Silica Nanoparticle-Reinforced Bioactive Oxidized Alginate/Polyacrylamide–Gelatin Interpenetrating Polymer Network Composite Hydrogels
Abstract
1. Introduction
2. Results and Discussion
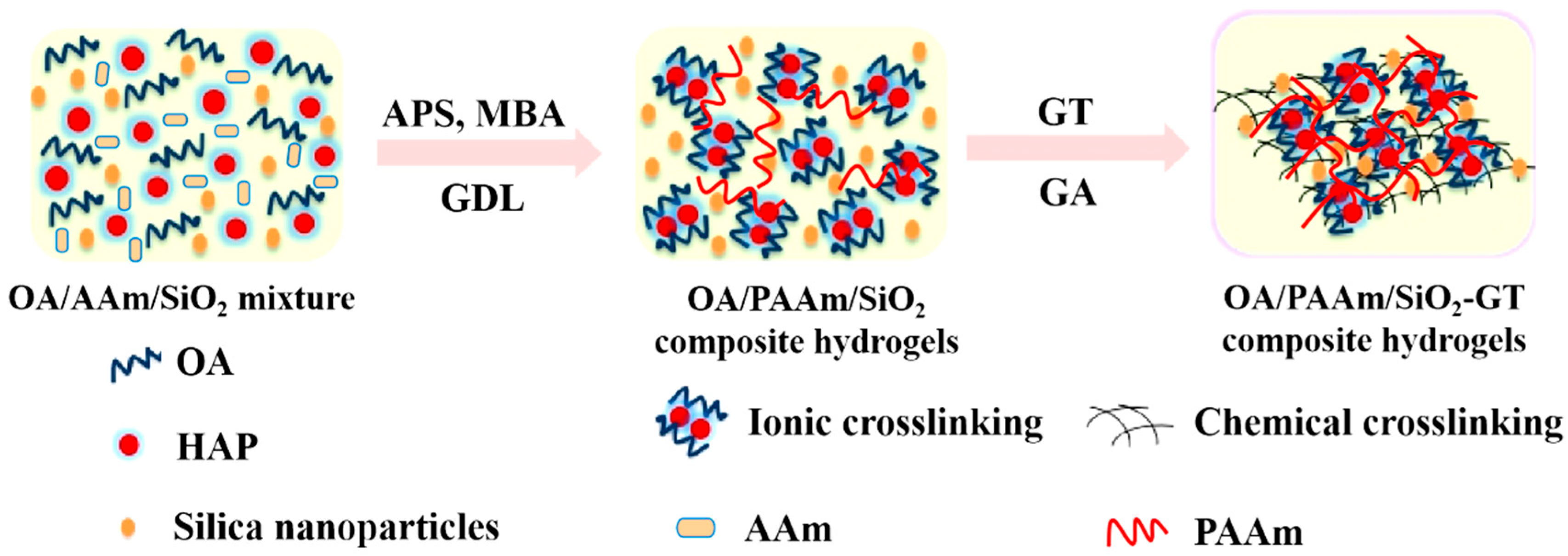
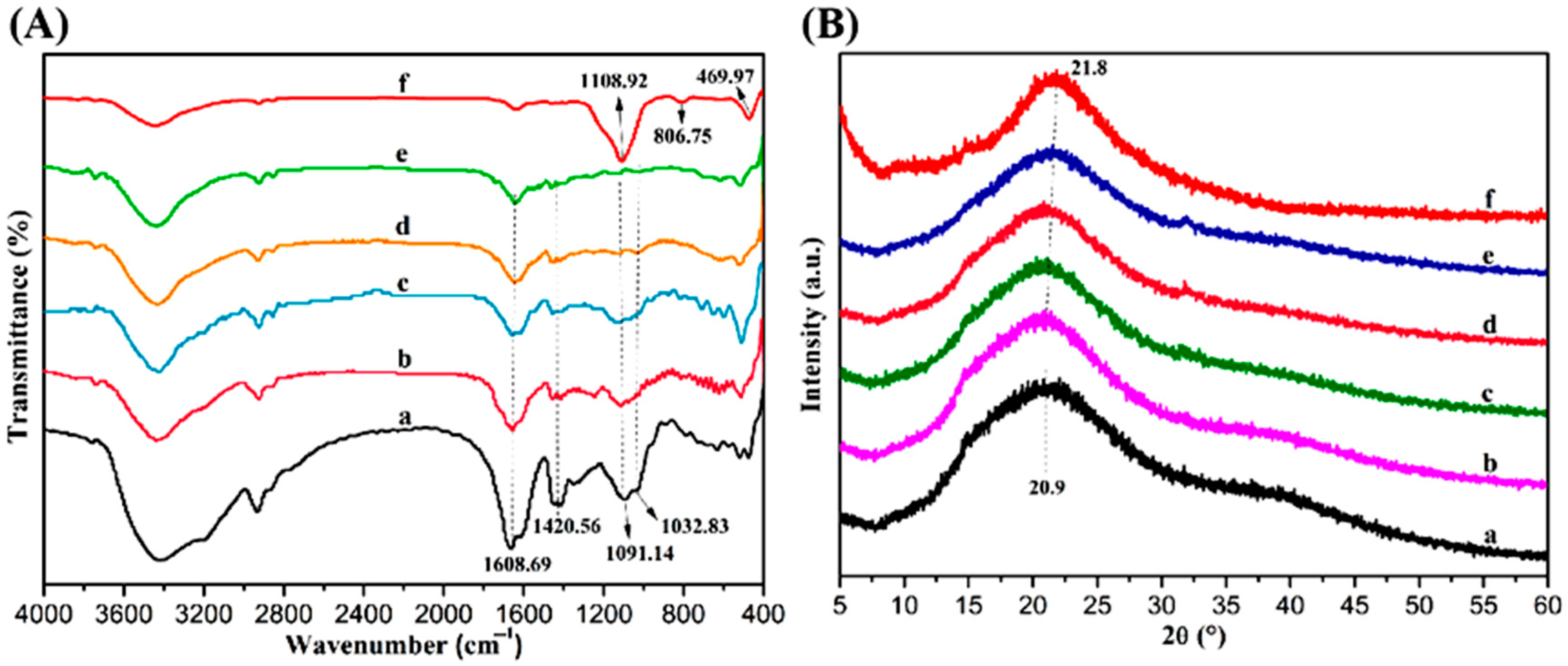
2.1. Interactions Among the Components of Composite Hydrogels
2.2. Morphological Characteristics and Mechanical Properties of Composite Hydrogels
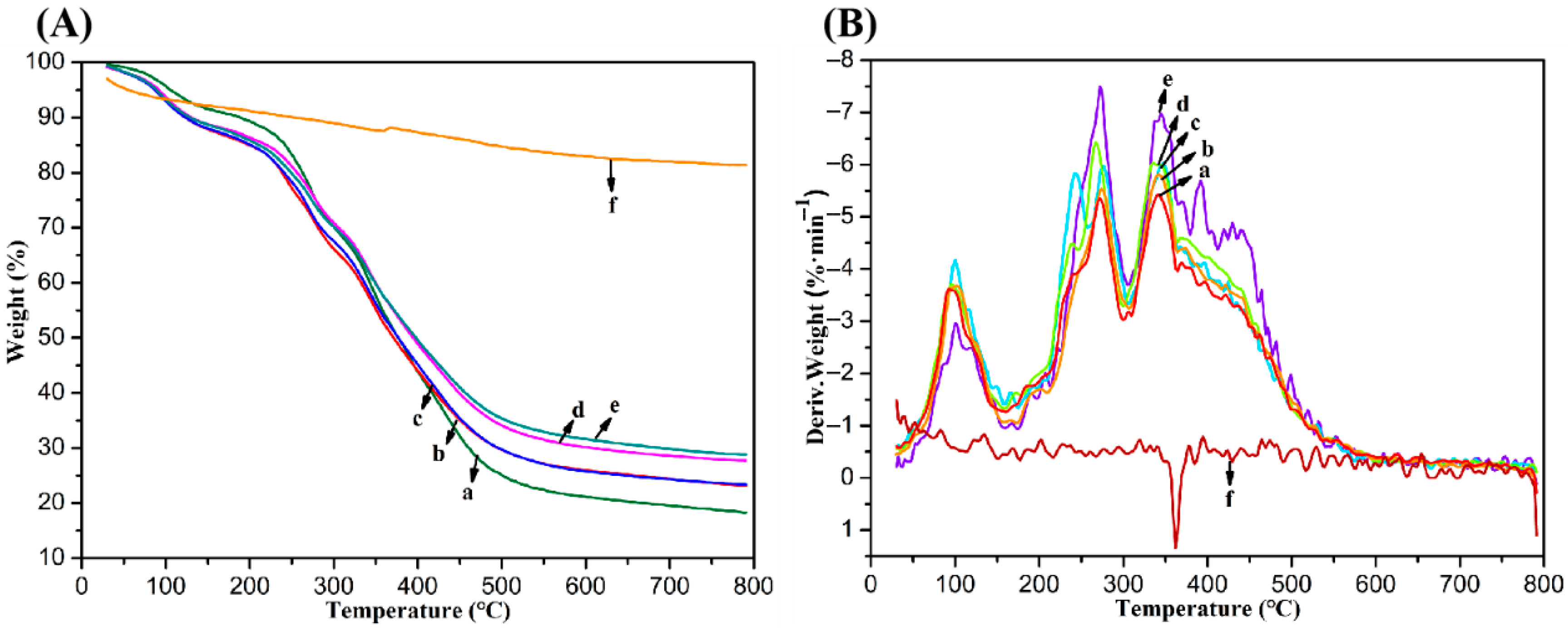
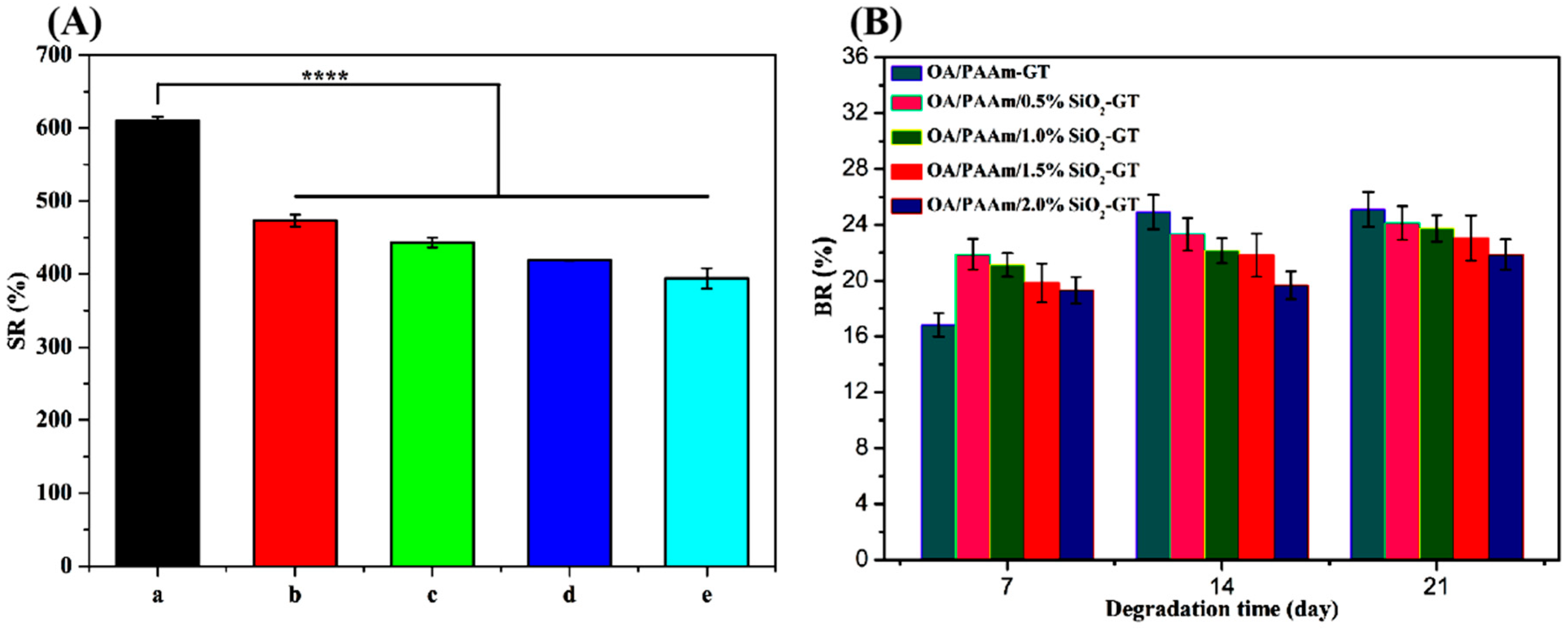
2.3. Swelling and Biodegradation Properties of Composite Hydrogels
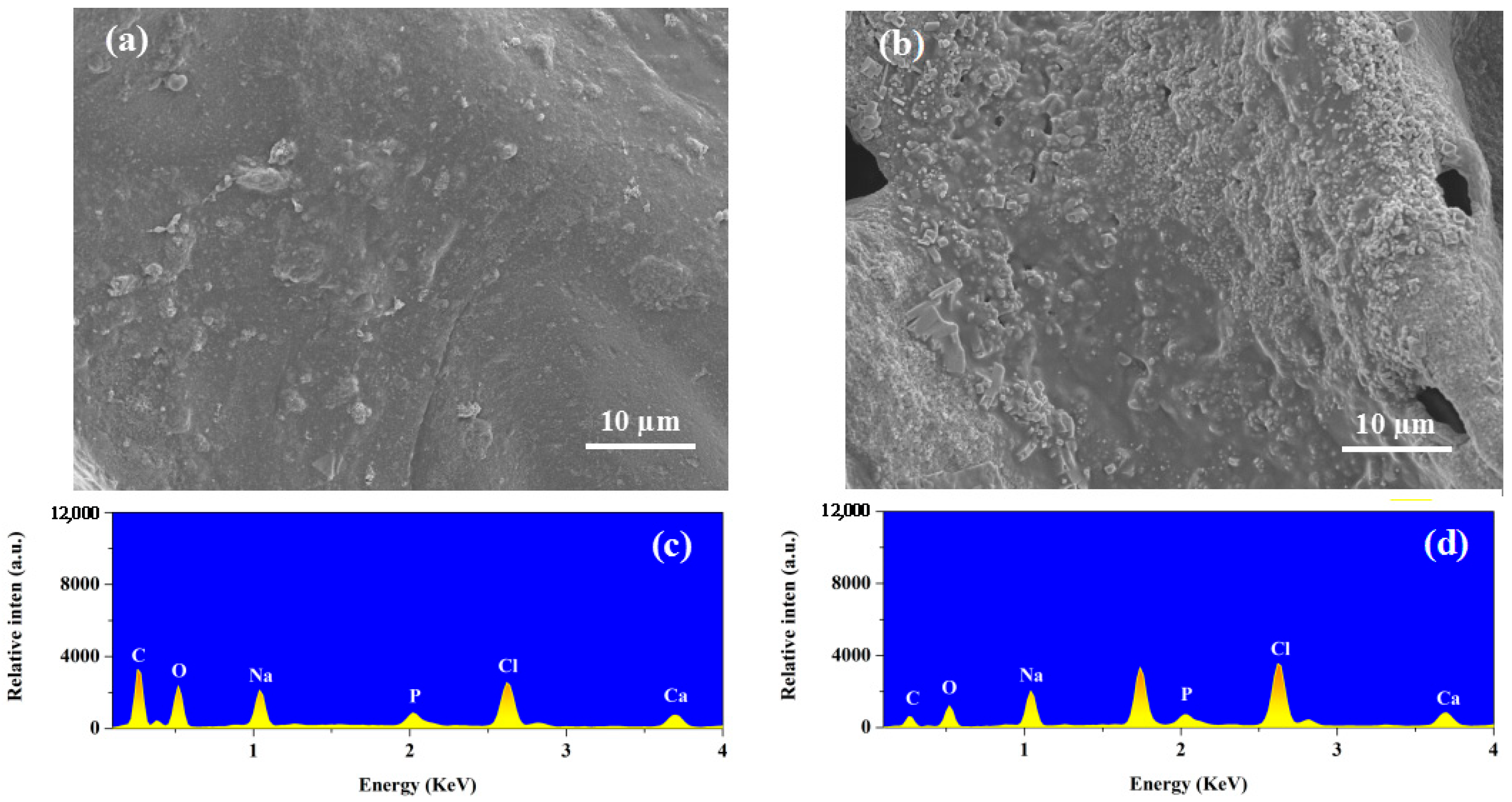
2.4. Biomineralization Study of Composite Hydrogels
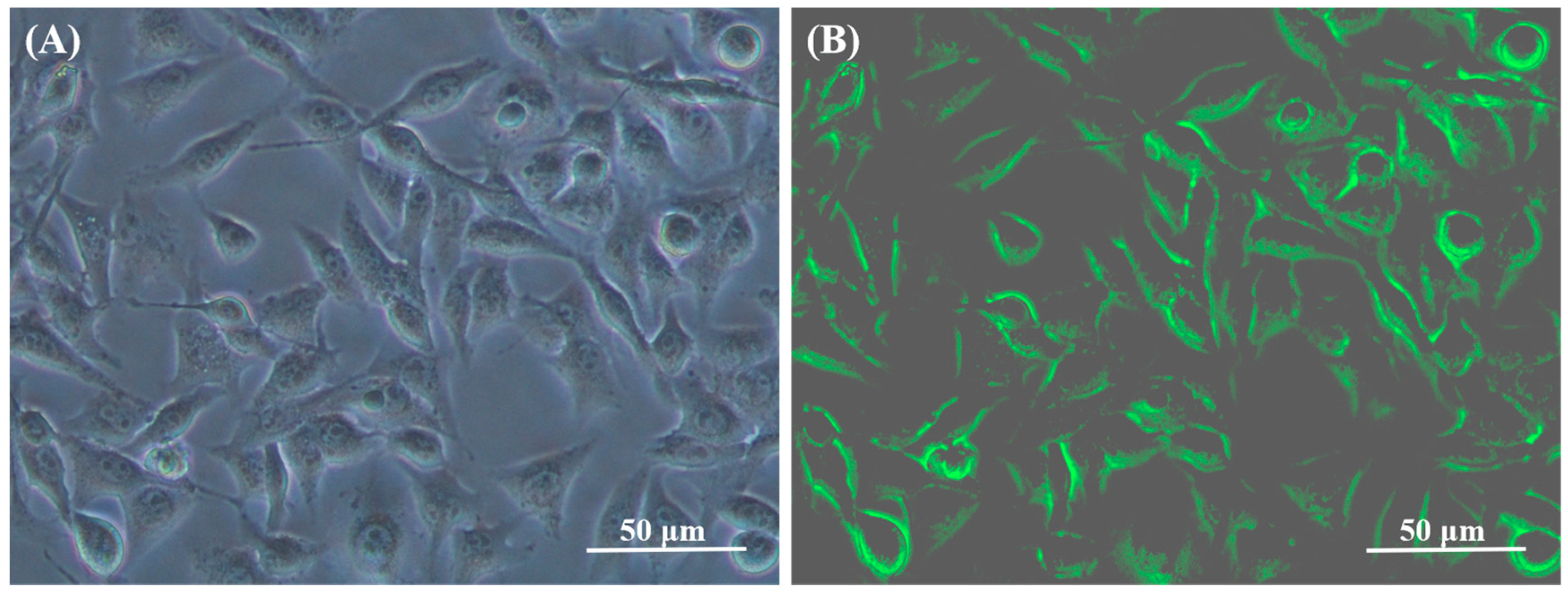
2.5. In Vitro Cytocompatibility of Composite Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Fabrication of OA/PAAm/SiO2-GT Composite Hydrogels
4.3. Characterization
4.4. In Vitro Swelling Behavior, Biodegradability, and Biomineralization Measurement
4.5. In Vitro Cytocompatibility Test
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, X.H.; Wen, Y.T.; Zhang, Z.X.; Zhu, J.L.; Song, X.; Phan, T.T.; Li, J. Recent advances in smart hydrogels derived from polysaccharides and their applications for wound dressing and healing. Biomaterials 2025, 318, 123134. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.P.; Huang, Y.; He, J.H.; Han, Y.; Guo, B. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(vinyl alcohol) hydrogel can autonomously self-heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef]
