Sustainable Durian Rind Carboxymethyl Cellulose/Poly(vinyl) Alcohol Hydrogels Synthesis for Enhancing Crosslinking and Release Kinetics Efficiency
Abstract
1. Introduction
2. Results and Discussion
2.1. Durian Rind Cellulose and CMCd Characterization
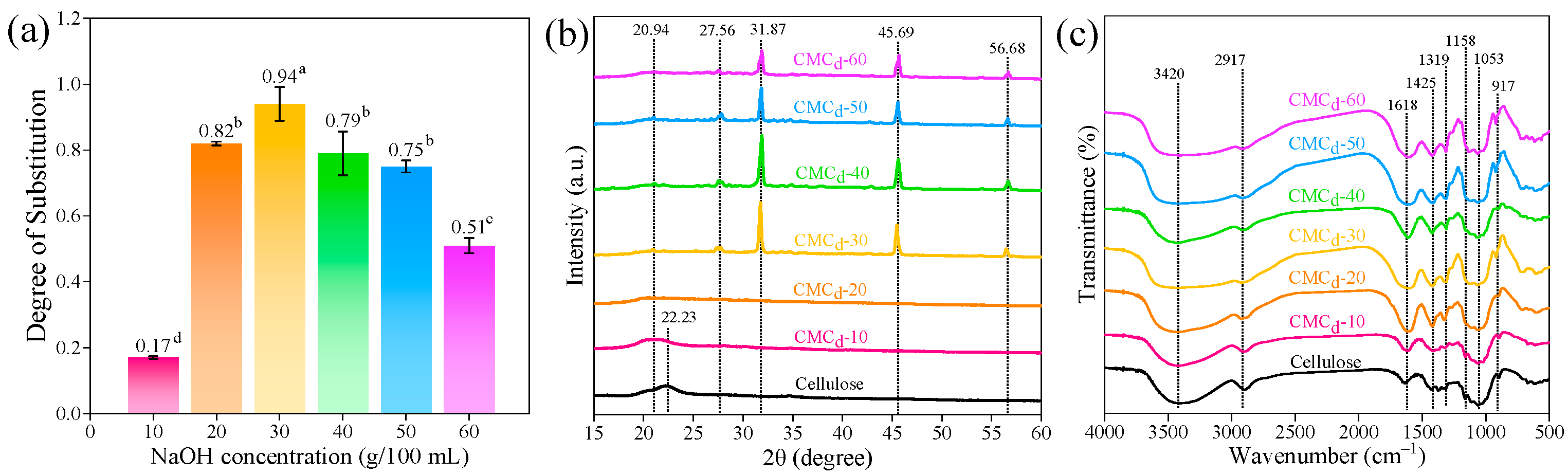
2.1.1. Degree of Substitution and Percent Yield of CMCd
2.1.2. XRD Analysis of CMCd
2.1.3. Analysis of the Chemical Functional Group of CMCd
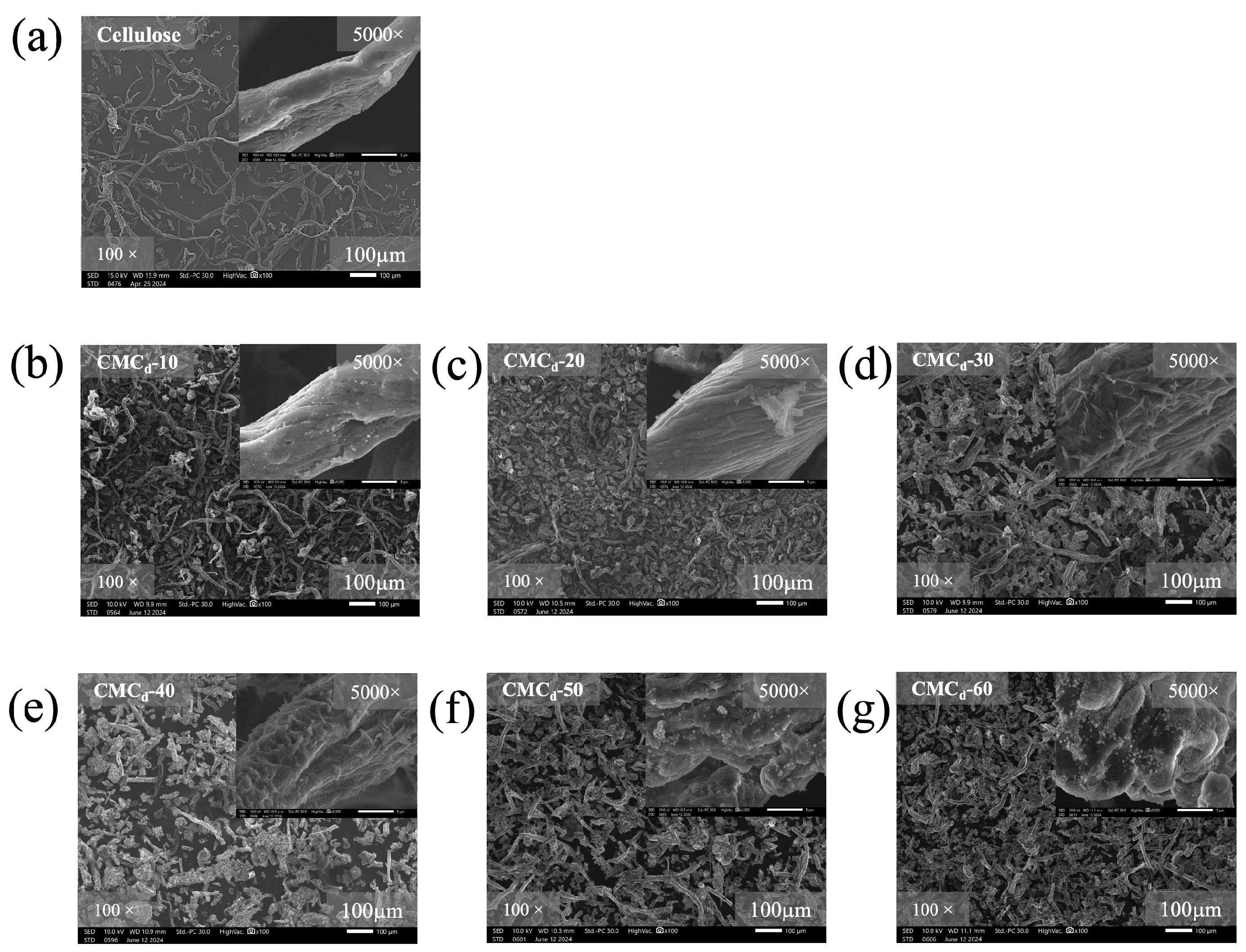
2.1.4. Morphology of CMCd
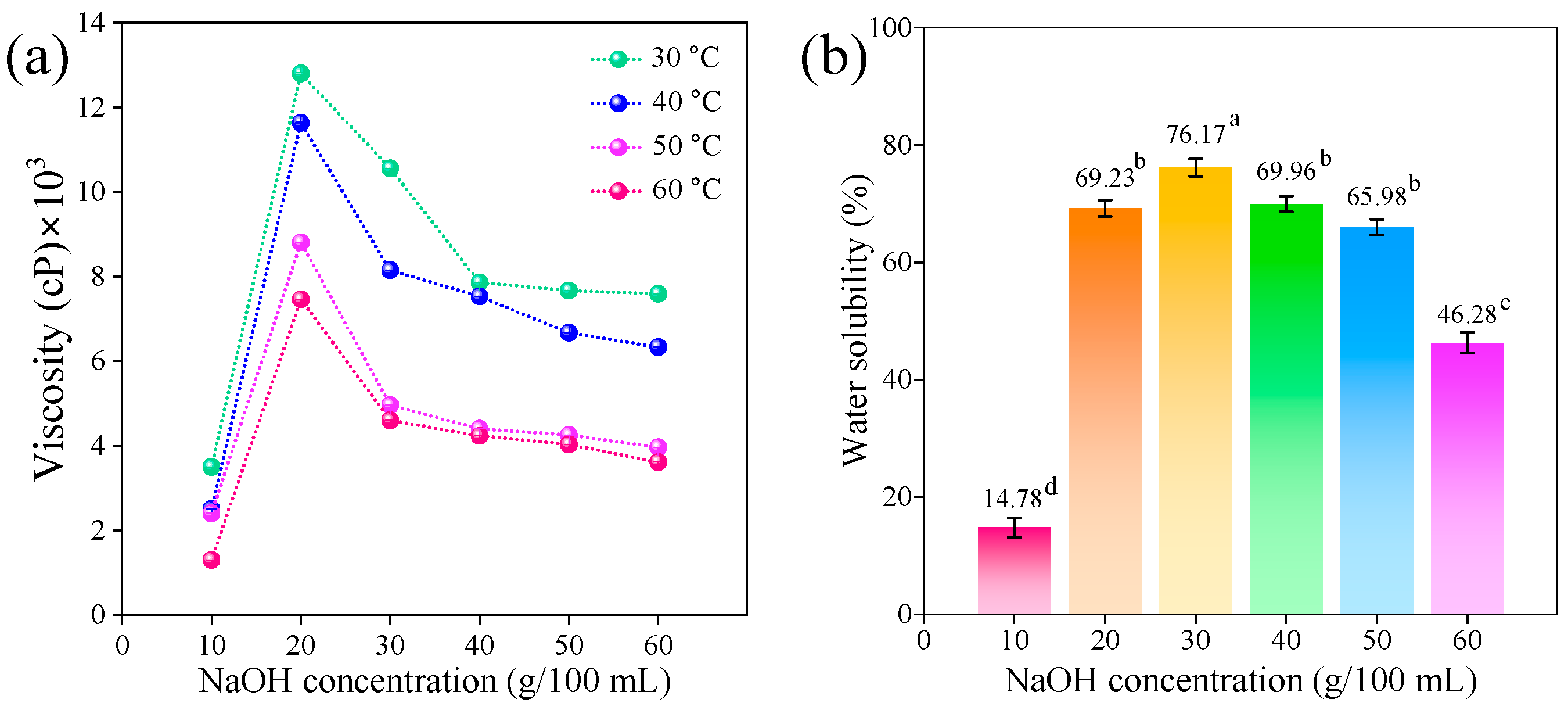
2.1.5. Viscosity and Water Solubility of CMCd
2.1.6. Color Measurement of Cellulose Fibers and CMCd
2.2. CMCd/PVA Hydrogel Characterization
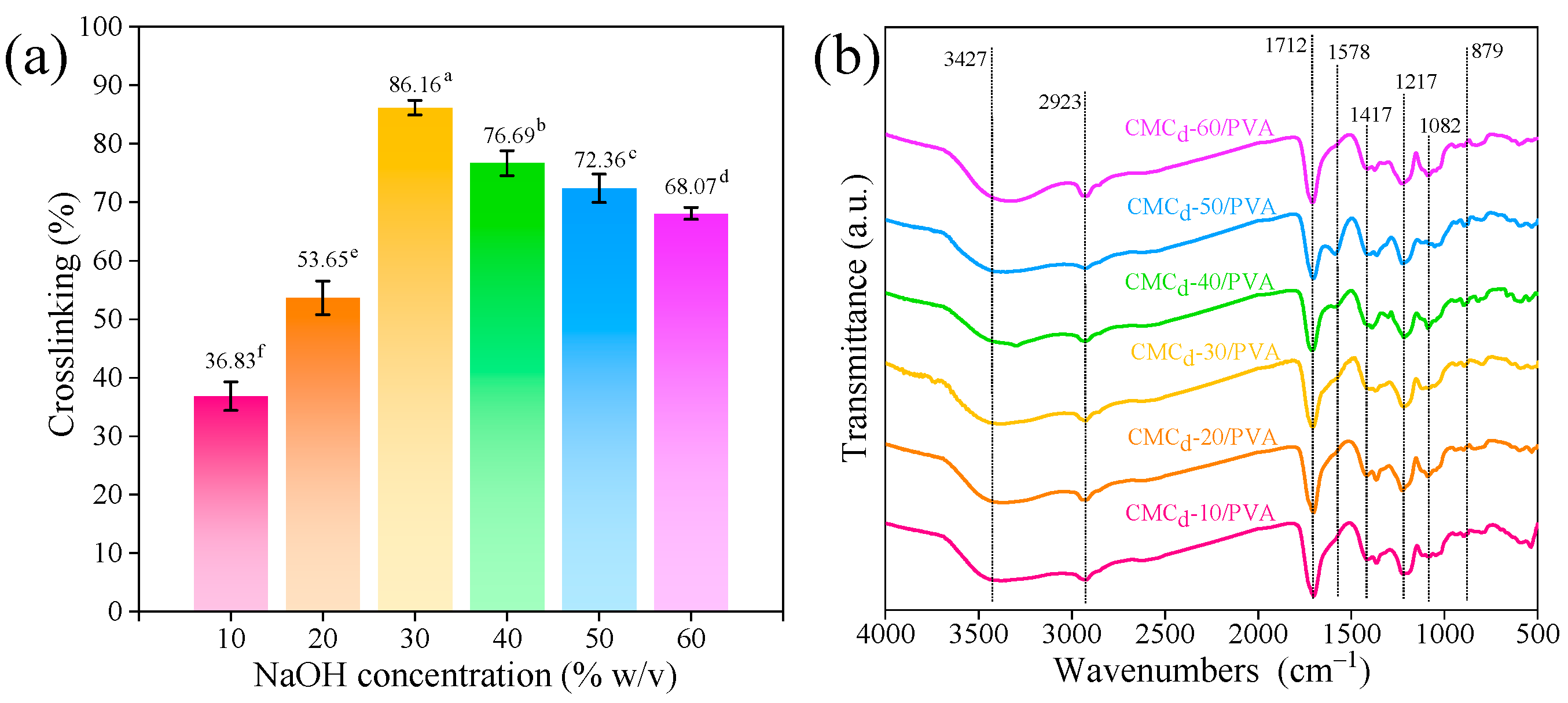
2.2.1. Crosslinking Percentage of CMCd/PVA Hydrogel
2.2.2. Chemical Functional Group of CMCd/PVA Hydrogels
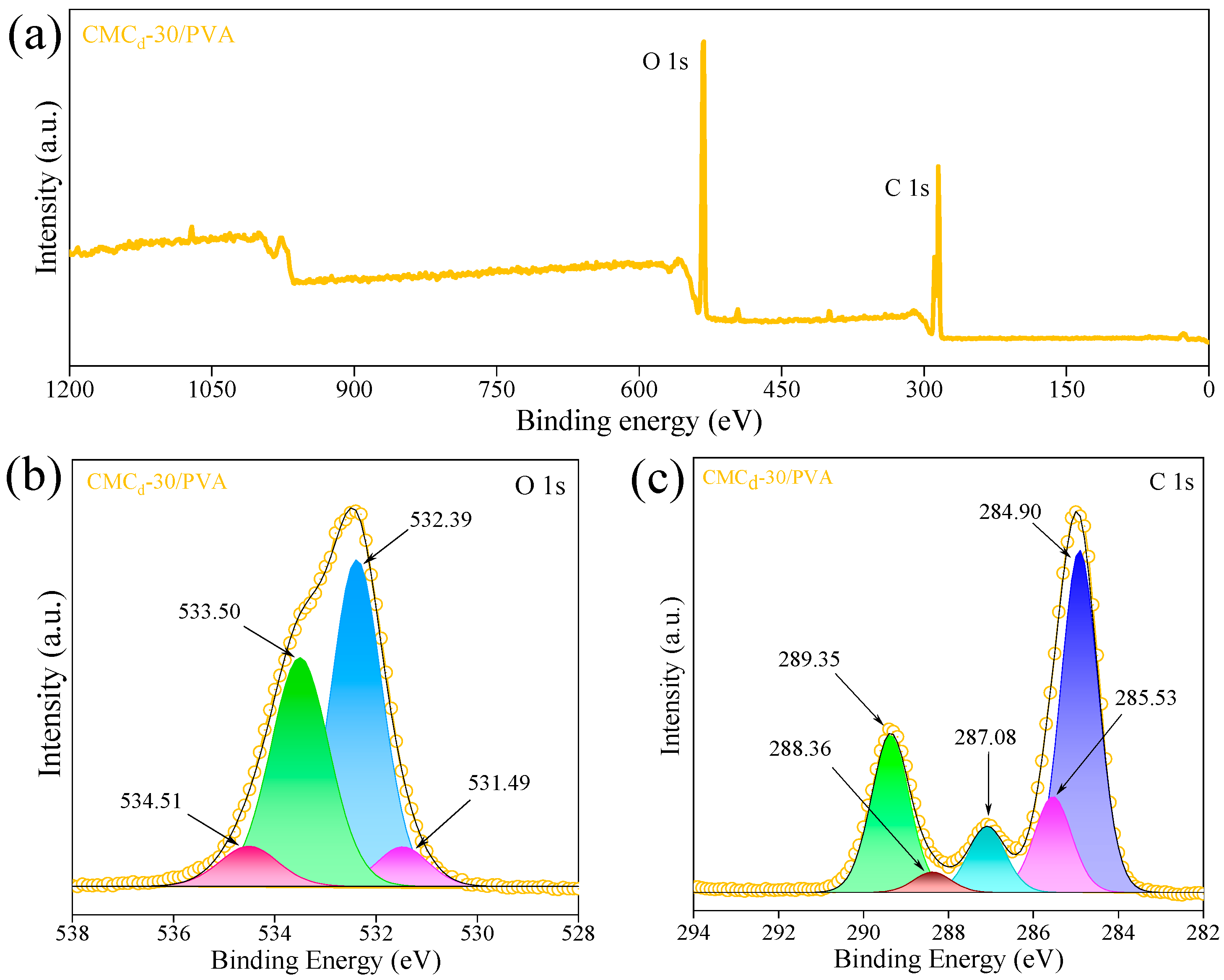
2.2.3. XPS Analysis of CMCd/PVA Hydrogel
2.2.4. Thermal Properties Comparison of CMCd and CMCd/PVA Hydrogels
2.3. MB Releasing Ability and Kinetic
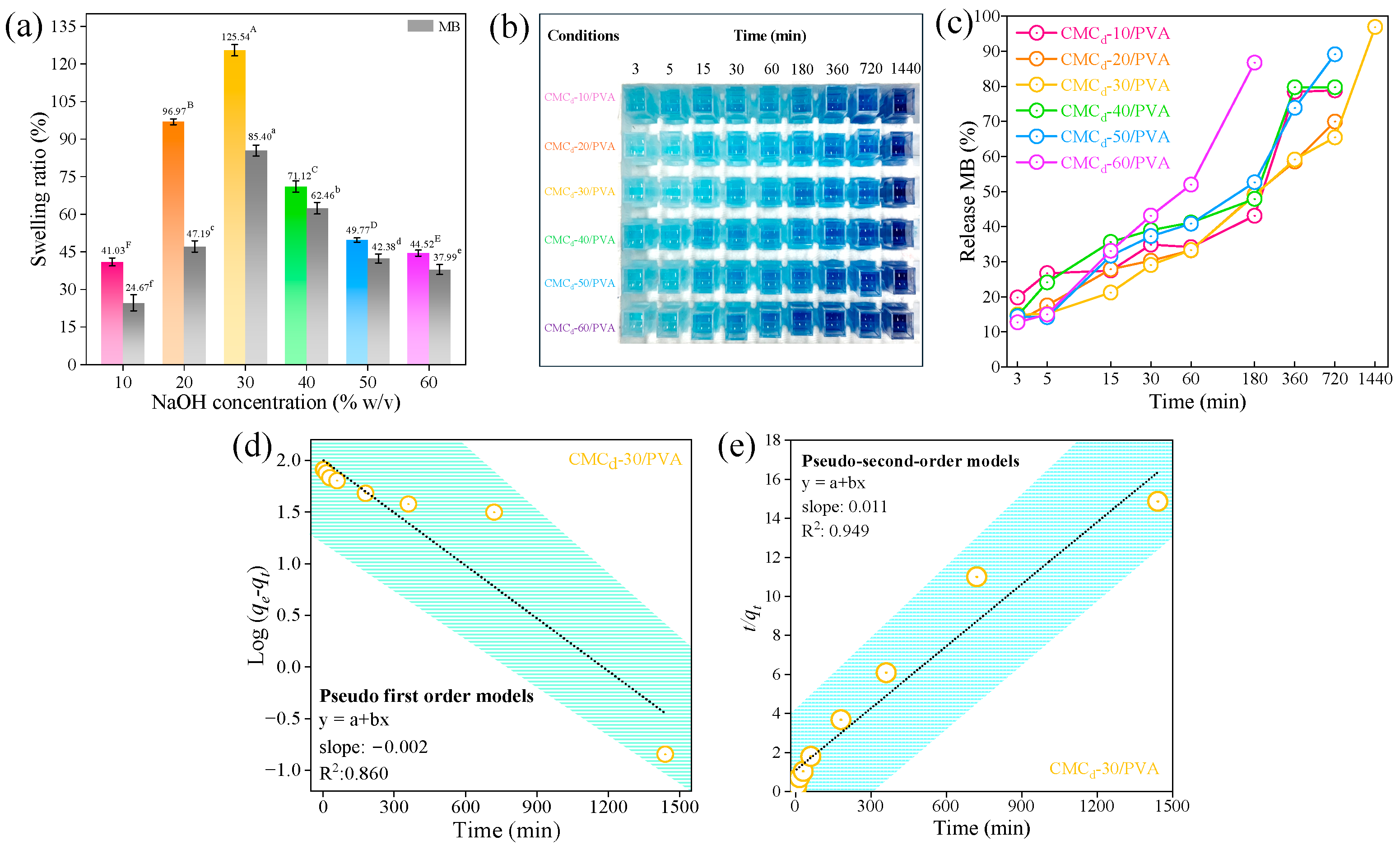
2.3.1. Swelling Ratio of CMCd/PVA Hydrogel Films
2.3.2. Methylene Blue Releasing
3. Conclusions
4. Materials and Methods
4.1. Materials
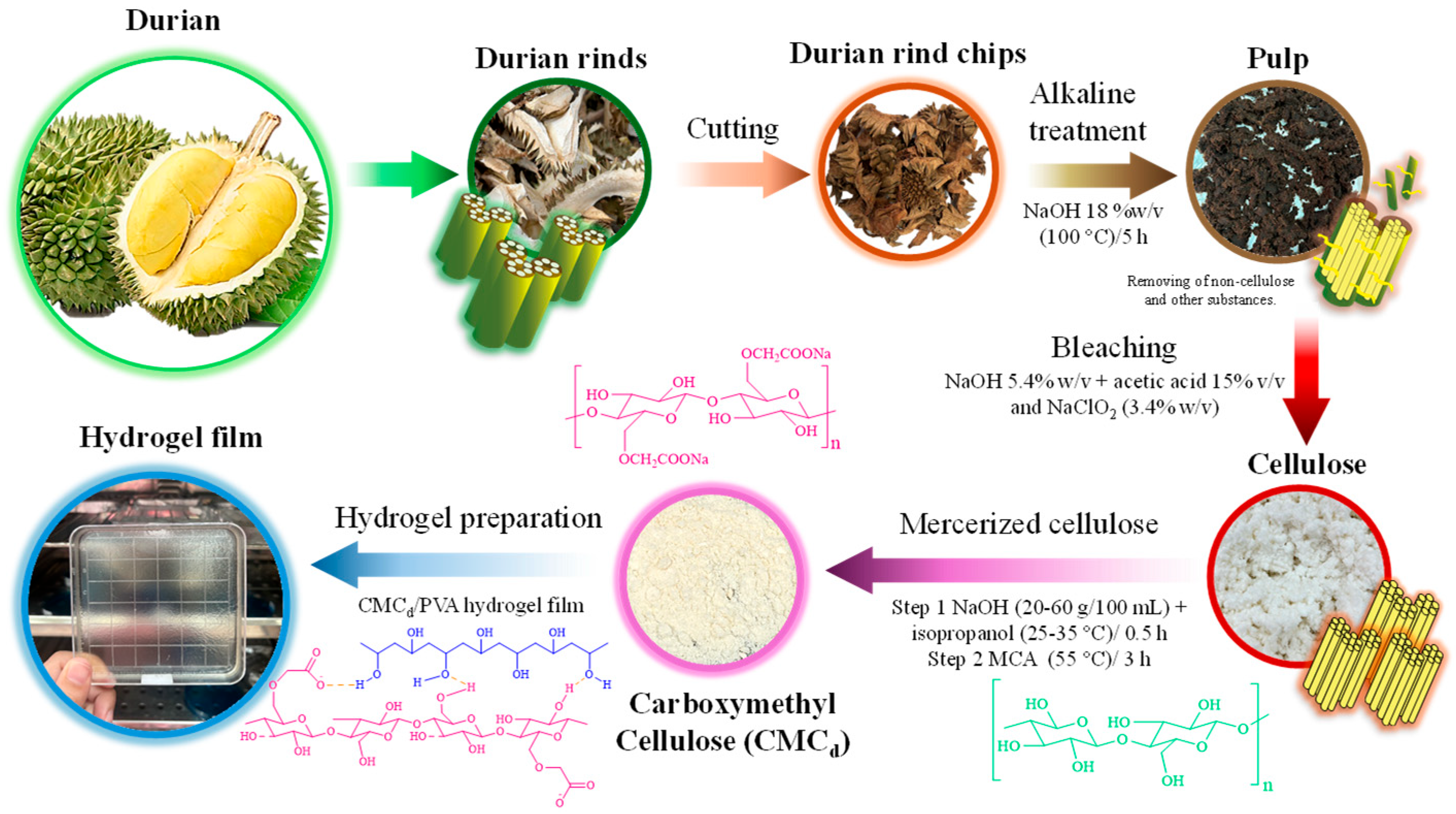
4.2. Durian Rind Cellulose and CMCd Preparation
4.2.1. Degree of Substitution
4.2.2. Crosslinking Percentage
4.3. Characterization of CMC
4.3.1. Morphology Investigation CMCd
4.3.2. Structural Analysis of CMCd
4.3.3. Functional Group of CMCd
4.3.4. X-Ray Photoelectron Spectroscopy
4.3.5. Viscosity and Water Solubility of CMCd Samples
4.3.6. Thermal Properties
4.3.7. Swelling Ratio Analysis
4.3.8. Color Measurement
4.4. Preparation of CMCd/PVA Hydrogel Films
4.5. In Vitro Release Study of Model Drugs
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Utilization of Carboxymethyl Cellulose from Durian Rind Agricultural Waste to Improve Physical Properties and Stability of Rice Starch-Based Film. J. Polym. Environ. 2018, 27, 286–298. [Google Scholar] [CrossRef]
- Togrul, H. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behaviour of carboxymethyl cellulose. Carbohydr. Polym. 2003, 54, 73–82. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Eitssayeam, S.; Pengpat, K. Study of carboxymethyl cellulose from papaya peels as binder in ceramics. Adv. Mater. Res. 2010, 93, 17–21. [Google Scholar] [CrossRef]
- Rachtanapun, P. Blended Films of Carboxymethyl Cellulose from Papaya Peel (CMCp) and Corn Starch. Kasetsart J. Nat. Sci. 2009, 43, 259–266. [Google Scholar]
- Rodsamran, P.; Sothornvit, R. Rice stubble as a new biopolymer source to produce carboxymethyl cellulose-blended films. Carbohydr. Polym. 2017, 171, 94–101. [Google Scholar] [CrossRef]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers 2020, 13, 81. [Google Scholar] [CrossRef]
- Kamthai, S.; Magaraphan, R. Mechanical and barrier properties of spray dried carboxymethyl cellulose (CMC) film from bleached bagasse pulp. Ind. Crops Prod. 2017, 109, 753–761. [Google Scholar] [CrossRef]
- Adinugraha, M.P.; Marseno, D.W.; Haryadi. Synthesis and characterization of sodium carboxymethylcellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Kumthai, S.; Mulkarat, N.; Pintajam, N.; Suriyatem, R. Value added of mulberry paper waste by carboxymethylation for preparation a packaging film. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012081. [Google Scholar] [CrossRef]
