Research Progress on the Application of Food Colloids in Precise Targeted Delivery of Drugs and Bioactive Compounds
Abstract
1. Introduction
2. Food Colloid Delivery Carriers
2.1. Definition and Classification of Food Colloids
2.2. Overview of Targeted Delivery Systems
2.3. The Physicochemical Properties of Food Colloids and Their Application Basis in Delivery Systems
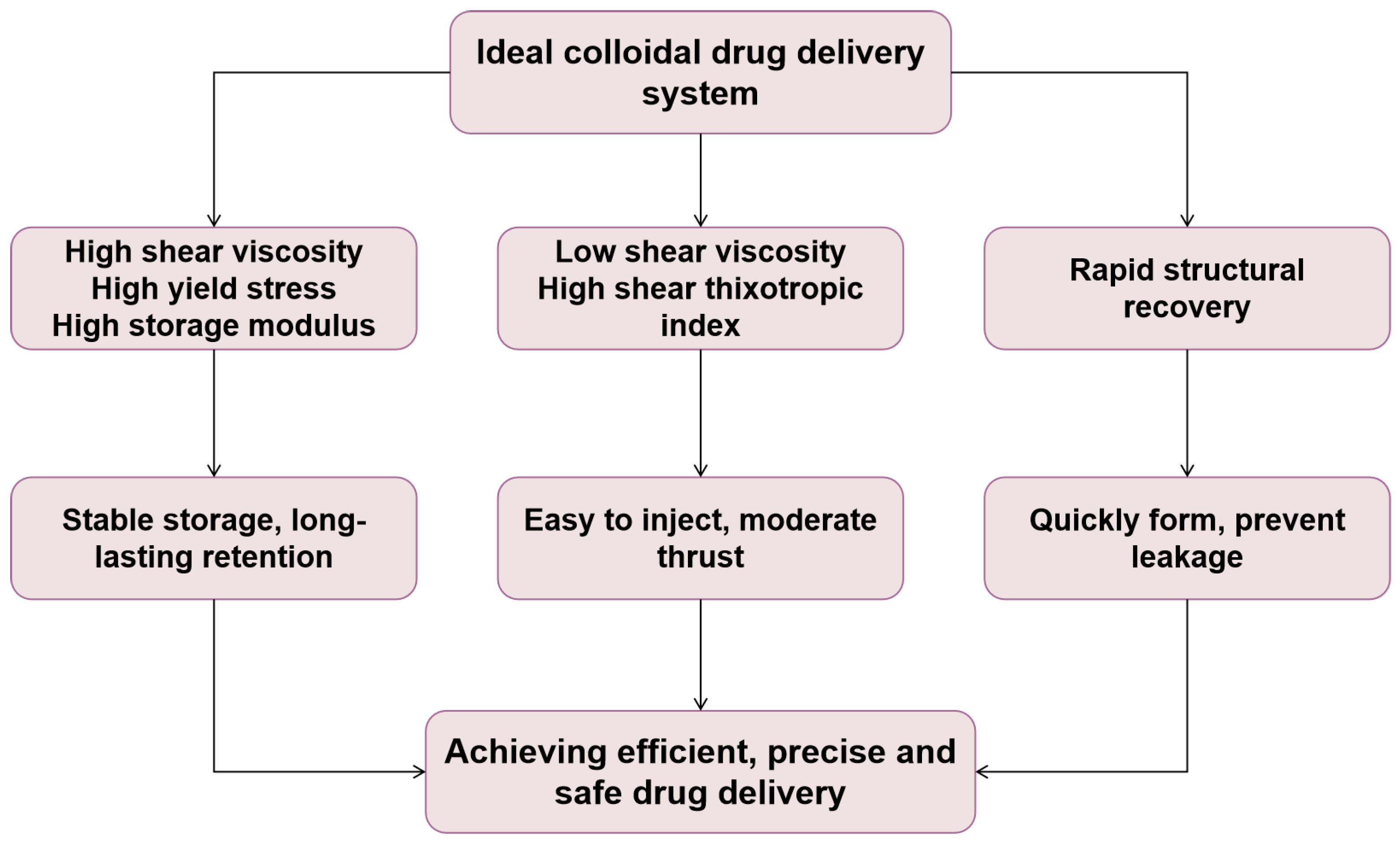
2.3.1. Rheological Properties of Food Colloids
2.3.2. Stability and Encapsulation Capacity of Food Colloids
2.3.3. Interaction Mechanisms Between Food Colloids and Drugs/Nutrients
2.3.4. Interfacial Behavior Has a Decisive Impact on Delivery Efficiency
2.4. Common Food-Grade Colloidal Materials and Their Characteristics
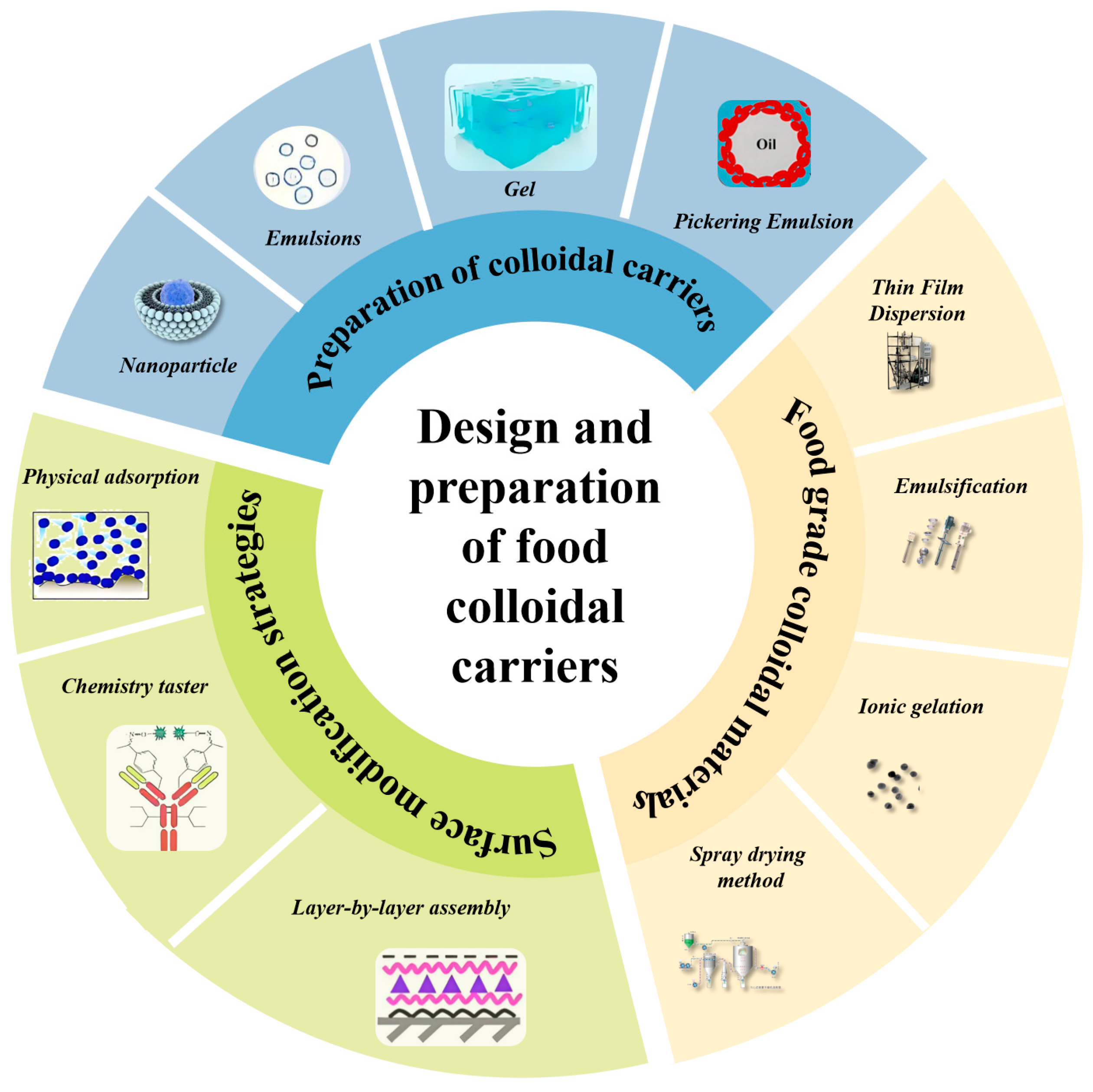
2.5. Preparation Methods of Colloidal Carriers
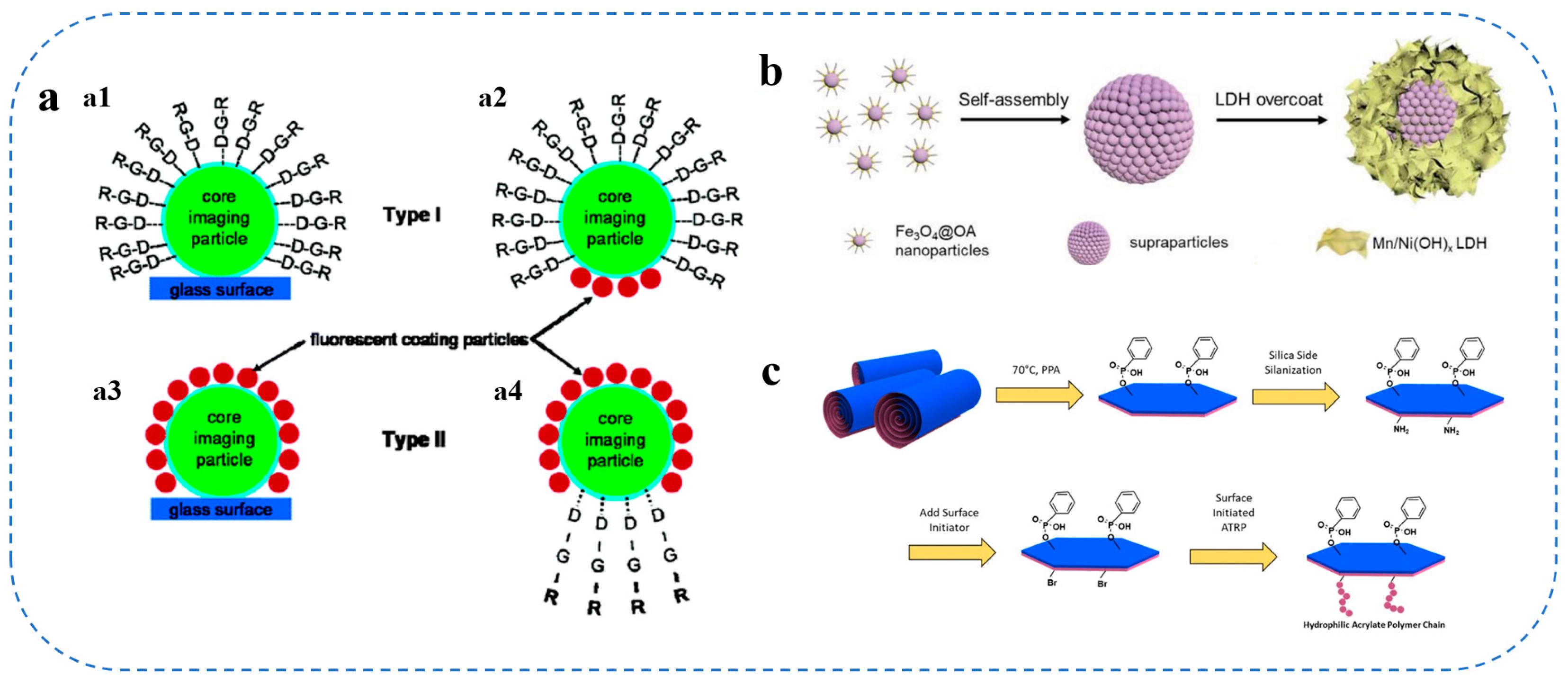
2.6. Surface Modification Strategies for Colloidal Carriers
3. Research Progress on Food-Based Colloidal Systems for Precise Targeted Delivery in Different Tissues
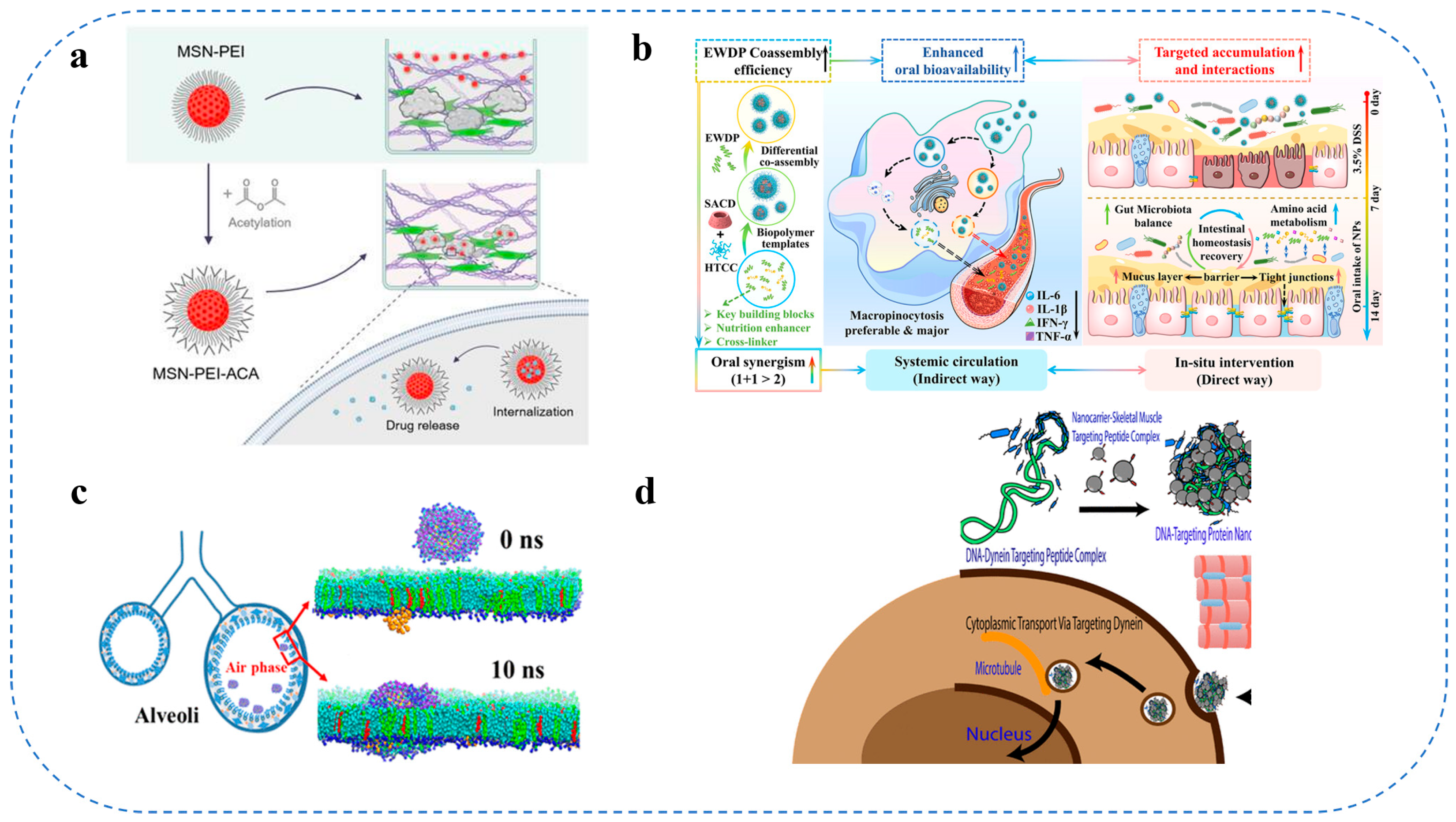
3.1. Colloidal Delivery Systems for Anticancer Drugs
3.2. Brain-Targeted Delivery Systems
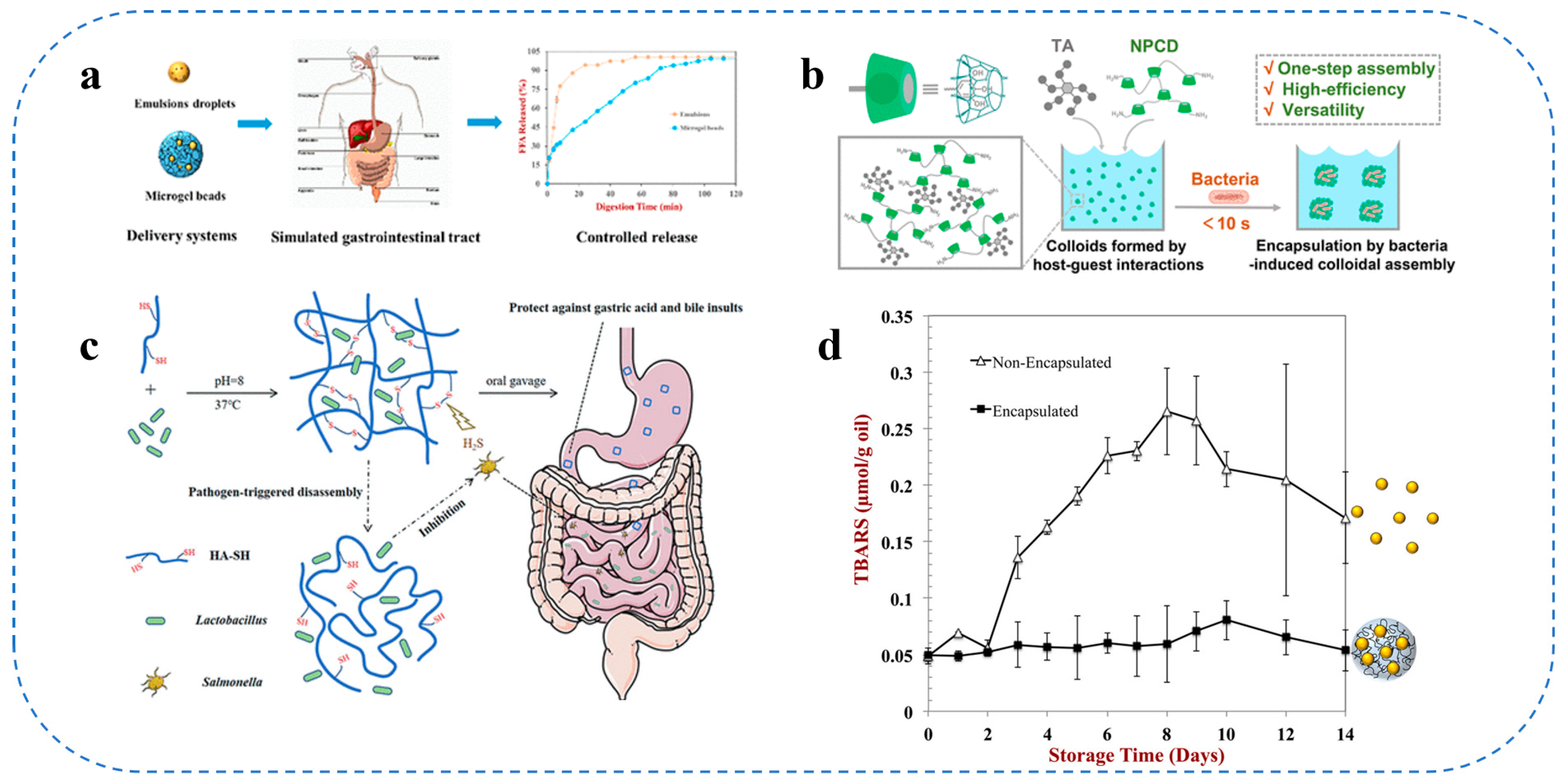
3.3. Intestinal Targeted Delivery Systems
3.4. Lung-Targeted Delivery Systems
3.5. Skeletal Muscle-Targeted Delivery Systems
3.6. Heart-Targeted Delivery Systems
3.7. Food Colloids in Application of Food Colloids in Oral Delivery Systems
4. Research Progress on Food-Based Colloidal Systems for Nutrient Delivery
4.1. Food Colloids in Delivery of Vitamins and Minerals
4.2. Food Colloids in Encapsulation and Delivery of Probiotics and Bioactive Peptides
4.3. Food Colloids in Targeted Release of Functional Lipids
5. Applications of Nanoliposomes in Delivery of Bioactive Substances
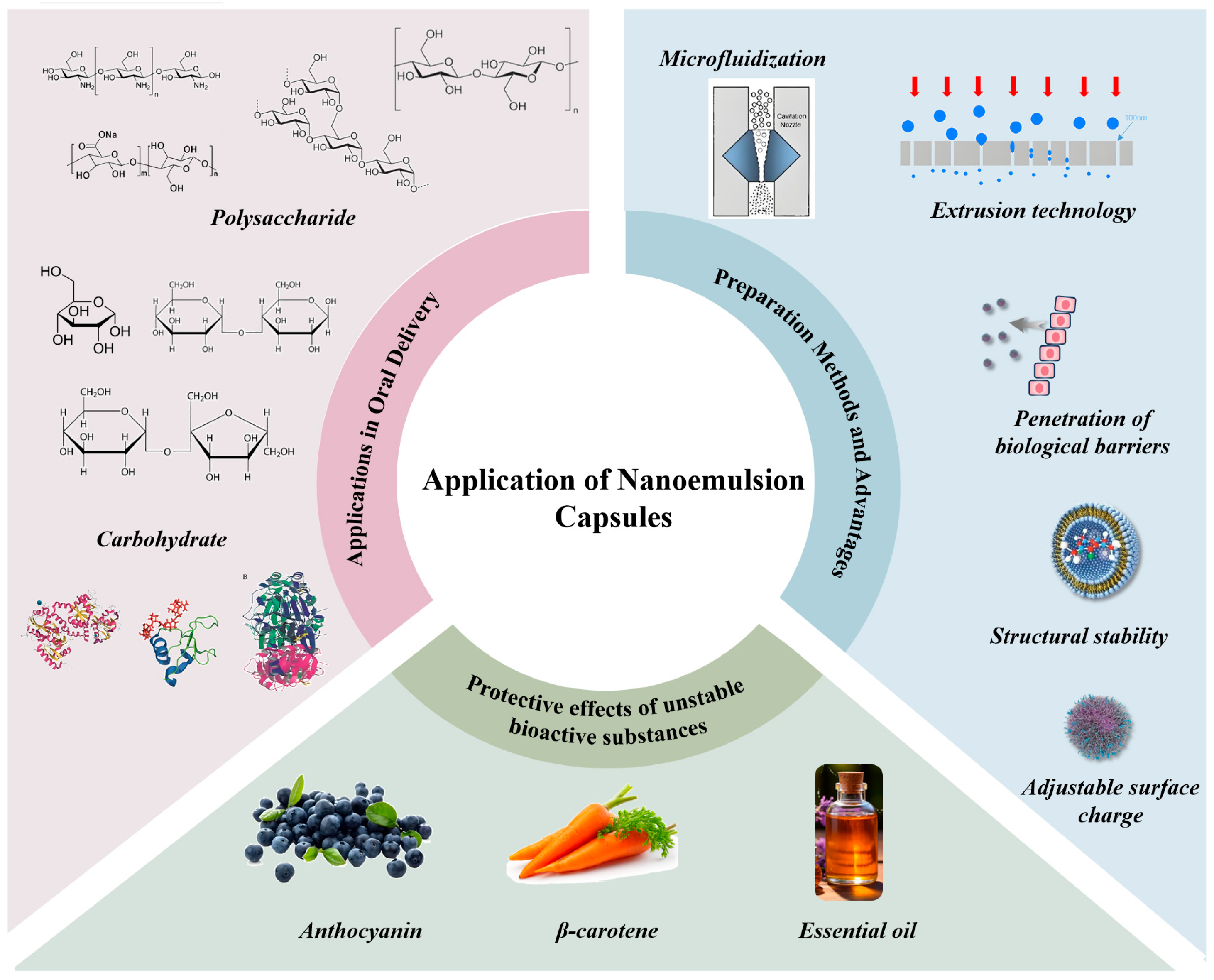
5.1. Preparation Methods and Advantages of Nanoliposomes
5.2. Protective Role of Nanoliposomes for Unstable Bioactive Substances
5.3. Application of Nanoliposomes in Oral Delivery of Peptides, Proteins, Polysaccharides, and Other Substances
6. Patents and Commercially Available Products Based on Food Gels
6.1. Patents Based on Food-Derived Colloidal Carriers
6.2. Commercially Available Products Based on Food-Grade Colloids
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J. The future of food colloids: Next-generation nanoparticle delivery systems. Curr. Opin. Colloid Interface Sci. 2017, 28, 7–14. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Sacco, P.; De Leonardis, A.; Ceglie, A.; Lopez, F. Progress in Colloid Delivery Systems for Protection and Delivery of Phenolic Bioactive Compounds: Two Study Cases-Hydroxytyrosol and Curcumin. Molecules 2022, 27, 921. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Alizadeh Sani, M.; Azizi-Lalabadi, M.; McClements, D.J. Recent progress in the application of plant-based colloidal drug delivery systems in the pharmaceutical sciences. Adv. Colloid Interface Sci. 2022, 307, 102734. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Fan, S.; Ma, J.; Yan, Y.; Wang, M.; Tang, X.; Lv, P.; Zhang, Y. Targeted delivery of food functional ingredients in precise nutrition: Design strategy and application of nutritional intervention. Crit. Rev. Food Sci. Nutr. 2023, 64, 7854–7877. [Google Scholar] [CrossRef]
- Liu, F.; McClements, D.J.; Ma, C.; Liu, X. Novel Colloidal Food Ingredients: Protein Complexes and Conjugates. Annu. Rev. Food Sci. Technol. 2023, 14, 35–61. [Google Scholar] [CrossRef]
- Song, Y.; Wu, J.; Liu, Y.; Xu, N.; Bai, H.; Wang, L.; Ai, J.; Li, K. The remodeling of ovarian function: Targeted delivery strategies for mesenchymal stem cells and their derived extracellular vesicles. Stem Cell Res. Ther. 2024, 15, 90. [Google Scholar] [CrossRef]
- Song, B.; Wu, M.; Qin, L.; Liang, W.; Wang, X. Smart Design of Targeted Drug Delivery System for Precise Drug Delivery and Visual Treatment of Brain Gliomas. Adv. Healthc. Mater. 2025, 14, 2402967. [Google Scholar] [CrossRef]
- Abdullah; Liu, L.; Javed, H.U.; Xiao, J. Engineering Emulsion Gels as Functional Colloids Emphasizing Food Applications: A Review. Front. Nutr. 2022, 9, 890188. [Google Scholar] [CrossRef]
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides Using Colloidal Systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Molecules, V.P.T.J. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Drost, E.; Adel, R.d.; Hazekamp, J.; Velikov, K.P. Temperature Responsive Colloidal Particles From Non-Covalently Interacting Small Molecular Weight Natural Bioactive Molecules. Soft Matter 2012, 8, 3515. [Google Scholar] [CrossRef]
- Shilpi, S.; Jain, A.; Gupta, Y.; Jain, S. Colloidosomes: An Emerging Vesicular System in Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 361–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; LvYe, J.; Yang, S.; Shi, Y.; Chen, Q. Critical Review of Food Colloidal Delivery System for Bioactive Compounds: Physical Characterization and Application. Foods 2024, 13, 2596. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Dave, A.; Singh, H. Nature-Assembled Structures for Delivery of Bioactive Compounds and Their Potential in Functional Foods. Front. Chem. 2020, 8, 564021. [Google Scholar] [CrossRef]
- Yu, X.; El-Aty, A.M.A.; Su, W.; Tan, M. Advancements in Precision Nutrition: Steady-state Targeted Delivery of Food Functional Factors for Nutrition Intervention of Chronic Diseases. Food Saf. Health 2023, 1, 22–40. [Google Scholar] [CrossRef]
- Tan, A.; Martin, A.L.; Nguyen, T.H.; Boyd, B.J.; Prestidge, C.A. Hybrid Nanomaterials That Mimic the Food Effect: Controlling Enzymatic Digestion for Enhanced Oral Drug Absorption. Angew. Chem. 2012, 51, 5475–5479. [Google Scholar] [CrossRef]
- McClements, D.J. Nano-enabled personalized nutrition: Developing multicomponent-bioactive colloidal delivery systems. Adv. Colloid Interface Sci. 2020, 282, 102211. [Google Scholar] [CrossRef]
