1. Introduction
The effective management of hard-to-treat skin wounds, including diabetic foot ulcers, venous leg ulcers, and pressure ulcers, remains a persistent challenge in clinical practice [
1]. These wounds are commonly associated with prolonged healing times, frequent recurrence, and an increased risk of infection, leading to substantial physical, psychological, and economic burdens for both patients and healthcare systems worldwide [
2,
3]. Unlike acute wounds, hard-to-treat wounds are characterized by dysregulated inflammatory responses, insufficient vascular support, and impaired extracellular matrix (ECM) remodeling, which together disrupt the normal progression of tissue repair [
4,
5]. Despite continuous advances in wound care strategies, there remains a significant unmet clinical need for safe and effective therapeutic approaches capable of supporting coordinated and durable wound healing [
6].
Wound healing is a dynamic and highly regulated biological process that involves overlapping and interdependent phases of inflammation, proliferation, and tissue remodeling [
4]. Throughout this process, the local wound microenvironment plays a critical role in governing cell migration, angiogenesis, immune regulation, and extracellular matrix deposition. In chronic or hard-to-treat wounds, disruption of this microenvironment often results in prolonged inflammation and ineffective tissue repair [
7]. Conventional wound dressings are primarily designed to act as passive physical barriers, providing protection against external contamination and moisture loss. However, such dressings generally lack the capacity to actively regulate cellular behavior or modulate the wound microenvironment [
8]. In recent years, biomaterial-based wound dressings and scaffolds have attracted increasing attention because they can offer temporary structural support while simultaneously interacting with cells and tissues [
9]. Nevertheless, the rational design of biomaterials that integrate mechanical stability, controlled degradation, and biologically relevant functionality remains a major challenge in the development of advanced wound healing strategies.
An ideal wound dressing or scaffold should possess sufficient mechanical integrity to maintain structural stability under physiological conditions, while degrading at a rate compatible with tissue regeneration [
10,
11]. Materials that degrade too rapidly may fail to provide sustained support for cell infiltration and extracellular matrix deposition, whereas materials that persist excessively may impede tissue remodeling and prolong inflammatory responses. Beyond mechanical strength and degradation behavior, wound dressings are also expected to create a favorable microenvironment that supports cell adhesion, migration, and proliferation, promotes angiogenesis, and modulates immune responses toward a regenerative phenotype [
12]. Balancing these often competing requirements within a single material system remains particularly challenging, reflecting the complexity and dynamic nature of the wound healing process.
Natural biomaterials derived from extracellular matrix (ECM) components have been extensively investigated for wound healing applications owing to their inherent biocompatibility and biological relevance [
13,
14]. Among them, gelatin, a denatured form of collagen, is one of the most widely used protein-based biomaterials in tissue engineering and regenerative medicine [
15]. Gelatin is abundant, cost-effective, and readily processable into hydrogels with tunable mechanical properties and degradation profiles, making it attractive for wound dressing and scaffold fabrication. Moreover, gelatin retains cell-interactive motifs that facilitate cell adhesion and migration, which are essential for tissue repair [
16,
17]. Importantly, gelatin-based materials have already been translated into commercially available products, including wound dressings and hemostatic agents, demonstrating their practical applicability and clinical acceptance. However, gelatin-based hydrogels alone often exhibit limited mechanical robustness and lack sufficient biological specificity to support the coordinated regulation of inflammation, angiogenesis, and matrix remodeling, particularly in complex or hard-to-treat wounds. These limitations highlight the need to further modify or combine gelatin with complementary components to enhance its functional performance.
Recombinant collagen has emerged as an attractive alternative to animal-derived collagen in biomedical applications, owing to its defined molecular composition, reduced batch-to-batch variability, and elimination of risks associated with animal-derived pathogens and immunogenic contaminants [
18]. Compared with collagen extracted from animal tissues, recombinant collagen offers improved reproducibility and quality control, which are particularly important for translational and clinical applications [
19]. Among different collagen subtypes, recombinant type III collagen (rColIII) is of special relevance to wound healing, as type III collagen is a predominant component of the provisional extracellular matrix formed during the early stages of tissue repair. Type III collagen plays a critical role in supporting cell migration, granulation tissue formation, and subsequent matrix remodeling during wound healing [
20,
21]. Its presence is closely associated with early regenerative responses and the transition toward more mature collagen architectures at later stages. rColIII scaffolds have been shown to promote fibroblast adhesion, proliferation, and migration while maintaining favorable biocompatibility and low immunogenicity, making them promising biomaterials for soft regenerative applications [
22,
23,
24]. Hybrid hydrogels incorporating rColIII have been shown to accelerate wound closure, enhance collagen deposition, and improve vascularization in full-thickness diabetic wound models without eliciting adverse systemic reactions, underscoring their in vivo efficacy in challenging healing environments [
25,
26]. In addition, rColIII has been reported to facilitate functional skin regeneration in both preclinical and clinical studies, exhibiting enhanced re-epithelialization, reduced inflammation, and improved dermal matrix organization in models of skin injury and UV-induced damage [
24,
27]. Despite these advantages, recombinant collagen-based matrices alone often provide limited control over the physicochemical microenvironment, highlighting the need for complementary components to further tailor material properties for wound healing.