- Jia, F.; Liu, H.-j.; Zhang, G.-g. Preparation of Carboxymethyl Cellulose from Corncob. Procedia Environ. Sci. 2016, 31, 98–102. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; et al. Carboxymethyl Bacterial Cellulose from Nata de Coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Leksawasdi, N.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Effect of Monochloroacetic Acid on Properties of Carboxymethyl Bacterial Cellulose Powder and Film from Nata de Coco. Polymers 2021, 13, 488. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C.; Rawdkuen, S.; Tongdeesoontorn, W.; Klunklin, W. Carboxymethyl cellulose from Young Palmyra palm fruit husk: Synthesis, characterization, and film properties. Food Hydrocoll. 2022, 124, 107277. [Google Scholar] [CrossRef]
- Klunklin, W.; Hinmo, S.; Thipchai, P.; Rachtanapun, P. Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir. Polymers 2023, 15, 3376. [Google Scholar] [CrossRef]
- Heinze, T.; Pfeiffer, K. Studies on the synthesis and characterization of carboxymethylcellulose. Angew. Makromol. Chem. 1999, 266, 37–45. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kim, J.T.; Roy, S.; Jayakumar, A. Recent advances in carboxymethyl cellulose-based active and intelligent packaging materials: A comprehensive review. Int. J. Biol. Macromol. 2024, 259, 129194. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pereira, C.F.; Ribeiro, A.B.; Casanova, F.; Freixo, R.; Pintado, M.; Ramos, O.L. Carboxymethyl Cellulose as a Food Emulsifier: Are Its Days Numbered? Polymers 2023, 15, 2408. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and Characterization of Low Methoxyl Pectin/Gelatin/Carboxymethyl Cellulose Absorbent Hydrogel Film for Wound Dressing Applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef] [PubMed]
- Paria, A.; Rai, V.K. The fate of carboxymethyl cellulose as a polymer of pharmaceutical importance. Biol. Sci. 2022, 2, 204–215. [Google Scholar] [CrossRef]
- Putri, D.A.; Kurniyati, Z. Effect of sodium chloroacetate towards the synthesis of CMC (carboxymethyl cellulose) from durian (Durio zibethinus) peel cellulose. Int. J. Innov. Res. Adv. Eng. (IJIRAE) 2016, 3, 28–32. [Google Scholar]
- Pratinthong, K.; Punyodom, W.; Jantrawut, P.; Jantanasakulwong, K.; Tongdeesoontorn, W.; Sriyai, M.; Panyathip, R.; Thanakkasaranee, S.; Worajittiphon, P.; Tanadchangsaeng, N.; et al. Modification of a Carboxymethyl Cellulose/Poly(vinyl alcohol) Hydrogel Film with Citric Acid and Glutaraldehyde Crosslink Agents to Enhance the Anti-Inflammatory Effectiveness of Triamcinolone Acetonide in Wound Healing. Polymers 2024, 16, 1798. [Google Scholar] [CrossRef] [PubMed]
- Lubis, R.; Wirjosentono, B.; Eddyanto; Septevani, A.A. Preparation, characterization and antimicrobial activity of grafted cellulose fiber from durian rind waste. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125311. [Google Scholar] [CrossRef]
- Browne, D.; Briggs, F.; Asuri, P. Role of Polymer Concentration on the Release Rates of Proteins from Single- and Double-Network Hydrogels. Int. J. Mol. Sci. 2023, 24, 16970. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Tyagi, V.; Thakur, A. Applications of biodegradable carboxymethyl cellulose-based composites. Results Mater. 2023, 20, 100481. [Google Scholar] [CrossRef]