- Van Drunen, R.; Jimenez-Vergara, A.C.; Tsai, E.H.; Tchen, R.; Cagle, T.; Agee, B.A.; Roberts, J.; Steele, J.M.; Munoz-Pinto, D.J. Collagen based multicomponent interpenetrating networks as promising scaffolds for 3D culture of human neural stem cells, human astrocytes, and human microglia. ACS Appl. Bio Mater. 2019, 2, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.M.; Bootsma, K.; Berberich, J.A.; Sparks, J.L. Tunable stress relaxation behavior of an alginate-polyacrylamide hydrogel: Comparison with muscle tissue. Biomacromolecules 2015, 16, 1497–1505. [Google Scholar] [CrossRef]
- Darnell, M.; Sun, J.Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.G.; Mooney, D.J. Performance and biocompatibility of extremely tough algi-nate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.H.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z.G. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 17. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.M.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Wang, H.C.; Sun, X.Y.; Bu, Y.N.; Yan, H.Q.; Lin, Q. Chemical characterization and biological properties of titania/hydroxyapatite-promoted biomimetic alginate-chitosan-gelatin composite hydrogels. Ceram. Int. 2023, 49, 25744–25756. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Mazurek, L.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; Stradczuk-Mazurek, M.; Konop, M. Biomedical potential of alginate wound dressings-From preclinical studies to clinical applications: A review. Int. J. Biol. Macromol. 2025, 309, 142908. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Sun, L.L.; Wang, H.C.; Cao, S.S.; Shang, T.; Yan, H.Q.; Lin, Q. Nano-SiO2 reinforced alginate-chitosan-gelatin nanocomposite hydrogels with improved physicochemical properties and biological activity. Colloids Surf. B Biointerfaces 2023, 228, 113413. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Liu, J.Y.; Bu, Y.N.; Wu, T.; Fan, J.J.; Yan, H.Q.; Lin, Q. Dodecyl glycoside intercalated organo-montmorillonite promoted biomimetic alginate/microcrystalline cellulose/nano-hydroxyapatite composite hydrogels for bone tissue engineering. Int. J. Biol. Macromol. 2025, 310, 143304. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.Y.; Chen, M.Y.; Zhou, J.X.; Suo, Z.H. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Int. 2013, 5, 10418–10422. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Liu, P.; Zhang, C.; Yang, Z.W.; Xiong, G.Y.; Zheng, X.R.; Luo, H.L. Synthesis of a three-dimensional network-structured scaffold built of silica nanotubes for potential bone tissue engineering applications. J. Alloys Compd. 2015, 647, 711–719. [Google Scholar] [CrossRef]
- Li, Z.X.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte multilayer assemblies on materials surfaces: From cell adhesion to tissue engineering. Chem. Mater. 2012, 24, 854–869. [Google Scholar] [CrossRef]
- Ha, S.W.; Viggeswarapu, M.; Habib, M.M.; Beck, G.R., Jr. Bioactive effects of silica nanoparticles on bone cells are size, surface, and composition dependent. Acta Biomater. 2018, 82, 184–196. [Google Scholar] [CrossRef]
