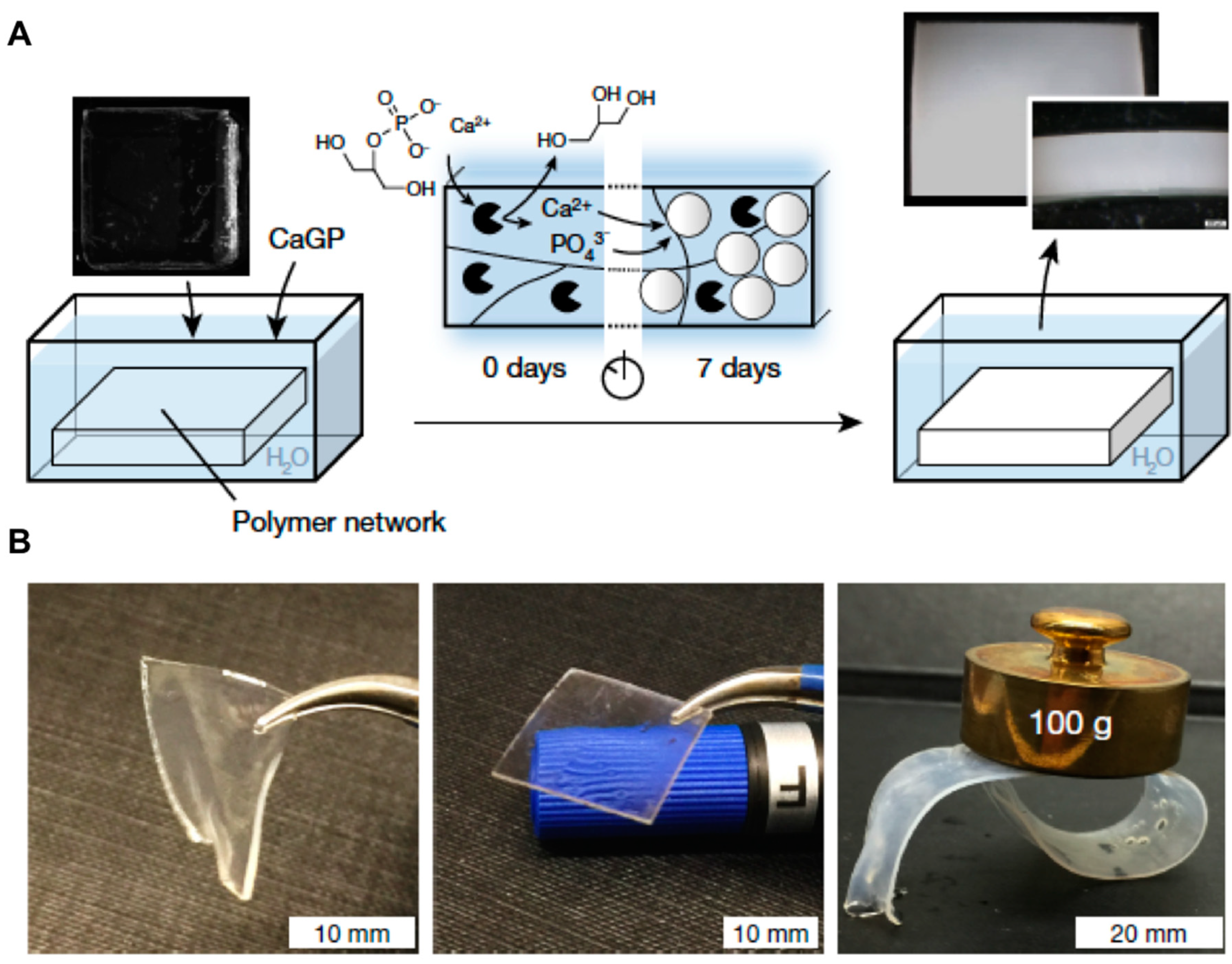
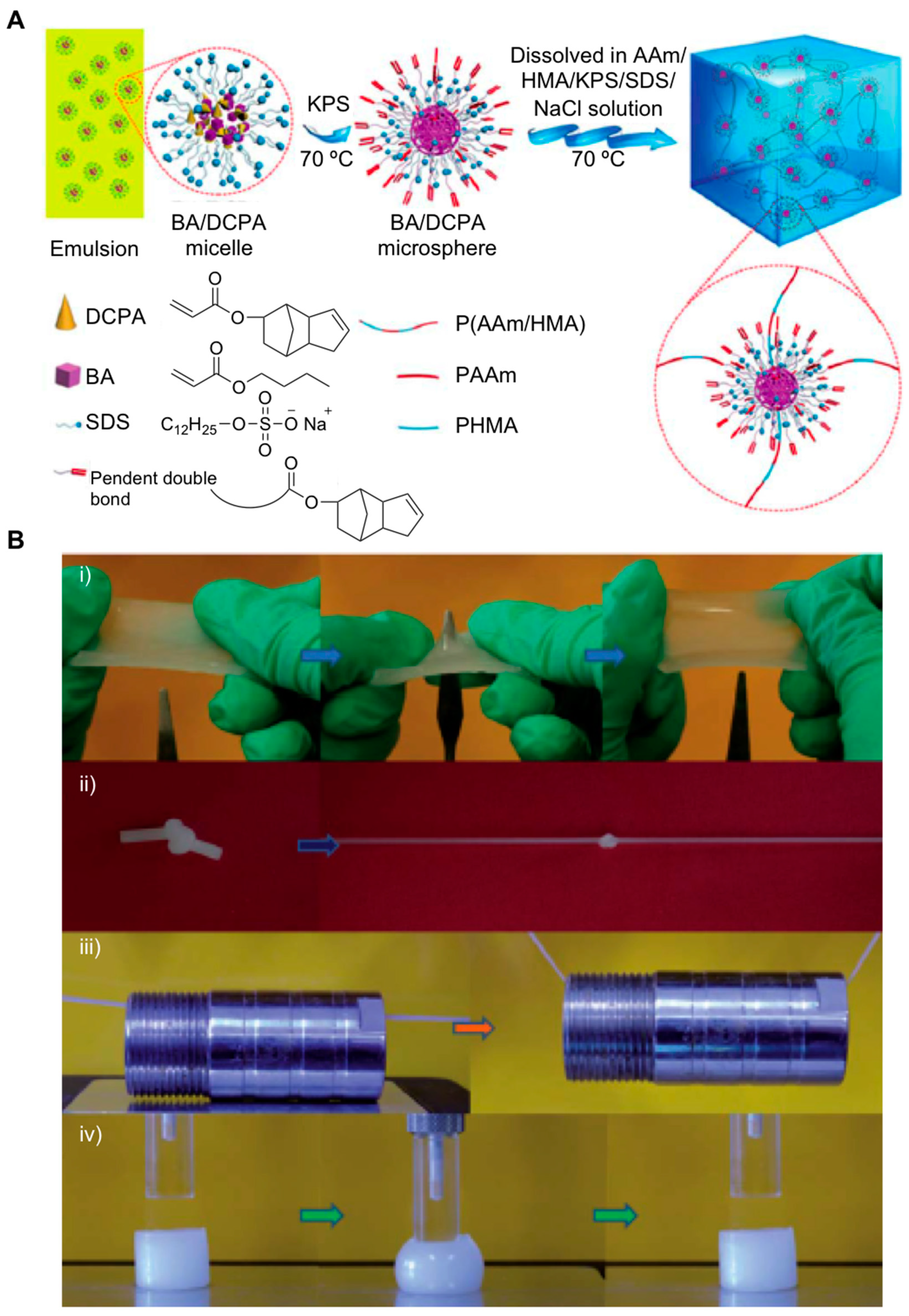
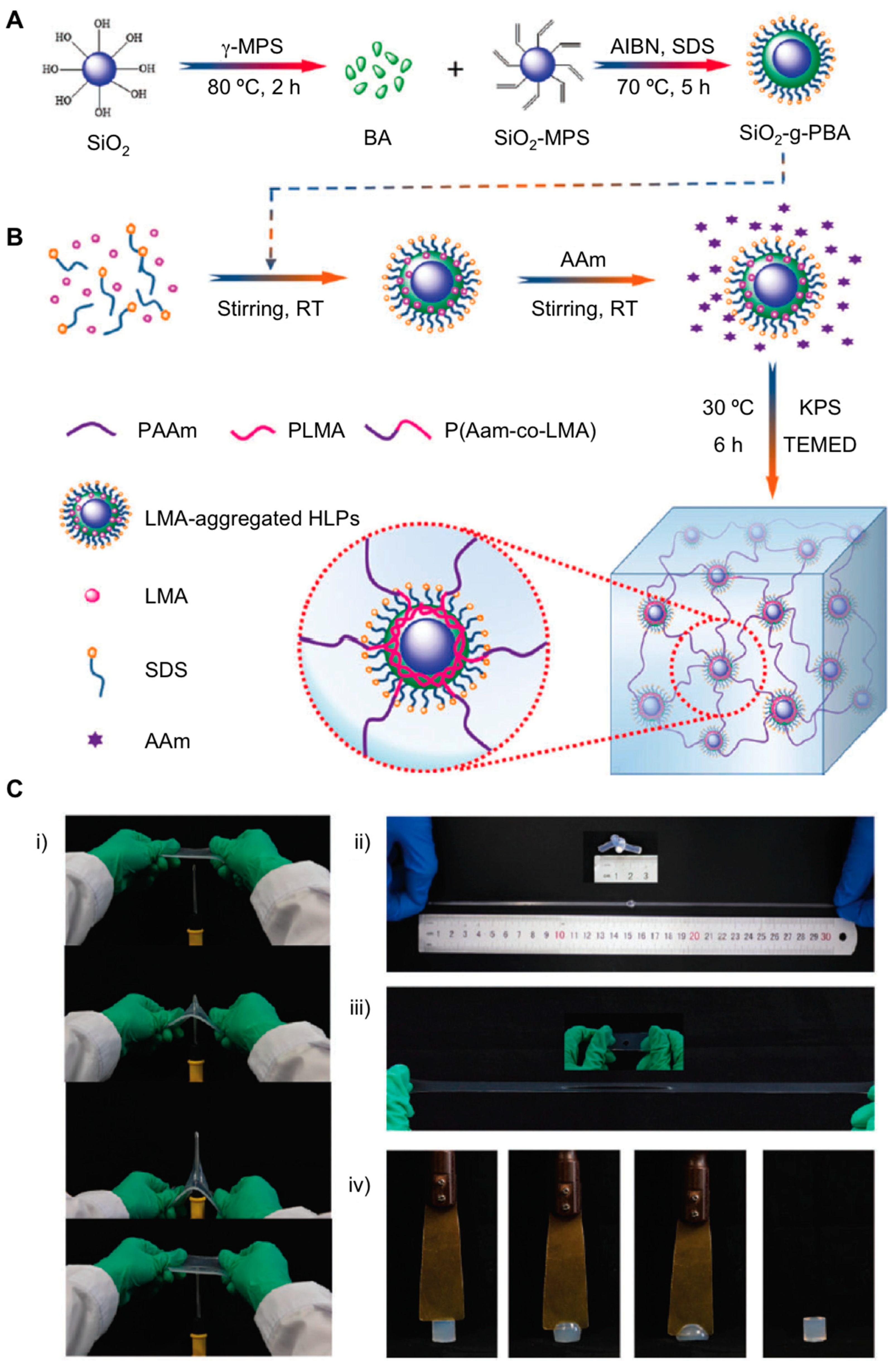
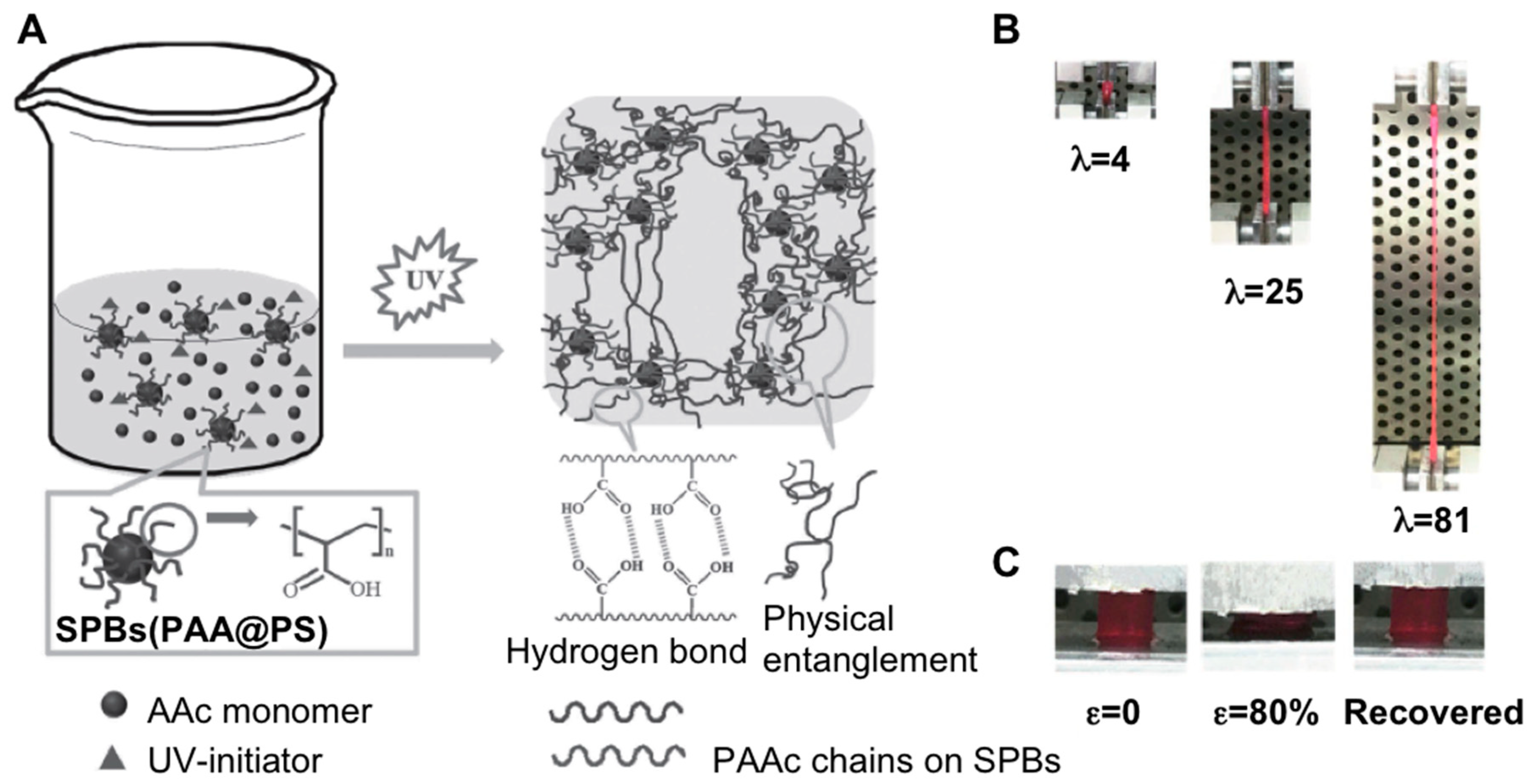
2.1. Nanocomposite Hydrogels
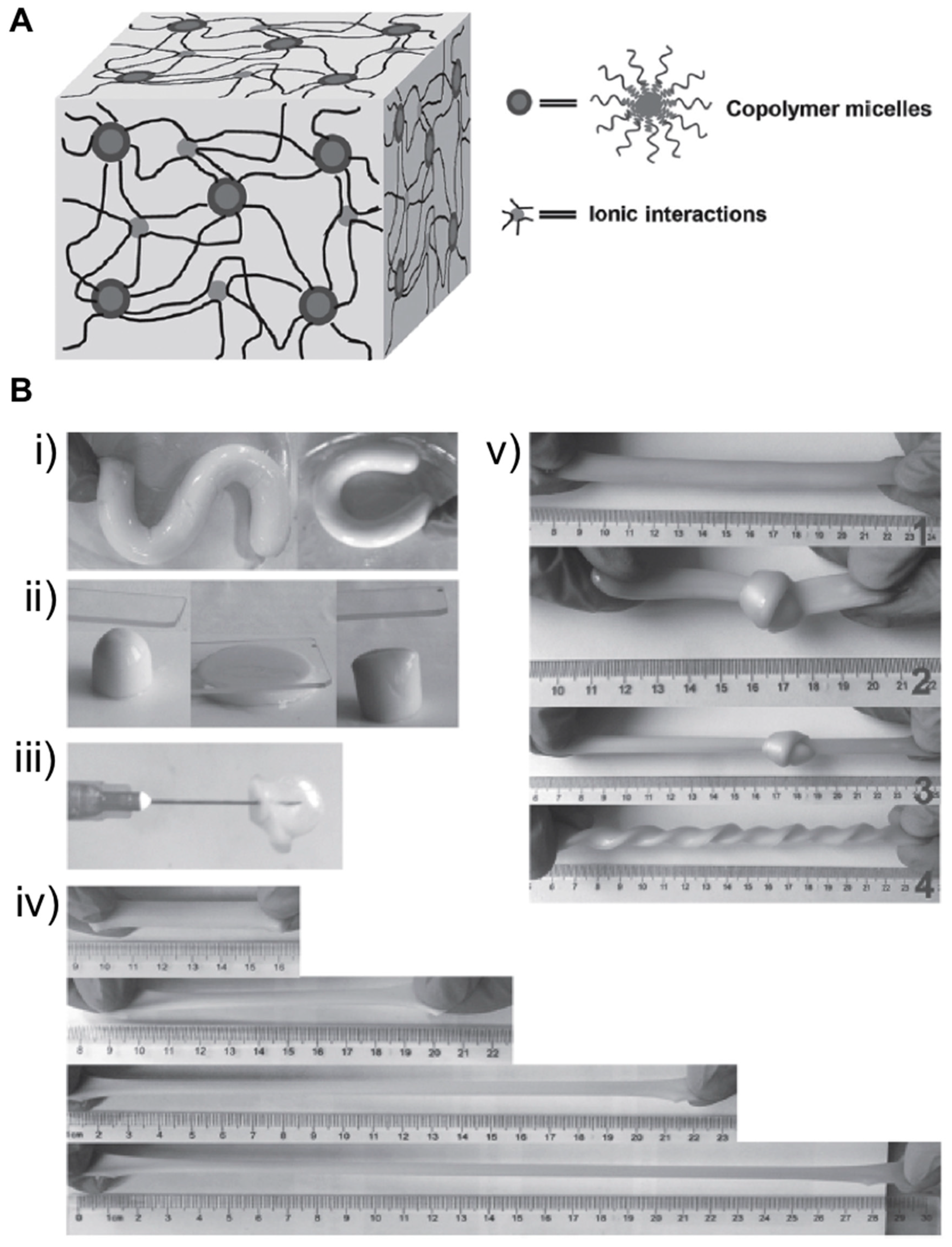
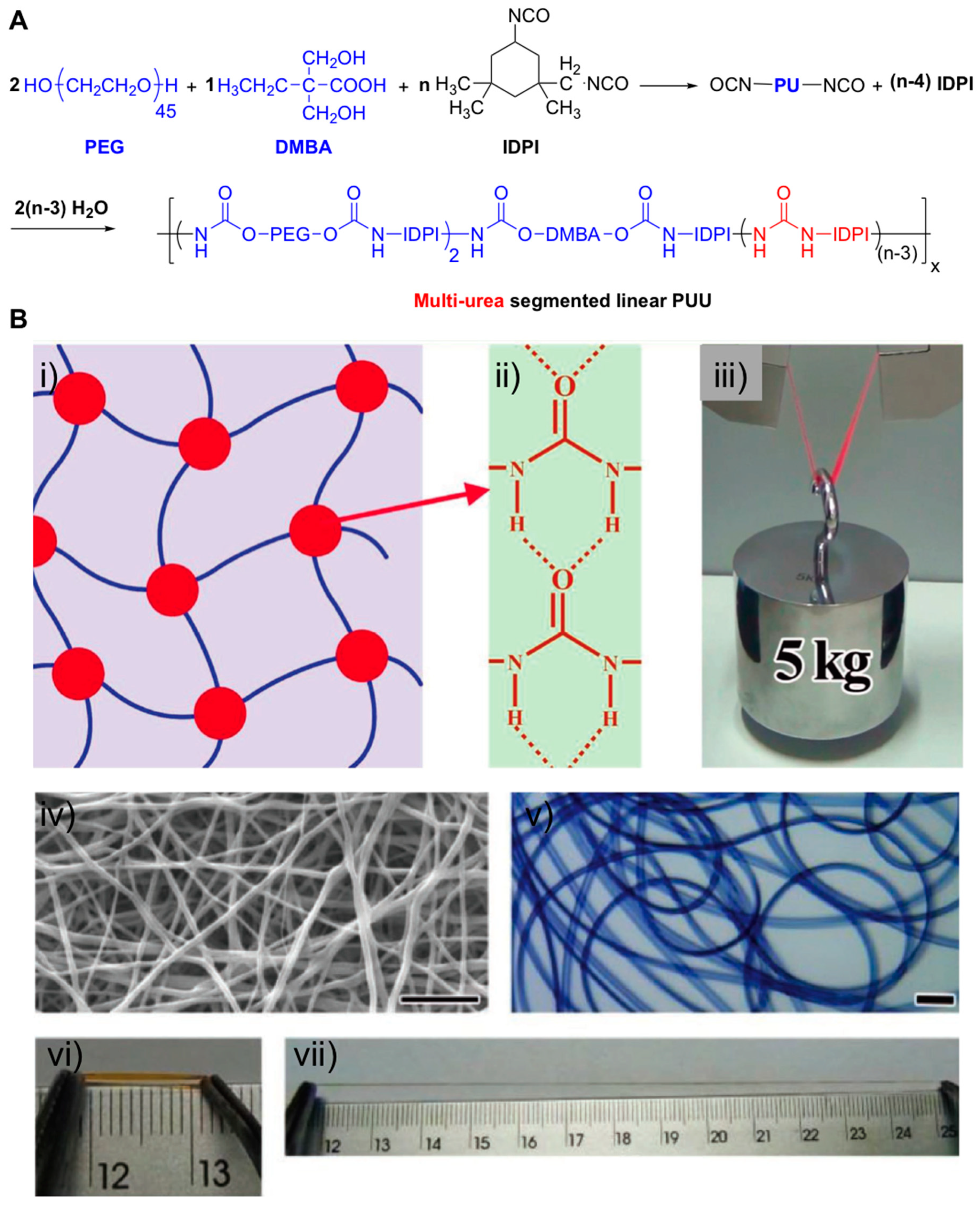
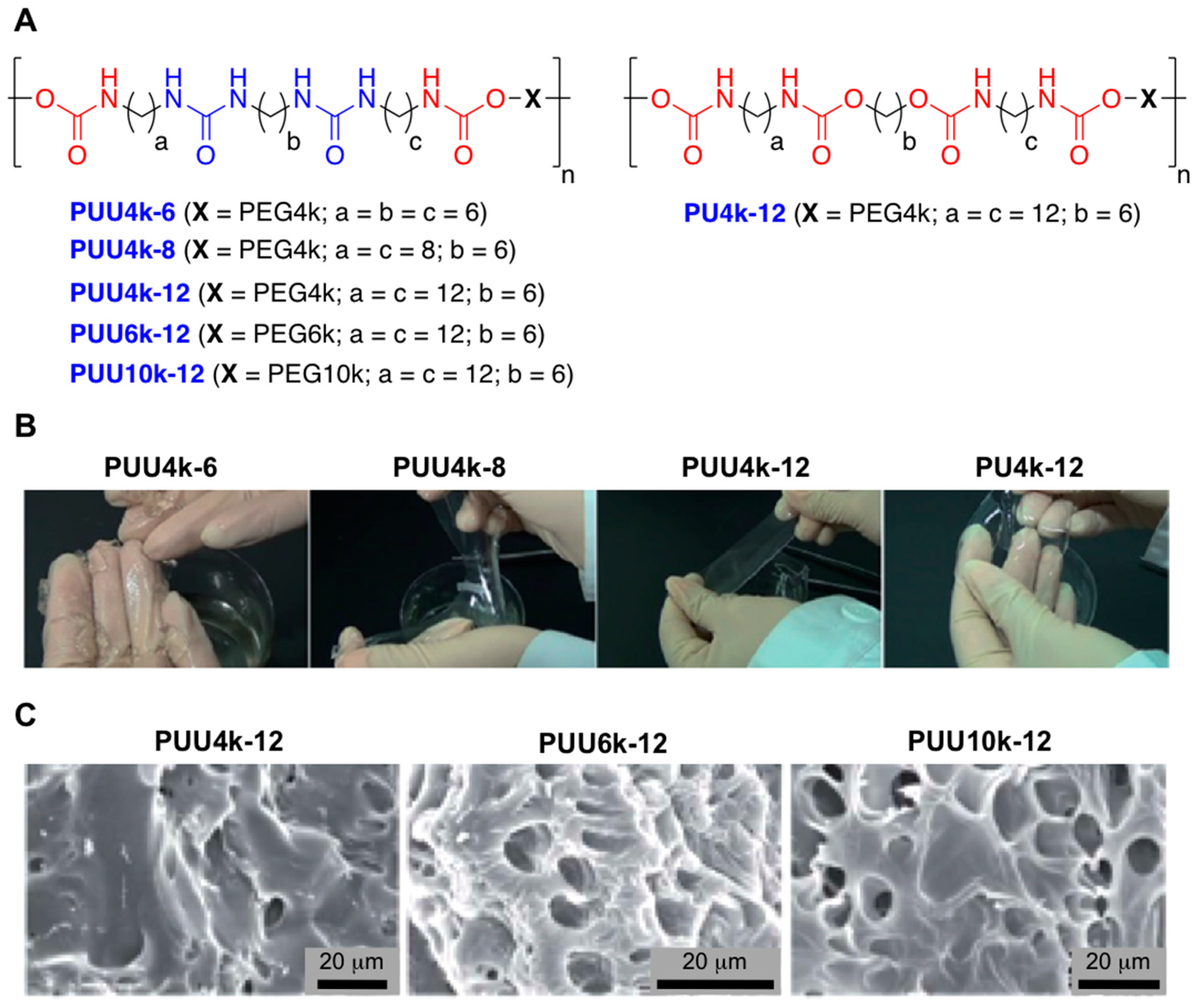
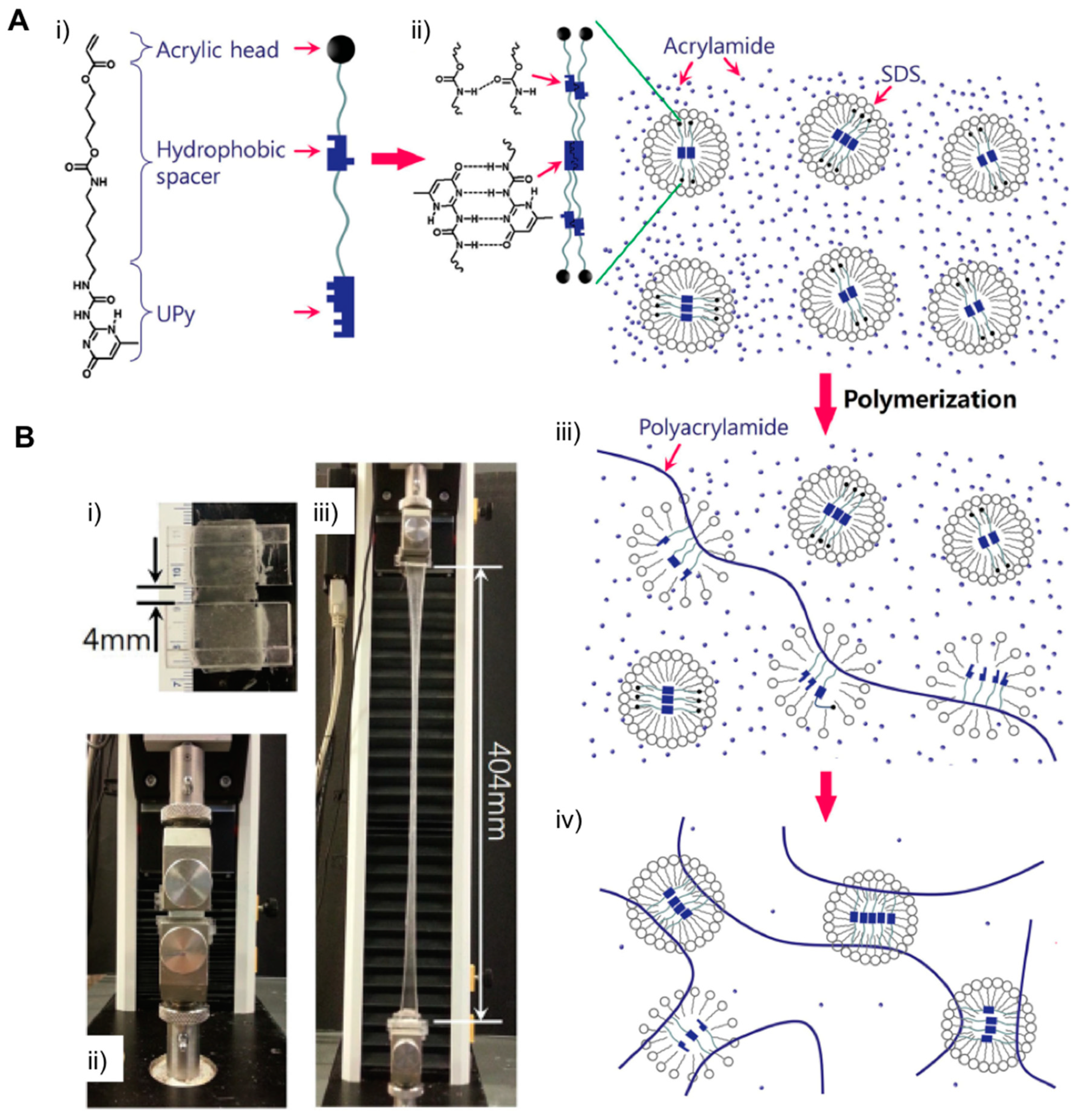
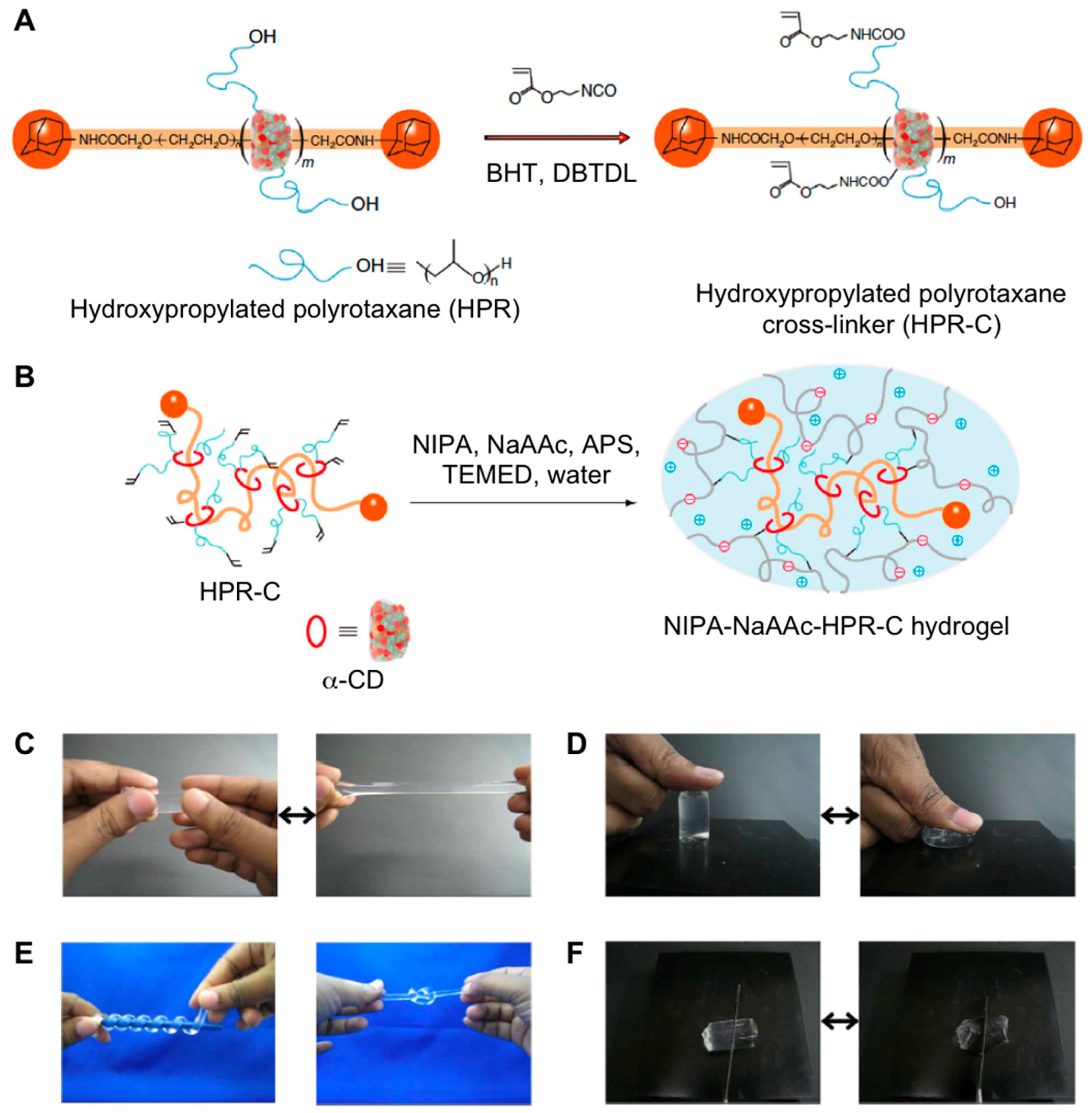
2.2. Supramolecular Hydrogels
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAc | acrylic acid |
| AAm | acrylamide |
| AIBN | 2,2′-azobis(2-methylpropionitrile) |
| BA | butyl acrylate |
| BHT | butyl hydroxyl toluene |
| CaGP | 2-glycerol phosphate |
| CB[8] | cucurbit[8]uril |
| α-CD | α-cyclodextrin |
| 3D | tridimensional |
| DAC | 2-(dimethylamino)ethylacrylatemethyl chloride |
| DC | double cross-linked (DBTDL: dibutyltin dilaurate |
| DCPA | dicyclopentyl acrylate |
| D-Gel | dual-cross-linked hydrogels |
| DLS | dynamic light scattering |
| DMBA | dimethylbutanoic acid |
| DN | double-network |
| DPC | dual physically cross-linked |
| EDPOA | ethyl-2-[4-(dihydroxyphosphoryl)-2-oxabutyl]-acrylate |
| G-6-P | glucose-6-phosphate |
| HMA | hexadecyl methacrylate |
| HPR | hydropropylated polyrotaxane |
| IPDI | isophorone diisocyanate |
| IPN | interpenetrating polymer network |
| KPS | potassium peroxodisulfate |
| MBAAm | N,N′-methylene-bis(acrylamide) |
| M-Gel | mono-cross-linked hydrogels |
| MMSPH | macromolecular microspheres |
| MPS | 3-methacryloxypropyltrimethoxysilane |
| NaAAc | sodium acrylic acid |
| NaMMT | sodium montmorillonite |
| NCHs | nanocomposite hydrogels |
| NIPA | N-isopropylacrylamide |
| LMWG | low molecular weight gelators |
| PAAc | polyacrylic acid |
| P(AAm-co-AAc) | poly(acrylamide-co-acrylic acid) |