Beyond protein-based components, polysaccharide materials are frequently introduced into wound dressings to modulate hydration, microstructure, and the local wound microenvironment [
28]. Such materials can absorb excess wound exudate, maintain a moist interface, and influence the viscoelastic behavior of hydrogel matrices, thereby supporting cell–material interactions during tissue repair. When integrated into composite hydrogels, polysaccharides may serve as effective modulators of network organization and physicochemical properties rather than primary sources of biological activity [
29,
30]. Among these materials, carboxymethyl cellulose (CMC) has been widely applied in tissue engineering field owing to its hydrophilicity, fluid absorption capacity, and established clinical use [
31,
32]. In composite systems, the contribution of CMC is highly dependent on its incorporation level and interaction with protein-based matrices [
29]. Excessive polysaccharide content may disrupt hydrogel network integrity and adversely affect mechanical performance or cellular responses, underscoring the importance of rational formulation design when integrating CMC into multifunctional wound matrices.
From a material design perspective, the integration of protein-based matrices with polysaccharide components provides a rational strategy to address the multifaceted requirements of wound healing materials. By combining gelatin as a structural scaffold, rColIII as a biologically relevant extracellular matrix component, and polysaccharide-based modulators of hydration and network organization, composite hydrogels offer the potential to achieve balanced physicochemical properties and coordinated biological responses. However, systematic investigations that correlate formulation design with material performance and biological outcomes in wound healing remain limited. Recent studies have demonstrated that rCol III-based hydrogels can enhance dermal regeneration through improved ECM mimicry and reduced inflammation, and that polysaccharide-based hydrogels, such as CMC, can provide tunable mechanics, hydration, and degradation [
25,
26,
33]. However, these systems are generally evaluated at isolated material or biological levels. Systematic integration of ECM components with tunable polysaccharide content, together with multi-scale evaluation linking formulation-dependent physicochemical properties to cellular responses and in vivo wound repair, remains largely unexplored.
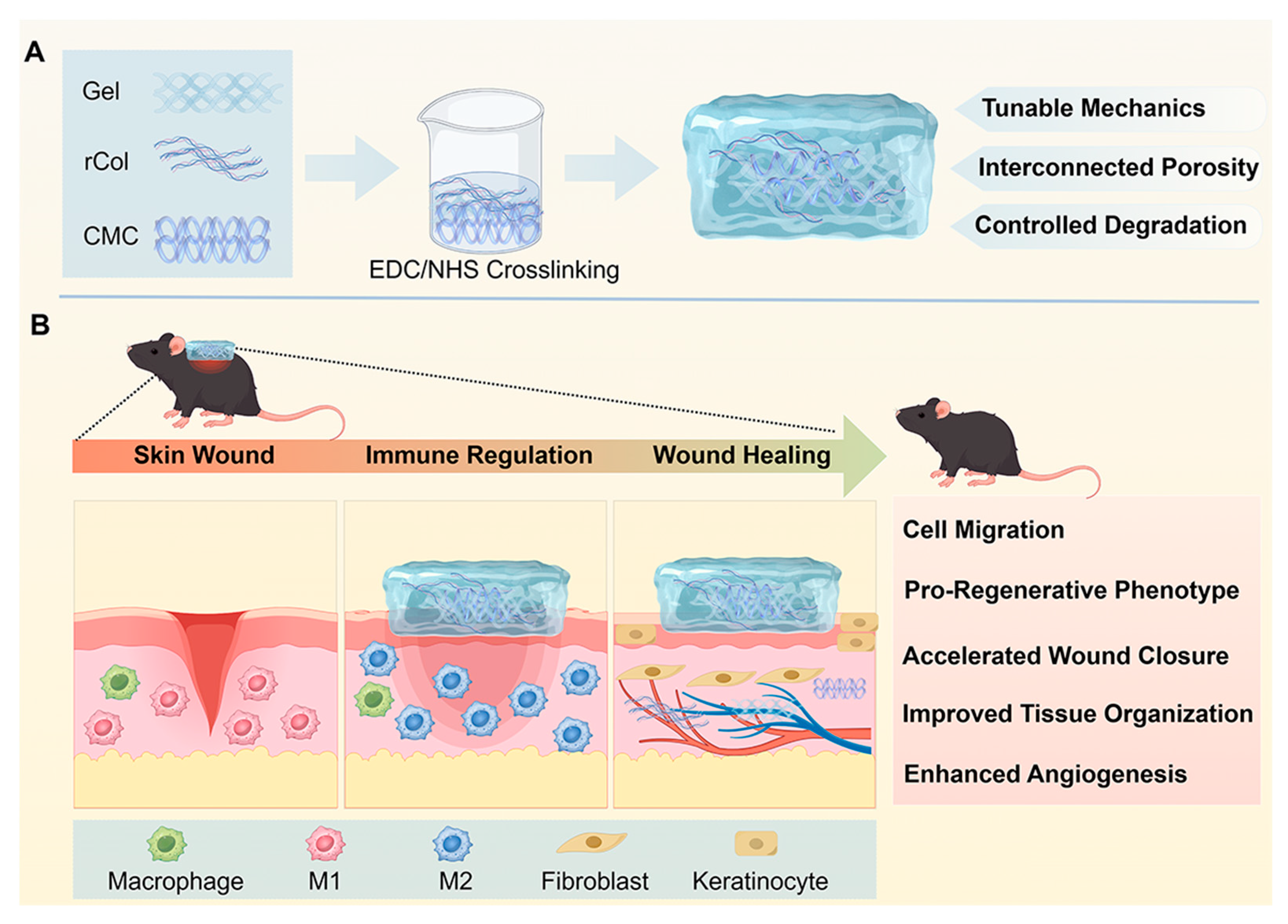
In this study, we developed a series of gelatin-based composite hydrogels incorporating rColIII and CMC via EDC/NHS-mediated crosslinking, aiming to integrate complementary structural and microenvironmental functions for wound healing, as schematically illustrated in