- Cheng, B.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Liu, F.G.; Ma, C.C.; Gao, Y.X.; McClements, D.J. Food-Grade Covalent Complexes and Their Application as Nutraceutical Delivery Systems: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Decker, E.A.; McClements, D.J. Development of food-grade filled hydrogels for oral delivery of lipophilic active ingredients: pH-triggered release. Food Hydrocoll. 2015, 44, 345–352. [Google Scholar] [CrossRef]
- Chai, J.; Jiang, P.; Wang, P.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Zhao, L.; Norde, W.; et al. The intelligent delivery systems for bioactive compounds in foods: Physicochemical and physiological conditions, absorption mechanisms, obstacles and responsive strategies. Trends Food Sci. Technol. 2018, 78, 144–154. [Google Scholar] [CrossRef]
- Tamkeen, J.; Kukatil, L.; Rahman, A.; Reddi, P.; Gundlapalli, S.P. The Applications of Food Hydrocolloids in Drug Delivery System. Ger. J. Pharm. Biomater. 2022, 1, 4–14. [Google Scholar] [CrossRef]
- Tan, Y.; McClements, D.J. Plant-Based Colloidal Delivery Systems for Bioactives. Molecules 2021, 26, 6895. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mansi, K.; Mehta, L.; Sharma, M. Nanoemulsions: Delivery System for Drugs. Webology 2021, 18. [Google Scholar] [CrossRef]
- Kumar, V.; Garg, V.; Saini, N.; Aggarwal, N.; Kumar, H.; Kumar, D.; Chopra, H.; Kamal, M.A.; Dureja, H. An Updated Review on Nanoemulsion: Factory for Food and Drug Delivery. Curr. Pharm. Biotechnol. 2024, 25, 2218–2252. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Román-Guerrero, A.; Cortés-Camargo, S.; Velázquez-Gutiérrez, S.K.; Pérez-Alonso, C. Recent Approaches in Alginate-Based Carriers for Delivery of Therapeutics and Biomedicine. In Polysaccharide-Based Biomaterials; Royal Society of Chemistry: London, UK, 2022; pp. 27–68. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Chitosan-Based Particles as Controlled Drug Delivery Systems. Drug Deliv. 2004, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, A.; Dalgleish, D.G.; Lakemond, C.M.M.; Pouzot, M.; Donato, L.; Kruif, C.G.d.; Ettelaie, R.; Gunning, P.T.; Stuart, M.A.C.; Miller, R.; et al. Food Colloids; Royal Society of Chemistry: London, UK, 2005. [Google Scholar] [CrossRef]
- Dickinson, E. Food Colloids Research: Historical Perspective and Outlook. Adv. Colloid Interface Sci. 2011, 165, 7–13. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Habib, A.; Yasmeen, E.; Yousaf, M. Recent Advances in Nanoparticle-Based Targeted Drug-Delivery Systems Against Cancer and Role of Tumor Microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 317–353. [Google Scholar] [CrossRef]
- Boyd, B.J. Past and Future Evolution in Colloidal Drug Delivery Systems. Expert Opin. Drug Deliv. 2007, 5, 69–85. [Google Scholar] [CrossRef]
- Simović, S.; Barnes, T.J.; Tan, A.; Prestidge, C.A. Assembling Nanoparticle Coatings to Improve the Drug Delivery Performance of Lipid Based Colloids. Nanoscale 2012, 4, 1220–1230. [Google Scholar] [CrossRef]
- Roudi, N.E.; Saraygord-Afshari, N.; Hemmaty, M. Protein Nano-Cages: Novel Carriers for Optimized Targeted Remedy. F1000research 2017, 6, 1541. [Google Scholar] [CrossRef]
- Qiu, J.; Camargo, P.H.C.; Jeong, U.; Xia, Y. Synthesis, Transformation, and Utilization of Monodispersed Colloidal Spheres. Acc. Chem. Res. 2019, 52, 3475–3487. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Stabilising Emulsion-based Colloidal Structures With Mixed Food Ingredients. J. Sci. Food Agric. 2012, 93, 710–721. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; McClements, D.J. Protein Encapsulation in Alginate Hydrogel Beads: Effect of pH on Microgel Stability, Protein Retention and Protein Release. Food Hydrocoll. 2016, 58, 308–315. [Google Scholar] [CrossRef]
- Douaire, M.; Norton, I.T. Designer Colloids in Structured Food for the Future. J. Sci. Food Agric. 2013, 93, 3147–3154. [Google Scholar] [CrossRef] [PubMed]
- Genovese, D.B.; Lozano, J.E.; Rao, M.A. The Rheology of Colloidal and Noncolloidal Food Dispersions. J. Food Sci. 2007, 72, R11–R20. [Google Scholar] [CrossRef]
- Pawar, A.B.; Caggioni, M.; Hartel, R.W.; Spicer, P.T. Arrested Coalescence of Viscoelastic Droplets with Internal Microstructure. Faraday Discuss. 2012, 158, 341. [Google Scholar] [CrossRef]
- Hsiao, L.C.; Newman, R.S.; Glotzer, S.C.; Solomon, M.J. Role of Isostaticity and Load-Bearing Microstructure in the Elasticity of Yielded Colloidal Gels. Proc. Natl. Acad. Sci. USA 2012, 109, 16029–16034. [Google Scholar] [CrossRef]
- Jafari, Z.; Shirazinejad, A.; Hashemi, S.M.B.; Fathi, M. Influence of Temperature, Ion Type, and Ionic Strength on Dynamic Viscoelastic, Steady-state, and Dilute-solution Behavior of Melissa Officinalis Seed Gum. J. Food Process Eng. 2023, 46, e14363. [Google Scholar] [CrossRef]
- Park, J.D.; Ahn, K.H.; Lee, S.J. Structural Change and Dynamics of Colloidal Gels Under Oscillatory Shear Flow. Soft Matter 2015, 11, 9262–9272. [Google Scholar] [CrossRef]
- Monasterio, A.; Osorio, F. Physicochemical Properties of Nanoliposomes Encapsulating Grape Seed Tannins Formed With Ultrasound Cycles. Foods 2024, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, M.B.; Castillo, S.; Uriarte, S.L.; González, M.P.B. Encapsulation of Lactiplantibacillus Plantarum And Beetroot Extract With Alginate and Effect of Capsules on Rheological Properties and Stability of an Oil-in-Water Emulsion Model Food. Pol. J. Food Nutr. Sci. 2023, 73, 242–252. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zhang, S.; Gu, Q.; McClements, D.J.; Chen, S.; Liu, X.; Liu, F. Lactoferrin-Based Ternary Composite Nanoparticles With Enhanced Dispersibility and Stability for Curcumin Delivery. ACS Appl. Mater. Interfaces 2023, 15, 18166–18181. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core–Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(lactic-co-Glycolic Acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Nwankwo, J.A.; Liu, W.; Guo, X.; Lin, Y.; Hussain, M.; Khan, I.; Joshua, M.; Ibrahim, A.N.; Ngozi, O.J.; Ali, A.; et al. Microemulsion Gel Systems: Formulation, Stability Studies, Biopolymer Interactions, and Functionality in Food Product Development. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70110. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Malacrida, C.R.; Nicoletti, V.R. Influence of Emulsification Methods and Use of Colloidal Silicon Dioxide on the Microencapsulation by Spray Drying of Turmeric Oleoresin in Gelatin-starch Matrices. Can. J. Chem. Eng. 2016, 94, 2210–2218. [Google Scholar] [CrossRef]
