Vanillin Beyond Flavor: Therapeutic Potentials and Emerging Applications in Hydrogel-Based Biomaterials
Abstract
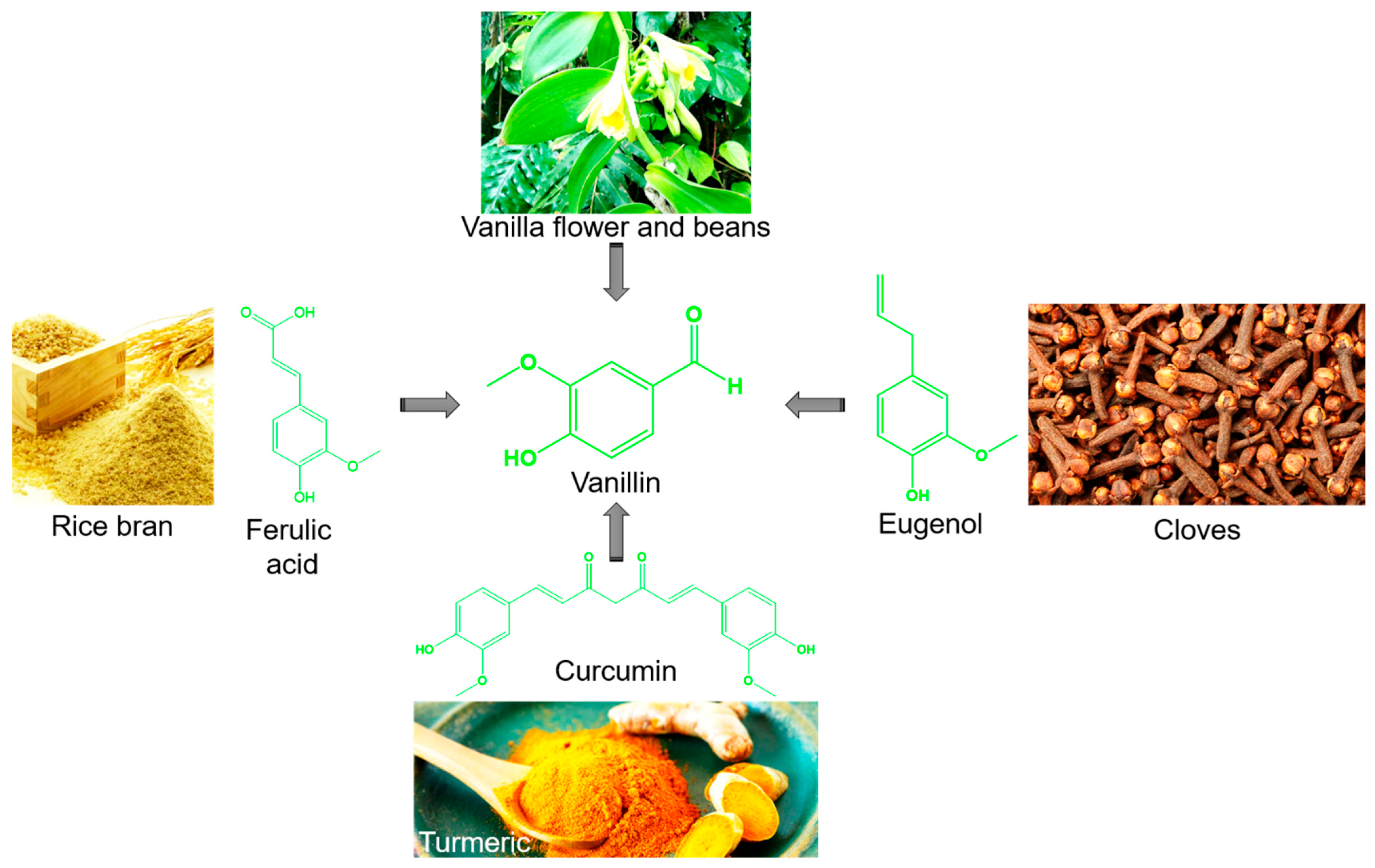
1. Introduction
2. Pharmacological Properties of Vanillin
2.1. Anticancer Properties
2.2. Antimicrobial Properties
2.3. Antioxidant and Anti-Inflammatory Properties
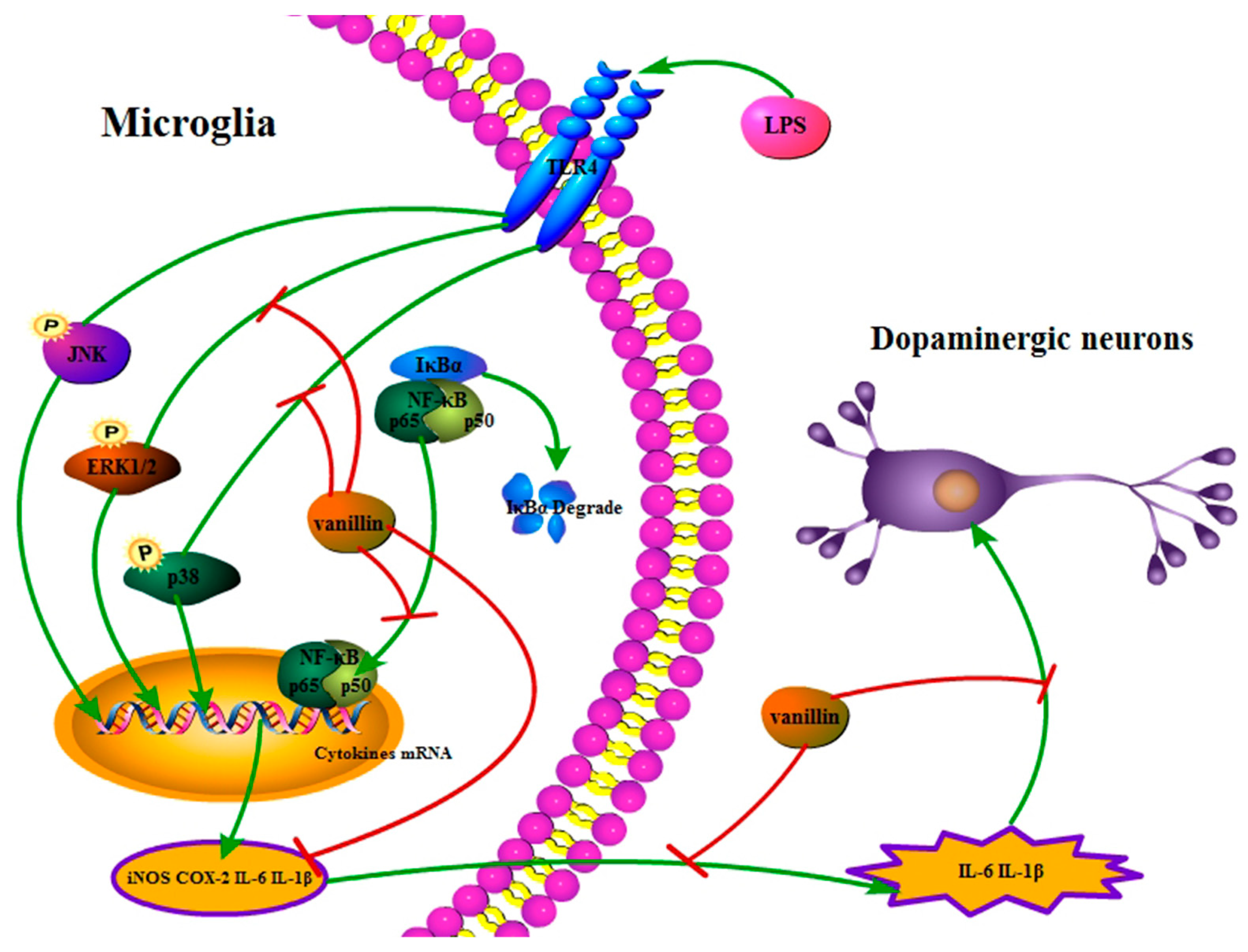
2.4. Neuroprotective Properties
2.5. Hepatoprotective and Nephroprotective Properties
2.6. Anti-Hyperglycemic and Anti-Hyperlipidemic Properties
3. Improving Pharmacological Properties of Vanillin Using Biomaterials
4. Vanillin-Based Hydrogels: Design, Properties, and Biomedical Applications
4.1. Overview of Hydrogels
4.2. Rheological and Mechanical Properties of Vanillin-Based Hydrogels
4.3. Vanillin-Based Hydrogels for Wound Healing
4.4. Vanillin-Based Hydrogels for Anticancer Therapy
4.5. Vanillin-Based Hydrogels for Anti-Inflammatory Applications
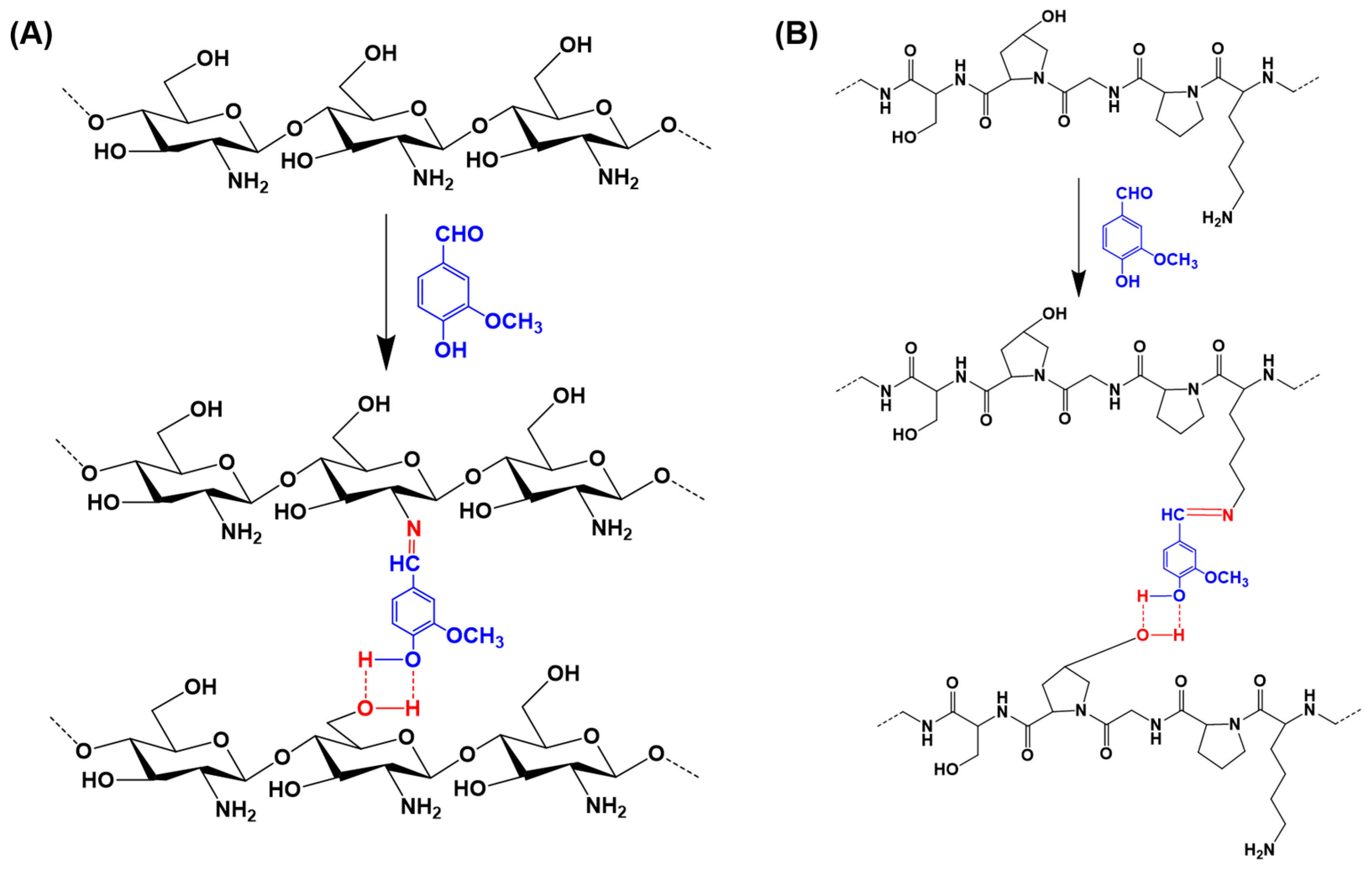
4.6. Vanillin as a Crosslinker or Functional Moiety in Hydrogel Networks
4.7. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Sun, L.; Huo, Y.-X.; Guo, S. Strategies for improving the production of bio-based vanillin. Microb. Cell Factories 2023, 22, 147. [Google Scholar] [CrossRef]
- Gallage, N.J.; Hansen, E.H.; Kannangara, R.; Olsen, C.E.; Motawia, M.S.; Jørgensen, K.; Holme, I.; Hebelstrup, K.; Grisoni, M.; Møller, B.L. Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat. Commun. 2014, 5, 4037. [Google Scholar] [CrossRef]
- Sano, K.; Uzawa, Y.; Kaneshima, I.; Nakasato, S.; Hashimoto, M.; Tanaka, Y.; Nakatani, S.; Kobata, K. Vanillin reduction in the biosynthetic pathway of capsiate, a non-pungent component of Capsicum fruits, is catalyzed by cinnamyl alcohol dehydrogenase. Sci. Rep. 2022, 12, 12384. [Google Scholar] [CrossRef] [PubMed]
- Subramani, G.; Manian, R. Optimizing bio-vanillin synthesis from ferulic acid via Pediococcus acidilactici: A systematic approach to process enhancement and yield maximization. J. Biotechnol. 2024, 393, 49–60. [Google Scholar] [CrossRef]
- Yan, L.; Chen, P.; Zhang, S.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci. Rep. 2016, 6, 34644. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Banerjee, G.; Sen, S.K. Cleaner production of vanillin through biotransformation of ferulic acid esters from agroresidue by Streptomyces sannanensis. J. Clean. Prod. 2018, 182, 272–279. [Google Scholar] [CrossRef]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A food additive with multiple biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Kafali, M.; Finos, M.A.; Tsoupras, A. Vanillin and Its Derivatives: A Critical Review of Their Anti-Inflammatory, Anti-Infective, Wound-Healing, Neuroprotective, and Anti-Cancer Health-Promoting Benefits. Nutraceuticals 2024, 4, 522–561. [Google Scholar] [CrossRef]
- Ngarmsak, M.; Delaquis, P.; Toivonen, P.; Ngarmsak, T.; Ooraikul, B.; Mazza, G. Antimicrobial Activity of Vanillin against Spoilage Microorganisms in Stored Fresh-Cut Mangoes. J. Food Prot. 2006, 69, 1724–1727. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef]
- Sindhu, G.; Nishanthi, E.; Sharmila, R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: A biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. [Google Scholar] [CrossRef]
- Gallage, N.J.; Møller, B.L. Vanillin–Bioconversion and Bioengineering of the Most Popular Plant Flavor and Its De Novo Biosynthesis in the Vanilla Orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef]
- Ciciliato, M.P.; de Souza, M.C.; Tarran, C.M.; de Castilho, A.L.T.; Vieira, A.J.; Rozza, A.L. Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation. Pharmaceutics 2022, 14, 755. [Google Scholar] [CrossRef]
- Turabee, M.H.; Thambi, T.; Lee, D.S. Development of an Injectable Tissue Adhesive Hybrid Hydrogel for Growth Factor-Free Tissue Integration in Advanced Wound Regeneration. ACS Appl. Bio Mater. 2019, 2, 2500–2510. [Google Scholar] [CrossRef]
- Santos, J.H.; Graf, U.; Reguly, M.L.; Rodrigues de Andrade, H.H. The synergistic effects of vanillin on recombination predominate over its antimutagenic action in relation to MMC-induced lesions in somatic cells of Drosophila melanogaster. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 444, 355–365. [Google Scholar] [CrossRef]