Scheme 1. By systematically varying the composition of the hydrogel network, we evaluated the resulting changes in mechanical properties, microstructure, cytocompatibility, and cellular responses in vitro, as well as wound healing performance in a full-thickness skin wound model in diabetic mice. Through this formulation-driven evaluation, the present work aims to provide insight into the rational design of composite hydrogels as regenerative wound matrices for hard-to-treat wounds.
4. Materials and Methods
4.1. Materials
Recombinant collagen type III (rCol) was purchased from Jiangsu Chuangjian Medical Technology Co., Ltd., Jiangsu, China. Dulbecco’s. Gelatin (porcine, type B) and CMC were purchased from Sigma-Aldrich, Beijing, China. N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich. Collagenase type II was purchased from Worthington, USA. All other chemicals were analytical grade and used without further purification. Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Invitrogen, Shanghai, China. CCK-8 solution and live/dead staining reagent were purchased from Beyotime, Shanghai, China. DAPI and Phalloidin were purchased from Solabio Science & Technology Co., Ltd., Beijing, China. Anti-Ki67 antibodies, anti-CD31 and α-SMA were purchased from Abcam, Cambridge, UK.
4.2. Preparation of Scaffolds with Different Formulations
Five types of scaffolds were fabricated using the EDC/NHS crosslinking method from previously reported work [
34]. To create the Gel/rCol/CMC scaffolds, CMC was first dissolved in MES solution and then mixed with EDC and NHS solutions. After 30 min of stirring, gelatin solution was added to the mixed solution and stirred before rCol was added to the mixture. Hydrogel scaffolds with different rCol concentrations were prepared. Pure gelatin and Gel/rCol hydrogel scaffolds were prepared using the same method as control groups. Different hydrogel formulations were prepared by mixing gelatin, rCol III, and CMC at fixed mass ratios of 10:1:x, where x = 0, 0.1, 1, or 2. Accordingly, the compositions were denoted as Gel/rCol/CMC-0.1 (10:1:0.1), Gel/rCol/CMC-1 (10:1:1), and Gel/rCol/CMC-2 (10:1:2). EDC and NHS were added at an EDC/NHS molar ratio of 2:1 at a 2-fold molar excess relative to CMC carboxyl groups for all Gel/rCol/CMC formulations. A Gel/rCol formulation (10:1) and a gelatin alone (Gel) formulation were also prepared. The detailed compositions of all five scaffolds are summarized in
Table S1. The formed hydrogels were immersed in DI water to exclude the unreacted agents.