- Chen, S.; Shi, X.; Chinnathambi, S.; Hanagata, N. Large-scale fabrication of freestanding, micropatterned silica nanotubes via a hybrid hydrogel-templated route. Adv. Healthc. Mater. 2013, 2, 1091–1095. [Google Scholar] [CrossRef]
- Chen, S.; Osaka, A.; Hanagata, N. Collagen-templated sol-gel fabrication, microstructure, in vitro apatite deposition, and osteoblastic cellMC3T3-E1 compatibility of novel silica nanotube compacts. J. Mater. Chem. 2011, 21, 4332–4338. [Google Scholar] [CrossRef]
- Luo, Y.X.; Wu, C.T.; Lode, A.; Gelinsky, M. Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication 2013, 5, 015005–015013. [Google Scholar] [CrossRef]
- Sowjanya, J.A.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf. B 2013, 109, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Reakasame, S.; Boccaccini, A.R. Oxidized alginate-based hydrogels for tissue engineering applications: A review. Biomacromolecules 2018, 19, 3–21. [Google Scholar] [CrossRef]
- Li, Z.Y.; Sun, X.Y.; Chen, X.Q.; Wang, H.C.; Li, D.Z.; Shang, T.; Qi, L.X.; Yan, H.Q.; Lin, Q. Effects of TiO2 nanoparticles on the physicochemical and biological properties of oxidized sodium alginate/polyacrylamide-gelatin composite hydrogels fabricated by interpenetrating network approach. React. Funct. Polym. 2023, 191, 105679. [Google Scholar] [CrossRef]
- Gungor, M.; Sagirli, M.N.; Calisir, M.D.; Selcuk, S.; Kilic, A. Developing centrifugal spun thermally cross-linked gelatin based fibrous biomats for antibacterial wound dressing applications. Polym. Eng. Sci. 2021, 61, 2311–2322. [Google Scholar] [CrossRef]