- Prakasha, R.; Vinay, G.M.; Srilatha, P.; Pandey, H. Nanoemulsions as Carriers of Bioactive Compounds in Functional Foods: Preparation and Application. Eur. J. Nutr. Food Saf. 2025, 17, 78–95. [Google Scholar] [CrossRef]
- Li, S.; Li, P.; Wang, J.; Lu, Y.; Chen, Y.; Zhen, Z.; Jiang, J.; Cheng, X.; Bi, L. Investigation of the Stability and Bioaccessibility of Β-Carotene Encapsulated in Emulsion Gels With Nonspherical Droplets. ACS Food Sci. Technol. 2025, 5, 770–779. [Google Scholar] [CrossRef]
- Nishimoto-Sauceda, D.; Robles, L.E.R.; Antunes-Ricardo, M. Biopolymer Nanoparticles: A Strategy to Enhance Stability, Bioavailability, and Biological Effects of Phenolic Compounds as Functional Ingredients. J. Sci. Food Agric. 2021, 102, 41–52. [Google Scholar] [CrossRef]
- McClements, D.J. Delivery by Design (DbD): A Standardized Approach to the Development of Efficacious Nanoparticle- and Microparticle-Based Delivery Systems. Compr. Rev. Food Sci. Food Saf. 2017, 17, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wu, Y.C.; Cai, K.; He, H.; Li, Y.; Lan, M.; Chen, X.; Cheng, J.; Yin, L. High Drug Loading and Sub-Quantitative Loading Efficiency of Polymeric Micelles Driven by Donor–Receptor Coordination Interactions. J. Am. Chem. Soc. 2018, 140, 1235–1238. [Google Scholar] [CrossRef]
- Pourhossein, A.; Rafizadeh, M.; Chen, P. Stimuli-responsive Zein-based Nanoparticles as a Potential Carrier for Ellipticine: Synthesis, Release, and in Vitro Delivery. Polym. Adv. Technol. 2020, 31, 2007–2019. [Google Scholar] [CrossRef]
- Neves, R.P.; Bronze-Uhle, E.S.; Santos, P.L.d.; Lisboa-Filho, P.N.; Magdalena, A.G. Salicylic acid incorporation in fe3o4-bsa nanoparticles for drug release. Quím. Nova 2021, 44, 824–829. [Google Scholar] [CrossRef]
- Dinsmore, A.D.; Hsu, M.F.; Nikolaides, M.G.; Márquez, M.; Bausch, A.R.; Weitz, D.A. Colloidosomes: Selectively Permeable Capsules Composed of Colloidal Particles. Science 2002, 298, 1006–1009. [Google Scholar] [CrossRef]
- Rajić, D.; Spasojević, L.; Cvjetković, V.G.; Bučko, S.; Fraj, J.; Budinčić, J.M.; Petrović, L.; Pilić, B.; Sharipova, A.; Babayev, A.; et al. Zein–resin Composite Nanoparticles with Coencapsulated Carvacrol. J. Food Process. Preserv. 2021, 46, e15741. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-Based Delivery Systems: Challenges and Opportunities. Curr. Top. Med. Chem. 2015, 16, 1026–1039. [Google Scholar] [CrossRef]
- Połomska, A.; Leroux, J.C.; Brambilla, D. Layer-by-Layer Coating of Solid Drug Cores: A Versatile Method to Improve Stability, Control Release and Tune Surface Properties. Macromol. Biosci. 2016, 17, 1600228. [Google Scholar] [CrossRef]
- Liu, J.; Song, Z.; Luo, J.; Ngai, T.; Sun, G. Programmable Control of Active Ingredient Release in Pickering Emulsions Using Light. Small 2025, 21, e2412361. [Google Scholar] [CrossRef]
- Dickinson, E. Colloids in Food: Ingredients, Structure, and Stability. Annu. Rev. Food Sci. Technol. 2015, 6, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Tarahi, M.; Ahmed, J. Recent Advances in Legume Protein-based Colloidal Systems. Legume Sci. 2023, 5, e185. [Google Scholar] [CrossRef]
- Peng, D.; Ye, J.; Jin, W.; Yang, J.; Geng, F.; Deng, Q. A Review on the Utilization of Flaxseed Protein as Interfacial Stabilizers for Food Applications. J. Am. Oil Chem. Soc. 2022, 99, 723–737. [Google Scholar] [CrossRef]
- Flanagan, J.G.; Singh, H. Microemulsions: A Potential Delivery System for Bioactives in Food. Crit. Rev. Food Sci. Nutr. 2006, 46, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, Y.; Huang, Q. Recent Advances on Food-Grade Particles Stabilized Pickering Emulsions: Fabrication, Characterization and Research Trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef]
- Xu, M.; Wan, Z.; Yang, X.Q. Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems. Molecules 2021, 26, 6075. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 864943. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering Emulsions: Versatility of Colloidal Particles and Recent Applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Patel, A.R.; Velikov, K.P. Colloidal Delivery Systems in Foods: A General Comparison With Oral Drug Delivery. LWT 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Tahir, A.; Ahmad, R.S.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Muhammad, N.; Nisa, M.; Nadeem, M.T.; Yasmin, A.; Tahir, H.S.; et al. Recent Approaches for Utilization of Food Components as Nano-Encapsulation: A Review. Int. J. Food Prop. 2021, 24, 1074–1096. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Oprea, I.; Fărcaş, A.C.; Leopold, L.; Diaconeasa, Z.; Coman, C.; Socaci, S. Nano-Encapsulation of Citrus Essential Oils: Methods and Applications of Interest for the Food Sector. Polymers 2022, 14, 4505. [Google Scholar] [CrossRef]
- Wang, Z.; Neves, M.A.; Isoda, H.; Nakajima, M. Preparation and Characterization of Micro/Nano-Emulsions Containing Functional Food Components. Jpn. J. Food Eng. 2015, 16, 263–276. [Google Scholar] [CrossRef]