| P(AAm-co-LMA) | poly(acrylamide-co-laurylmethacrylate) |
| PAAm-l-MBAm | Polyacrylamide-l-N,N′-methylenebis-(acrylamide) |
| PDMA-l-TEG | poly-N,N-dimethyl acrylamide-l-TEG |
| PEG | polyethylene glycol |
| PF127 | pluronic F127 |
| PHEA-l-TEG | triethylene glycol dimethacrylate |
| PNIPAm | poly(N-isopropyl acrylamide) |
| PUU | polyurethane-urea |
| Q-CNCs | quaternized cellulose nanocrystals |
| TEMED | N,N,N,N-tetramethylethylenediamine |
| SEM | scanning electron microscopy |
| SDS | sodium dodecyl sulfate |
| SMA | stearyl methacrylate |
| SPBs | spherical polymer brushes |
| TEM | transmission electron microscopy |
References
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 758–780. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable Polysaccharides for Controlled Drug Delivery. Chempluschem 2016, 81, 504–514. [Google Scholar] [CrossRef]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. 2016, 555–574. [Google Scholar] [CrossRef]
- Marsich, E.; Travan, A.; Donati, I.; Di Luca, A.; Benincasa, M.; Crosera, M.; Paoletti, S. Biological response of hydrogels embedding gold nanoparticles. Colloids Surf. B Biointerfaces 2011, 83, 331–339. [Google Scholar] [CrossRef]
- Kopeĉek, J. Hydrogels: From soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Yan, H.; Wang, Y.; Zhao, Y.; Feng, B.; Duan, K.; Weng, J. An injectable supramolecular self-healing bio-hydrogel with high stretchability, extensibility and ductility, and a high swelling ratio. J. Mater. Chem. B 2017, 5, 7021–7034. [Google Scholar] [CrossRef]
- Sun, G.; Li, Z.; Liang, R.; Weng, L.T.; Zhang, L. Super stretchable hydrogel achieved by non-aggregated spherulites with diameters <5 nm. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Le, X.; Lu, W.; Zheng, J.; Tong, D.; Zhao, N.; Ma, C.; Xiao, H.; Zhang, J.; Huang, Y.; Chen, T. Stretchable supramolecular hydrogels with triple shape memory effect. Chem. Sci. 2016, 7, 6715–6720. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Simon, J.R.; Ghoorchian, A.; Scholl, Z.; Lin, S.; Rubinstein, M.; Marszalek, P.; Chilkoti, A.; López, G.P.; Zhao, X. Strong, Tough, Stretchable, and Self-Adhesive Hydrogels from Intrinsically Unstructured Proteins. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.P.; Kholiya, F.; Vadodariya, N.; Budheliya, V.M.; Gogda, A.; Meena, R. Carboxymethylagarose-based multifunctional hydrogel with super stretchable, self-healable having film and fiber forming properties. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Mano, J.F. Extremely strong and tough hydrogels as prospective candidates for tissue repair—A review. Eur. Polym. J. 2015, 72, 344–364. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer (Guildf) 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Fares, M.M.; Shirzaei Sani, E.; Portillo Lara, R.; Oliveira, R.B.; Khademhosseini, A.; Annabi, N. Interpenetrating network gelatin methacryloyl (GelMA) and pectin-g-PCL hydrogels with tunable properties for tissue engineering. Biomater. Sci. 2018, 6, 2938–2950. [Google Scholar] [CrossRef] [PubMed]
- Rennerfeldt, D.A.; Renth, A.N.; Talata, Z.; Gehrke, S.H.; Detamore, M.S. Tuning mechanical performance of poly(ethylene glycol) and agarose interpenetrating network hydrogels for cartilage tissue engineering. Biomaterials 2013, 34, 8241–8257. [Google Scholar] [CrossRef]
- Truong, V.X.; Ablett, M.P.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P. Simultaneous Orthogonal Dual-Click Approach to Tough, in-Situ-Forming Hydrogels for Cell Encapsulation. J. Am. Chem. Soc. 2015, 137, 1618–1622. [Google Scholar] [CrossRef]