- Bernstein-Levi, O.; Ochbaum, G.; Bitton, R. The effect of covalently linked RGD peptide on the conformation of polysaccharides in aqueous solutions. Colloids Surf. B 2016, 137, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.Q.; Chen, X.Q.; Feng, M.X.; Shi, Z.F.; Zhang, W.; Wang, Y.; Ke, C.R.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloids Surf. B 2019, 177, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Devara Venkata Krishna, D.V.; Sankar, M.R. Synthesis and characterization of SiO2 nanoparticles reinforced 3D printable gelatin/PVA/guar gum/ hydroxypropyl methylcellulose-based biocomposite hydrogel. Ind. Crop. Prod. 2024, 218, 118977. [Google Scholar] [CrossRef]
- Dutta, S.D.; Jin, H.X.; Patel, D.K.; Ganguly, K.; Lim, K.T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ji, L.; Yin, X.; Zhu, L.; Lin, Q.; Qin, J. Synthesis and characterization of bacterial cellulose sulfates usinga SO3/pyridine complex in DMAc/LiCl. Carbohydr. Polym. 2014, 101, 947–953. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, X.Q.; Bao, C.L.; Yi, J.L.; Lei, M.Y.; Ke, C.R.; Zhang, W.; Lin, Q. Synthesis and assessment of CTAB and NPE modified organo-montmorillonite for the fabrication of organo-montmorillonite/alginate based hydrophobic pharmaceutical controlled-release formulation. Colloids Surf. B 2020, 191, 110983. [Google Scholar] [CrossRef]
- Kang, H.L.; Shu, Y.; Li, Z.; Guan, B.; Peng, S.J.; Huang, Y.; Liu, R.G. An effect of alginate on the stability of LDH nanosheets in aqueous solution and preparation of alginate/LDH nanocomposites. Carbohydr. Polym. 2014, 100, 158–165. [Google Scholar] [CrossRef]
