The Influence of Differently Shaped Gold Nanoparticles Functionalized with NIPAM-Based Hydrogels on the Release of Cytochrome C
Abstract
:1. Introduction
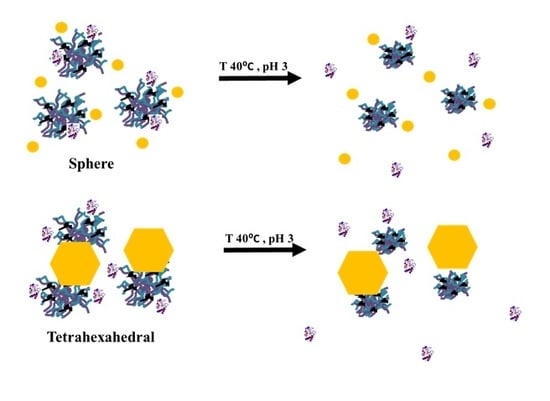
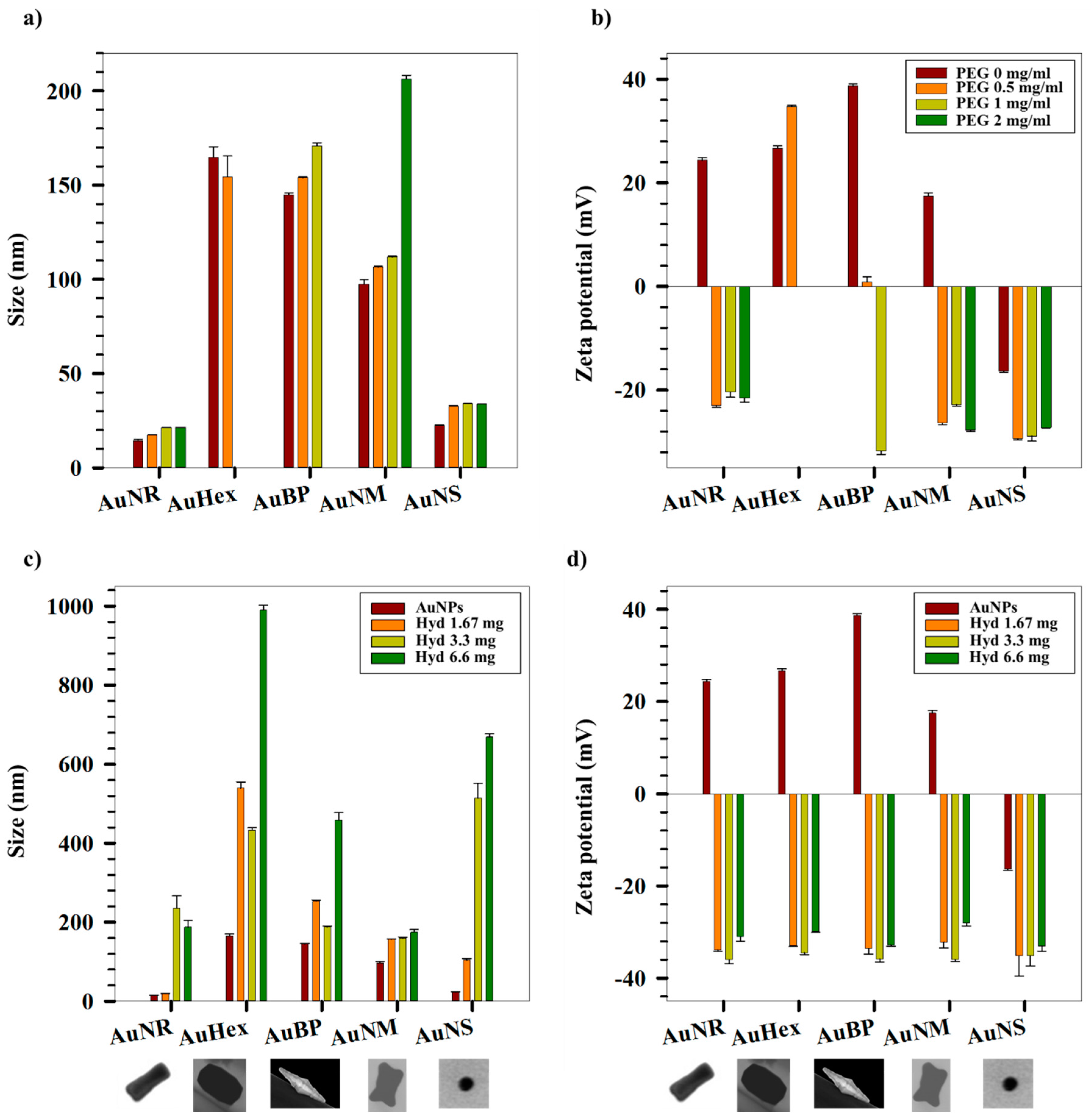
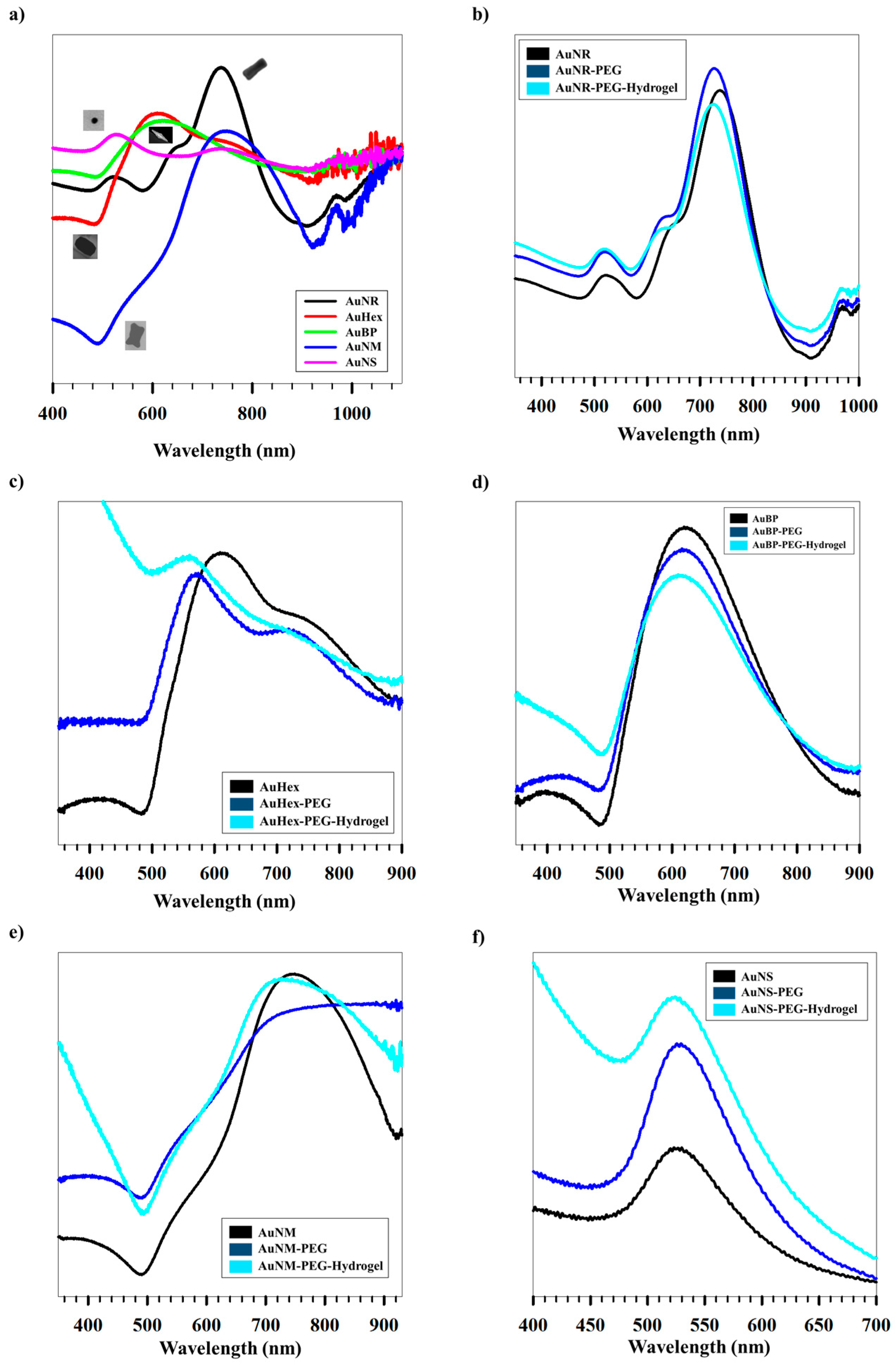
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Hydrogel
4.2. Synthesis of Spherical AuNPs
4.3. Synthesis of Au Seed
4.4. Synthesis of Anisotropic AuNPs
4.5. Coating of AuNPs
4.6. Loading and Release with Cyt C
4.7. Characterization Techniques
4.7.1. Scanning (Transmission) Electron Microscopy (S(T)EM)
4.7.2. Dynamic Light Scattering (DLS)
4.7.3. Ultraviolet-Visible Spectroscopy (UV-Vis)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Jiang, L.; Zhan, Q.; Qian, J.; He, S. Localized surface plasmon resonance (LSPR) of polyelectrolyte-functionalized gold-nanoparticles for bio-sensing. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 172–179. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Niidome, T.; Nariai, A.; Niidome, Y.; Yamada, S. Gold nanorod-sensitized cell death: Microscopic observation of single living cells irradiated by pulsed near-infrared laser light in the presence of gold nanorods. Chem. Lett. 2006, 35, 500–501. [Google Scholar] [CrossRef]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Petrova, H.; Chen, J.; McLellan, J.M.; Siekkinen, A.R.; Marquez, M.; Li, X.; Xia, Y.; Hartland, G.V. Ultrafast laser studies of the photothermal properties of gold nanocages. J. Phys. Chem. B 2006, 110, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, R.; Zhang, G.; Zhong, M.; Liu, Y.; Van Pelt, C.S.; Liang, D.; Wei, W.; Sood, A.K.; Li, C. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: A platform for near-infrared light-trigged drug release. J. Control. Release 2012, 158, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, Y.; Niidome, T.; Yamada, S.; Nakashima, N.; Niidome, Y. Expression of plasmid DNA released from DNA conjugates of gold nanorods. Chem. Lett. 2007, 36, 952–953. [Google Scholar] [CrossRef]