- Gu, Z.; Huang, K.; Luo, Y.; Zhang, L.; Kuang, T.; Chen, Z.; Liao, G. Double network hydrogel for tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1520. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef]
- Rodell, C.B.; Dusaj, N.N.; Highley, C.B.; Burdick, J.A. Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv. Mater. 2016, 28, 8419–8424. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Samanta, S.K. Soft-Nanocomposites of Nanoparticles and Nanocarbons with Supramolecular and Polymer Gels and Their Applications. Chem. Rev. 2016, 116, 11967–12028. [Google Scholar] [CrossRef] [PubMed]
- Rahman, C.V.; Saeed, A.; White, L.J.; Gould, T.W.A.; Kirby, G.T.S.; Sawkins, M.J.; Alexander, C.; Rose, F.R.A.J.; Shakesheff, K.M. Chemistry of polymer and ceramic-based injectable scaffolds and their applications in regenerative medicine. Chem. Mater. 2012, 24, 781–795. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Zhou, C.H.; Wang, J.; Tong, D.S.; Yu, W.H.; Wang, H. Recent advances in clay mineral-containing nanocomposite hydrogels. Soft Matter 2015, 11, 9229–9246. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.-S.; Hu, X.-H.; Chu, L.-Q. Recent Advances in Antimicrobial Hydrogels Containing Metal Ions and Metals/Metal Oxide Nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Takehisa, T.; Fan, S. Effects of clay content on the properties of nanocomposite hydrogels composed of poly(N-isopropylacrylamide) and clay. Macromolecules 2002, 35, 10162–10171. [Google Scholar] [CrossRef]
- Song, F.; Li, X.; Wang, Q.; Liao, L.; Zhang, C. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J. Biomed. Nanotechnol. 2015, 11, 40–52. [Google Scholar] [CrossRef]
