Color Development in Carotenoid-Enriched Bigels: Effects of Extraction Method, Saponification, and Oleogel-to-Hydrogel Ratios on CIELAB Parameters
Abstract
1. Introduction
2. Results and Discussion
2.1. Pequi Carotenoid Profile
2.2. Color of Carotenoid-Enriched Bigels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Pequi Carotenoid Extraction
4.3. HPLC-PDA-MS Analysis
4.4. Hydrogels (HG), Oleogels (OG), and Bigel Formulations
4.5. Color on Bigels
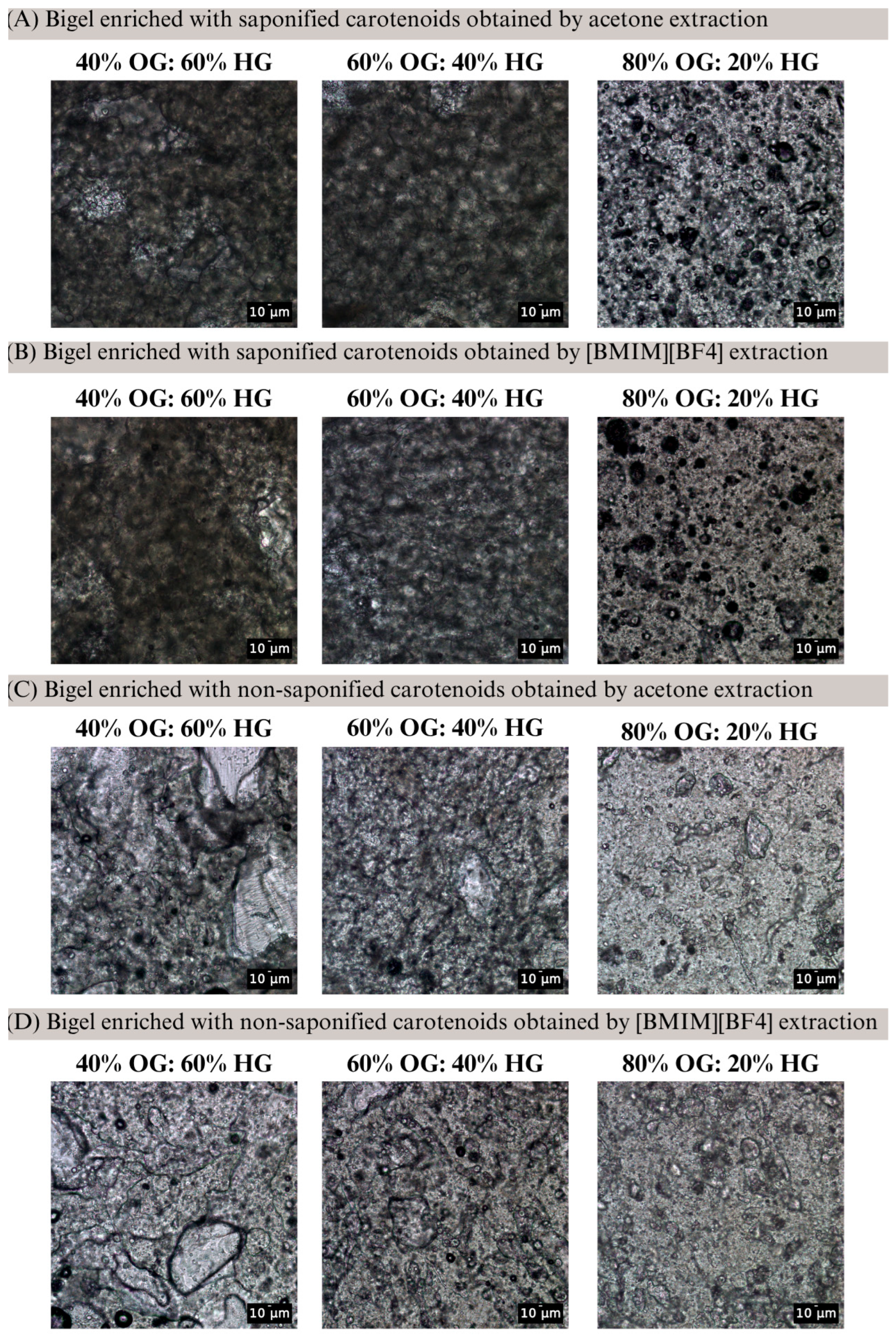
4.6. Bright-Field Microscopy Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ac NS | Carotenoids extracted with acetone and non-saponified |
| Ac S | Carotenoids extracted with acetone and saponified |
| B NS | Carotenoids extracted with [BMIM][BF4] and non-saponified |
| B S | Carotenoids extracted with [BMIM][BF4] and saponified |
| [BMIM][BF4] | 1-butyl-3-methylimidazolium tetrafluoroborate |
| CI | Confidence Interval |
| HG | Hydrogel |
| HPLC | High-Performance Liquid Chromatography |
| KOH | Potassium hydroxide |
| MS | Mass spectrometry |
| NS | Non-saponified |
| OG | Oleogel |
| S | Saponified |
| PDA | photodiode array detector |
| UV/Vis | Ultraviolet–Visible spectral characteristics |
References
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; da S. Petruci, J.F.; Batista, A.D. Novel Approaches for Colorimetric Measurements in Analytical Chemistry—A Review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 12.1–12.20. [Google Scholar] [CrossRef] [PubMed]
- Wannasin, D.; McClements, D.J. Optimizing the Appearance of Plant-Based Foods: Impact of Pigment and Droplet Characteristics on Optical Properties of Model Oil-in-Water Emulsions. Food Biophys. 2023, 18, 289–301. [Google Scholar] [CrossRef]
- Li, L.; Feng, X.; Teng, F.; Geng, M.; Li, Y. Insights into Beeswax-Gelatin/Carboxymethyl Chitosan Bigel Systems: Structural-Property Relationships Governing Dual Encapsulation of Hydrophilic and Hydrophobic Bioactive Compounds. Food Chem. 2025, 493, 145942. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold Carotenoids: Much More than Lutein Esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Nomenclature, Structures, and Physical and Chemical Properties. In Food Carotenoids: Chemistry, Biology and Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 1–23. ISBN 9781466591240. [Google Scholar]