- Bardhan, R.; Chen, W.; Perez-Torres, C.; Bartels, M.; Huschka, R.M.; Zhao, L.L.; Morosan, E.; Pautler, R.G.; Joshi, A.; Halas, N.J. Nanoshells with targeted simultaneous enhancement of magnetic and optical imaging and photothermal therapeutic response. Adv. Funct. Mater. 2009, 19, 3901–3909. [Google Scholar] [CrossRef]
- Cobley, C.M.; Au, L.; Chen, J.; Xia, Y. Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery. Expert Opin. Drug Deliv. 2010, 7, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Beziere, N.; del Pino, P.; Pelaz, B.; Estrada, G.; Tian, F.; Ntziachristos, V.; de la Fuente, J.M.; Cui, D. Gold Nanoprisms as Optoacoustic Signal Nanoamplifiers for In Vivo Bioimaging of Gastrointestinal Cancers. Small 2013, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yong, K.T.; Roy, I.; Pudavar, H.E.; Law, W.C.; Bergey, E.J.; Prasad, P.N. Gold nanorods coated with multilayer polyelectrolyte as contrast agents for multimodal imaging. J. Phys. Chem. C 2007, 111, 12552–12557. [Google Scholar] [CrossRef]
- Qin, H.; Zhao, Y.; Zhang, J.; Pan, X.; Yang, S.; Xing, D. Inflammation-targeted gold nanorods for intravascular photoacoustic imaging detection of matrix metalloproteinase-2 (MMP2) in atherosclerotic plaques. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Sasaki, D.Y.; Perroud, T.D.; Yoo, D.; Patel, K.D.; Lee, L.P. Biologically Functional Cationic Phospholipid-Gold Nanoplasmonic Carriers of RNA. J. Am. Chem. Soc. 2009, 131, 14066–14074. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Boulos, S.P.; Lohse, S.E.; Thompson, L.B.; Murphy, C.J. Homing Peptide-Conjugated Gold Nanorods: The Effect of Amino Acid Sequence Display on Nanorod Uptake and Cellular Proliferation. Bioconjugate Chem. 2014, 25, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Oyelere, A.K.; Chen, P.C.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Peptide-conjugated gold nanorods for nuclear targeting. Bioconjugate Chem. 2007, 18, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Bai, X.; Zheng, L. Ultrafast Preparation of Three-Dimensional Dendritic Gold Nanostructures in Aqueous Solution and Their Applications in Catalysis and SERS. J. Phys. Chem. C 2011, 115, 14641–14647. [Google Scholar] [CrossRef]