- Sinigaglia, M.; Reguly, M.L.; de Andrade, H.H.R. Effect of vanillin on toxicant-induced mutation and mitotic recombination in proliferating somatic cells of Drosophila melanogaster. Environ. Mol. Mutagen. 2004, 44, 394–400. [Google Scholar] [CrossRef]
- Ho, K.; Yazan, L.S.; Ismail, N.; Ismail, M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009, 33, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Song, P.; Zhang, Q.; Wu, Z.; Wang, J.; Li, X.; Xu, R.; Zhao, W.; Liu, Y.; et al. A vanillin derivative suppresses the growth of HT29 cells through the Wnt/β-catenin signaling pathway. Eur. J. Pharmacol. 2019, 849, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Srinual, S.; Chanvorachote, P.; Pongrakhananon, V. Suppression of cancer stem-like phenotypes in NCI-H460 lung cancer cells by vanillin through an Akt-dependent pathway. Int. J. Oncol. 2017, 50, 1341–1351. [Google Scholar] [CrossRef]
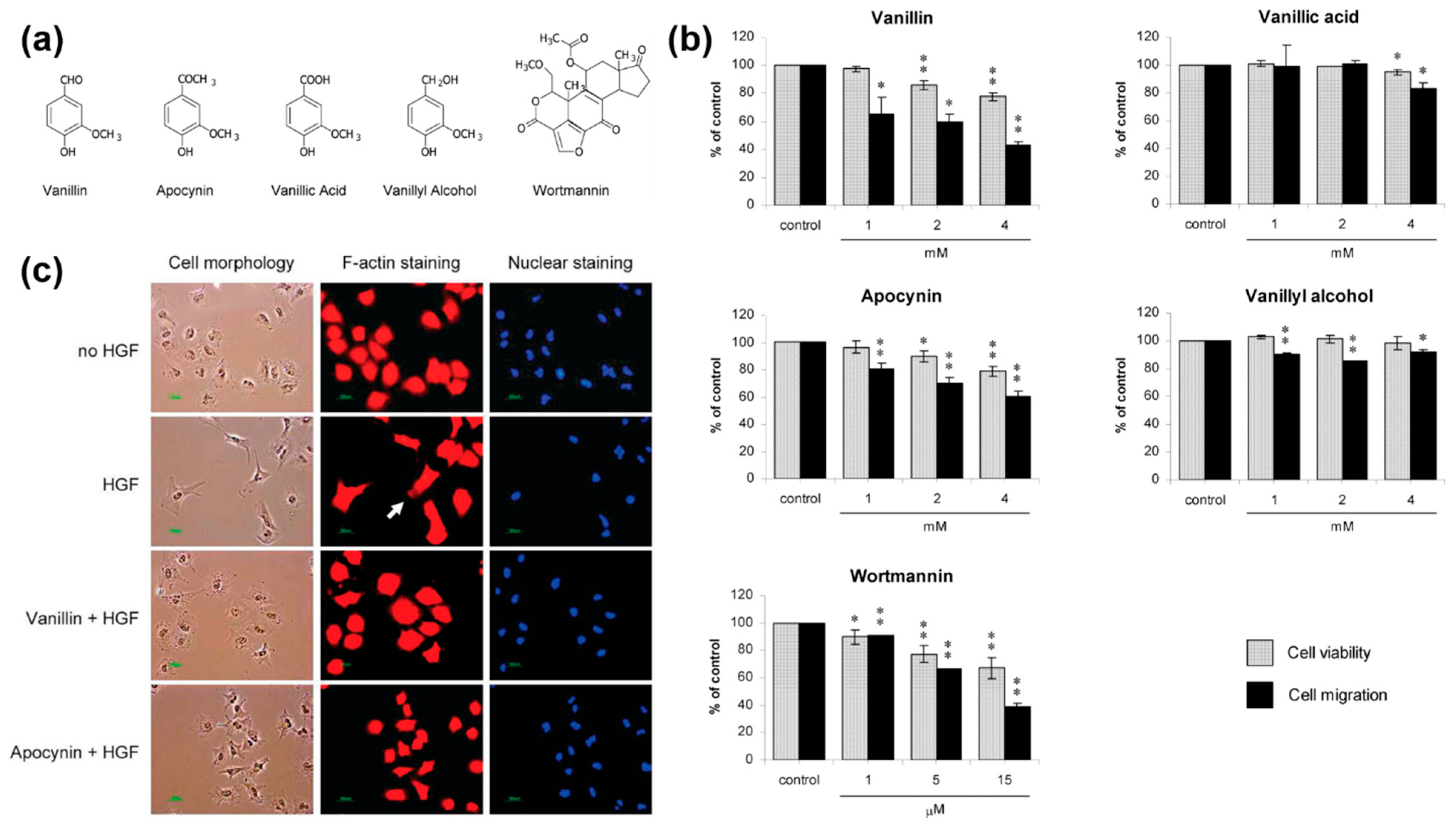
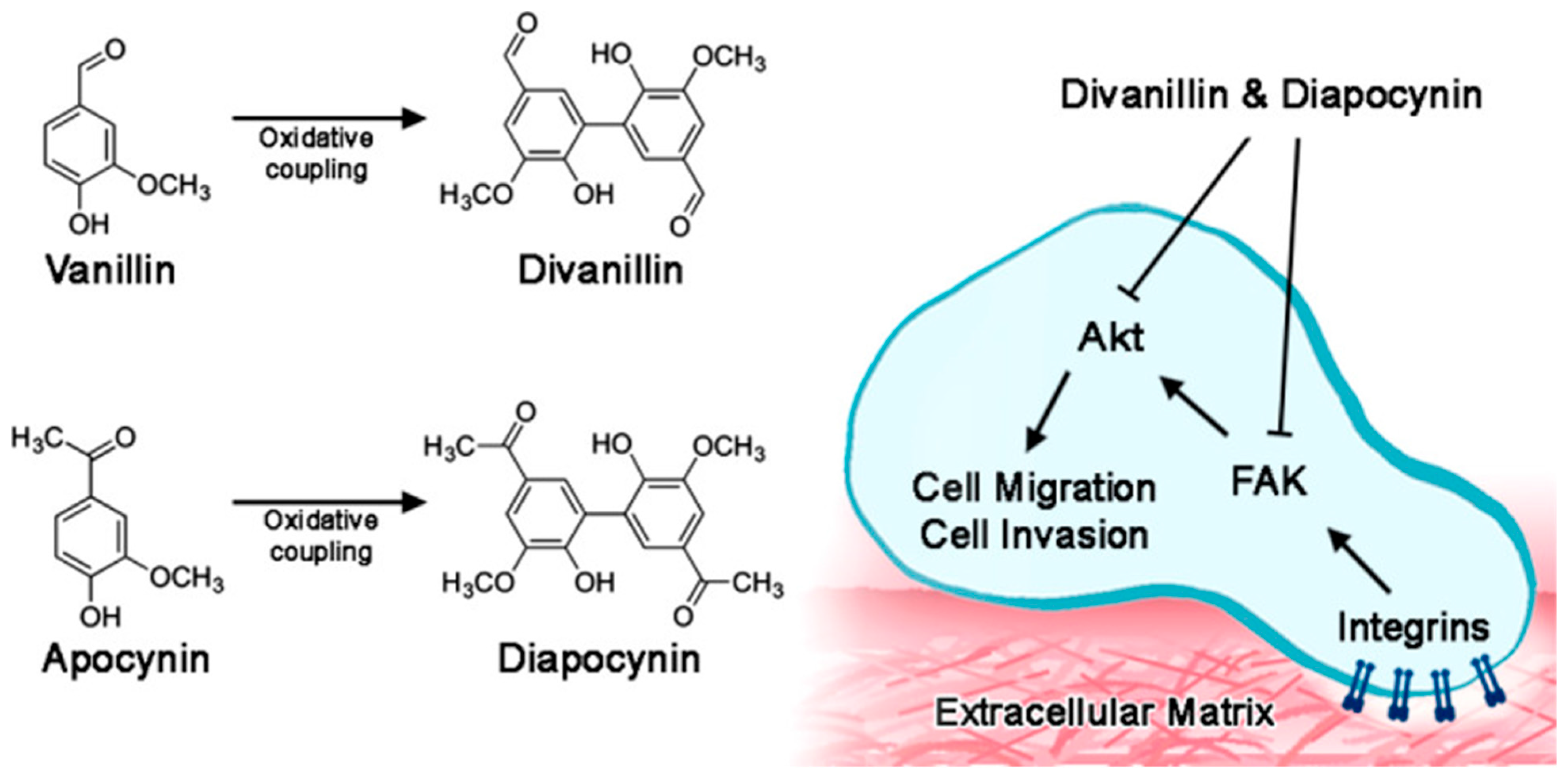
- Jantaree, P.; Lirdprapamongkol, K.; Kaewsri, W.; Thongsornkleeb, C.; Choowongkomon, K.; Atjanasuppat, K.; Ruchirawat, S.; Svasti, J. Homodimers of Vanillin and Apocynin Decrease the Metastatic Potential of Human Cancer Cells by Inhibiting the FAK/PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2017, 65, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Naz, H.; Tarique, M.; Khan, P.; Luqman, S.; Ahamad, S.; Islam, A.; Ahmad, F.; Hassan, M.I. Evidence of vanillin binding to CAMKIV explains the anti-cancer mechanism in human hepatic carcinoma and neuroblastoma cells. Mol. Cell Biochem. 2018, 438, 35–45. [Google Scholar] [CrossRef]
- Akagi, K.; Hirose, M.; Hoshiya, T.; Mizoguchi, Y.; Ito, N.; Shirai, T. Modulating effects of ellagic acid, vanillin and quercetin in a rat medium term multi-organ carcinogenesis model. Cancer Lett. 1995, 94, 113–121. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Younis, N.N.; Shaheen, M.A.; Elseweidy, M.M. The synergistic effect between vanillin and doxorubicin in ehrlich ascites carcinoma solid tumor and MCF-7 human breast cancer cell line. Pathol. Res. Pract. 2016, 212, 767–777. [Google Scholar] [CrossRef]
- Li, M.; Lang, Y.; Gu, M.-M.; Shi, J.; Chen, B.P.C.; Yu, L.; Zhou, P.-K.; Shang, Z.-F. Vanillin derivative VND3207 activates DNA-PKcs conferring protection against radiation-induced intestinal epithelial cells injury in vitro and in vivo. Toxicol. Appl. Pharmacol. 2020, 387, 114855. [Google Scholar] [CrossRef]
- Maurya, D.K.; Adhikari, S.; Nair, C.K.K.; Devasagayam, T.P.A. DNA protective properties of vanillin against γ-radiation under different conditions: Possible mechanisms. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 634, 69–80. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure—Function Analysis of the Vanillin Molecule and Its Antifungal Properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. The Potential Application of Vanillin in Preventing Yeast Spoilage of Soft Drinks and Fruit Juices. J. Food Prot. 2004, 67, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cortes, T.; Pérez España, V.H.; López Pérez, P.A.; Rodríguez-Jimenes, G.D.C.; Robles-Olvera, V.J.; Aparicio Burgos, J.E.; Cuervo-Parra, J.A. Antifungal activity of vanilla juice and vanillin against Alternaria alternata. CyTA J. Food 2019, 17, 375–383. [Google Scholar] [CrossRef]
- Sánchez, J.C.; García, R.F.; Cors, M.T. 1,1-Diphenyl-2-picrylhydrazyl radical and superoxide anion scavenging activity of Rhizophora mangle (L.) bark. Pharmacogn. Res. 2010, 2, 279–284. [Google Scholar] [CrossRef]
- Kadeche, L.; Bourogaa, E.; Saoudi, M.; Boumendjel, A.; Djeffal, A.; Feki, A.E.; Messarah, M. Ameliorative Effects of Vanillin Against Metribuzin-Induced Oxidative Stress and Toxicity in Rats. Int. J. Pharm. Pharm. Sci. 2017, 9, 56–62. [Google Scholar] [CrossRef]
- Ben Saad, H.; Kharrat, N.; Driss, D.; Gargouri, M.; Marrakchi, R.; Jammoussi, K.; Magné, C.; Boudawara, T.; Ellouz Chaabouni, S.; Zeghal, K.M.; et al. Effects of vanillin on potassium bromate-induced neurotoxicity in adult mice: Impact on behavior, oxidative stress, genes expression, inflammation and fatty acid composition. Arch. Physiol. Biochem. 2017, 123, 165–174. [Google Scholar] [CrossRef]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui, E.M.; Barkallah, M.; Marouani, C.; Kallel, C.; Zeghal, N. Erythrocyte oxidative damage in rat treated with CCl4: Protective role of vanillin. Toxicol. Ind. Health 2012, 28, 908–916. [Google Scholar] [CrossRef]