- Ahamefule, C.S.; Osilo, C.; Ahamefule, B.C.; Madueke, S.N.; Moneke, A.N. Simultaneous production of biofuel from agricultural wastes and bioremediation of the waste substrates: A review. Curr. Res. Microb. Sci. 2024, 7, 100305. [Google Scholar] [CrossRef]
- Xing, H.; Fei, Y.; Cheng, J.; Wang, C.; Zhang, J.; Niu, C.; Fu, Q.; Cheng, J.; Lu, L. Green Preparation of Durian Rind-Based Cellulose Nanofiber and Its Application in Aerogel. Molecules 2022, 27, 6507. [Google Scholar] [CrossRef] [PubMed]
- Mohd Kamal, N.S.A.; Mohd Fuzi, S.F.Z.; Mohd Ghazali, M.I.; Dailin, D.J. Development of Photosensitive Hydrogel-based 3-dimensional Bioprinting Using Locally Extracted Pectin from Durian Rind Waste and Cellulose for Pharmaceutical Application. Malays. J. Med. Health Sci. 2023, 19, 152–161. [Google Scholar] [CrossRef]
- Panraksa, P.; Rachtanapun, P.; Thipchai, P.; Lesniewska, E.; Brachais, C.H.; Debeaufort, F.; Chambin, O.; Jantrawut, P. Sustainable 3D printing of oral films with tunable characteristics using CMC-based inks from durian rind wastes. Eur. J. Pharm. Biopharm. 2023, 186, 30–42. [Google Scholar] [CrossRef]
- Siswanta, D.; Wahyuni, R.; Mudasir, M. Synthesis of Glutaraldehyde-Crosslinked Carboxymethyl Cellulose-Polyvinyl Alcohol Film as an Adsorbent for Methylene Blue. Key Eng. Mater. 2020, 840, 35–42. [Google Scholar] [CrossRef]
- Churam, T.; Usubharatana, P.; Phungrassami, H. Sustainable Production of Carboxymethyl Cellulose: A Biopolymer Alternative from Sugarcane (Saccharum officinarum L.) Leaves. Sustainability 2024, 16, 2352. [Google Scholar] [CrossRef]
- Golbaghi, L.; Khamforoush, M.; Hatami, T. Carboxymethyl cellulose production from sugarcane bagasse with steam explosion pulping: Experimental, modeling, and optimization. Carbohydr. Polym. 2017, 174, 780–788. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Yao, W.; Weng, Y.; Catchmark, J.M. Improved cellulose X-ray diffraction analysis using Fourier series modeling. Cellulose 2020, 27, 5563–5579. [Google Scholar] [CrossRef]
- Montoya-Escobar, N.; Ospina-Acero, D.; Velasquez-Cock, J.A.; Gomez-Hoyos, C.; Serpa Guerra, A.; Ganan Rojo, P.F.; Velez Acosta, L.M.; Escobar, J.P.; Correa-Hincapie, N.; Triana-Chavez, O.; et al. Use of Fourier Series in X-ray Diffraction (XRD) Analysis and Fourier-Transform Infrared Spectroscopy (FTIR) for Estimation of Crystallinity in Cellulose from Different Sources. Polymers 2022, 14, 5199. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Miao, X.; Hu, X.; Zhang, Q.; Jie, X.; Zheng, X. Novel Synthesis of Plasmonic Ag/AgCl@TiO2 Continues Fibers with Enhanced Broadband Photocatalytic Performance. Catalysts 2017, 7, 117. [Google Scholar] [CrossRef]
- Ahmad, K.; Kakakhel, M.B.; Hayat, S.; Wazir-Ud-Din, M.; Mahmood, M.M.; Ur Rehman, S.; Siddique, M.T.; Mirza, S.M. Thermoluminescence study of pellets prepared using NaCl from Khewra Salt Mines in Pakistan. Radiat. Env. Biophys. 2021, 60, 365–375. [Google Scholar] [CrossRef]
- Ismail, N.M.; Bono, A.; Valintinus, A.C.R.; Nilus, S.; Chng, L.M. Optimization of Reaction Conditions for Preparing Carboxymethylcellulose. J. Appl. Sci. 2010, 10, 2530–2536. [Google Scholar] [CrossRef][Green Version]