- Yu, Y.; Kant, K.; Shapter, J.G.; Addai-Mensah, J.; Losic, D. Gold nanotube membranes have catalytic properties. Microporous Mesoporous Mater. 2012, 153, 131–136. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Guix, M.; Madrid, R.E.; Merkoçi, A. Bimetallic nanowires as electrocatalysts for nonenzymatic real-time impedancimetric detection of glucose. Chem. Commun. 2012, 48, 1686–1688. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Z.; Zong, S.; Huang, Z.; Zhang, P.; Cui, Y. A SERS-based immunoassay with highly increased sensitivity using gold/silver core-shell nanorods. Biosens. Bioelectron. 2012, 38, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Lee, J.; Wark, A.W.; Lee, H.J. Nanoparticle-enhanced surface plasmon resonance detection of proteins at attomolar concentrations: Comparing different nanoparticle shapes and sizes. Anal. Chem. 2012, 84, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Castellana, E.T.; Gamez, R.C.; Russell, D.H. Label-free biosensing with lipid-functionalized gold nanorods. J. Am. Chem. Soc. 2011, 133, 4182–4185. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.J.; Menichetti, S.; Rotello, V.M. Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Xu, X.; Lam, R.; Giljohann, D.; Ho, D.; Mirkin, C.A. Strategy for Increasing Drug Solubility and Efficacy through Covalent Attachment to Polyvalent DNA–Nanoparticle Conjugates. ACS Nano 2011, 5, 6962–6970. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Miranda, O.R.; Thompson, M.A.; Pabelick, C.M.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Prakash, Y.S.; Mukherjee, P. Effect of Nanoparticle Surface Charge at the Plasma Membrane and Beyond. Nano Lett. 2010, 10, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bexiga, M.G.; Anguissola, S.; Boya, P.; Simpson, J.C.; Salvati, A.; Dawson, K.A. Time resolved study of cell death mechanisms induced by amine-modified polystyrene nanoparticles. Nanoscale 2013, 5, 10868–10876. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Thompson, L.B.; Alkilany, A.M.; Sisco, P.N.; Boulos, S.P.; Sivapalan, S.T.; Yang, J.A.; Chernak, D.J.; Huang, J. The many faces of gold nanorods. J. Phys. Chem. Lett. 2010, 1, 2867–2875. [Google Scholar] [CrossRef]