- Eroglu, A.; Al’Abri, I.S.; Kopec, R.E.; Crook, N.; Bohn, T. Carotenoids and Their Health Benefits as Derived via Their Interactions with Gut Microbiota. Adv. Nutr. 2023, 14, 238–255. [Google Scholar] [CrossRef]
- Lombardo, M.; Serrao, S.; Lombardo, G. Challenges in Age-Related Macular Degeneration: From Risk Factors to Novel Diagnostics and Prevention Strategies. Front. Med. 2022, 9, 887104. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration— Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef]
- Kraak, V.I.; Story, M. Influence of Food Companies’ Brand Mascots and Entertainment Companies’ Cartoon Media Characters on Children’s Diet and Health: A Systematic Review and Research Needs. Obes. Rev. 2015, 16, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Souza, C.; Nass, P.; Jacob-Lopes, E.; Zepka, L.Q.; Braga, A.R.C.; De Rosso, V.V. Changing Despicable Me: Potential Replacement of Azo Dye Yellow Tartrazine for Pequi Carotenoids Employing Ionic Liquids as High-Performance Extractors. Food Res. Int. 2023, 174, 113593. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An In Vitro Digestion Method Adapted for Carotenoids and Carotenoid Esters: Moving Forward towards Standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid Esters in Foods—A Review and Practical Directions on Analysis and Occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Abdala, A.F.; Gallardo, A.P.; Olvera, L.G.; Silva, E.M.E. Hydrolysis of Carotenoid Esters from Tagetes Erecta by the Action of Lipases from Yarrowia Lipolytica. Bioresour. Bioprocess. 2017, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Souza, C.; Fernandes, A.S.; Mazzo, T.M.; Perrechil, F.; De Rosso, V.V. Fine-Tuning Carotenoid-Enriched Bigel Formulations: Exploring the Influence of Oleogel: Hydrogel Ratio on Physicochemical Properties and 3D Food Printing. Food Hydrocoll. 2025, 164, 111202. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Zhang, P.; Tu, Z.; Tan, M. Tailoring the Properties of Bigels by Beeswax Content for 3D Food Printing, Astaxanthin Delivery, and Freshness Sensing. Food Hydrocoll. 2026, 170, 111703. [Google Scholar] [CrossRef]