- Rani, M.; Rudhziah, S.; Ahmad, A.; Mohamed, N. Biopolymer Electrolyte Based on Derivatives of Cellulose from Kenaf Bast Fiber. Polymers 2014, 6, 2371–2385. [Google Scholar] [CrossRef]
- Alizadeh Asl, S.; Mousavi, M.; Labbafi, M. Synthesis and Characterization of Carboxymethyl Cellulose from Sugarcane Bagasse. J. Food Process. Technol. 2017, 8, 1000687. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Kaur, P.; Bohidar, H.B.; Williams, R.; Pfeffer, F.M.; Agrawal, R. Turning trash into treasure: Conversion of agroresidue rice straw into carboxymethylcellulose biopolymer. Biofuels Bioprod. Biorefining 2024, 19, 139–150. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the role of hydrogen bonds: Not in charge of everything. Cellulose 2021, 29, 1–23. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Gieroba, B.; Kalisz, G.; Krysa, M.; Khalavka, M.; Przekora, A. Application of Vibrational Spectroscopic Techniques in the Study of the Natural Polysaccharides and Their Cross-Linking Process. Int. J. Mol. Sci. 2023, 24, 2630. [Google Scholar] [CrossRef] [PubMed]
- Panyathip, R.; Witthayapak, M.; Thuephloi, P.; Sukunta, J.; Thipchai, P.; Thanakkasaranee, S.; Jantanasakulwong, K.; Rachtanapun, P. Characterization of corn husks carboxymethyl cellulose formation using Raman spectroscopy. Ind. Crops Prod. 2025, 228, 120887. [Google Scholar] [CrossRef]
- Nordin, N.A.; Rahman, N.A.; Talip, N.; Yacob, N. Citric Acid Cross-Linking of Carboxymethyl Sago Starch Based Hydrogel for Controlled Release Application. Macromol. Symp. 2018, 382, 1800086. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Dhane, N.S.; Ghorpade, V.S. Citric Acid Crosslinked Carboxymethyl Cellulose-based Composite Hydrogel Films for Drug Delivery. Indian. J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Dahlan, N.A.; Pushpamalar, J.; Veeramachineni, A.K.; Muniyandy, S. Smart Hydrogel of Carboxymethyl Cellulose Grafted Carboxymethyl Polyvinyl Alcohol and Properties Studied for Future Material Applications. J. Polym. Environ. 2017, 26, 2061–2071. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Abd El-Mohdy, H.L.; Abd El-Rehim, H.A. Radiation preparation of PVA/CMC copolymers and their application in removal of dyes. J. Hazard. Mater. 2009, 168, 68–75. [Google Scholar] [CrossRef]
- Gulati, I.; Park, J.; Maken, S.; Lee, M.-G. Production of carboxymethylcellulose fibers from waste lignocellulosic sawdust using NaOH/NaClO2 pretreatment. Fibers Polym. 2014, 15, 680–686. [Google Scholar] [CrossRef]
- Nurfajriani; Pulungan, A.N.; Yusuf, M.; Tampubolon, M.D.; Bukit, N. The Effects of Sodium Hydroxide Concentrations on Synthesis of Carboxymethyl Cellulose from Bacterial Cellulosa. J. Phys. Conf. Ser. 2020, 1485, 012055. [Google Scholar] [CrossRef]
- Penjumras, P.; Rahman, R.B.A.; Talib, R.A.; Abdan, K. Extraction and Characterization of Cellulose from Durian Rind. Agric. Agric. Sci. Procedia 2014, 2, 237–243. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.; Conradzhang, Z.; Brown, H.; Arey, B.; Holladay, J. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohydr. Polym. 2007, 68, 235–241. [Google Scholar] [CrossRef]
- Zhang, J.; Tao, L.; Zhang, X.; Sui, X.; Song, S.; Wei, Y.; Yu, L. Carboxymethylation enhances the low oil absorption of freeze-thawed tapioca starch in fried ham sausage batter. LWT 2023, 184, 115050. [Google Scholar] [CrossRef]