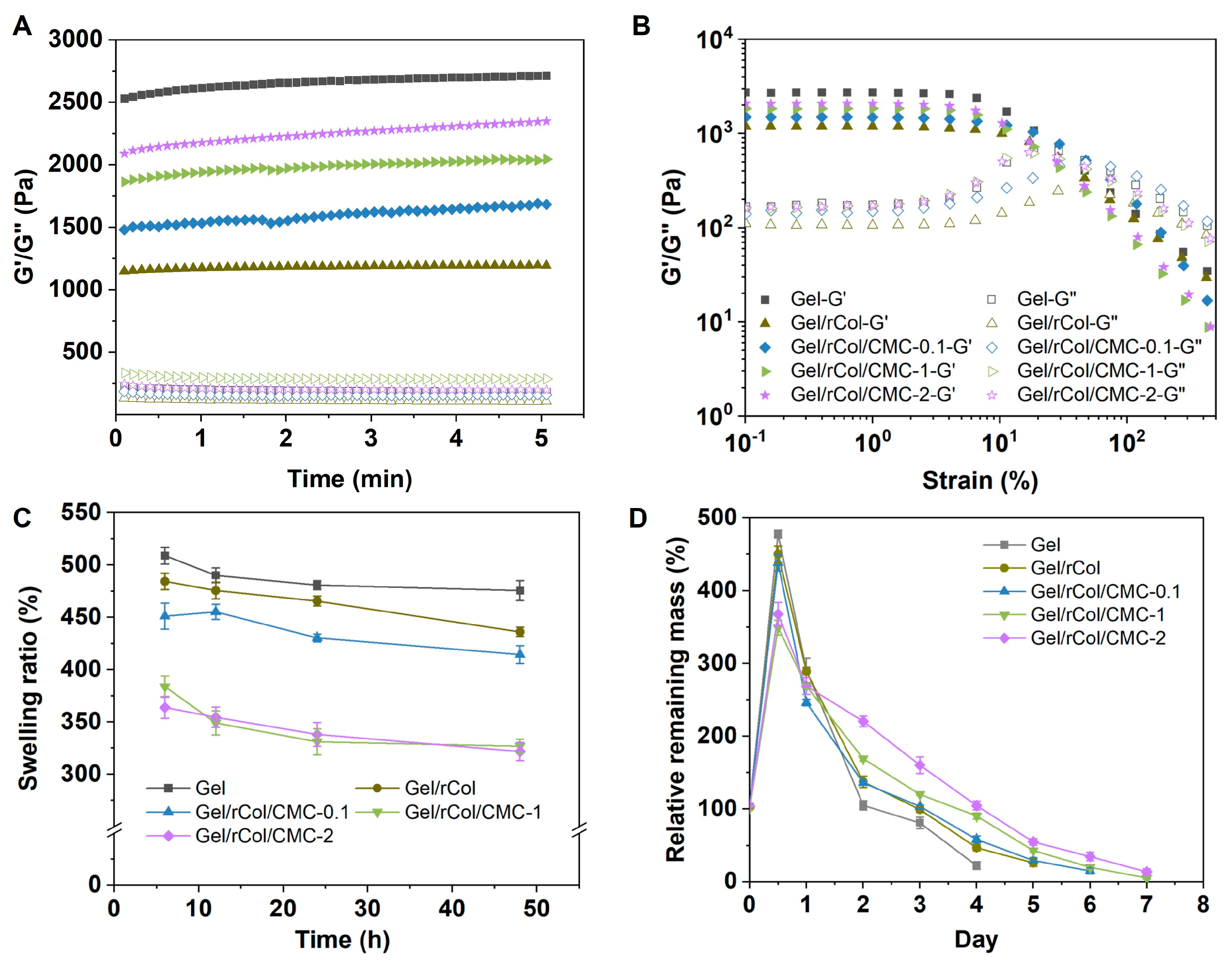
4.3. Rheological Analysis of the Hydrogel Scaffolds
Rheological analysis was performed to determine the mechanical properties of the scaffolds using a TA rheometer (HR-2, Shanghai, China) equipped with an 8 mm parallel plate. The hydrogel scaffolds (200 μL each sample) prepared as mentioned above were added to the HR-2 plate. The upper plate was lowered to a gap size of 1 mm, and samples were tested. The storage modulus (G′) and loss modulus (G″) were recorded at 25 °C under a frequency of 1.0 Hz and a strain of 1%.
