Nanocomposite Cryogels Based on Chitosan for Efficient Removal of a Triphenylmethane Dye from Aqueous Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Cross-Linker Concentration and Zeolite Content on Network Formation
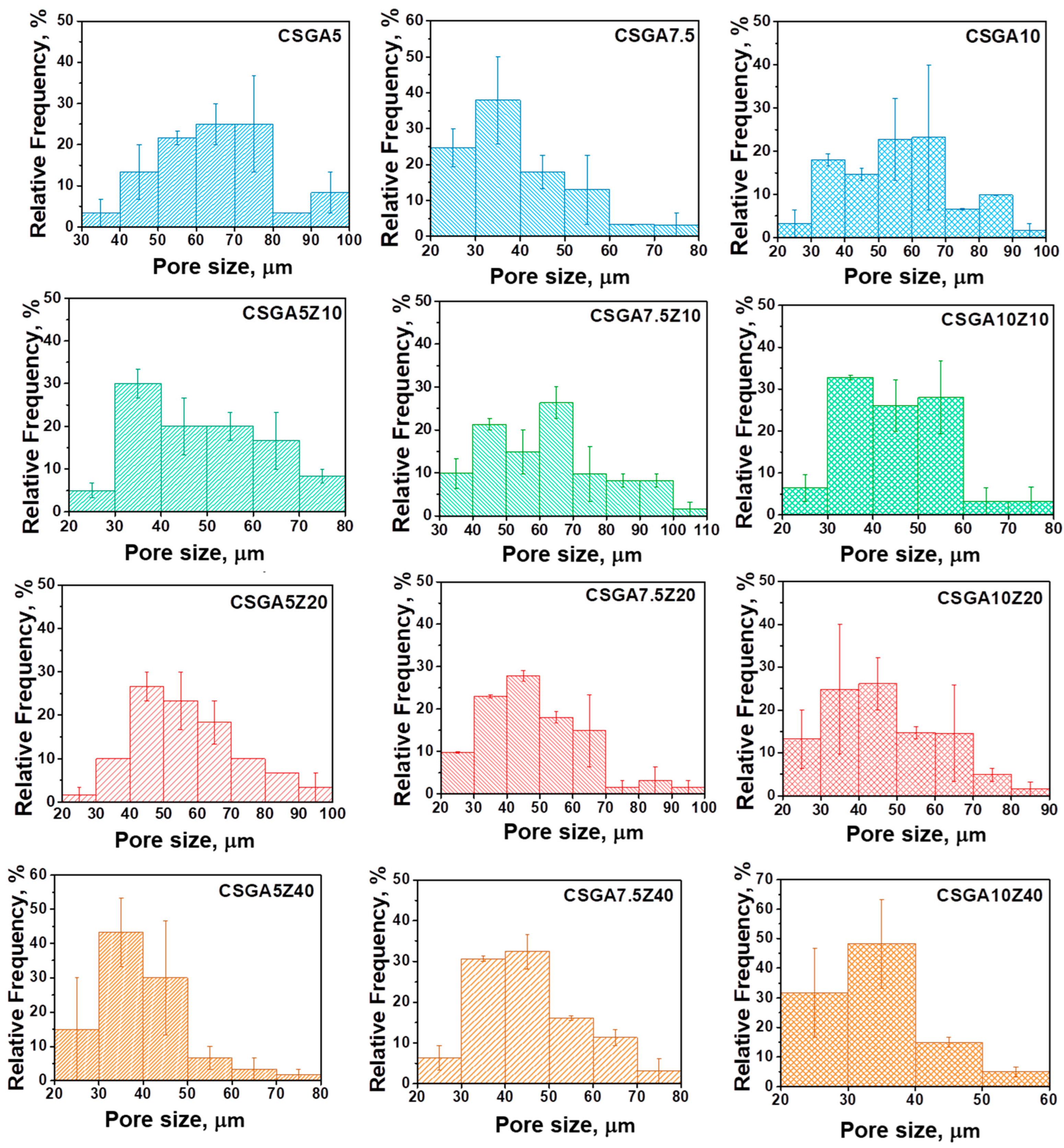
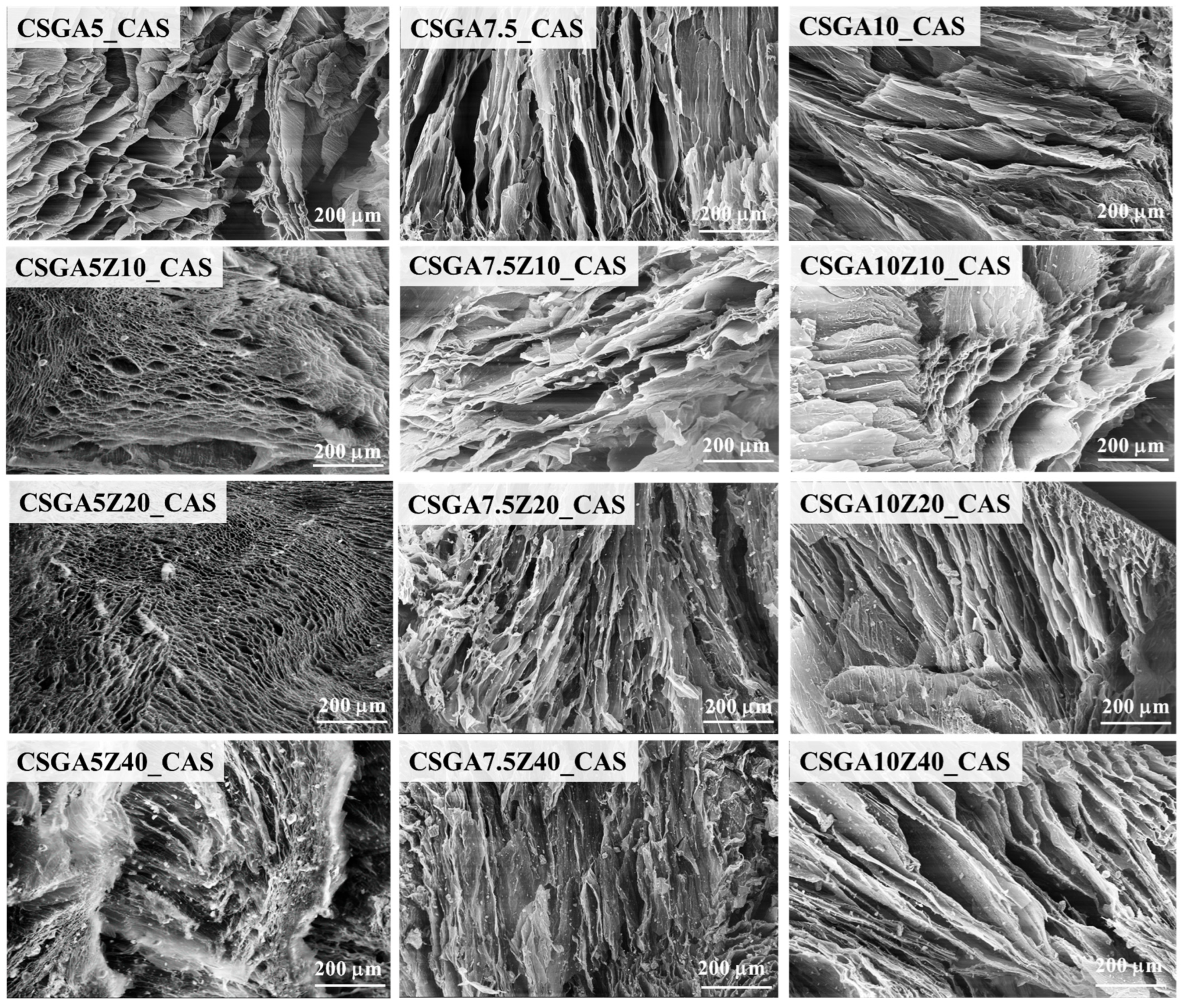
2.2. Internal Morphology and Pore Size Districution of the Nanocomposite Cryogels
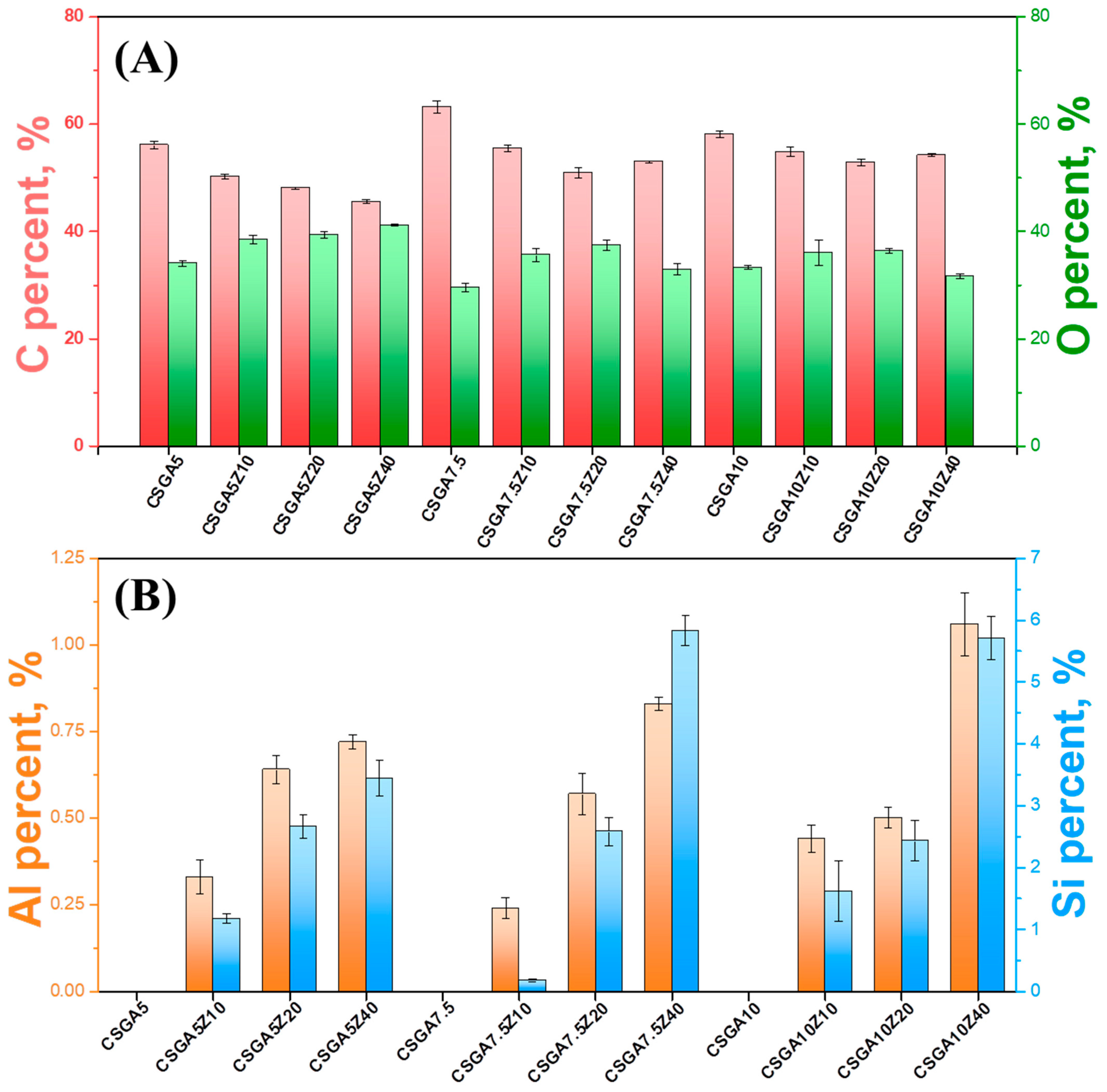
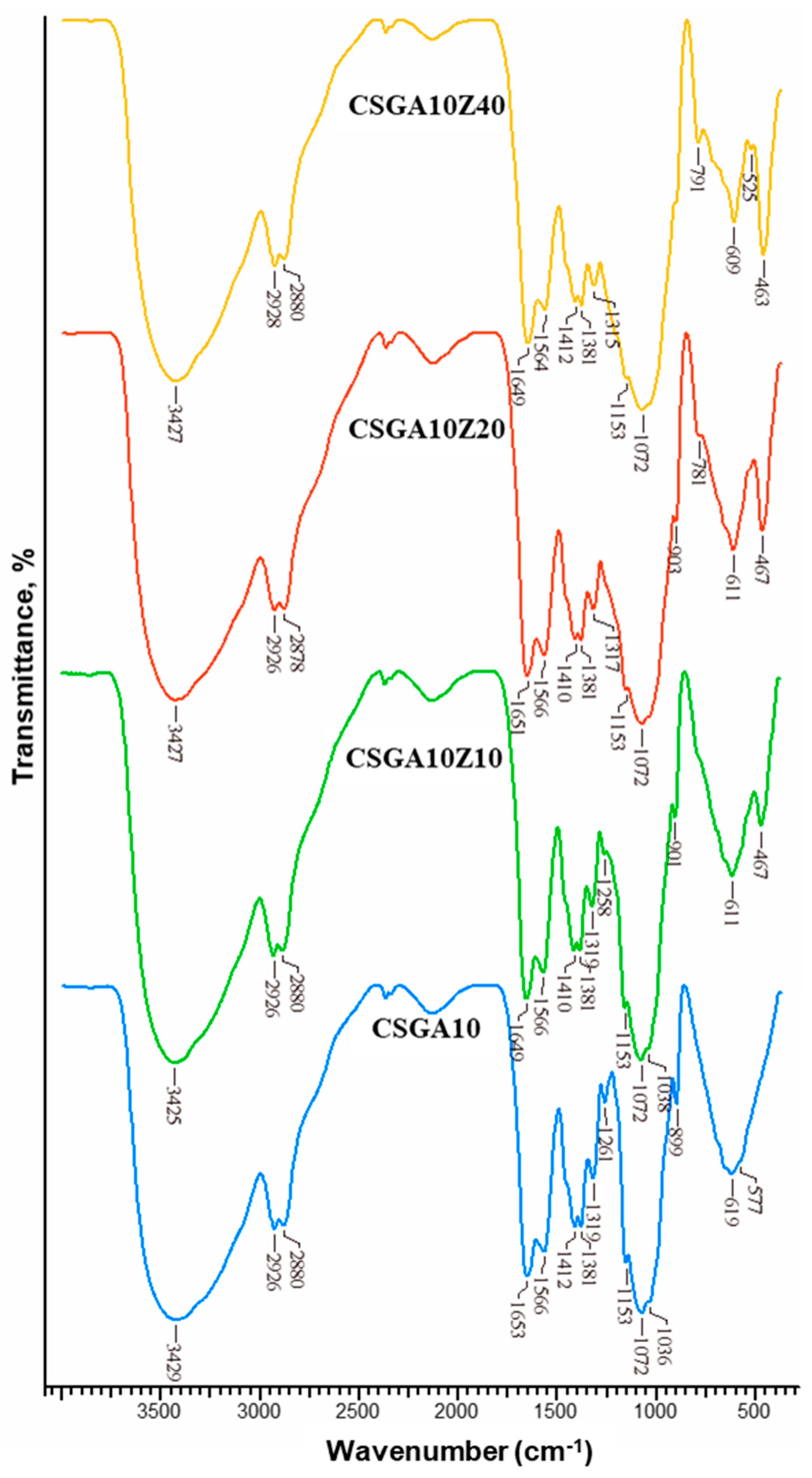