- Ben Sghaier, A.E.O.; Chaabouni, Y.; Msahli, S.; Sakli, F. Morphological and crystalline characterization of NaOH and NaOCl treated Agave americana L. fiber. Ind. Crops Prod. 2012, 36, 257–266. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, F.; Liu, R.; Yu, Y.; Yu, W. Morphology and supramolecular structure characterization of cellulose isolated from heat-treated moso bamboo. Cellulose 2019, 26, 7067–7078. [Google Scholar] [CrossRef]
- Singh, S.C.; Murthy, Z.V.P. Study of cellulosic fibres morphological features and their modifications using hemicelluloses. Cellulose 2017, 24, 3119–3130. [Google Scholar] [CrossRef]
- Abdelrahim, K.A.; Ramaswamy, H.S.; Doyon, G.; Toupin, C. Effects of concentration and temperature on carboxymethylcellulose rheology. Int. J. Food Sci. Technol. 2007, 29, 243–253. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhu, W.L. Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 2007, 14, 409–417. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Carboxymethyl Ethers of Cellulose and Starch—A Review. Macromol. Symp. 2005, 223, 13–40. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Çelikçi, N.; Ziba, C.A.; Dolaz, M. Synthesis and Characterization of Carboxymethyl Cellulose (Cmc) from Different Waste Sources Containing Cellulose and Investigation of Its Use in the Construction Industry. Cellul. Chem. Technol. 2022, 56, 55–68. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Kadnaim, A.; Janvikul, W.; Wichai, U.; Rutnakornpituk, M. Synthesis and properties of carboxymethylchitosan hydrogels modified with poly(ester-urethane). Carbohydr. Polym. 2008, 74, 257–267. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Ishida, J.; Syuu, G.; Sekine, Y.; Ikeda-Fukazawa, T. Structural changes of water in poly(vinyl alcohol) hydrogel during dehydration. J. Chem. Phys. 2014, 140, 044909. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Oxford, UK, 2014; pp. 67–89. [Google Scholar]
- Ibrahim, M.M.; Koschella, A.; Kadry, G.; Heinze, T. Evaluation of cellulose and carboxymethyl cellulose/poly(vinyl alcohol) membranes. Carbohydr. Polym. 2013, 95, 414–420. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, S.; Fang, G.; Huang, C.; Wu, T. Directionally-Grown Carboxymethyl Cellulose/Reduced Graphene Oxide Aerogel with Excellent Structure Stability and Adsorption Capacity. Polymers 2020, 12, 2219. [Google Scholar] [CrossRef]
- Zuo, B.; Hu, Y.; Lu, X.; Zhang, S.; Fan, H.; Wang, X. Surface Properties of Poly(vinyl alcohol) Films Dominated by Spontaneous Adsorption of Ethanol and Governed by Hydrogen Bonding. J. Phys. Chem. C 2013, 117, 3396–3406. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Wang, Y.; Lv, Y.; Wu, H.; Ma, X.; Tan, M. Highly fluorescent carbon dots for visible sensing of doxorubicin release based on efficient nanosurface energy transfer. Biotechnol. Lett. 2016, 38, 191–201. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Zou, X.; Xing, L.; Liu, W.; Huang, Z.; Feng, Y.; Wang, J. Biomass-Based Ferric Tannate Hydrogel with a Photothermal Conversion Function for Solar Water Evaporation. ACS Appl. Polym. Mater. 2023, 5, 9574–9584. [Google Scholar] [CrossRef]