- Liu, M.X.; Dai, L.B.; Shi, H.Z.; Xiong, S.; Zhou, C.R. In vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering. Mater. Sci. Eng. C 2015, 49, 700–712. [Google Scholar] [CrossRef]
- Valente, J.F.A.; Valente, T.A.M.; Alves, P.; Ferreira, P.; Silva, A.; Correia, I.J. Alginate based scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2012, 32, 2596–2603. [Google Scholar] [CrossRef]
- Li, Z.Y.; Chen, X.Q.; Bao, C.L.; Liu, C.; Liu, C.Y.; Li, D.Z.; Yan, H.Q.; Lin, Q. Fabrication and evaluation of alginate/bacterial cellulose nanocrystals-chitosan-gelatin composite scaffolds. Molecules 2021, 26, 5003. [Google Scholar] [CrossRef]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Sergio, S. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef]
- Montanheiro, T.L.; Montagna, L.S.; Patrulea, V.; Jordan, O.; Borchard, G.; Lobato, G.M.M.; Catalani, L.H.; Lemes, A.P. Evaluation of cellulose nanocrystal addition on morphology, compression modulus and cytotoxicity of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds. J. Mater. Sci. 2019, 54, 7198–7210. [Google Scholar] [CrossRef]
- Özarslan, A.C.; Yücel, S. Fabrication and characterization of strontiumincorporated 3-D bioactive glass scaffolds for bone tissue from biosilica. Mater Sci. Eng. C 2016, 68, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Singhmar, R.; Son, Y.; Jo, Y.J.; Zo, S.; Min, B.K.; Sood, A.; Han, S.S. Fabrication of alginate composite hydrogel encapsulated retinoic acid and nano Se doped biphasic CaP to augment in situ mineralization and osteoimmunomodulation for bone regeneration. Int. J. Biol. Macromol. 2024, 275, 133597. [Google Scholar] [CrossRef] [PubMed]