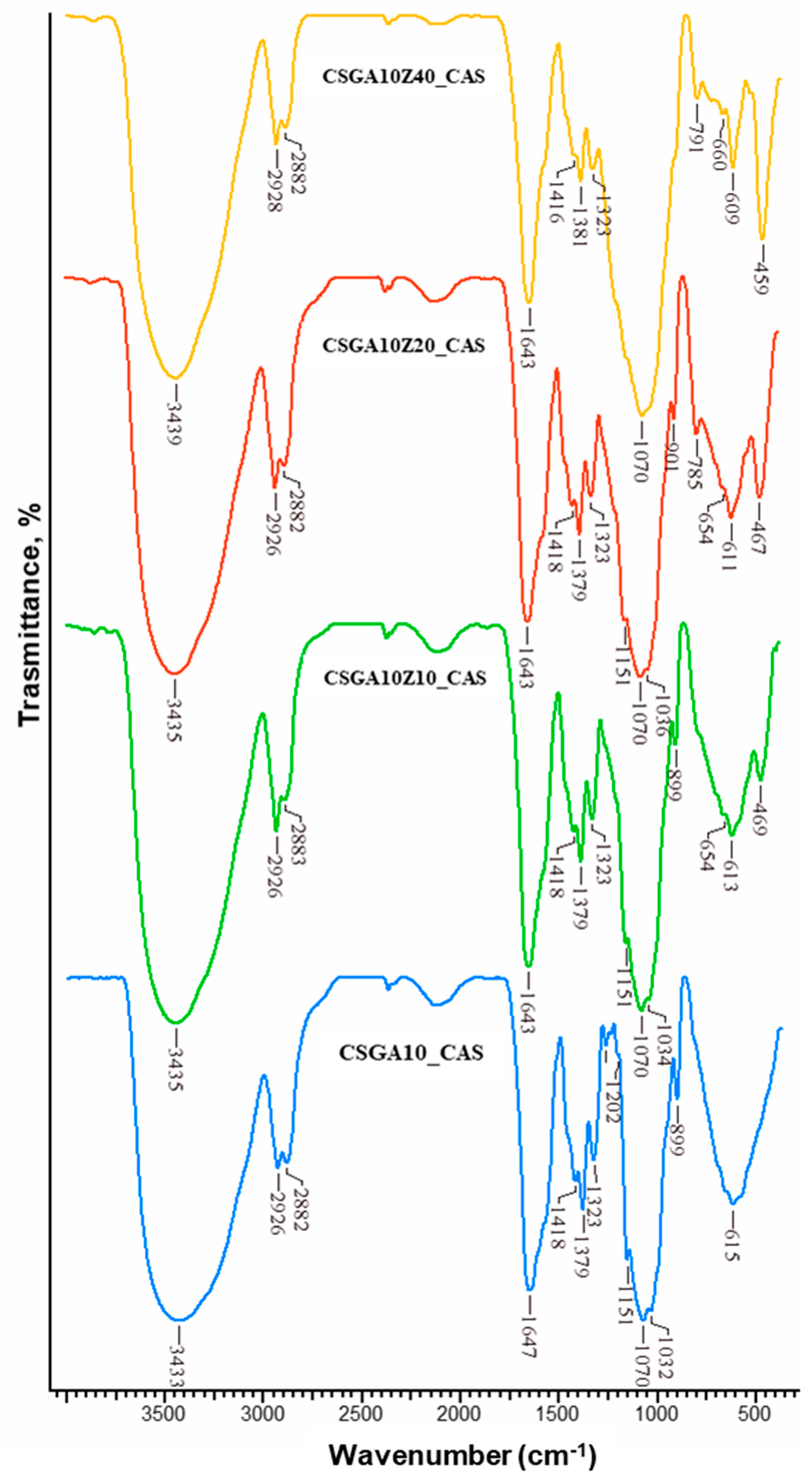
2.3. Structural Characterization
2.4. Swelling Behavior
2.5. Adsorption of CAS Dye
2.5.1. Sorption Isotherms
2.5.2. Mechanism of CAS Sorption
- (i)
- A red-shift of the –OH/–NH stretching from 3429 to 3433 cm−1 indicates formation of hydrogen bonds between CAS dye molecules and –OH/–NH groups of the cryogel matrix.
- (ii)
- A blue-shift of the amide I band from 1653 to 1647 cm−1 suggests direct involvement of C=O groups