- Kaimal, A.M.; Singhal, R.S. A Bigel Based Formulation Protects Lutein Better in the Gastric Environment with Controlled Release and Antioxidant Profile than Other Gel Based Systems. Food Chem. 2023, 423, 136304. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and Characterization of Novel Bigels Based on Monoglyceride-Beeswax Oleogel and High Acyl Gellan Gum Hydrogel for Lycopene Delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef]
- Qiu, R.; Liu, X.; Tian, H.; Hu, Z.; Wang, K.; Zhao, L. Exploration of Bigel 4D Printing with Spontaneous Colour Change for Monitoring Bio-Actives Kinetic Behaviour Based on the Dual-Units 3D Printer. J. Food Eng. 2024, 367, 111861. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of Food-Grade Bigels Based on κ-Carrageenan Hydrogel and Monoglyceride Oleogels as Carriers for β-Carotene: Roles of Oleogel Fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Gurak, P.D.; Ferreira Marczak, L.D.; Tessaro, I.C. Tracking Bioactive Compounds with Colour Changes in Foods—A Review. Dyes Pigments 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Neves, B.V.; Fernandes, A.S.; Nass, P.; Jacob-Lopes, E.; Zepka, L.Q.; Mazzo, T.M.; Longo, E.; Cavalcante Braga, A.R.; de Rosso, V.V. Exploring Different Food-Grade Bigel Systems for Delivering Bioactive Carotenoids: Part 1—Evaluation of Color Parameters. Food Hydrocoll. 2025, 169, 111625. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Qualitative and Quantitative Analyses. In Food Carotenoids: Chemistry, Biology and Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 47–81. ISBN 978-1-118-73330-1. [Google Scholar]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the Colour and the Chemical Structure of Carotenoid Pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Karimidastjerd, A.; Cetinkaya, T.; Tarahi, M.; Singh, L.; Konar, N.; Khiabani, A.H.; Toker, O.S. Novel Approaches in Food Grade Bigels Properties and Applications: A Review. Int. J. Biol. Macromol. 