- Li, S.; Dong, L.; Wei, Z.; Sheng, G.; Du, K.; Hu, B. Adsorption and mechanistic study of the invasive plant-derived biochar functionalized with CaAl-LDH for Eu(III) in water. J Environ. Sci. 2020, 96, 127–137. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network hydrogel for removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127262. [Google Scholar] [CrossRef]
- Han, C.; Cao, M.; Yu, J.; Wang, S.; Zhou, X.; Chen, Y.; Yang, F. Carboxymethyl Cellulose-Based Composite Polymer Hydrogels Cross-Linked with Epichlorohydrin and Application for Cu(II) Removal. ACS Appl. Polym. Mater. 2023, 5, 2070–2078. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Hantsche, H. High resolution XPS of organic polymers, the scienta ESCA300 database. By G. Beamson and D. Briggs, Wiley, Chichester 1992, 295 pp., hardcover, £ 65.00, ISBN 0-471-93592-1. Adv. Mater. 1993, 5, 778. [Google Scholar] [CrossRef]
- Castro-Cabado, M.; Parra-Ruiz, F.J.; Casado, A.L.; Román, J.S. Thermal Crosslinking of Maltodextrin and Citric Acid. Methodology to Control the Polycondensation Reaction under Processing Conditions. Polym. Polym. Compos. 2016, 24, 643–654. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Chang, C.; Lue, A.; Zhang, L. Effects of Crosslinking Methods on Structure and Properties of Cellulose/PVA Hydrogels. Macromol. Chem. Phys. 2008, 209, 1266–1273. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, M.; Shen, Y. Enhanced swelling ratio and water retention capacity for novel super-absorbent hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123972. [Google Scholar] [CrossRef]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef]
- Jin, X.; Hsieh, Y.-L. pH-responsive swelling behavior of poly(vinyl alcohol)/poly(acrylic acid) bi-component fibrous hydrogel membranes. Polymer 2005, 46, 5149–5160. [Google Scholar] [CrossRef]
- Gohil, J.M.; Bhattacharya, A.; Ray, P. Studies on the Crosslinking of Poly (Vinyl Alcohol). J. Polym. Res. 2005, 13, 161–169. [Google Scholar] [CrossRef]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Guerry, A.; Cottaz, S.; Fleury, E.; Bernard, J.; Halila, S. Redox-stimuli responsive micelles from DOX-encapsulating polycaprolactone-g-chitosan oligosaccharide. Carbohydr. Polym. 2014, 112, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Parameswaranpillai, J.; Siengchin, S. Release of toxic methylene blue from water by mesoporous silicalite-1: Characterization, kinetics and isotherm studies. Appl. Water Sci. 2021, 11, 110. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Lokuge, H.; Vigneswaran, S. Kinetics and Thermodynamics Study of Methylene Blue Adsorption to Sucrose- and Urea-Derived Nitrogen-Enriched, Hierarchically Porous Carbon Activated by KOH and H3PO4. ACS Omega 2023, 8, 16158–16173. [Google Scholar] [CrossRef]
- Cesco, C.T.; Valente, A.J.M.; Paulino, A.T. Methylene Blue Release from Chitosan/Pectin and Chitosan/DNA Blend Hydrogels. Pharmaceutics 2021, 13, 842. [Google Scholar] [CrossRef]
- Mohd Kanafi, N.; Abdul Rahman, N.; Rosdi, N.H.; Bahruji, H.; Maarof, H. Hydrogel Nanofibers from Carboxymethyl Sago Pulp and Its Controlled Release Studies as a Methylene Blue Drug Carrier. Fibers 2019, 7, 56. [Google Scholar] [CrossRef]
- Altam, A.A.; Zhu, L.; Babiker, D.; Yagoub, H.; Yang, S. Loading and releasing behavior of carboxymethyl cellulose and chitosan complex beads. Prog. Nat. Sci. Mater. Int. 2022, 32, 715–723. [Google Scholar] [CrossRef]