- Takadama, H.; Kim, H.M.; Kokubo, T.; Nakamura, T. X-ray photoelectron spectroscopy study on the process of apatite formation on a sodium silicate glass in simulated body fluid. J. Am. Ceram. Soc. 2002, 85, 1933–1936. [Google Scholar] [CrossRef]
- Yang, M.; Zhen, W.J.; Chen, H.; Shan, Z.H. Biomimetic design of oxidized bacterial cellulose-gelatin-hydroxyapatite nanocomposites. J. Bionic Eng. 2016, 13, 631–640. [Google Scholar] [CrossRef]
- Shi, X.T.; Wang, Y.J.; Ren, L.; Zhao, N.R.; Gong, Y.H.; Wang, D.A. Novel mesoporous silica-based antibiotic releasing scaffold for bone repair. Acta Biomater. 2009, 5, 1697–1707. [Google Scholar] [CrossRef]
- Tchobanian, A.; Oosterwyck, H.V.; Fardim, P. Polysaccharides for tissue engineering: Current land-scape and future prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, P.; Lagrèze, W.A.; Noack, C.; Boehringer, D.; Biermann, J. Staining of fluorogold-prelabeled retinal ganglion cells with calcein-AM: A new method for assessing cell vitality. J. Neurosci. Methods 2010, 192, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.Y.; Liu, X.J.; Huang, Q.L. The stimulatory effect of silica nanoparticles on osteogenic differentiation of human mesenchymal stem cells. Biomed. Mater. 2016, 12, 015001. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.X.; Xiao, G.Z.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal–regulated kinase–MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef] [PubMed]