- Díaz-Ruiz, R.; Valdeón, I.; Álvarez, J.R.; Matos, M.; Gutiérrez, G. Simultaneous Encapsulation of trans-resveratrol and Vitamin D3 in Highly Concentrated Double Emulsions. J. Sci. Food Agric. 2020, 101, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Bouwens, E.C.M.; Velikov, K.P. Sodium Caseinate Stabilized Zein Colloidal Particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Narsaiah, K.; Jha, S.N.; Wilson, R.A.; Mandge, H.M.; Manikantan, M.R. Optimizing Microencapsulation of Nisin With Sodium Alginate and Guar Gum. J. Food Sci. Technol. 2012, 51, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Yang, W.; Suleria, H.A.R.; Song, Y. Microencapsulation and the Characterization of Polyherbal Formulation (PHF) Rich in Natural Polyphenolic Compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef]
- Tolve, R.; Galgano, F.; Caruso, M.C.; Tchuenbou-Magaia, F.; Condelli, N.; Favati, F.; Zhang, Z. Encapsulation of Health-Promoting Ingredients: Applications in Foodstuffs. Int. J. Food Sci. Nutr. 2016, 67, 888–918. [Google Scholar] [CrossRef]
- Murase, S.K.; Lv, L.P.; Kaltbeitzel, A.; Landfester, K.; Valle, L.J.d.; Katsarava, R.; Puiggalí, J.; Crespy, D. Amino Acid-Based Poly(ester Amide) Nanofibers for Tailored Enzymatic Degradation Prepared by Miniemulsion-Electrospinning. RSC Adv. 2015, 5, 55006–55014. [Google Scholar] [CrossRef]
- Jiang, S.; Lv, L.P.; Landfester, K.; Crespy, D. Nanocontainers in and Onto Nanofibers. Acc. Chem. Res. 2016, 49, 816–823. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Martín-González, M.F.S. Characterization of Ergocalciferol Loaded Solid Lipid Nanoparticles. J. Food Sci. 2012, 77, N8–N13. [Google Scholar] [CrossRef] [PubMed]
- Bollhorst, T.; Rezwan, K.; Maas, M. Colloidal Capsules: Nano- And Microcapsules With Colloidal Particle Shells. Chem. Soc. Rev. 2017, 46, 2091–2126. [Google Scholar] [CrossRef]
- Escareño, N.; Topete, A.; Taboada, P.; Daneri-Navarro, A. Rational Surface Engineering of Colloidal Drug Delivery Systems for Biological Applications. Curr. Top. Med. Chem. 2018, 18, 1224–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, Q.; Luo, J.; Zeng, M.; Wang, L.; Huang, D.; Wang, X.; Mannan, S.; Peng, B.; Cheng, Z. Natural Halloysites-Based Janus Platelet Surfactants for the Formation of Pickering Emulsion and Enhanced Oil Recovery. Sci. Rep. 2019, 9, 163. [Google Scholar] [CrossRef]
- Xu, A.Y.; Melton, L.D.; Williams, M.A.K.; McGillivray, D.J. Protein and Polysaccharide Conjugates as Emerging Scaffolds for Drug Delivery Systems. Int. J. Nanotechnol. 2017, 14, 470. [Google Scholar] [CrossRef]
- Pinelli, F.; Perale, G.; Rossi, F. Coating and Functionalization Strategies for Nanogels and Nanoparticles for Selective Drug Delivery. Gels 2020, 6, 6. [Google Scholar] [CrossRef]
- Yake, A.M.; Zahr, A.S.; Jerri, H.A.; Pishko, M.V.; Velegol, D. Localized Functionalization of Individual Colloidal Carriers for Cell Targeting and Imaging. Biomacromolecules 2007, 8, 1958–1965. [Google Scholar] [CrossRef]
- Chakraborty, A.; Dalal, C.; Jana, N.R. Colloidal Nanobioconjugate With Complementary Surface Chemistry for Cellular and Subcellular Targeting. Langmuir 2018, 34, 13461–13471. [Google Scholar] [CrossRef]
- Maity, A.R.; Chakraborty, A.; Mondal, A.; Jana, N.R. Carbohydrate Coated, Folate Functionalized Colloidal Graphene as a Nanocarrier for Both Hydrophobic and Hydrophilic Drugs. Nanoscale 2014, 6, 2752. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface Modification, Functionalization and Bioconjugation of Colloidal Inorganic Nanoparticles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Wang, W.; Peng, J.; Li, Y.; Shangguan, Y.; Ouyang, G.; Xu, M.; Wang, S.; Wei, J.; et al. Colloidal Surface Engineering: Growth of Layered Double Hydroxides With Intrinsic Oxidase-Mimicking Activities to Fight Against Bacterial Infection in Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000092. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L. Challenges and Solutions to Incorporation of Nutraceuticals in Foods. Annu. Rev. Food Sci. Technol. 2015, 6, 463–477. [Google Scholar] [CrossRef]
- Rizzo, S.; Zingale, E.; Romeo, A.; Lombardo, R.; Pignatello, R. Colon Delivery of Nutraceutical Ingredients by Food-Grade Polymeric Systems: An Overview of Technological Characterization and Biological Evaluation. Appl. Sci. 2023, 13, 5443. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoscale Nutrient Delivery Systems for Food Applications: Improving Bioactive Dispersibility, Stability, and Bioavailability. J. Food Sci. 2015, 80, N1602–N1611. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Liu, Y.; Zou, L.; McClements, D.J.; Liu, W. A Review of Recent Progress in Improving the Bioavailability of Nutraceutical-loaded Emulsions After Oral Intake. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3963–4001. [Google Scholar] [CrossRef]
- Park, D.; Lee, S.J.; Park, J.-W. Aptamer-Based Smart Targeting and Spatial Trigger–Response Drug-Delivery Systems for Anticancer Therapy. Biomedicines 2024, 12, 187. [Google Scholar] [CrossRef]
- Kushwaha, P.; Aqeel, R.; Srivastava, N. Micelles in Cancer Therapy: An Update on Preclinical and Clinical Status. Recent Pat. Nanotechnol. 2022, 16, 283–294. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Zhong, T.; Li, L.; Shen, H.; Jia, N. Functionalized Multiwalled Carbon Nanotubes-Anticancer Drug Carriers: Synthesis, Targeting Ability and Antitumor Activity. Nano Biomed. Eng. 2011, 3, 157–162. [Google Scholar] [CrossRef]
- Holgado, M.A.; Álvarez-Fuentes, J.; Arévalo, M.F.; Arias, J.L. Possibilities of Poly(D,L-Lactide-Co-Glycolide) in the Formulation of Nanomedicines Against Cancer. Curr. Drug Targets 2011, 12, 1096–1111. [Google Scholar] [CrossRef]
- Singh, I.; Swami, R.; Pooja, D.; Jeengar, M.K.; Khan, W.; Sistla, R. Lactoferrin Bioconjugated Solid Lipid Nanoparticles: A New Drug Delivery System for Potential Brain Targeting. J. Drug Target. 2015, 24, 212–223. [Google Scholar] [CrossRef]