- Pham, B.T.T.; Nguyen, D.V.; Phung, T.K.; Nguyen, T.T. Adsorptive removal of methylene blue dye using durian peel waste reinforced polyvinyl alcohol-based composite membrane. J. Appl. Polym. Sci. 2024, 141, e55470. [Google Scholar] [CrossRef]
- Khatooni, H.; Peighambardoust, S.J.; Foroutan, R.; Mohammadi, R.; Ramavandi, B. Adsorption of methylene blue using sodium carboxymethyl cellulose-g-poly (acrylamide-co-methacrylic acid)/Cloisite 30B nanocomposite hydrogel. J. Polym. Environ. 2022, 31, 297–311. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Pan, Y.; Wang, L.; Xiao, H. Redox-responsive carboxymethyl cellulose hydrogel for adsorption and controlled release of dye. Eur. Polym. J. 2020, 123, 109447. [Google Scholar] [CrossRef]
- Pacaphol, K.; Aht-Ong, D. The influences of silanes on interfacial adhesion and surface properties of nanocellulose film coating on glass and aluminum substrates. Surf. Coat. Technol. 2017, 320, 70–81. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, Z.; Kapu, N.S.; Chang, X.F.; Beatson, R.; Trajano, H.L.; Martinez, D.M. Increasing efficiency of enzymatic hemicellulose removal from bamboo for production of high-grade dissolving pulp. Bioresour. Technol. 2017, 223, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Thipchai, P.; Punyodom, W.; Jantanasakulwong, K.; Thanakkasaranee, S.; Hinmo, S.; Pratinthong, K.; Kasi, G.; Rachtanapun, P. Preparation and Characterization of Cellulose Nanocrystals from Bamboos and Their Application in Cassava Starch-Based Film. Polymers 2023, 15, 2622. [Google Scholar] [CrossRef]
- Lee, H.; Park, I. The Influence of Starch Modification with Amylosucrase Treatment on Morphological Features. Processes 2020, 8, 1409. [Google Scholar] [CrossRef]
- Surdu, V.-A.; Győrgy, R. X-ray Diffraction Data Analysis by Machine Learning Methods—A Review. Appl. Sci. 2023, 13, 9992. [Google Scholar] [CrossRef]
- Poralan, G.M.; Gambe, J.E.; Alcantara, E.M.; Vequizo, R.M. X-ray diffraction and infrared spectroscopy analyses on the crystallinity of engineered biological hydroxyapatite for medical application. IOP Conf. Ser. Mater. Sci. Eng. 2015, 79, 012028. [Google Scholar] [CrossRef]
- Thipchai, P.; Sringarm, K.; Punyodom, W.; Jantanasakulwong, K.; Thanakkasaranee, S.; Panyathip, R.; Arjin, C.; Rachtanapun, P. Production of Nanocellulose from Sugarcane Bagasse and Development of Nanocellulose Conjugated with Polylysine for Fumonisin B1 Toxicity Absorption. Polymers 2024, 16, 1881. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Poly(vinyl alcohol) hydrogels: Reinforcement of radiation-crosslinked networks by crystallization. J. Polym. Sci. Polym. Chem. Ed. 2003, 14, 441–457. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Zamani-Babgohari, F.; Irannejad, A.; Khayati, G.R.; Kalantari, M. Non-isothermal decomposition kinetics of commercial polyacrylamide hydrogel using TGA and DSC techniques. Thermochim. Acta 2023, 725, 221–230. [Google Scholar] [CrossRef]
- Sarkar, K.; Sen, K. Polyvinyl alcohol based hydrogels for urea release and Fe(III) uptake from soil medium. J. Environ. Chem. Eng. 2018, 6, 736–744. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Banach, M. Photocatalytic properties of zirconium oxide–zinc oxide nanoparticles synthesised using microwave irradiation. Appl. Nanosci. 2019, 10, 941–954. [Google Scholar] [CrossRef]