- Gao, G.; Du, G.; Sun, Y.; Fu, J. Self-healable, tough, and ultrastretchable nanocomposite hydrogels based on reversible polyacrylamide/montmorillonite adsorption. ACS Appl. Mater. Interfaces 2015, 7, 5029–5037. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Deng, X.; Wang, T.; Yang, Z.; Zhou, W.; Wang, C. Dual Physically Cross-Linked Hydrogels with High Stretchability, Toughness, and Good Self-Recoverability. Macromolecules 2016, 49, 5660–5668. [Google Scholar] [CrossRef]
- Qin, Z.; Niu, R.; Tang, C.; Xia, J.; Ji, F.; Dong, D.; Zhang, H.; Zhang, S.; Li, J.; Yao, F. A Dual-Crosslinked Strategy to Construct Physical Hydrogels with High Strength, Toughness, Good Mechanical Recoverability, and Shape-Memory Ability. Macromol. Mater. Eng. 2018, 303, 1–12. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, T.; Hu, D.; Chang, C. Dual Physically Cross-Linked Nanocomposite Hydrogels Reinforced by Tunicate Cellulose Nanocrystals with High Toughness and Good Self-Recoverability. ACS Appl. Mater. Interfaces 2017, 9, 24230–24237. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, M.; Cao, J.; Yan, B.; Yang, W.; Jin, X.; Ma, A.; Chen, W.; Ding, X.; Zhang, G.; et al. Highly Flexible, Tough, and Self-Healable Hydrogels Enabled by Dual Cross-Linking of Triblock Copolymer Micelles and Ionic Interactions. Macromol. Mater. Eng. 2017, 302, 1–5. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Lu, B.; Li, T.; Zhao, H.; Li, X.; Gao, C.; Zhang, S.; Xie, E. Graphene-based composite materials beneficial to wound healing. Nanoscale 2012, 4, 2978. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, D.; Liu, L.; Gai, G. Recent achievements of self-healing graphene/polymer composites. Polymers (Basel) 2018, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, L.; Chen, Q.; Zhang, Q.; Guo, G. Tough, Stretchable, Compressive Novel Polymer/Graphene Oxide Nanocomposite Hydrogels with Excellent Self-Healing Performance. ACS Appl. Mater. Interfaces 2017, 9, 38052–38061. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, Y.-T.; Xie, X.-M. Self-healable, super tough graphene oxide–poly(acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar] [CrossRef]
- Rauner, N.; Meuris, M.; Zoric, M.; Tiller, J.C. Enzymatic mineralization generates ultrastiff and tough hydrogels with tunable mechanics. Nature 2017, 543, 407–410. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, C.; Liu, X.; Chen, Q.; Zhang, G.; Yang, M.; Liu, F. Network structure and compositional effects on tensile mechanical properties of hydrophobic association hydrogels with high mechanical strength. Polymer 2010, 51, 1507–1515. [Google Scholar] [CrossRef]
- Huang, T.; Xu, H.; Jiao, K.; Zhu, L.; Brown, H.R.; Wang, H. A novel hydrogel with high mechanical strength: A macromolecular microsphere composite hydrogel. Adv. Mater. 2007, 19, 1622–1626. [Google Scholar] [CrossRef]
- Jiang, F.; Huang, T.; He, C.; Brown, H.R.; Wang, H. Interactions affecting the mechanical properties of macromolecular microsphere composite hydrogels. J. Phys. Chem. B 2013, 117, 13679–13687. [Google Scholar] [CrossRef]
- Ren, X.Y.; Yu, Z.; Liu, B.; Liu, X.J.; Wang, Y.J.; Su, Q.; Gao, G.H. Highly tough and puncture resistant hydrogels driven by macromolecular microspheres. RSC Adv. 2016, 6, 8956–8963. [Google Scholar] [CrossRef]
- Shibayama, M. Structure-mechanical property relationship of tough hydrogels. Soft Matter 2012, 8, 8030. [Google Scholar] [CrossRef]
- Haraguchi, K. Nanocomposite hydrogels. Curr. Opin. Solid State Mater. Sci. 2007, 11, 47–54. [Google Scholar] [CrossRef]