2024, 283, 137424. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Mass Spectrometry. In Carotenoids; Enzell, C.R., Back, S., Eds.; Birkhäuser: Basel, Switzerland, 1995; pp. 261–320. [Google Scholar]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Analysis in Carotenoid; ILSI Press: Washington, DC, USA, 2001; ISBN 1578810728/9781578810727. [Google Scholar]
- Fernandes, A.S.; Jacob-Lopez, E.; Zepka, L.Q.; Roca, M.; de Rosso, V.V. Bioactive Compound-Loaded Food-Grade Bigels: (I) Characterization and Study of Colorimetry and 3d-Printing Capability. Food Hydrocoll. 2025, 168, 111486. [Google Scholar] [CrossRef]
- Li, D.; Fan, Z.; Tang, W.K.S. Domain Learning Naming Game for Color Categorization. PLoS ONE 2017, 12, e0188164. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. Two-Step Cleanup Procedure for the Identification of Carotenoid Esters by Liquid Chromatography-Atmospheric Pressure Chemical Ionization-Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1457, 116–124. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A.Z. Identification and Quantification of Carotenoids, by HPLC-PDA-MS/MS, from Amazonian Fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of New Carotenoid Esters in Mango and Citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.L.; Rodrigues, D.B.; Chitchumroonchokchai, C. Bioavailability and Metabolism of Carotenoid Esters: Physical, Chemical and Biological Properties. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2019; Volume 2019, pp. 390–420. ISBN 9781782628309. [Google Scholar]
- McLaren, K. The Development of the CIE 1976 (L*a*b) Uniform Colour Space and Color Difference Formula. J. Stud. Dyn. Change 2008, 92, 338–341. [Google Scholar]
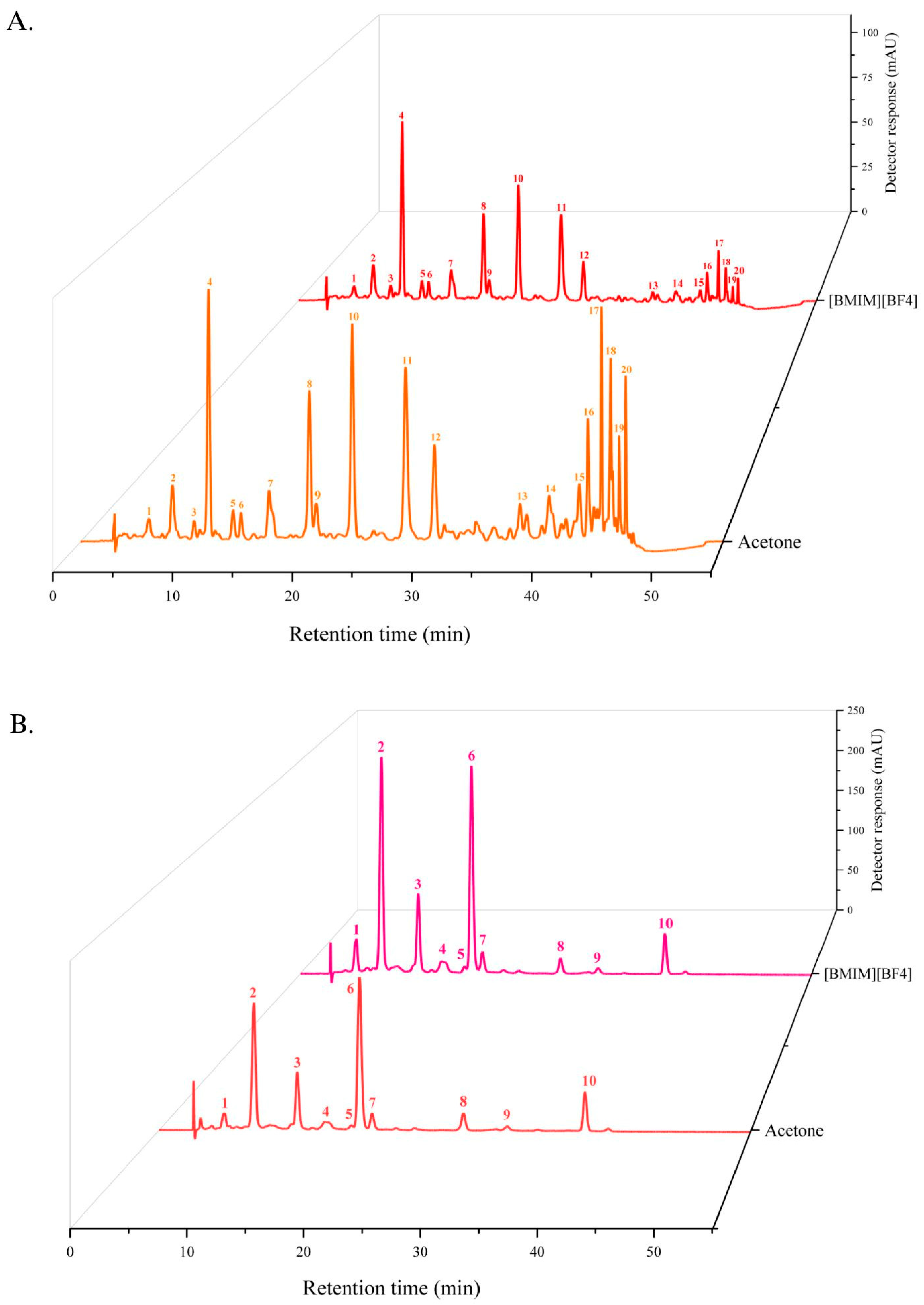

| UV-Vis Characteristics | |||||
|---|---|---|---|---|---|
| Peak a | Carotenoids | tR (min) b | λ(max) c | %III/II d | %AB/II e |
| 1 | cis-antheraxanthin | 5.8 | 419, 444, 471 | 52 | n.d. |
| 2 | all-trans-antheraxanthin | 7.8 | 420, 445, 473 | 60 | 0 |