4.4. Swelling and Enzymatic Degradation of Hydrogel Scaffolds
The swelling behavior was measured by monitoring the change in mass of dry and wet scaffolds. Freeze-dried scaffolds were used for the swelling and enzymatic degradation experiments. The hydrogel samples were placed in containers and pre-frozen in a −80 °C freezer for 24 h to ensure complete solidification. Subsequently, the pre-frozen samples are promptly transferred to the pre-cooled sample chamber (typically below −50 °C) of a lyophilizer vacuum freeze dryer (SCIENTZ-10N/A, Ningbo, China) and subjected to lyophilization for approximately 3 days. Freeze-dried scaffolds were first weighed and fully immersed in PBS buffer. The scaffolds were wiped with filter paper to remove extra buffer before weighing the wet mass. The swelling ratio was calculated as the ratio of swollen gel weight to dry protein weight.
The enzymatic degradation of hydrogel scaffolds was investigated by immersing 10 mg of each matrix in D-PBS buffer containing Collagenase II. The in vitro degradation rate was determined by measuring the remaining amount (Wt) of the matrix after incubation with 0.5 U/mL Collagenase II for a specified time period, until the matrix was completely degraded. To ensure accurate measurements, the microtubes containing the samples were inverted and checked for any excessive media along the inner walls of the tube for at least 1 min before weighing. The same amount of fresh ultrapure water was added to the tubes after each weighing.
The relative remaining mass of the scaffold during enzymatic degradation was calculated as follows:
where W0 is the initial dry weight of the scaffold, and Wt is the remaining dry weight at each time point.
4.5. Morphology of the Scaffolds
The morphology and porosity of the hydrogel scaffolds were characterized by scanning electron microscopy (SEM). Prior to SEM imaging, the hydrogel samples were frozen at −80 °C, followed by freeze-drying (0.05 mbar, −50 °C, 24 h), and sputter-coated with gold. The scaffolds were cut in half using a razor blade and mounted on an aluminum stub. The samples were coated with a thin layer of gold before observation using an AMETEK® Quanta 3D FEG machine (Berwyn, IL, USA). Quantitative analysis of the pore microstructure of the SEM image was carried out by Image J software (Version 1.54p).