- Ye, E.; Loh, X.J. Polymeric hydrogels and nanoparticles: A merging and emerging field. Aust. J. Chem. 2013, 66, 997–1007. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Ren, X.; Gao, G. Highly tough, anti-fatigue and rapidly self-recoverable hydrogels reinforced with core–shell inorganic–organic hybrid latex particles. Soft Matter 2017, 13, 6059–6067. [Google Scholar] [CrossRef] [PubMed]
- Reza Saboktakin, M.; Tabatabaei, R.M. Supramolecular hydrogels as drug delivery systems. Int. J. Biol. Macromol. 2015, 75, 426–436. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Liu, W.; Zhao, X.; Lu, W. Intermolecular hydrogen bonding strategy to fabricate mechanically strong hydrogels with high elasticity and fatigue resistance. Soft Matter 2013, 9, 6331. [Google Scholar] [CrossRef]
- Yang, N.; Yang, H.; Shao, Z.; Guo, M. Ultrastrong and Tough Supramolecular Hydrogels from Multiurea Linkage Segmented Copolymers with Tractable Processablity and Recyclability. Macromol. Rapid Commun. 2017, 38, 1–6. [Google Scholar] [CrossRef]
- Cui, Y.; Tan, M.; Zhu, A.; Guo, M. Non-covalent interaction cooperatively induced stretchy, tough and stimuli-responsive polyurethane–urea supramolecular (PUUS) hydrogels. J. Mater. Chem. B 2015, 3, 2834–2841. [Google Scholar] [CrossRef]
- Jeon, I.; Cui, J.; Illeperuma, W.R.K.; Aizenberg, J.; Vlassak, J.J. Extremely Stretchable and Fast Self-Healing Hydrogels. Adv. Mater. 2016, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Wenz, E.; Lindner, P. Density profile of spherical polymer brushes. Phys. Rev. Lett. 1996, 77, 95–98. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Shen, Z.; Li, M.; Guo, X.; Yao, Y. Ultrastretchable, Tough, and Notch-Insensitive Hydrogels Formed with Spherical Polymer Brush Crosslinker. Macromol. Rapid Commun. 2017, 38, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Loh, X.J.; Jones, S.T.; Dreiss, C.A.; Scherman, O.A. Sustained release of proteins from high water content supramolecular polymer hydrogels. Biomaterials 2012, 33, 4646–4652. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Harada, A. Highly Flexible, Tough, and Self-Healing Supramolecular Polymeric Materials Using Host-Guest Interaction. Macromol. Rapid Commun. 2016, 37, 86–92. [Google Scholar] [CrossRef]
- Mealy, J.E.; Rodell, C.B.; Burdick, J.A. Sustained small molecule delivery from injectable hyaluronic acid hydrogels through host-guest mediated retention. J. Mater. Chem. B 2015, 3, 8010–8019. [Google Scholar] [CrossRef]
- Rodell, C.B.; Kaminski, A.L.; Burdick, J.A. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 2013, 14, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J. Supramolecular host–guest polymeric materials for biomedical applications. Mater. Horiz. 2014, 1, 185–195. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host-Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Li, N.; Abell, C.; Scherman, O.A. Tough Supramolecular Polymer Networks with Extreme Stretchability and Fast Room-Temperature Self-Healing. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijalvo, S.; Eritja, R.; Díaz Díaz, D. On the Race for More Stretchable and Tough Hydrogels. Gels 2019, 5, 24. https://doi.org/10.3390/gels5020024
Grijalvo S, Eritja R, Díaz Díaz D. On the Race for More Stretchable and Tough Hydrogels. Gels. 2019; 5(2):24. https://doi.org/10.3390/gels5020024
Chicago/Turabian StyleGrijalvo, Santiago, Ramon Eritja, and David Díaz Díaz. 2019. "On the Race for More Stretchable and Tough Hydrogels" Gels 5, no. 2: 24. https://doi.org/10.3390/gels5020024
APA StyleGrijalvo, S., Eritja, R., & Díaz Díaz, D. (2019). On the Race for More Stretchable and Tough Hydrogels. Gels, 5(2), 24. https://doi.org/10.3390/gels5020024