- Yan, X.; Liu, D.F.; Zhang, X.Y.; Liu, D.; Xu, S.Y.; Chen, G.X.; Huang, B.X.; Ren, W.Z.; Wang, W.; Fu, S.P.; et al. Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Na, J.Y.; Park, Y.D.; Lee, J.S. Anti-Neuroinflammatory Effects of Vanillin Through the Regulation of Inflammatory Factors and NF-κB Signaling in LPS-Stimulated Microglia. Appl. Biochem. Biotechnol. 2019, 187, 884–893. [Google Scholar] [CrossRef]
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Takahashi, Y.; Matsumoto, Y.; Okamoto, M.; Fujiwara, Y.; et al. Comprehensive behavioral study of the effects of vanillin inhalation in mice. Biomed. Pharmacother. 2019, 115, 108879. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, C.; Manivasagam, T.; Nataraj, J.; Justin Thenmozhi, A.; Essa, M.M. Neurosupportive Role of Vanillin, a Natural Phenolic Compound, on Rotenone Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 626028. [Google Scholar] [CrossRef]
- Dhanalakshmi, C.; Janakiraman, U.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M.; Kalandar, A.; Khan, M.A.; Guillemin, G.J. Vanillin Attenuated Behavioural Impairments, Neurochemical Deficts, Oxidative Stress and Apoptosis Against Rotenone Induced Rat Model of Parkinson’s Disease. Neurochem. Res. 2016, 41, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.L.; Antunes, J.S.; Lovison, K.; Kunz, R.I.; Castor, L.R.G.; Brancalhão, R.M.C.; Bertolini, G.R.F.; Ribeiro, L.F.C. Action of vanillin (Vanilla planifolia) on the morphology of tibialis anterior and soleus muscles after nerve injury. Einstein (Sao Paulo) 2017, 15, 186–191. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef]
- Beaudry, F.; Ross, A.; Lema, P.P.; Vachon, P. Pharmacokinetics of vanillin and its effects on mechanical hypersensitivity in a rat model of neuropathic pain. Phytother. Res. 2010, 24, 525–530. [Google Scholar] [CrossRef]
- Makni, M.; Chtourou, Y.; Barkallah, M.; Fetoui, H. Protective effect of vanillin against carbon tetrachloride (CCl4)-induced oxidative brain injury in rats. Toxicol. Ind. Health 2012, 28, 655–662. [Google Scholar] [CrossRef]
- Fouad, A.A.; Al-Melhim, W.N. Vanillin mitigates the adverse impact of cisplatin and methotrexate on rat kidneys. Hum. Exp. Toxicol. 2018, 37, 937–943. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S. Gastrodia elata Blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: Vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur. J. Nutr. 2011, 50, 107–118. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.; Msomi, N.Z.; Ijomone, O.M.; Islam, M.S. Vanillin improves glucose homeostasis and modulates metabolic activities linked to type 2 diabetes in fructose–streptozotocin induced diabetic rats. Arch. Physiol. Biochem. 2021, 130, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Zabad, I.E.M.; Amin, M.N.; El-Shishtawy, M.M. Protective effect of vanillin on diabetic nephropathy by decreasing advanced glycation end products in rats. Life Sci. 2019, 239, 117088. [Google Scholar] [CrossRef] [PubMed]
- Belagali, Y.; Ullal, S.D.; Shoeb, A.; Bhagwath, V.; Ramya, K.; Maskeri, R. Effect of vanillin on lipid profile in a model of hyperlipidemia, a preliminary study. Indian J. Exp. Biol. 2013, 51, 288–291. [Google Scholar]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.-H.; Son, S.; Jo, D.-G.; Ahn, C.-H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Saravanakumar, G.; Chu, J.-U.; Heo, R.; Ko, H.; Deepagan, V.G.; Kim, J.-H.; Park, J.H. Synthesis and physicochemical characterization of reduction-sensitive block copolymer for intracellular delivery of doxorubicin. Macromol. Res. 2013, 21, 100–107. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Phan, V.H.G.; Lee, D.S.; Thambi, T.; Huynh, D.P. Bioresorbable pH- and temperature-responsive injectable hydrogels-incorporating electrosprayed particles for the sustained release of insulin. Polym. Degrad. Stab. 2019, 162, 36–46. [Google Scholar] [CrossRef]