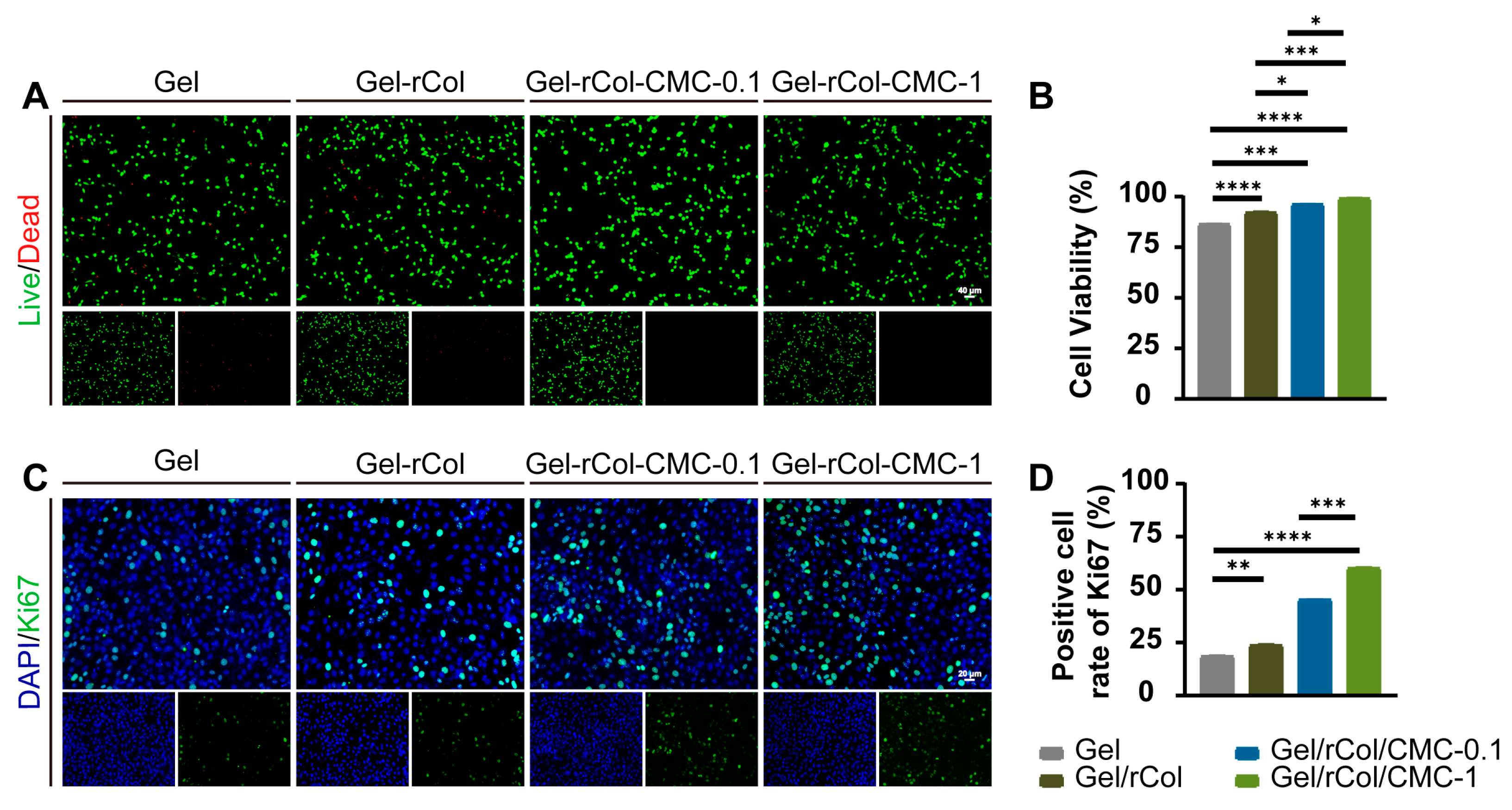
4.6. In Vitro Biocompatibility and Proliferation
CCK-8 assay: The in vitro cytotoxicity of the extract of the scaffolds was tested by a standard CCK-8 assay using 3T3 cells according to the instructions of ISO 10993. First, the hydrogel extract was prepared by immersing 1 g of hydrogel scaffold in 5 mL of full DMEM cell culture media at 37 °C for 24 h. In the meantime, 3T3 cells were seeded in a 96-well plate with a concentration of 8000 per well and left to attach overnight. The hydrogel extract was diluted to a series of concentrations (0.625, 1.25, 2.5, 10 mg/mL). The hydrogel extracts were added to the wells and co-cultured with 3T3 cells for 24 h and 48 h at 37 °C. Full cell culture with no extract was used as the control. Subsequently, 100 μL of fresh DMEM and 10 μL of CCK-8 solution (Beyotime, Shanghai, China) were added to each well for a 1 h of incubation at 37 °C, and the absorbance at 450 nm was measured using a microplate reader. The viability was calculated as follows.
Live/Dead staining: The 3T3 cells were co-cultured with the hydrogel extracts at a concentration of 1 × 105/mL, respectively. Live/dead staining was performed after 24 h of culture at 37 °C following the instructions of the manufacture. Images were taken by an inverted fluorescent microscope. The viability was calculated as follows.
Ki67 immunohistochemistry: Ki67 immunohistochemistry was used to evaluate the proliferation of 3T3 cells. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, stained with anti-Ki67 antibodies (1:200, Abcam, Cambridge, UK) at 4 °C for one night, and then incubated with AF488-conjugated secondary antibody (1:100, Abcam, Cambridge, UK) for 1 h at room temperature. 4′,6-diamidino-2-phenylindole (DAPI) was used to detect the nuclei in immunofluorescence staining. The number of Ki67-positive cells was counted using Image J software. The calculation formula for the positive cell ratio was as follows.