- Çaban, S.; Çapan, Y.; Couvreur, P.; Dalkara, T. Preparation and Characterization of Biocompatible Chitosan Nanoparticles for Targeted Brain Delivery of Peptides. In Neurotrophic Factors: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; pp. 321–332. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.; Jain, S.K. Nasal-Nanotechnology: Revolution for Efficient Therapeutics Delivery. Drug Deliv. 2014, 23, 671–683. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Multifunctional Nanoparticles for Successful Targeted Drug Delivery Across the Blood-Brain Barrier. In Molecular Insight of Drug Design; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Patel, A.R.; Heussen, P.C.; Hazekamp, J.; Velikov, K.P. Stabilisation and Controlled Release of Silibinin From pH Responsive Shellac Colloidal Particles. Soft Matter 2011, 7, 8549. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Liu, C.; Zhang, H.; Li, S.; Zhang, T.; Yu, Z.; Chi, X.; Zhang, Z.; Du, Z. Programmable Food-Derived Peptide Coassembly Strategies for Boosting Targeted Colitis Therapy by Enhancing Oral Bioavailability and Restoring Gut Microenvironment Homeostasis. ACS Nano 2025, 19, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Pawara, Y.S.; Mahajan, H.S. Spray Dried Nanoparticle for Pulmonary Delivery: Current Developments and Future Perspectives. Indian J. Pharm. Educ. Res. 2024, 58, s1156–s1168. [Google Scholar] [CrossRef]
- Chattopadhyay, S. Aerosol Generation Using Nanometer Liposome Suspensions for Pulmonary Drug Delivery Applications. J. Liposome Res. 2013, 23, 255–267. [Google Scholar] [CrossRef]
- Mishra, B.J.; Kaul, A.; Trivedi, P. l-Cysteine Conjugated Poly l-lactide Nanoparticles Containing 5-Fluorouracil: Formulation, Characterization, Release and Uptake by Tissues in Vivo. Drug Deliv. 2014, 22, 214–222. [Google Scholar] [CrossRef]
- Chikazawa, M.; Sato, R. Identification of Functional Food Factors as Β2-Adrenergic Receptor Agonists and Their Potential Roles in Skeletal Muscle. J. Nutr. Sci. Vitaminol. 2018, 64, 68–74. [Google Scholar] [CrossRef]
- Tacchi, F.; Orozco-Aguilar, J.; Gutiérrez, D.; Simón, F.; Salazar, J.; Vilos, C.; Cabello-Verrugio, C. Scaffold Biomaterials and Nano-Based Therapeutic Strategies for Skeletal Muscle Regeneration. Nanomedicine 2021, 16, 2521–2538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Pan, J. Baicalin-Loaded PEGylated Lipid Nanoparticles: Characterization, Pharmacokinetics, and Protective Effects on Acute Myocardial Ischemia in Rats. Drug Deliv. 2016, 23, 3696–3703. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, S.; Hao, R.; Dong, X.; Fu, L.; Han, B. RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection. Pharmaceutics 2020, 12, 575. [Google Scholar] [CrossRef]
- Sahoo, R.; Mukherjee, N.; Paramanik, S.; Jana, N.R. Vegetable Oil–Based Pickering Nanoemulsions as Carriers for Cytosolic Drug Delivery. ACS Appl. Nano Mater. 2024, 7, 15702–15709. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Xue, C. Oral Cell-Targeted Delivery Systems Constructed of Edible Materials: Advantages and Challenges. Molecules 2022, 27, 7991. [Google Scholar] [CrossRef]
- Prabhakar, N.; Långbacka, E.; Özliseli, E.; Mattsson, J.; Mahran, A.; Suleymanova, I.; Sahlgren, C.; Rosenholm, J.M.; Åkerfelt, M.; Nees, M. Surface Modification of Mesoporous Silica Nanoparticles as a Means to Introduce Inherent Cancer-Targeting Ability in a 3D Tumor Microenvironment. Small Sci. 2024, 4, 2400084. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Bioresponsive Hyaluronic Acid-Capped Mesoporous Silica Nanoparticles for Targeted Drug Delivery. Chem.-A Eur. J. 2013, 19, 1778–1783. [Google Scholar] [CrossRef]
- Rahikkala, A.; Pereira, S.A.P.; Figueiredo, P.; Passos, M.L.; André, R.T.S.A.; Saraiva, M.L.M.; Santos, H.A. Mesoporous Silica Nanoparticles for Targeted and Stimuli-Responsive Delivery of Chemotherapeutics: A Review. Adv. Biosyst. 2018, 2, 1800020. [Google Scholar] [CrossRef]
- Oyeniyi, Y.J.; Abdurahma, A. Fabrication and Design of Dendrimers for Cancer Chemotherapy. Bio-Research 2019, 14, 926. [Google Scholar] [CrossRef]
- Naeem, S.; Viswanathan, G.; Misran, M. Liposomes as Colloidal Nanovehicles: On the Road to Success in Intravenous Drug Delivery. Rev. Chem. Eng. 2018, 34, 365–383. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-Delivery of Baicalein and Doxorubicin by Hyaluronic Acid Decorated Nanostructured Lipid Carriers for Breast Cancer Therapy. Drug Deliv. 2015, 23, 1364–1368. [Google Scholar] [CrossRef]
- Macierzanka, A.; Mackie, A.R.; Krupa, Ł. Permeability of the Small Intestinal Mucus for Physiologically Relevant Studies: Impact of Mucus Location and Ex Vivo Treatment. Sci. Rep. 2019, 9, 17516. [Google Scholar] [CrossRef] [PubMed]
- Macierzanka, A.; Mackie, A.R.; Bajka, B.; Rigby, N.M.; Nau, F.; Dupont, D. Transport of Particles in Intestinal Mucus Under Simulated Infant and Adult Physiological Conditions: Impact of Mucus Structure and Extracellular DNA. PLoS ONE 2014, 9, e95274. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Xiao, H.; McClements, D.J. Delivery of Lipophilic Bioactives: Assembly, Disassembly, and Reassembly of Lipid Nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.I.; Gandhi, N.S.; Hughes, Z.E.; Saha, S.C. Computational Studies of Lipid-Wrapped Gold Nanoparticle Transport Through Model Lung Surfactant Monolayers. J. Phys. Chem. B 2021, 125, 1392–1401. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-Mediated Pulmonary Drug Delivery: A Review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Mneimneh, A.T.; Elmaradny, H.A. A Review on Aerosol Drug Delivery: Fundamentals, Classifications, Particle Size Analysis and the Engagement of Nanoparticulate Systems. Drug Deliv. Lett. 2022, 12, 258–275. [Google Scholar] [CrossRef]
- Jativa, S.D.; Thapar, N.; Broyles, D.; Dikici, E.; Daftarian, P.; Jiménez, J.J.; Daunert, S.; Deo, S.K. Enhanced Delivery of Plasmid DNA to Skeletal Muscle Cells Using a DLC8-Binding Peptide and ASSLNIA-Modified PAMAM Dendrimer. Mol. Pharm. 2019, 16, 2376–2384. [Google Scholar] [CrossRef]