- Dalmolin, L.F.; Khalil, N.M.; Mainardes, R.M. Delivery of vanillin by poly(lactic-acid) nanoparticles: Development, characterization and in vitro evaluation of antioxidant activity. Mater. Sci. Eng. C 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Mathiyalagan, R.; Murugesan, M.; Ramadhania, Z.M.; Nahar, J.; Manivasagan, P.; Boopathi, V.; Jang, E.-S.; Yang, D.C.; Conde, J.; Thambi, T. Triterpenoid saponin-based supramolecular host-guest injectable hydrogels inhibit the growth of melanoma via ROS-mediated apoptosis. Mater. Sci. Eng. R Rep. 2024, 160, 100824. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Nguyen, P.P.T.; Luu, C.H.; Le, N.-H.H.; Nguyen, H.T.; Manivasagan, P.; Jang, E.-S.; Li, Y.; Thambi, T. Biomimetic injectable hydrogel based on silk fibroin/hyaluronic acid embedded with methylprednisolone for cartilage regeneration. Colloids Surf. B Biointerfaces 2022, 219, 112859. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Le, T.M.D.; Janarthanan, G.; Ngo, P.-K.T.; Lee, D.S.; Thambi, T. Development of bioresorbable smart injectable hydrogels based on thermo-responsive copolymer integrated bovine serum albumin bioconjugates for accelerated healing of excisional wounds. J. Ind. Eng. Chem. 2021, 96, 345–355. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, S.H.; Giang Phan, V.H.; Thambi, T.; Lee, D.S. Therapeutic effects of boronate ester cross-linked injectable hydrogels for the treatment of hepatocellular carcinoma. Biomater. Sci. 2021, 9, 7275–7286. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, C.; Li, Z.; Gu, Z.; Yang, J.; Luo, K. Chitosan-based hydrogel dressings for diabetic wound healing via promoting M2 macrophage-polarization. Carbohydr. Polym. 2024, 331, 121873. [Google Scholar] [CrossRef] [PubMed]
- Marchianò, V.; Matos, M.; López, M.; Weng, S.; Serrano-Pertierra, E.; Luque, S.; Blanco-López, M.C.; Gutiérrez, G. Nanovesicles as Vanillin Carriers for Antimicrobial Applications. Membranes 2023, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Castan, L.; Del Toro, G.; Fernández, A.A.; González, M.; Ortíz, E.; Lobo, D. Biological activity of liposomal vanillin. J. Med. Food 2013, 16, 551–557. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Solid Inclusion Complexes of Vanillin with Cyclodextrins: Their Formation, Characterization, and High-Temperature Stability. J. Agric. Food Chem. 2011, 59, 11772–11778. [Google Scholar] [CrossRef]
- Zhao, D.; Shi, D.; Sun, J.; Li, H.; Zhao, M.; Sun, B. Quantification and cytoprotection by vanillin, 4-methylguaiacol and 4-ethylguaiacol against AAPH-induced abnormal oxidative stress in HepG2 cells. RSC Adv. 2018, 8, 35474–35484. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Thambi, T.; Giang Phan, V.H.; Kim, S.H.; Duy Le, T.M.; Duong, H.T.T.; Lee, D.S. Smart injectable biogels based on hyaluronic acid bioconjugates finely substituted with poly(β-amino ester urethane) for cancer therapy. Biomater. Sci. 2019, 7, 5424–5437. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Thambi, T.; Giang Phan, V.H.; Lee, D.S. Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Carbohydr. Polym. 2020, 233, 115832. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Jung, J.M.; Lee, D.S. Recent strategies to develop pH-sensitive injectable hydrogels. Biomater. Sci. 2023, 11, 1948–1961. [Google Scholar] [CrossRef]
- Ngo, P.-K.T.; Nguyen, D.N.; Nguyen, H.-P.; Tran, T.-H.H.; Nguyen, Q.-N.D.; Luu, C.H.; Phan, T.-H.; Le, P.K.; Phan, V.H.G.; Ta, H.T.; et al. Silk fibroin/chitosan/montmorillonite sponge dressing: Enhancing hemostasis, antimicrobial activity, and angiogenesis for advanced wound healing applications. Int. J. Biol. Macromol. 2024, 279, 135329. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Giang Phan, V.H.; Pan, Z.; Xuan, X.; Yang, H.Y.; Luu, C.H.; Phan, T.-H.; Le, T.M.D.; Thambi, T. Integrated and hyaluronic acid-coated mesoporous silica nanoparticles conjugated with cisplatin and chlorin e6 for combined chemo and photodynamic cancer therapy. Eur. Polym. J. 2024, 220, 113426. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Min Jung, J.; Lip Jung, Y.; Han Kim, S.; Sung Lee, D.; Thambi, T. Injectable hydrogel imbibed with camptothecin-loaded mesoporous silica nanoparticles as an implantable sustained delivery depot for cancer therapy. J. Colloid Interface Sci. 2023, 636, 328–340. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Duong, H.-S.; Le, Q.-G.T.; Janarthanan, G.; Vijayavenkataraman, S.; Nguyen, H.-N.H.; Nguyen, B.-P.T.; Manivasagan, P.; Jang, E.-S.; Li, Y.; et al. Nanoengineered injectable hydrogels derived from layered double hydroxides and alginate for sustained release of protein therapeutics in tissue engineering applications. J. Nanobiotechnol. 2023, 21, 405. [Google Scholar] [CrossRef] [PubMed]