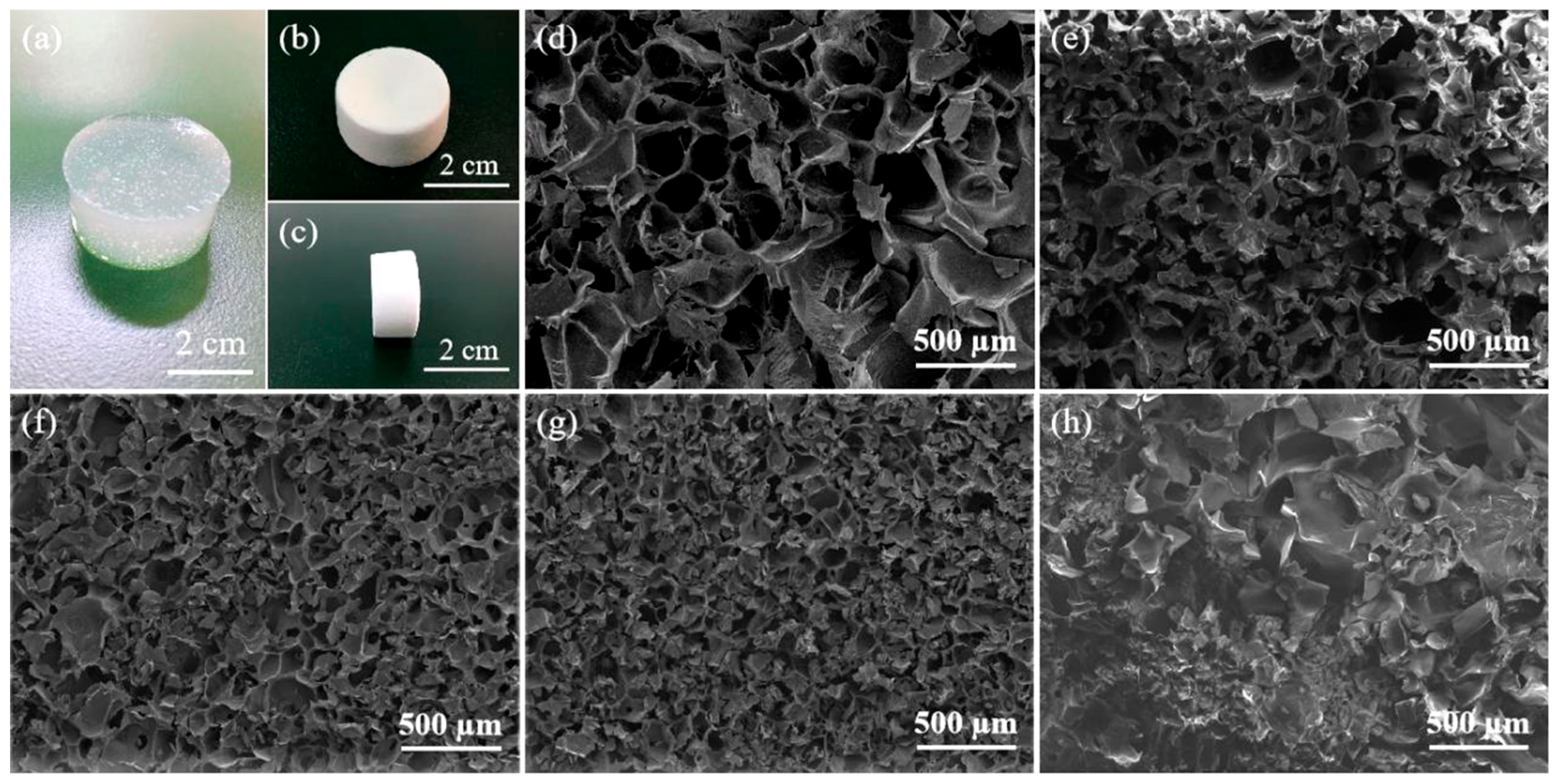



| Samples | Porosity (%) | Density (mg/mL) |
|---|---|---|
| OA/PAAm-GT | 88.44 | 0.133 ± 0.002 |
| OA/PAAm/0.5%SiO2-GT | 89.35 | 0.137 ± 0.001 |
| OA/PAAm/1.0%SiO2-GT | 83.55 | 0.147 ± 0.002 |
| OA/PAAm/1.5%SiO2-GT | 80.57 | 0.154 ± 0.002 |
| OA/PAAm/2.0%SiO2-GT | 79.64 | 0.163 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Y.; Liu, J.; Fan, J.; Chen, X.; Yan, H.; Lin, Q. Silica Nanoparticle-Reinforced Bioactive Oxidized Alginate/Polyacrylamide–Gelatin Interpenetrating Polymer Network Composite Hydrogels. Gels 2025, 11, 748. https://doi.org/10.3390/gels11090748
Bu Y, Liu J, Fan J, Chen X, Yan H, Lin Q. Silica Nanoparticle-Reinforced Bioactive Oxidized Alginate/Polyacrylamide–Gelatin Interpenetrating Polymer Network Composite Hydrogels. Gels. 2025; 11(9):748. https://doi.org/10.3390/gels11090748
Chicago/Turabian StyleBu, Yanan, Jiayi Liu, Jiji Fan, Xiuqiong Chen, Huiqiong Yan, and Qiang Lin. 2025. "Silica Nanoparticle-Reinforced Bioactive Oxidized Alginate/Polyacrylamide–Gelatin Interpenetrating Polymer Network Composite Hydrogels" Gels 11, no. 9: 748. https://doi.org/10.3390/gels11090748
APA StyleBu, Y., Liu, J., Fan, J., Chen, X., Yan, H., & Lin, Q. (2025). Silica Nanoparticle-Reinforced Bioactive Oxidized Alginate/Polyacrylamide–Gelatin Interpenetrating Polymer Network Composite Hydrogels. Gels, 11(9), 748. https://doi.org/10.3390/gels11090748


.png)