- Hersh, J.; Capcha, J.M.C.; Irion, C.I.; Lambert, G.; Noguera, M.; Singh, M.P.; Kaur, A.; Dikici, E.; Jiménez, J.J.; Shehadeh, L.A.; et al. Peptide-Functionalized Dendrimer Nanocarriers for Targeted Microdystrophin Gene Delivery. Pharmaceutics 2021, 13, 2159. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.R.; Liu, X.; Young, C.S.; Saleh, K.K.; Ji, Y.; Jiang, J.; Emami, M.R.; Mokhonova, E.; Spencer, M.J.; Meng, H.; et al. Nanoparticles Systemically Biodistribute to Regenerating Skeletal Muscle in DMD. J. Nanobiotechnol. 2023, 21, 303. [Google Scholar] [CrossRef]
- Wen, J.; Du, Y.; Li, D.; Alany, R.G. Development of Water-in-Oil Microemulsions With the Potential of Prolonged Release for Oral Delivery of L-Glutathione. Pharm. Dev. Technol. 2013, 18, 1424–1429. [Google Scholar] [CrossRef]
- Zhang, R.; McClements, D.J. Enhancing Nutraceutical Bioavailability by Controlling the Composition and Structure of Gastrointestinal Contents: Emulsion-Based Delivery and Excipient Systems. Food Struct. 2016, 10, 21–36. [Google Scholar] [CrossRef]
- Maurya, V.K.; Shakya, A.; Bashir, K.; Jan, K.; McClements, D.J. Fortification by Design: A Rational Approach to Designing Vitamin D Delivery Systems for Foods and Beverages. Compr. Rev. Food Sci. Food Saf. 2022, 22, 135–186. [Google Scholar] [CrossRef]
- Gu, L.; Su, Y.; Zhang, Z.; Zheng, B.; Zhang, R.; McClements, D.J.; Yang, Y. Modulation of Lipid Digestion Profiles Using Filled Egg White Protein Microgels. J. Agric. Food Chem. 2017, 65, 6919–6928. [Google Scholar] [CrossRef]
- Martins, A.C.; Mariana Vieira dos Santos, K.; Rodrigues, V.M.; Hinnig, P.d.F.; Fernandes, A.C.; Bernardo, G.L.; Rossana Pacheco da Costa, P.; Uggioni, P.L. Market-driven Fortification of Vitamins and Minerals in Packaged Foods Targeted at Children in Brazil. Nutr. Bull. 2024, 49, 209–219. [Google Scholar] [CrossRef]
- Shatnyuk, L.N.; Vrzhesinskaya, O.A.; Kodencova, V.; Matveeva, A.V. Prospects for Increasing the Vitamin Value of Food Concentrates: Bouillon Cubes. Food Process. Tech. Technol. 2020, 50, 296–305. [Google Scholar] [CrossRef]
- Cirican, C.-A.; Ognean, F.; Ognean, M. The Effects of Fortification With a Commercial Mixture of Vitamins and Minerals on Bread Characteristics. Jpn. Assoc. Pet. Technol. 2025, 30, 477–482. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Liu, S.; Tang, J. Bacterial Cellulose Nanofibril-Based Pickering Emulsions: Recent Trends and Applications in the Food Industry. Foods 2022, 11, 4064. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.X.; Xiao, H.; Wu, F.G. Intestinal Delivery of Probiotics: Materials, Strategies, and Applications. Adv. Mater. 2024, 36, e2310174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Ren, X.; Xu, T.; Peng, Q.; Zhang, Y.; Chao, Z.; Jiang, W.; Jia, L.; Han, L. Bacteria-Induced Colloidal Encapsulation for Probiotic Oral Delivery. ACS Nano 2023, 17, 6886–6898. [Google Scholar] [CrossRef]
- Mamvura, C.I.; Moolman, S.; Kalombo, L.; Hall, A.N.; Thantsha, M.S. Characterisation of the Poly-(Vinylpyrrolidone)-Poly-(Vinylacetate-Co-Crotonic Acid) (PVP:PVAc-CA) Interpolymer Complex Matrix Microparticles Encapsulating a Bifidobacterium Lactis Bb12 Probiotic Strain. Probiotics Antimicrob. Proteins 2011, 3, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lu, C.; Liu, Y.; Kong, L.; Bai, H.; Mu, H.; Li, Z.; Geng, H.; Duan, J. Encapsulation of Lactobacillus Rhamnosus in Hyaluronic Acid-Based Hydrogel for Pathogen-Targeted Delivery to Ameliorate Enteritis. ACS Appl. Mater. Interfaces 2020, 12, 36967–36977. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.M.; Zahoor, T.; Sagheer, A.; Nadeem, M.; Khaliq, A.; Iqbal, R.; Ahsan, S.; Ahmad, Z. Assessment of Antagonistic Activity of Free and Encapsulated Bifidobacterium Bifidum Against Salmonella. J. Food Saf. 2018, 38, e12546. [Google Scholar] [CrossRef]
- Farahmand, A.; Ghorani, B.; Emadzadeh, B.; Sarabi-Jamab, M.; Emadzadeh, M.; Modiri, A.; Tucker, N. Millifluidic-Assisted Ionic Gelation Technique for Encapsulation of Probiotics in Double-Layered Polysaccharide Structure. Food Res. Int. 2022, 160, 111699. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, K.; Wang, L.; Lin, X.; Tan, M.; Su, W. Microfluidic Strategies for Encapsulation, Protection, and Controlled Delivery of Probiotics. J. Agric. Food Chem. 2024, 72, 15092–15105. [Google Scholar] [CrossRef]
- Kil, B.J.; Yoon, S.J.; Yun, C.H.; Huh, C.S. The Effect of Milk Protein on the Biological and Rheological Properties of Probiotic Capsules. J. Microbiol. Biotechnol. 2020, 30, 1870–1875. [Google Scholar] [CrossRef]
- McClements, D.J. Design of Nano-Laminated Coatings to Control Bioavailability of Lipophilic Food Components. J. Food Sci. 2010, 75, R30–R42. [Google Scholar] [CrossRef]
- Chen, F.; Liang, L.; Zhang, Z.; Deng, Z.; Decker, E.A.; McClements, D.J. Inhibition of Lipid Oxidation in Nanoemulsions and Filled Microgels Fortified With Omega-3 Fatty Acids Using Casein as a Natural Antioxidant. Food Hydrocoll. 2017, 63, 240–248. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Ajeeshkumar, K.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and Analysis. In Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers; Humana Press: Totowa, NJ, USA, 2009; pp. 29–50. [Google Scholar] [CrossRef]
- Elkhoury, K.; Kahn, C.J.; Sánchez-González, L.; Arab-Tehrany, E. Liposomes for Biomedical Applications. In Soft Matter for Biomedical Applications; Royal Society of Chemistry: London, UK, 2021; pp. 392–404. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Medina, D.I.; Parra-Saldívar, R.; Iqbal, H.M. Insight Into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First Century Biomedical Settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Mahmood, K.; Shah, Y.A.; Ateeq, H.; Sibat, A.; Khan, M.R.; Busquets, R. Encapsulation of Probiotic Bacteria Using Polyelectrolytes Stabilized Nanoliposomes for Improved Viability Under Hostile Conditions. J. Food Sci. 2023, 88, 3839–3848. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Gaspari, M.; Celano, M.; Wolfram, J.; Voce, P.; Puxeddu, E.; Filetti, S.; Celia, C.; Ferrari, M.; et al. Targeting the Thyroid Gland With Thyroid-Stimulating Hormone (TSH)-nanoliposomes. Biomaterials 2014, 35, 7101–7109. [Google Scholar] [CrossRef]