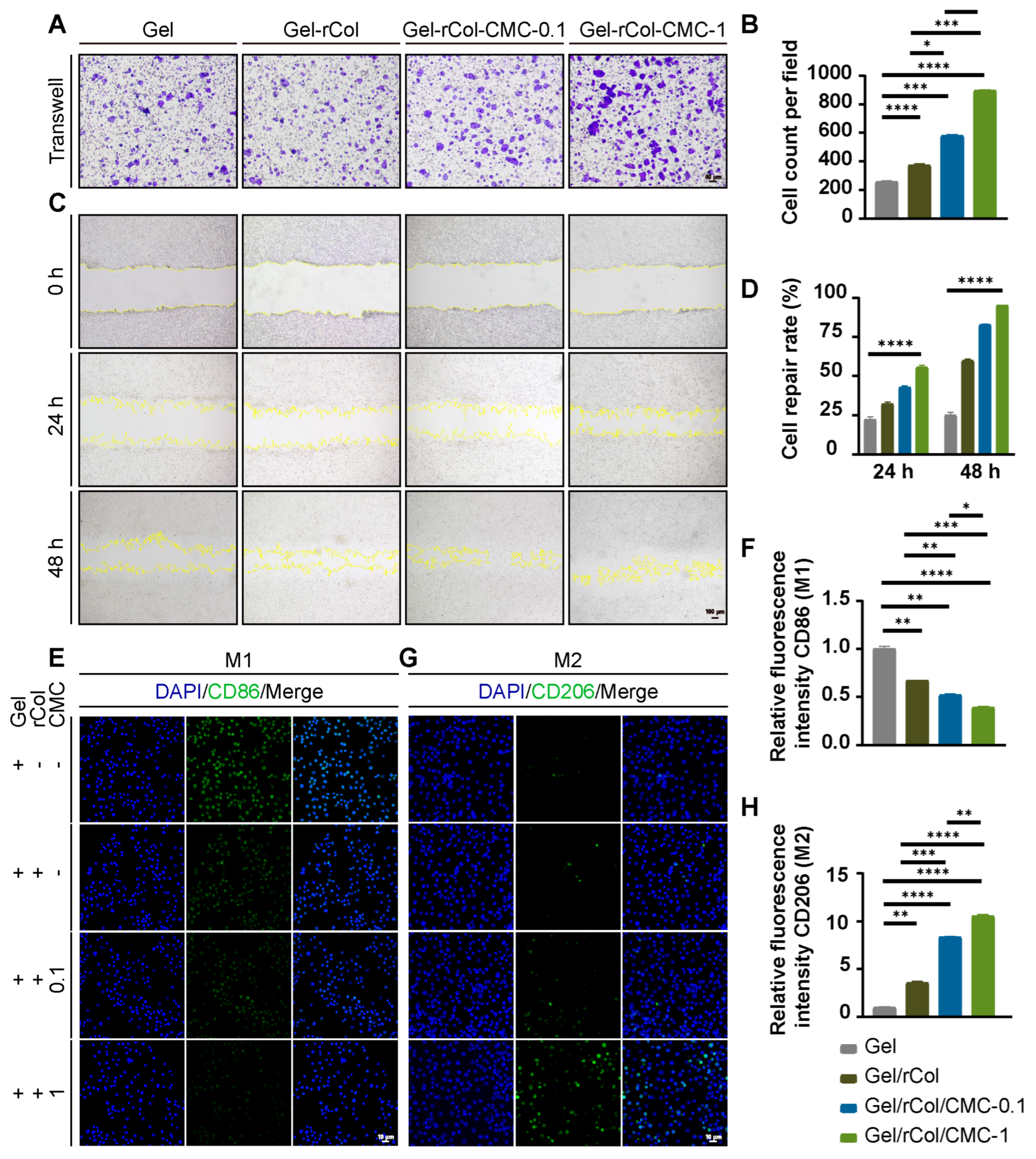
Transwell assay: We used 6-well Transwell inserts with 8 μm pore size filters and 6-well culture plates. Cells were suspended in medium containing 10% FBS and plated at 5 × 105 cells per well in the upper chamber. Then, 1 mL of medium containing hydrogel extract was added to the lower chamber. After 24 h of incubation, cells on the upper surface of the filter membrane were removed with a cotton swab, and the migrated cells on the lower side of the filter membrane were stained with crystal violet. Photos were taken at every time point in each group. Positive cells were counted by Image J software.
Cells wound scratch assay: The proliferation of 3T3 cells was recorded using an FSX100 microscope. The proliferation distance of cells at every indication time was observed and analyzed as a ratio of proliferation area to original area. The cell scratch assay was performed using 3T3 cells. First, 1 mL of 3T3 cells (1 × 105 cells/well) was seeded in a 12-well plate and placed in a 37 °C incubator with 5% CO2 for 24 h. A 200 μL pipette tip was used to induce a scratch in the 3T3 cells at a density of 70–80% per well. The 70–80% confluence allowed for both migration and proliferation over the 48 h of observation, avoiding contact inhibition of 3T3 cells at 90–100% confluence. Cell closures were recorded by microscope at each time point. The proliferation rate was determined as the ratio of proliferated area to original area. The fusion of cells was recorded by a microscope with an initial cell density of 1 × 105, and photos were taken at every time point in each group. The percentage of wound closure was calculated for each field using the following formula.