- Johnson, C.J.; Dujardin, E.; Davis, S.A.; Murphy, C.J.; Mann, S. Growth and form of gold nanorods prepared by seed-mediated, surfactant-directed synthesis. J. Mater. Chem. 2002, 12, 1765–1770. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Singh, G.; Glomm, W.R. Shape control of gold nanostructures using binary surfactant mixtures. Chem. Mater. 2016. submitted. [Google Scholar]
- Chithrani, D.B. Intracellular uptake, transport, and processing of gold nanostructures. Mol. Membr. Biol. 2010, 27, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control. Release 2006, 114, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Ng, J.; Krieg, T.; Griesser, H.J. A robust procedure for the functionalization of gold nanorods and noble metal nanoparticles. Chem. Commun. 2009, 13, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Shiotani, A.; Mori, T.; Niidome, T.; Niidome, Y.; Katayama, Y. Stable incorporation of gold nanorods into N-isopropylacrylamide hydrogels and their rapid shrinkage induced by near-infrared laser irradiation. Langmuir 2007, 23, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, T.R. Thermo-and pH-responsive hydrogel-coated gold nanoparticles. Chem. Mater. 2004, 16, 3647–3651. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, T.R. Thermo-and pH-responsive hydrogel-coated gold nanoparticles prepared from rationally designed surface-confined initiators. J. Nanopart. Res. 2011, 13, 2909–2918. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Blackburn, W.H.; Smith, M.H.; Kapa, L.B.; Lyon, L.A.; McDonald, J.F. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC Cancer 2010, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Lee, H.; Chmielewski, J.; Lyon, L.A. Folate-mediated cell targeting and cytotoxicity using thermoresponsive microgels. J. Am. Chem. Soc. 2004, 126, 10258–10259. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; McDonagh, B.H.; Singh, G.; Sandvig, I.; Sandvig, A.; Glomm, W.R. Growing gold nanostructures for shape-selective cellular uptake. Mater. Today Chem. 2017. submitted. [Google Scholar]