- Zhang, P.; Bao, Z.; Jiang, P.; Zhang, S.; Zhang, X.; Lin, S.; Sun, N. Nanoliposomes for Encapsulation and Calcium Delivery of Egg White Peptide–calcium Complex. J. Food Sci. 2021, 86, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, X.; Yang, J.; Tan, C. Hyaluronic Acid-Coated Nanoliposomes as Delivery Systems for Fisetin: Stability, Membrane Fluidity, and Bioavailability. Foods 2024, 13, 2406. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Almeda, R.A.; Vidallon, M.L.P.; Reyes, C.T. Enhanced Bioactivity and Efficient Delivery of Quercetin Through Nanoliposomal Encapsulation Using Rice Bran Phospholipids. J. Sci. Food Agric. 2018, 99, 1980–1989. [Google Scholar] [CrossRef]
- Rohilla, S.; Dureja, H. Recent Patents, Formulation and Characterization of Nanoliposomes. Recent Pat. Drug Deliv. Formul. 2015, 11, 213–224. [Google Scholar] [CrossRef]
- Aniya; Zhang, L.; Li, Y.; Fu, X. Nanolipsome Modified With Inulin and Pea Protein Isolate Improve the Thermal Stability and Slow the Release of Anthocyanin at Simulated in Vitro Digestion and Hot Cocoa Beverage. Foods 2025, 14, 731. [Google Scholar] [CrossRef]
- Oksztulska, E. Methods of Nanoliposomes Preparation. Curr. Issues Pharm. Med. Sci. 2013, 26, 203–205. [Google Scholar] [CrossRef]
- Chabib, L.; Rodli, F.H.A.; Nugroho, B.H.; Suryani, A.; Firmansyah, F. Development of Nanoliposome Formulation of Beta-Carotene Using High Speed Homogeniser Method. Pharm. Educ. 2024, 24, 1–8. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H.M. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes With Antifungal Potentialities. Molecules 2021, 26, 2880. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, F.; McClements, D.J.; Xie, B.; Sun, Z.; Deng, Q. Oligomeric Procyanidin Nanoliposomes Prevent Melanogenesis and UV Radiation-Induced Skin Epithelial Cell (HFF-1) Damage. Molecules 2020, 25, 1458. [Google Scholar] [CrossRef]
- Cao, S.; Hao, J.; Wang, Y.; Zhou, X.; Wang, F. Chitosan-coated Nanoliposomes for the Enhanced Stability of Walnut Angiotensin-converting Enzyme (ACE) Inhibitory Peptide. J. Food Sci. 2023, 88, 2130–2140. [Google Scholar] [CrossRef]
- Khatib, N.; Varidi, M.J.; Mohebbi, M.; Varidi, M.; Hosseini, S.M.H. Co-encapsulation of Lupulon and Xanthohumol in Lecithin-based Nanoliposomes Developed by Sonication Method. J. Food Process. Preserv. 2019, 43, e14075. [Google Scholar] [CrossRef]
- Zarrabi, A.; Abadi, M.A.A.; Khorasani, S.; Mohammadabadi, M.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M. Fractionation of Flaxseed-Derived Bioactive Peptides and Their Influence on Nanoliposomal Carriers. J. Agric. Food Chem. 2020, 68, 15097–15106. [Google Scholar] [CrossRef]
- Yokota, D.; Moraes, M.B.d.; Pinho, S.C. Characterization of Lyophilized Liposomes Produced With Non-Purified Soy Lecithin: A Case Study of Casein Hydrolysate Microencapsulation. Braz. J. Chem. Eng. 2012, 29, 325–335. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Tan, C.P.; Saari, N. Comparative Physicochemical Stability and Efficacy Study of Lipoid S75-Biopeptides Nanoliposome Composite Produced by Conventional and Direct Heating Methods. Int. J. Food Prop. 2018, 21, 1646–1660. [Google Scholar] [CrossRef]

| Target Position | Design Strategy | Advantages | Reference |
|---|---|---|---|
| Tumors | Aptamer-based systems | Designed to target the acidic tumor microenvironment or external stimuli (e.g., light, temperature) to promote drug penetration, prolong circulation time, and achieve selective accumulation through enhanced permeation retention (EPR) effects | [100,101,102] |
| Dendritic macromolecular system | Precise delivery of drugs directly to cancer cells through multiple interactions with cellular receptors | [102] | |
| Other colloidal systems (e.g., functionalized multi-walled carbon nanotubes (f-MWNTs)) | Introduction of folic acid or polyethylene glycol modification enhances solubility, biocompatibility and tumor targeting ability | [103,104] | |
| Brain | Utilization of a receptor-mediated trans-BBB transport system | Improved targeting and sustained release of anticancer drugs showing enhanced cytotoxicity against brain tumors | [105,106] |
| Intranasal drug delivery system | Colloidal carriers such as liposomes, nanoemulsions, and polymeric nanoparticles prepared from food-grade materials can be administered intranasally to enable rapid and efficient drug transport to deep brain structures while minimizing systemic exposure | [107,108] | |
| Surface Modification and Smart Response Architecture Design for Multifunctional Nanoparticle Systems | Combining long-cycling properties with targeting moieties (e.g., peptides, aptamers, carbohydrate ligands) not only encapsulates lipophilic anticancer or neurotherapeutic drugs, but also responds to stimuli (e.g., pH, enzymes) in the brain parenchyma. These modifications improve the precision of drug release, reduce off-target effects, and facilitate in vivo imaging and monitoring | [109] | |
| Intestinal tract | Development of food colloidal systems using pH-responsive and enzyme-resistant materials | Enhanced ability to protect and strategically release bioactives under intestinal conditions. | [110] |
| Programmable Co-Assembly Methods | Food-derived peptide co-assembly enhances targeted therapy for colitis by minimizing degradation and retention challenges posed by the intestinal barrier | [111] | |