- Luu, C.H.; Nguyen, G.; Le, T.-T.; Nguyen, T.-M.N.; Giang Phan, V.H.; Murugesan, M.; Mathiyalagan, R.; Jing, L.; Janarthanan, G.; Yang, D.C.; et al. Graphene Oxide-Reinforced Alginate Hydrogel for Controlled Release of Local Anesthetics: Synthesis, Characterization, and Release Studies. Gels 2022, 8, 246. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Mathiyalagan, R.; Nguyen, M.-T.; Tran, T.-T.; Murugesan, M.; Ho, T.-N.; Huong, H.; Yang, D.C.; Li, Y.; Thambi, T. Ionically cross-linked alginate-chitosan core-shell hydrogel beads for oral delivery of insulin. Int. J. Biol. Macromol. 2022, 222, 262–271. [Google Scholar] [CrossRef]
- Murugesan, M.; Mathiyalagan, R.; Ramadhania, Z.M.; Nahar, J.; Luu, C.H.; Phan, V.H.G.; Yang, D.C.; Zhou, Q.; Chan Kang, S.; Thambi, T. Tailoring hyaluronic acid hydrogels: Impact of cross-linker length and density on skin rejuvenation as injectable dermal fillers and their potential effects on the MAPK signaling pathway suppression. Bioact. Mater. 2025, 49, 154–171. [Google Scholar] [CrossRef]
- Sarisuta, K.; Iwami, M.; Martín-Vaca, B.; Chanthaset, N.; Ajiro, H. pH Effect on Particle Aggregation of Vanillin End-Capped Polylactides Bearing a Hydrophilic Group Connected by a Cyclic Acetal Moiety. Langmuir 2023, 39, 3994–4004. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Nguyen, B.-P.T.; Nguyen, N.Y.; Tran, C.-N.D.; Nguyen, Q.-N.D.; Luu, C.H.; Manivasagan, P.; Jang, E.-S.; Yang, D.C.; Yang, D.U.; et al. Longan-inspired chitosan-pectin core-shell hydrogel beads for oral delivery of biodrugs to enhance osteoporosis therapy. Int. J. Biol. Macromol. 2025, 308, 142254. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Luu, C.H.; Murugesan, M.; Nguyen, T.-N.-Q.; Ha, N.-Y.N.; Ngo, H.L.; Nguyen, N.-D.H.; Pan, Z.; Phan, V.H.G.; Li, Y.; et al. Injectable gelatin-pectin hydrogel for dental tissue engineering: Enhanced angiogenesis and antibacterial efficacy for pulpitis therapy. Int. J. Biol. Macromol. 2025, 284, 137939. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, B.-P.T.; Ho, T.-N.; Tran, C.-N.D.; Tran, T.-H.H.; Nguyen, H.-P.H.; Nguyen, H.-P.; Huynh, N.-T.; Li, Y.; Phan, V.H.G.; et al. Orally ingestible medication utilizing layered double hydroxide nanoparticles strengthened alginate and hyaluronic acid-based hydrogel bead for bowel disease management. Int. J. Biol. Macromol. 2024, 269, 132122. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Giang Phan, V.H.; Kim, S.H.; Duy Le, T.M.; Lee, D.S. Hyaluronic acid decorated pH- and temperature-induced injectable bioconjugates for sustained delivery of bioactive factors and highly efficient wound regeneration. New J. Chem. 2019, 43, 18979–18982. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Thambi, T.; Yin, Y.; Kim, S.H.; Nguyen, T.L.; Phan, V.H.G.; Kim, J.; Jeong, J.H.; Lee, D.S. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials 2020, 230, 119599. [Google Scholar] [CrossRef]
- Le, T.M.D.; Duong, H.T.T.; Thambi, T.; Giang Phan, V.H.; Jeong, J.H.; Lee, D.S. Bioinspired pH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules 2018, 19, 3536–3548. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, J.; Hwang, K.; Chun, S.-J.; Kim, J.H.; Lee, T.-J.; Lee, B.-T.; Cho, H.-J.; Kim, B.-J.; Wu, Q.; et al. Poly(vinyl alcohol) Hydrogels Reinforced with Cellulose Nanocrystals for Sustained Delivery of Salicylic Acid. ACS Appl. Nano Mater. 2024, 7, 3918–3930. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Thambi, T.; Kim, B.S.; Huynh, D.P.; Lee, D.S. Engineering highly swellable dual-responsive protein-based injectable hydrogels: The effects of molecular structure and composition in vivo. Biomater. Sci. 2017, 5, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
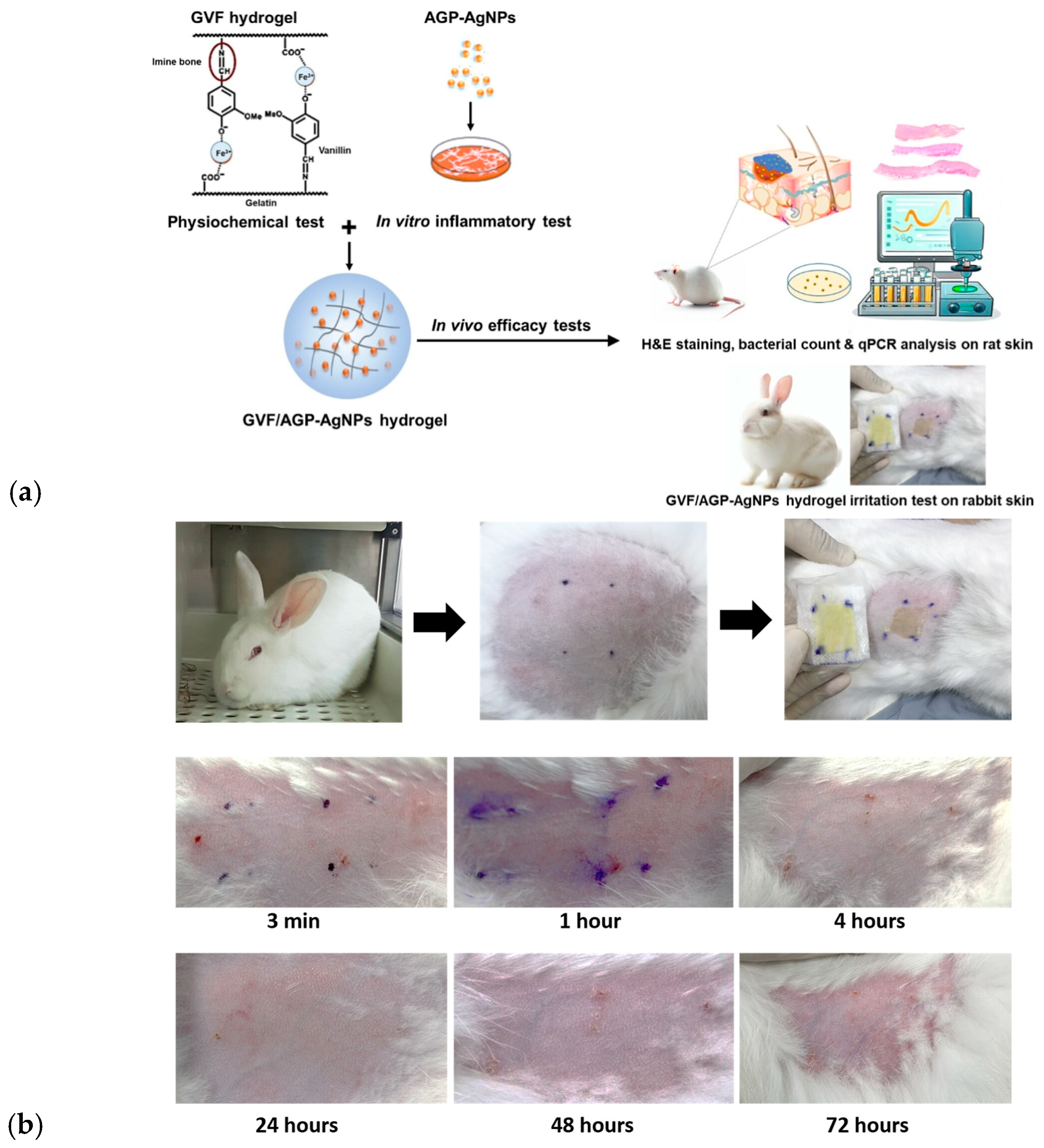
- Talodthaisong, C.; Patramanon, R.; Thammawithan, S.; Lapmanee, S.; Maikaeo, L.; Sricharoen, P.; Khongkow, M.; Namdee, K.; Jantimaporn, A.; Kayunkid, N.; et al. A Shear-Thinning, Self-Healing, Dual-Cross Linked Hydrogel Based on Gelatin/Vanillin/Fe3+/AGP-AgNPs: Synthesis, Antibacterial, and Wound-Healing Assessment. Macromol. Biosci. 2023, 23, 2300250. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Pyun, D.G.; Choi, H.J.; Yoon, H.S.; Thambi, T.; Lee, D.S. Polyurethane foam containing rhEGF as a dressing material for healing diabetic wounds: Synthesis, characterization, in vitro and in vivo studies. Colloids Surf. B Biointerfaces 2015, 135, 699–706. [Google Scholar] [CrossRef]