Phenotype Assessment of Macrophage Polarization: For immunofluorescence, macrophages were seeded in confocal microscopy dishes for 24 h and subsequently activated with LPS (1 mg/mL) for 24 h. Macrophages were incubated with different hydrogels for another 24 h before fixing with 4% paraformaldehyde. Then, macrophages were incubated with primary antibodies against CD86, and CD206 at 4 °C for 12 h, and then incubated with respective fluorescent-labeled secondary antibodies for 1 h. Cell nuclei were stained with DAPI. Finally, samples were observed by confocal microscopy, and the fluorescence intensity was calculated with ImageJ software (Version 1.54p). The calculation formula for the positive cell ratio was as follows:
4.7. Animal Experiments
Male C57BL/6 mice weighing 20–25 g were utilized to establish STZ-induced diabetic wounds and to create an excisional skin wound model. Sprague–Dawley (SD) male rats 8–10 weeks in age with body weights ranging from 220 to 260 g were used for subcutaneous hydrogel implantation for biodegradation and biosafety tests. The animals were fed ad libitum water and a rodent diet. All procedures were approved by the Laboratory Animal Resources Center of Wenzhou Medical College (China), Approval No. xmsq2023-0462.
4.8. In Vivo Degradation and Tissue Integration of the Hydrogel Scaffolds
SD rats were used to test the in vivo degradation and tissue integration of the hydrogel scaffolds by subcutaneous implantation. A total of 36 SD rats were randomly assigned to three groups (Gel/rCol, Gel/rCol/CMC-0.1, and Gel/rCol/CMC-1), with n = 12 animals per group.
Rats were anesthetized with intraperitoneal injection of 10% chloral hydrate (3.0 mg/kg). Two subcutaneous pockets were made on the dorsum of each animal by blunt dissection, with two on each side. Each hydrogel weighing 200 mg was implanted in each pocket, and the incision was sutured with silk stitches. Implants were sampled every week until no obvious hydrogel remained (five samples for each time point). Gross images were recorded to observe the remaining hydrogels and the potential abnormal response.
4.9. In Vivo Wound Healing Tests
The wound healing effects of the scaffolds were tested in STZ-induced diabetic C57BL/6 mice. A total of 48 STZ-induced diabetic mice were randomly assigned into four groups (Blank, Gel/rCol, Gel/rCol/CMC-0.1, and Gel/rCol/CMC-1), with n = 12 animals per groups.
One wound on the dorsal surface of each mouse were created using a sterile 1 cm punch biopsy tool. Full-thickness wounds extending through the panniculus carnosus were excised using a sterile scissor. The treatment was then applied to the wounds. There were three treatment groups, including a saline control. The wounds were covered with sterile gauze after treatment. Digital photographs of the wounds were taken on the day of surgery and every 3 days thereafter. Wound closure rate was determined by measuring the wound area and calculated as the percentage of the original wound.
Hematoxylin and eosin (H&E) and Masson’s trichrome were used to visualize the in vivo degradation, tissue integration, and neo-vessel formation of the samples following the manufacturer’s instruction for use. After staining, the prepared slides were examined under an optical microscope to gain insight into the structure and composition of the tissue.
Epidermal thickness was measured on H&E-stained vertical skin sections. For each sample, five random fields were selected. In each field, three perpendicular measurements from the basal layer to the stratum corneum were taken using Image J software. The mean epidermal thickness for each sample was calculated by averaging all individual measurements. Group means were then derived from multiple biological replicates.
Quantification of collagen deposition: Collagen deposition was assessed in Masson’s trichrome-stained sections. Five randomly selected fields per sample were captured under high magnification. Using ImageJ software with the color deconvolution plugin, the blue-stained collagen fibers were specifically isolated and thresholded. The collagen volume fraction (CVF) was calculated as follows.
The mean CVF from all fields was used for statistical analysis.
CD31 and α-SMA immunohistochemistry: The sections were fixed in acetone at −20 °C for immunofluorescence treatment. The sections were stained with CD31 and α-SMA antibodies. After washing with PBS, the sections were incubated with secondary antibody. For double immunofluorescence staining of CD31 and α-SMA, paraffin sections were first dewaxed and washed with PBS. Then, the sections were blocked with serum and incubated with anti-CD31 primary antibody and anti-α-SMA antibody. Finally, the sections were stained with DAPI and photographed. Image J was used to count the number of CD31+-stained vessels and the number of mature vessels stained with both CD31 and α-SMA.
4.10. Statistical Analysis
All values are expressed as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism (Version 8.0.2), Origin (Version 2025b), and Image J software (Version 1.54p). We analyzed the comparisons between groups using Student’s t test, one-way ANOVA followed by Tukey’s multiple comparisons test, and Dunnett’s multiple comparisons test. Differences were considered statistically significant when ns, not significant, * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.