| Lungs | Powder systems designed as nanostructured | Enhanced deposition in the alveolar region of the lung while overcoming challenges of particle aggregation and rapid clearance by alveolar macrophages | [112] |
| Generation of nanoliposome suspension liquid systems | Encapsulates both hydrophilic and lipophilic drugs, thus providing dual functionality for local targeting in the lungs and systemic delivery through the alveolar epithelium | [113] | |
| Targeted chemical modifications coupled to L-cysteine or other targeting groups | Improved lung distribution and significant increase in lung deposition | [114] | |
| Striated muscle | Encapsulation from plasmid DNA to small molecule drugs using carriers derived from natural macromolecules such as proteins, polysaccharides and lipids | Addressing cellular uptake, endosomal escape, and nuclear entry challenges in gene delivery | [3] |
| Embedding of bioactive substances of food origin | Stabilizes against gastrointestinal degradation and enhances its effects on skeletal muscle by improving bioavailability | [115,116] | |
| Heart | Polyethylene glycolized lipid nanoparticles | Reduced systemic toxicity while facilitating a controlled delayed drug release profile for cardiac therapies | [117] |
| RGD-functionalized polymer nanocarriers | Significantly enhanced uptake of nanoparticles into infarcted lesions, resulting in increased local concentrations of therapeutic molecules and improved cardioprotection. | [118] | |
| Vegetable oil-based Pickering nanoemulsion | Promoting more direct cytoplasmic release of cardioprotective agents in the myocardium has the potential to reduce immune cell clearance and increase therapeutic payloads | [119] | |
| Oral | Emulsions, microemulsions, liposomes and emulsion gels | Enhanced solubility and stability of hydrophobic drugs, gastrointestinal (GI) targeted protection, and improved interaction with intestinal epithelial cells | [3,120] |
| Classification | Structure | Function | Representative Substance |
|---|---|---|---|
| Functional Lipids | Triglycerides | Functional energy storage, providing essential fatty acids, and promoting the absorption of fat-soluble vitamins. | EPA/DHA |
| Carotenoids | Isoprene derivatives | Antioxidant, immunomodulatory, photoprotective | β-carotene, lutein, lycopene |
| Phytosterols | Cyclopentane-fused fully hydrogenated phenanthrene (Steron) | Lower cholesterol | β-sitosterol, stigmasterol, campesterol |
| Carrier System | Materials | Characteristics | Representative Patents |
|---|---|---|---|
| Protein Colloid Systems | Whey protein, casein, soy protein, zein, pea protein | Utilizing the amphiphilic nature of proteins and their self-assembly properties under specific conditions (such as pH changes, heating, or enzymatic digestion) to form nanoparticles or micelles. | WO2021174007A1 |
| Polysaccharide Colloid Systems | Chitosan, Alginate, Pectin, Starch, Cellulose | Utilizing sodium alginate to form an egg-carton-like gel structure through Ca2+ cross-linking, which remains stable in gastric acid and swells and dissolves at the neutral pH of the intestine to release the drug. Chitosan can open the tight junctions of the intestinal epithelium to promote absorption. | EP2890396B1 |
| Liposome Colloid Systems | Solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), liposomes. | Using emulsifiers (such as lecithin + Tween) and homogenization processes, hydrophobic substances are encapsulated within a lipid core to facilitate their lymphatic absorption in the small intestine. | US2018002859A1 |
| Composite Colloid Systems | Constructing colloidal systems such as emulsion gels/high-core-phase emulsions, lipid-polymer hybrid nanoparticles, and layer-by-layer self-assembled microcapsules to enhance drug stability and achieve targeted controlled release. | US2019032147A1 |
| Technology | Raw Materials | Targeted Mechanism | Product | Application |
|---|---|---|---|---|
| Microemulsion | Fats, phospholipids, polyglycerides | Lymphatic absorption targeting, significantly enhancing bioavailability | Accucor® (Lonza, Basel, Switzerland), VESIsorb® (VesiFact, Zug, Switzerland) | CoQ10, Omega-3, vitamins, CBD, and other hydrophobic supplements |
| Polysaccharide Gel System | Alginate, pectin, chitosan | pH-triggered or microbial enzyme-triggered | Various probiotic products, COLAL® (IFT Pharma, Berwyn, PA, USA) | Probiotics (intestinal colonization), prebiotics, colon disease medications |
| Enteric-coated system | Alginate | pH-triggered, targeted intestinal release | Eudragit® (Evonik, Essen, Germany), ACRYL-EZE® (Colorcon, Harleysville, PA, USA) | Loading ibuprofen and other medications |
| Protein-based embedding system | Whey protein, Casein, Zein | Physical protection, enhancing intestinal absorption | Various premium functional foods and supplements | Encapsulate active substances such as vitamins, minerals, and polyphenols |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Ma, C.; Du, L.; Xu, Y.; Yang, X. Research Progress on the Application of Food Colloids in Precise Targeted Delivery of Drugs and Bioactive Compounds. Gels 2025, 11, 746. https://doi.org/10.3390/gels11090746
Guo Y, Ma C, Du L, Xu Y, Yang X. Research Progress on the Application of Food Colloids in Precise Targeted Delivery of Drugs and Bioactive Compounds. Gels. 2025; 11(9):746. https://doi.org/10.3390/gels11090746
Chicago/Turabian StyleGuo, Yong, Chao Ma, Lianxin Du, Yan Xu, and Xin Yang. 2025. "Research Progress on the Application of Food Colloids in Precise Targeted Delivery of Drugs and Bioactive Compounds" Gels 11, no. 9: 746. https://doi.org/10.3390/gels11090746
APA StyleGuo, Y., Ma, C., Du, L., Xu, Y., & Yang, X. (2025). Research Progress on the Application of Food Colloids in Precise Targeted Delivery of Drugs and Bioactive Compounds. Gels, 11(9), 746. https://doi.org/10.3390/gels11090746