| 3 | all-trans-zeaxanthin | 10.9 | 423, 450, 480 | 25 | 0 |
| 4 | cis-neoxanthin-ester | 13.0 | 330, 417, 441, 470 | 82 | 12 |
| 5 | all-trans-antheraxanthin-ester | 13.7 | 421, 445, 474 | 60 | 0 |
| 6 | cis-neoxanthin-ester | 16.1 | 330, 417, 441, 470 | 81 | 12 |
| 7 | all-trans-antheraxanthin-ester | 19.6 | 421, 445, 474 | 60 | 0 |
| 8 | cis-neoxanthin-ester | 20.2 | 330, 417, 441, 470 | 81 | 12 |
| 9 | all-trans-antheraxanthin-ester | 23.2 | 421, 445, 474 | 60 | 0 |
| 10 | all-trans-antheraxanthin-ester | 27.8 | 421, 445, 474 | 60 | 0 |
| 11 | all-trans-β-carotene | 30.3 | 425, 452, 478 | 25 | 0 |
| 12 | all-trans-antheraxanthin -ester | 37.6 | 421, 445, 474 | 60 | 0 |
| 13 | all-trans-antheraxanthin-ester | 40.1 | 420, 445, 473 | 60 | 0 |
| 14 | all-trans-antheraxanthin-ester | 42.7 | 420, 445, 473 | 60 | 0 |
| 15 | all-trans-zeaxanthin-ester | 43.3 | 423, 450, 480 | 25 | 0 |
| 16 | all-trans-zeaxanthin-ester | 44.6 | 423, 450, 480 | 25 | 0 |
| 17 | cis-zeaxanthin-ester | 45.3 | 332, 421, 447, 474 | 20 | 18 |
| 18 | all-trans-zeaxanthin-ester | 45.5 | 423, 450, 480 | 25 | 0 |
| 19 | all-trans-zeaxanthin-ester | 46.1 | 423, 450, 480 | 25 | 0 |
| 20 | all-trans-zeaxanthin-ester | 46.6 | 423, 450, 480 | 25 | 0 |
| UV-Vis Characteristics | Mass Spectrometry Characteristics (m/z) | ||||||
|---|---|---|---|---|---|---|---|
| Peak a | Carotenoids | tR b | λ(max) c | %III/II d | %AB/II e | [M+H] | MS/MS |
| 1 | all-trans-neoxanthin | 6.0 | 416, 439, 469 | 82 | 0 | 601 | 583[M+H-18]+, 565[M+ H-18-18]+, 547[M+H-18-18-18]+, 491[M+H-18-18-18-56]+, 221 |
| 2 | all-trans-antheraxanthin | 8.7 | 420, 444, 471 | 50 | 0 | 585 | 567[M+H-18]+, 549[M+H-18-18]+, 493[M+H-18-18-56]+, 221 |
| 3 | 9-cis-antheraxanthin | 12.7 | 332, 420, 442, 470 | 32 | 18 | 585 | 567[M+H-18]+, 549[M+H-18-18]+, 493[M+H-18-18-56]+, 221 |
| 4 | 13-cis-zeaxanthin | 15.2 | 336, 418, 443, 468 | n.c. | 43 | 569 | 551[M+H-18]+, 533[M+H-18-18]+, 463[M+H-106]+ |
| 5 | not identified | 17.6 | 335, 419, 441, 469 | 50 | 18 | n.d. | n.d. |
| 6 | all-trans-zeaxanthin | 18.3 | 423, 449, 476 | 25 | 0 | 569 | 551[M+H-18]+, 533[M+H-18-18]+, 463[M+H-106]+ |