- Bandyopadhyay, S.; Andersen, M.K.; Alvi, M.A.A.; Sharma, A.; Raju, R.; McDonagh, B.H.; Glomm, W.R. Incorporation of Fe@Au nanoparticles into multiresponsive pNIPAM-AAc colloidal gels modulates drug uptake and release. Colloid Polym. Sci. 2016, 294, 1929–1942. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Singh, G.; Sandvig, I.; Sandvig, A.; Mathieu, R.; Kumar, P.A.; Glomm, W.R. Synthesis and in vitro cellular interactions of superparamagnetic iron nanoparticles with a crystalline gold shell. Appl. Surf. Sci. 2014, 316, 171–178. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Singh, G.; Glomm, W.R. Shape tunable synthesis of anisotropic gold nanostructures through binary surfactant mixtures. Mater. Today Chem. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Van Lehn, R.C.; Alexander-Katz, A. Penetration of lipid bilayers by nanoparticles with environmentally-responsive surfaces: Simulations and theory. Soft Matter 2011, 7, 11392–11404. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef] [PubMed]
- Reynwar, B.J.; Illya, G.; Harmandaris, V.A.; Müller, M.M.; Kremer, K.; Deserno, M. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 2007, 447, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Niidome, Y.; Niidome, T.; Kaneko, K.; Kawasaki, H.; Yamada, S. Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity. Langmuir 2006, 22, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, M.T.; Fattal, E.; Desmaele, D.; Besnard, M.; Noel, J.P.; Gomis, J.M.; Appel, M.; Couvreur, P. Stealth® PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J. Control. Release 1999, 60, 121–128. [Google Scholar] [CrossRef]
- Larson-Smith, K.; Pozzo, D.C. Competitive adsorption of thiolated poly (ethylene glycol) and alkane-thiols on gold nanoparticles and its effect on cluster formation. Langmuir 2012, 28, 13157–13165. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Skobeleva, V.B.; Zinchenko, A.V.; Rogacheva, V.B.; Zezin, A.B.; Kabanov, V.A. Interaction of hydrogels of acrylic acid-acrylamide copolymers with cytochrome C. Polym. Sci. Ser. A 2001, 43, 315–322. [Google Scholar]