- Pyun, D.G.; Yoon, H.S.; Chung, H.Y.; Choi, H.J.; Thambi, T.; Kim, B.S.; Lee, D.S. Evaluation of AgHAP-containing polyurethane foam dressing for wound healing: Synthesis, characterization, in vitro and in vivo studies. J. Mater. Chem. B 2015, 3, 7752–7763. [Google Scholar] [CrossRef]
- Choi, H.J.; Thambi, T.; Yang, Y.H.; Bang, S.I.; Kim, B.S.; Pyun, D.G.; Lee, D.S. AgNP and rhEGF-incorporating synergistic polyurethane foam as a dressing material for scar-free healing of diabetic wounds. RSC Adv. 2017, 7, 13714–13725. [Google Scholar] [CrossRef]
- Eghbali, H.; Sadeghi, M.; Noroozi, M.; Movahedifar, F. Vanillin crosslinked 3D porous chitosan hydrogel for biomedicine applications: Preparation and characterization. J. Mech. Behav. Biomed. Mater. 2023, 145, 106044. [Google Scholar] [CrossRef]
- Amir, F.; Niazi, M.B.K.; Malik, U.S.; Jahan, Z.; Andleeb, S.; Ahmad, T.; Mustansar, Z. A multifunctional vanillin-infused chitosan-PVA hydrogel reinforced by nanocellulose and CuO-Ag nanoparticles as antibacterial wound dressing. Int. J. Biol. Macromol. 2024, 258, 128831. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Kamoun, E.A.; Badawi, N.M.; El-Moslamy, S.H.; Kh, M.; Salim, S.A. Cutting-edge biomaterials for advanced biomedical uses: Self-gelation of l-arginine-loaded chitosan/PVA/vanillin hydrogel for accelerating topical wound healing and skin regeneration. RSC Adv. 2024, 14, 31126–31142. [Google Scholar] [CrossRef] [PubMed]
- Lapmanee, S.; Bhubhanil, S.; Khongkow, M.; Namdee, K.; Yingmema, W.; Bhummaphan, N.; Wongchitrat, P.; Charoenphon, N.; Hutchison, J.A.; Talodthaisong, C.; et al. Application of Gelatin/Vanillin/Fe3+/AGP–AgNPs Hydrogels Promotes Wound Contraction, Enhances Dermal Growth Factor Expression, and Minimizes Skin Irritation. ACS Omega 2025, 10, 10530–10545. [Google Scholar] [CrossRef]
- Xiong, S.; Li, R.; Ye, S.; Ni, P.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.; Zhang, X. Vanillin enhances the antibacterial and antioxidant properties of polyvinyl alcohol-chitosan hydrogel dressings. Int. J. Biol. Macromol. 2022, 220, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, M.; Hemalatha, D.; Kim, H.; Rao, K.S.V.K.; Han, S.S. Vanillin/fungal-derived carboxy methyl chitosan/polyvinyl alcohol hydrogels prepared by freeze-thawing for wound dressing applications. Int. J. Biol. Macromol. 2024, 266, 130910. [Google Scholar] [CrossRef]
- Hao, X.; Gao, Z.; Hu, M. Anti-tumor role and molecular mechanism of vanillic acid. Discov. Oncol. 2025, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Kramb, J.-P.; Suthiphongchai, T.; Surarit, R.; Srisomsap, C.; Dannhardt, G.; Svasti, J. Vanillin Suppresses Metastatic Potential of Human Cancer Cells through PI3K Inhibition and Decreases Angiogenesis in Vivo. J. Agric. Food Chem. 2009, 57, 3055–3063. [Google Scholar] [CrossRef]
- Salahuddin, N.; Awad, S.; Elfiky, M. Vanillin-crosslinked chitosan/ZnO nanocomposites as a drug delivery system for 5-fluorouracil: Study on the release behavior via mesoporous ZrO2–Co3O4 nanoparticles modified sensor and antitumor activity. RSC Adv. 2022, 12, 21422–21439. [Google Scholar] [CrossRef]
- Lirdprapamongkol, K.; Sakurai, H.; Suzuki, S.; Koizumi, K.; Prangsaengtong, O.; Viriyaroj, A.; Ruchirawat, S.; Svasti, J.; Saiki, I. Vanillin enhances TRAIL-induced apoptosis in cancer cells through inhibition of NF-kappaB activation. In Vivo 2010, 24, 501–506. [Google Scholar]
- Kim, M.C.; Kim, S.J.; Kim, D.S.; Jeon, Y.D.; Park, S.J.; Lee, H.S.; Um, J.Y.; Hong, S.H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, L. Injectable smart-blended hydrogel cross-linked with Vanillin to accelerate differentiation of intervertebral disc-derived stem cells (IVDSCs) for promoting degenerative nucleolus pulposus in a rat model. Inflammopharmacology 2024, 32, 3443–3459. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Q.; Li, H.; Han, F.; Guo, Q.; Sun, H.; Zhao, H.; Tu, Z.; Liu, Z.; Zhu, C.; et al. Vanillin-based functionalization strategy to construct multifunctional microspheres for treating inflammation and regenerating intervertebral disc. Bioact. Mater. 2023, 28, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kim, E.; Abdelghani, H.T.M.; Han, S.S. Gelatin methacrylate/vanillin-based hydrogels: Synthesis, bio-compatibility and antibacterial evaluation for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2025, 721, 137161. [Google Scholar] [CrossRef]