| 7 | cis-5,6-epoxy-β-carotene | 19.5 | 332, 415, 440, 467 | 60 | 12 | 553 | 535[M+H-18]+, 461[M+H-92]+, 205 |
| 8 | all-trans-β-cryptoxanthin | 28.0 | 423, 450, 476 | 25 | 0 | 553 | 535[M+H-18]+, 495, 461[M+H-92]+ |
| 9 | 5,8-epoxy-β-carotene | 32.0 | 402, 424, 447 | 40 | 0 | 553 | 535[M+H-18]+, 461[M+H-92]+, 205 |
| 10 | all-trans-β-carotene | 39.2 | 425, 451, 477 | 33 | 0 | 537 | 444[M-92]+ |
| Color Parameters | OG | A NS | A S | B NS | B S |
|---|---|---|---|---|---|
| L* | 40% 1 h | 76.15 ± 0.05 | 75.89 ± 0.04 | 73.47 ± 0.10 | 73.16 ± 0.08 |
| 40% 12 h | 75.15 ± 0.31 | 74.52 ± 0.80 | 72.40 ± 0.39 | 71.77 ± 0.64 | |
| 60% 1 h | 76.91 ± 0.15 | 76.80 ± 0.01 | 74.94 ± 0.28 | 76.26 ± 0.04 | |
| 60% 12 h | 76.96 ± 0.01 | 75.96 ± 0.11 | 75.20 ± 0.01 | 76.17 ± 0.01 | |
| 80% 1 h | 79.04 ± 0.05 | 78.51 ± 0.04 | 77.73 ± 0.01 | 73.45 ± 0.01 | |
| 80% 12 h | 78.52 ± 0.03 | 77.42 ± 0.01 | 76.69 ± 0.24 | 73.36 ± 0.01 | |
| a* | 40% 1 h | 0.94 ± 0.01 | 1.76 ± 0.01 | 0.28 ± 0.01 | −0.10 ± 0.05 |
| 40% 12 h | 0.92 ± 0.02 | 1.55 ± 0.27 | 0.03 ± 0.16 | −0.06 ± 0.03 | |
| 60% 1 h | 0.44 ± 0.01 | 0.24 ± 0.00 | −0.04 ± 0.04 | −0.23 ± 0.00 | |
| 60% 12 h | 0.60 ± 0.00 | 0.37 ± 0.01 | 0.10 ± 0.00 | −0.31 ± 0.01 | |
| 80% 1 h | 0.89 ± 0.01 | −0.44 ± 0.00 | −0.16 ± 0.01 | 0.02 ± 0.00 | |
| 80% 12 h | 0.91 ± 0.02 | −0.40 ± 0.01 | −0.15 ± 0.05 | 0.00 ± 0.00 | |
| b* | 40% 1 h | 27.42 ± 0.03 | 32.26 ± 0.13 | 28.38 ± 0.05 | 27.63 ± 0.20 |
| 40% 12 h | 27.32 ± 0.09 | 30.95 ± 1.40 | 27.63 ± 0.17 | 28.32 ± 0.15 | |
| 60% 1 h | 27.95 ± 0.02 | 27.71 ± 0.01 | 26.52 ± 0.36 | 24.62 ± 0.01 | |
| 60% 12 h | 27.76 ± 0.00 | 28.12 ± 0.01 | 26.92 ± 0.00 | 24.38 ± 0.01 | |
| 80% 1 h | 30.84 ± 0.03 | 29.48 ± 0.01 | 25.76 ± 0.01 | 28.53 ± 0.01 | |
| 80% 12 h | 30.47 ± 0.06 | 29.21 ± 0.00 | 25.17 ± 0.40 | 27.96 ± 0.01 | |
| Chroma* | 40% 1 h | 27.44 ± 0.03 | 32.31 ± 0.13 | 28.38 ± 0.05 | 27.63 ± 0.20 |
| 40% 12 h | 27.33 ± 0.08 | 30.99 ± 1.41 | 27.63 ± 0.17 | 28.32 ± 0.15 | |
| 60% 1 h | 27.95 ± 0.02 | 27.71 ± 0.01 | 26.52 ± 0.36 | 24.62 ± 0.01 | |