- Soomherun, N.; Kreua-ongarjnukool, N.; Niyomthai, S.T.; Chumnanvej, S. Kinetics of Drug Release via Nicardipine Hydrochloride-loaded Carboxymethyl Cellulose/Poly(D,L-lactic-co-glycolic acid) Nanocarriers Using a Contemporary Emulsion Process. ChemNanoMat 2020, 6, 1754–1769. [Google Scholar] [CrossRef]
- Sauder, D.C.; DeMars, C.E. An Updated Recommendation for Multiple Comparisons. Adv. Methods Pract. Psychol. Sci. 2019, 2, 26–44. [Google Scholar] [CrossRef]
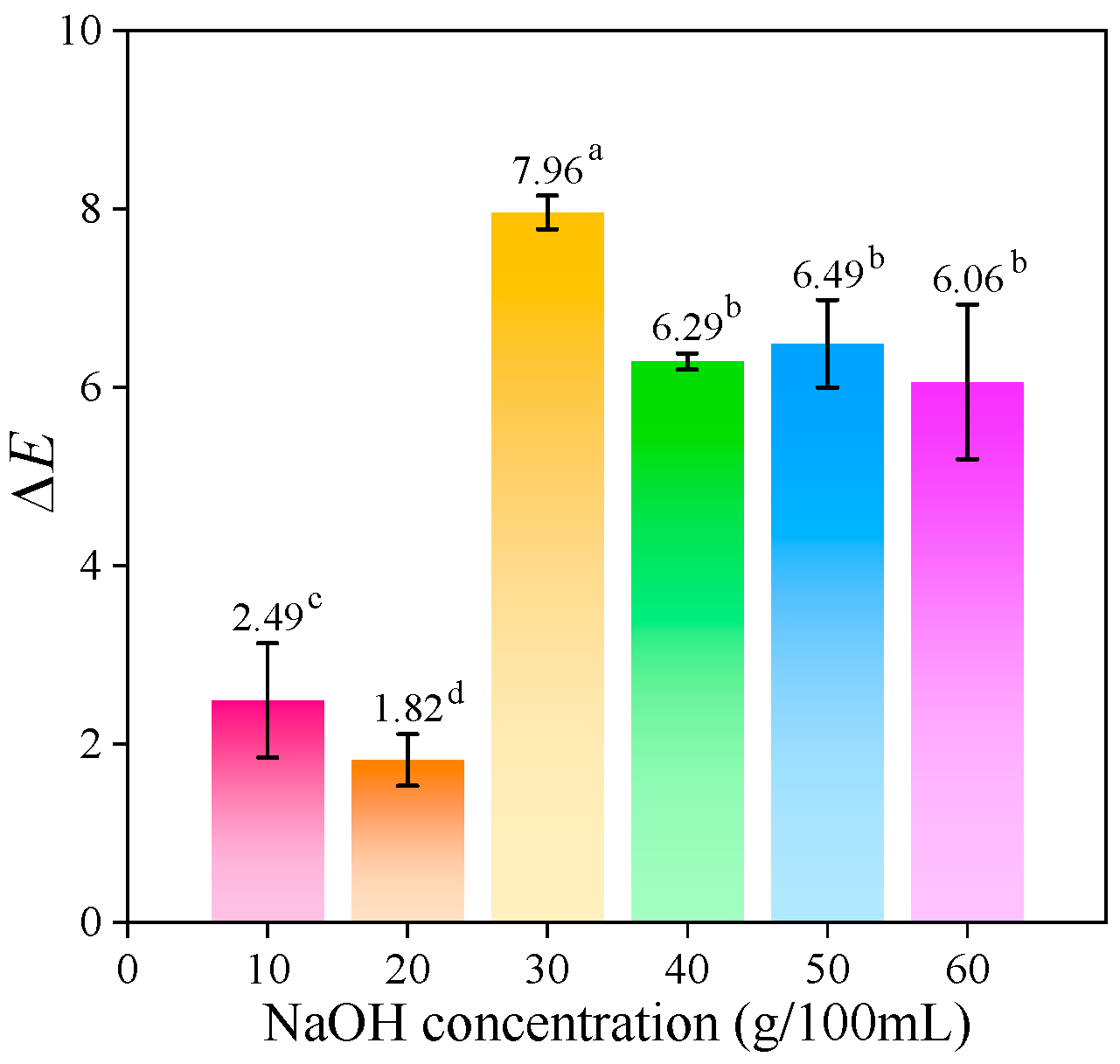

| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Cellulose | 93.49 ± 0.14 | 0.64 ± 0.01 | 6.49 ± 0.13 | - |
| CMCd-10 | 92.14 ± 0.78 | 0.94 ± 0.07 | 8.56 ± 0.18 | 2.49 c ± 0.64 |
| CMCd-20 | 92.37 ± 0.43 | 0.77 ± 0.02 | 7.92 ± 0.16 | 1.82 d ± 0.29 |
| CMCd-30 | 91.22 ± 0.32 | 0.69 ± 0.04 | 14.12 ± 0.19 | 7.96 a ± 0.19 |
| CMCd-40 | 91.95 ± 0.06 | 1.12 ± 0.04 | 12.57 ± 0.12 | 6.29 b ± 0.09 |
| CMCd-50 | 90.94 ± 0.62 | 0.97 ± 0.04 | 12.45 ± 0.02 | 6.49 b ± 0.49 |
| CMCd-60 | 92.29 ± 0.85 | 0.86 ± 0.06 | 12.39 ± 0.51 | 6.06 b ± 0.87 |
| Samples | Structure | Releasing Time (min) | Reference |
|---|---|---|---|
| Carboxymethyl sago pulp (CMSP) | CMSP hydrogel nanofibers | 2880 | [99] |
| Carboxymethyl cellulose and chitosan | Complex beads | 360 | [100] |
| Polyvinyl alcohol/agarose/maltodextrin composite and durian peel (DP) | Composite membrane | 300 | [101] |
| Carboxymethyl cellulose-g-poly (acrylamide-co-methacrylic acid)/ Cloisite 30B | Nanocomposite hydrogel | 120 | [102] |
| Carboxymethyl cellulose nanocrystal | Hydrogel | 360 | [103] |
| Carboxymethyl cellulose/Poly(vinyl) Alcohol | Hydrogel films | 1440 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratinthong, K.; Panyathip, R.; Thanakkasaranee, S.; Jantanasakulwong, K.; Tongdeesoontorn, W.; Noiwan, D.; Karbowiak, T.; Rachtanapun, C.; Rachtanapun, P. Sustainable Durian Rind Carboxymethyl Cellulose/Poly(vinyl) Alcohol Hydrogels Synthesis for Enhancing Crosslinking and Release Kinetics Efficiency. Gels 2025, 11, 728. https://doi.org/10.3390/gels11090728
Pratinthong K, Panyathip R, Thanakkasaranee S, Jantanasakulwong K, Tongdeesoontorn W, Noiwan D, Karbowiak T, Rachtanapun C, Rachtanapun P. Sustainable Durian Rind Carboxymethyl Cellulose/Poly(vinyl) Alcohol Hydrogels Synthesis for Enhancing Crosslinking and Release Kinetics Efficiency. Gels. 2025; 11(9):728. https://doi.org/10.3390/gels11090728
Chicago/Turabian StylePratinthong, Kanticha, Rangsan Panyathip, Sarinthip Thanakkasaranee, Kittisak Jantanasakulwong, Wirongrong Tongdeesoontorn, Duangjai Noiwan, Thomas Karbowiak, Chitsiri Rachtanapun, and Pornchai Rachtanapun. 2025. "Sustainable Durian Rind Carboxymethyl Cellulose/Poly(vinyl) Alcohol Hydrogels Synthesis for Enhancing Crosslinking and Release Kinetics Efficiency" Gels 11, no. 9: 728. https://doi.org/10.3390/gels11090728
APA StylePratinthong, K., Panyathip, R., Thanakkasaranee, S., Jantanasakulwong, K., Tongdeesoontorn, W., Noiwan, D., Karbowiak, T., Rachtanapun, C., & Rachtanapun, P. (2025). Sustainable Durian Rind Carboxymethyl Cellulose/Poly(vinyl) Alcohol Hydrogels Synthesis for Enhancing Crosslinking and Release Kinetics Efficiency. Gels, 11(9), 728. https://doi.org/10.3390/gels11090728