- Nadim, E.; Major, I.; Devine, D.; Paraskar, P. Biobased self-healing functional composites and their applications. J. Mater. Sci. Compos. 2025, 6, 3. [Google Scholar] [CrossRef]
- Yasar, M.; Oktay, B.; Dal Yontem, F.; Haciosmanoglu Aldogan, E.; Kayaman Apohan, N. Development of self-healing vanillin/PEI hydrogels for tissue engineering. Eur. Polym. J. 2023, 188, 111933. [Google Scholar] [CrossRef]
- da Silva, R.L.C.G.; Bezjak, D.; Corrales, T.P.; Kappl, M.; Petri, D.F.S. Chitosan/vanillin/polydimethylsiloxane scaffolds with tunable stiffness for muscle cell proliferation. Int. J. Biol. Macromol. 2025, 286, 138445. [Google Scholar] [CrossRef]
- Kou, Z.; Hu, Y.; Ma, Y.; Yuan, L.; Hu, L.; Zhou, Y.; Jia, P. Bio-based dynamically crosslinked networks/Fe3O4 nanoparticles composites with self-healing ability driven by multiple stimuli for non-contact welding. Ind. Crops Prod. 2023, 195, 116401. [Google Scholar] [CrossRef]
- Gover, E.; Rondinella, A.; Marino, M.; Brovedani, A.; Tavani, A.; Lopriore, M.; Alongi, M.; Manzocco, L.; Fedrizzi, L.; Goi, D.; et al. Vanillin-based photoactive materials: A sustainable approach to antimicrobial solutions. J. Photochem. Photobiol. A Chem. 2025, 468, 116501. [Google Scholar] [CrossRef]
- Zou, Q.; Li, J.; Li, Y. Preparation and characterization of vanillin-crosslinked chitosan therapeutic bioactive microcarriers. Int. J. Biol. Macromol. 2015, 79, 736–747. [Google Scholar] [CrossRef]
- Michailidou, G.; Koukaras, E.N.; Bikiaris, D.N. Vanillin chitosan miscible hydrogel blends and their prospects for 3D printing biomedical applications. Int. J. Biol. Macromol. 2021, 192, 1266–1275. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Houreld, N.N. Vanillin Enhances Photobiomodulation Wound Healing by Modulating Glyco-Oxidative Stress and Glucose Dysmetabolism in Diabetic Wounded Fibroblast Cells. J. Cell Mol. Med. 2025, 29, e70537. [Google Scholar] [CrossRef]
- Rakoczy, K.; Szlasa, W.; Saczko, J.; Kulbacka, J. Therapeutic role of vanillin receptors in cancer. Adv. Clin. Exp. Med. 2021, 30, 1293–1301. [Google Scholar] [CrossRef]
- Wang, J.; An, W.; Wang, Z.; Zhao, Y.; Han, B.; Tao, H.; Wang, J.; Wang, X. Vanillin Has Potent Antibacterial, Antioxidant, and Anti-Inflammatory Activities In Vitro and in Mouse Colitis Induced by Multidrug-Resistant Escherichia coli. Antioxidants 2024, 13, 1544. [Google Scholar] [CrossRef]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The evolving role of investigative toxicology in the pharmaceutical industry. Nat. Rev. Drug Discov. 2023, 22, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, J.W.; Thambi, T.; Yoon, A.R.; Choi, J.-W.; Li, Y.; Bui, Q.N.; Lee, D.S.; Yun, C.-O. Optimizing Active Tumor Targeting Biocompatible Polymers for Efficient Systemic Delivery of Adenovirus. Cells 2021, 10, 1896. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Yoo, C.K.; Park, J.H. Synthesis and physicochemical characterization of amphiphilic block copolymers bearing acid-sensitive orthoester linkage as the drug carrier. Polymer 2011, 52, 4753–4759. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Manivasagan, P.; Nguyen, T.L.; Phan, T.-H.; Luu, C.H.; Ho, D.-K.; Li, Y.; Kim, J.; Lee, D.S.; et al. Injectable Hydrogel Based on Protein-Polyester Microporous Network as an Implantable Niche for Active Cell Recruitment. Pharmaceutics 2022, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; You, D.G.; Han, H.S.; Deepagan, V.G.; Jeon, S.M.; Suh, Y.D.; Choi, K.Y.; Kim, K.; Kwon, I.C.; Yi, G.-R.; et al. Bioreducible Carboxymethyl Dextran Nanoparticles for Tumor-Targeted Drug Delivery. Adv. Healthc. Mater. 2014, 3, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Shao, Y.F.; Vu, Q.L.; Wu, P.C. Designing of pH-Sensitive Hydrogels for Colon Targeted Drug Delivery; Characterization and In Vitro Evaluation. Gels 2022, 8, 155. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Ko, H.; Lee, D.S.; Park, J.H. Bioreducible polymersomes for intracellular dual-drug delivery. J. Mater. Chem. 2012, 22, 22028–22036. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in natural polymer-based hydrogels: Synthesis, applications, and future directions in biomedical and environmental fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Ngo, P.-K.T.; Luu, C.H.; Nguyen, H.-P.; Nguyen, D.N.; Nguyen, K.D.; Van Luu, T.; Le, P.K.; Pan, Z.; Phan, V.H.G.; Li, Y.; et al. Multifunctional haemostatic and antibacterial wound dressing: Chitosan-silk fibroin composite with green-synthesised silver nanoparticles and deferoxamine. Surf. Interfaces 2025, 72, 107112. [Google Scholar] [CrossRef]