- Dumetz, A.C.; Snellinger-O’Brien, A.M.; Kaler, E.W.; Lenhoff, A.M. Patterns of protein-protein interactions in salt solutions and implications for protein crystallization. Protein Sci. 2007, 16, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.S.; Peppas, N.A. Modeling of drug release from swellable polymers. Eur. J. Pharm. Biopharm. 2000, 49, 47–58. [Google Scholar] [CrossRef]

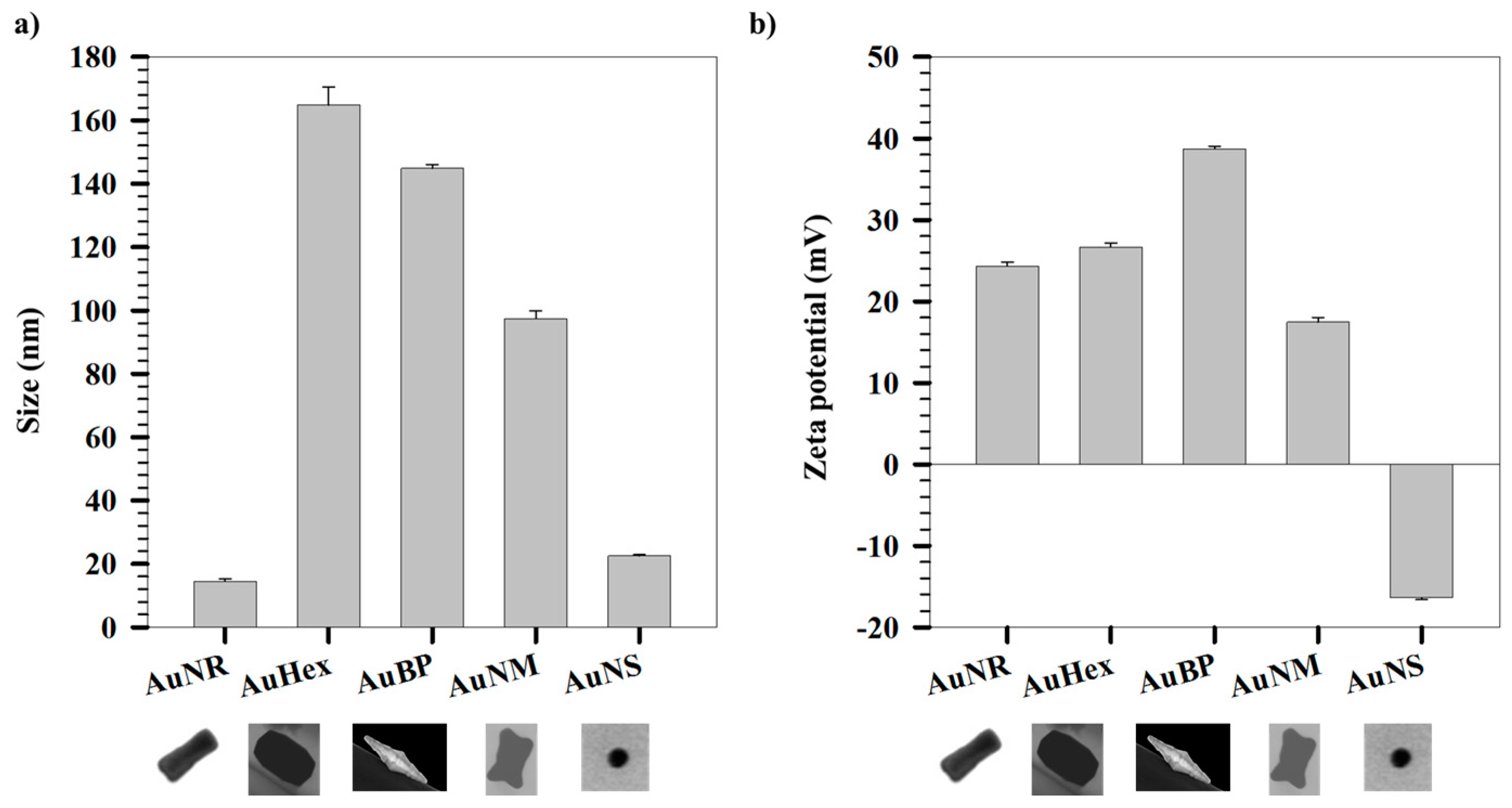


| AuNPs | Aspect Ratio (avg ± st. dev) | S(T)EM Size (nm) Length/Width | DLS Size (nm) |
|---|---|---|---|
| AuNR | 3.4 ± 0.5 | 42 ± 4/13 ± 2 | 14.4 ± 0.7 |
| AuHex | 1.4 ± 0.2 | 233 ± 25/171 ± 20 | 164.8 ± 5.6 |
| AuBP | 3.8 ± 0.5 | 382 ± 107/107 ± 45 | 144.9 ± 1.1 |
| AuNM | 1.5 ± 0.4 | 118 ± 15/83 ± 14 | 97.4 ± 2.4 |
| AuNS | – | 17 ± 2 | 22.5 ± 0.4 |
| Sample Name | Co-Surfactant | Moles of CTAB | Moles of Co-Surfactant |
|---|---|---|---|
| AuNR | Oleic acid | 3.3 × 10−3 | 6.3 × 10−5 |
| AuHex | Oleic acid | 3.3 × 10−3 | 9.4 × 10−4 |
| AuBP | DDAB | 3.3 × 10−3 | 4.3 × 10−4 |
| AuNM | Oleic acid | 3.3 × 10−3 | 9.4 × 10−5 |
| A* | Oleic acid | 3.3 × 10−3 | 9.4 × 10−5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandyopadhyay, S.; Sharma, A.; Glomm, W.R. The Influence of Differently Shaped Gold Nanoparticles Functionalized with NIPAM-Based Hydrogels on the Release of Cytochrome C. Gels 2017, 3, 42. https://doi.org/10.3390/gels3040042
Bandyopadhyay S, Sharma A, Glomm WR. The Influence of Differently Shaped Gold Nanoparticles Functionalized with NIPAM-Based Hydrogels on the Release of Cytochrome C. Gels. 2017; 3(4):42. https://doi.org/10.3390/gels3040042
Chicago/Turabian StyleBandyopadhyay, Sulalit, Anuvansh Sharma, and Wilhelm Robert Glomm. 2017. "The Influence of Differently Shaped Gold Nanoparticles Functionalized with NIPAM-Based Hydrogels on the Release of Cytochrome C" Gels 3, no. 4: 42. https://doi.org/10.3390/gels3040042
APA StyleBandyopadhyay, S., Sharma, A., & Glomm, W. R. (2017). The Influence of Differently Shaped Gold Nanoparticles Functionalized with NIPAM-Based Hydrogels on the Release of Cytochrome C. Gels, 3(4), 42. https://doi.org/10.3390/gels3040042