| 60% 12 h | 27.77 ± 0.00 | 28.13 ± 0.01 | 26.92 ± 0.00 | 24.38 ± 0.01 | |
| 80% 1 h | 30.85 ± 0.03 | 29.48 ± 0.01 | 25.76 ± 0.01 | 28.53 ± 0.01 | |
| 80% 12 h | 30.48 ± 0.06 | 29.21 ± 0.00 | 25.17 ± 0.39 | 27.96 ± 0.01 | |
| hue* (°) | 40% 1 h | 88.04 ± 0.01 | 86.87 ± 0.01 | 89.43 ± 0.02 | 90.20 ± 0.10 |
| 40% 12 h | 88.07 ± 0.04 | 87.15 ± 0.37 | 89.74 ± 0.13 | 90.11 ± 0.07 | |
| 60% 1 h | 89.10 ± 0.02 | 89.50 ± 0.00 | 90.09 ± 0.08 | 90.38 ± 0.00 | |
| 60% 12 h | 88.76 ± 0.00 | 89.24 ± 0.01 | 89.79 ± 0.00 | 90.38 ± 0.02 | |
| 80% 1 h | 88.35 ± 0.01 | 90.86 ± 0.00 | 90.35 ± 0.01 | 89.96 ± 0.00 | |
| 80% 12 h | 88.29 ± 0.03 | 90.78 ± 0.01 | 90.34 ± 0.11 | 90.00 ± 0.00 |
| Color Parameter | Crosslinking (1 vs. 12 h) | Solvent (Acetone vs. [BMIM][BF4]) | Saponification Process | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | CI | p | Β | CI | p | β | CI | p | |
| L* | −0.681 | −1.60; 0.238 | 0.144 | −2.269 | −3.030; −1.509 | 0.000 | 0.830 | −0.082; 1.743 | 0.074 |
| a* | −0.003 | −0.281; 0.275 | 0.986 | −0.699 | −0.922; −0.476 | 0.000 | 0.140 | −0.136; 0.417 | 0.315 |
| b* | −0.240 | −1.156; 0.675 | 0.602 | −2.304 | −3.039; −1.569 | 0.000 | −0.586 | −1.493; 0.321 | 0.202 |
| Chroma* | −0.237 | −1.157; 0.682 | 0.608 | −2.321 | −3.057; −1.584 | 0.000 | −0.594 | −1.505; 0.316 | 0.197 |
| hue* | −0.065 | −0.588; 0.458 | 0.805 | 1.339 | 0.925; 1.753 | 0.000 | −0.282 | −0.801; 0.237 | 0.282 |
| Color Parameter | Effect of Different Oleogel-to-Hydrogel Ratios 1 | ||
|---|---|---|---|
| Β | CI | p | |
| L* | |||
| 60% OG | 2.087 | 1.168; 3.006 | 0.000 |
| 80% OG | 2.774 | 1.85; 3.692 | 0.000 |
| a* | |||
| 60% OG | −0.520 | −0.827; −0.213 | 0.001 |
| 80% OG | −0.582 | −0.889; −0275 | 0.000 |
| b* | |||
| 60% OG | −1.990 | −2.998; −0.981 | 0.000 |
| 80% OG | −0.311 | −1.319; 0.698 | 0.541 |
| Chroma* | |||
| 60% OG | −2.009 | −3.021; −0.997 | 0.000 |
| 80% OG | −0.329 | −1.341; 0.683 | 0.518 |
| hue* | |||
| 60% OG | 0.991 | 0.421; 1.561 | 0.001 |
| 80% OG | 1.164 | 0.594; 1.734 | 0.000 |
| Color Parameter | L* | a* | b* | Chroma* | hue* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | CI | p | β | CI | p | β | CI | p | β | CI | p | β | CI | p |