- Chand, R.; Janarthanan, G.; Elkhoury, K.; Vijayavenkataraman, S. Digital light processing 3D bioprinting of biomimetic corneal stroma equivalent using gelatin methacryloyl and oxidized carboxymethylcellulose interpenetrating network hydrogel. Biofabrication 2025, 17, 025011. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, F.; Han, Z.; Qu, X.; Li, J.; Zhou, Z.; Chen, S.; Wang, H.; Lv, X. Bacterial cellulose-based hydrogel with regulated rehydration and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2024, 267, 131291. [Google Scholar] [CrossRef]
- Lagneau, N.; Tournier, P.; Halgand, B.; Loll, F.; Maugars, Y.; Guicheux, J.; Le Visage, C.; Delplace, V. Click and bioorthogonal hyaluronic acid hydrogels as an ultra-tunable platform for the investigation of cell-material interactions. Bioact. Mater. 2023, 24, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Magiera, A.; Kołodziejczyk-Czepas, J.; Olszewska, M.A. Antioxidant and Anti-Inflammatory Effects of Vanillic Acid in Human Plasma, Human Neutrophils, and Non-Cellular Models In Vitro. Molecules 2025, 30, 467. [Google Scholar] [CrossRef]
- Salih, T.; Caputo, M.; Ghorbel, M.T. Recent Advances in Hydrogel-Based 3D Bioprinting and Its Potential Application in the Treatment of Congenital Heart Disease. Biomolecules 2024, 14, 861. [Google Scholar] [CrossRef]
- Kamaraj, S.; Palanisamy, U.M.; Kadhar Mohamed, M.S.B.; Gangasalam, A.; Maria, G.A.; Kandasamy, R. Curcumin drug delivery by vanillin-chitosan coated with calcium ferrite hybrid nanoparticles as carrier. Eur. J. Pharm. Sci. 2018, 116, 48–60. [Google Scholar] [CrossRef]
- Li, L.; Yu, M.; Li, Y.; Li, Q.; Yang, H.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2021, 6, 1255–1266. [Google Scholar] [CrossRef]
- Yun, H.-M.; Kim, E.; Kwon, Y.-J.; Park, K.-R. Vanillin Promotes Osteoblast Differentiation, Mineral Apposition, and Antioxidant Effects in Pre-Osteoblasts. Pharmaceutics 2024, 16, 485. [Google Scholar] [CrossRef]
- Al-Baqami, N.; Hamza, R. Synergistic antioxidant capacities of vanillin and chitosan nanoparticles against reactive oxygen species, hepatotoxicity, and genotoxicity induced by aging in male Wistar rats. Hum. Exp. Toxicol. 2021, 40, 183–202. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, H.; Zhang, Y.; Huang, X.; Ke, Q.; Kou, X. Vanillin strengthened complex coacervation behavior between gelatin and sodium carboxymethyl cellulose endowed improved mechanical properties of microcapsules. Int. J. Biol. Macromol. 2025, 306, 141386. [Google Scholar] [CrossRef] [PubMed]
- Trezza, A.; Mahboob, L.; Visibelli, A.; Geminiani, M.; Santucci, A. Lignin Waste Valorization in the Bioeconomy Era: Toward Sustainable Innovation and Climate Resilience. Appl. Sci. 2025, 15, 8038. [Google Scholar] [CrossRef]
- Khare, S.; Kumar, S.; Urmaliya, P.; Yadav, S. Recent advancement and applications of green hydrogels: Revolutionizing biomedicine and environmental sustainability. Results Chem. 2025, 18, 102857. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Cui, L.; Yang, D.U.; Liu, J.; Mathiyalagan, R.; Kim, J.-H.; Subramaniyam, S.; Chen, C.; Yang, D.-C.; Li, L. Vanillin Beyond Flavor: Therapeutic Potentials and Emerging Applications in Hydrogel-Based Biomaterials. Gels 2026, 12, 16. https://doi.org/10.3390/gels12010016
Cui L, Yang DU, Liu J, Mathiyalagan R, Kim J-H, Subramaniyam S, Chen C, Yang D-C, Li L. Vanillin Beyond Flavor: Therapeutic Potentials and Emerging Applications in Hydrogel-Based Biomaterials. Gels. 2026; 12(1):16. https://doi.org/10.3390/gels12010016
Chicago/Turabian StyleCui, Lei, Dong Uk Yang, Jing Liu, Ramya Mathiyalagan, Jong-Hoon Kim, Sathiyamoorthy Subramaniyam, Changbao Chen, Deok-Chun Yang, and Ling Li. 2026. "Vanillin Beyond Flavor: Therapeutic Potentials and Emerging Applications in Hydrogel-Based Biomaterials" Gels 12, no. 1: 16. https://doi.org/10.3390/gels12010016
APA StyleCui, L., Yang, D. U., Liu, J., Mathiyalagan, R., Kim, J.-H., Subramaniyam, S., Chen, C., Yang, D.-C., & Li, L. (2026). Vanillin Beyond Flavor: Therapeutic Potentials and Emerging Applications in Hydrogel-Based Biomaterials. Gels, 12(1), 16. https://doi.org/10.3390/gels12010016