| Crosslinking after 12 h | −0.659 | −1.121; −0.197 | 0.006 | 0.003 | −0.183; 0.188 | 0.978 | −0.254 | −0.863 0.356 | 0.409 | −0.251 | −0.860; 0.359 | 0.414 | −0.075 | −0.408; 0.258 | 0.654 |
| Solvent [BMIM][BF4] | −2.291 | −2.753; −1.829 | 0.000 | −0.704 | −0.889; −0.519 | 0.000 | −2.290 | −2.900; −1.681 | 0.000 | −2.307 | −2.916; −1.697 | 0.000 | 1.349 | 1.016; 1.682 | 0.000 |
| Non-saponified carotenoids | 0.787 | 0.324; 1.250 | 0.001 | 0.181 | −0.005; 0.366 | 0.056 | −0.488 | −1.098; 0.123 | 0.116 | −0.495 | −1.105; 0.116 | 0.110 | −0.362 | −0.695; −0.0288 | 0.034 |
| 60% OG * | 2.054 | 1.488; 2.620 | 0.000 | −0.527 | −0.754; −0.300 | 0.000 | −1.969 | −2.716; −1.222 | 0.000 | −1.989 | −2.735; −1.242 | 0.000 | 1.006 | 0.598; 1.413 | 0.000 |
| 80% OG * | 2.741 | 2.175; 3.307 | 0.000 | −0.589 | −0.816; −0.362 | 0.000 | −0.291 | −1.038; 0.457 | 0.440 | −0.309 | −1.055; 0.438 | 0.412 | 1.179 | 0.771; 1.586 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Souza, C.; Bandoni, D.H.; de Rosso, V.V. Color Development in Carotenoid-Enriched Bigels: Effects of Extraction Method, Saponification, and Oleogel-to-Hydrogel Ratios on CIELAB Parameters. Gels 2025, 11, 823. https://doi.org/10.3390/gels11100823
Ramos-Souza C, Bandoni DH, de Rosso VV. Color Development in Carotenoid-Enriched Bigels: Effects of Extraction Method, Saponification, and Oleogel-to-Hydrogel Ratios on CIELAB Parameters. Gels. 2025; 11(10):823. https://doi.org/10.3390/gels11100823
Chicago/Turabian StyleRamos-Souza, Caroline, Daniel Henrique Bandoni, and Veridiana Vera de Rosso. 2025. "Color Development in Carotenoid-Enriched Bigels: Effects of Extraction Method, Saponification, and Oleogel-to-Hydrogel Ratios on CIELAB Parameters" Gels 11, no. 10: 823. https://doi.org/10.3390/gels11100823
APA StyleRamos-Souza, C., Bandoni, D. H., & de Rosso, V. V. (2025). Color Development in Carotenoid-Enriched Bigels: Effects of Extraction Method, Saponification, and Oleogel-to-Hydrogel Ratios on CIELAB Parameters. Gels, 11(10), 823. https://doi.org/10.3390/gels11100823