in dye binding, possibly through dipole–dipole or hydrogen bonding interactions.
- (iii)
- A down-shift of the band at 1566 cm−1 is attributed to the imine bond stretching vibrations (just a shoulder in the spectra of CSGA10_CAS).
- (iv)
- A down-shift and a red-shift of the –CH2 bending band from 1412 to 1418 cm−1 are observed.
- (v)
- The intensity of the –CH bending band at 1379 cm−1 increases.
- (vi)
- A blue-shift of the C6–OH stretching band from 1036 to 1032 cm−1 points to participation of hydroxyl groups in binding with the dye.
- (i)
- A red-shift of the –OH/–NH band from 3427 to 3435 cm−1, suggesting even stronger hydrogen bonding interactions facilitated by the zeolite-modified surface;
- (ii)
- A blue-shift of the amide I band from 1651 to 1643 cm−1, further supporting involvement of C=O in dye binding;
- (iii)
- A down-shift of the band at 1566 cm−1, attributed to the imine bond stretching vibrations (just a shoulder in the spectra of CSGA10Z20_CAS);
- (iv)
- A down-shift and red-shift of the –CH2 bending from 1410 to 1418 cm−1;
- (v)
- Increased intensity of the –CH bending at 1379 cm−1;
- (vi)
- A decrease in the intensity of the Al–O bands at 467 and 611 cm−1, observed after dye sorption, indicating that the zeolite’s surface –OH and Al–O groups are directly involved, likely via an ion-exchange mechanism.
2.5.3. Effect of Competing Ions on CAS Sorption
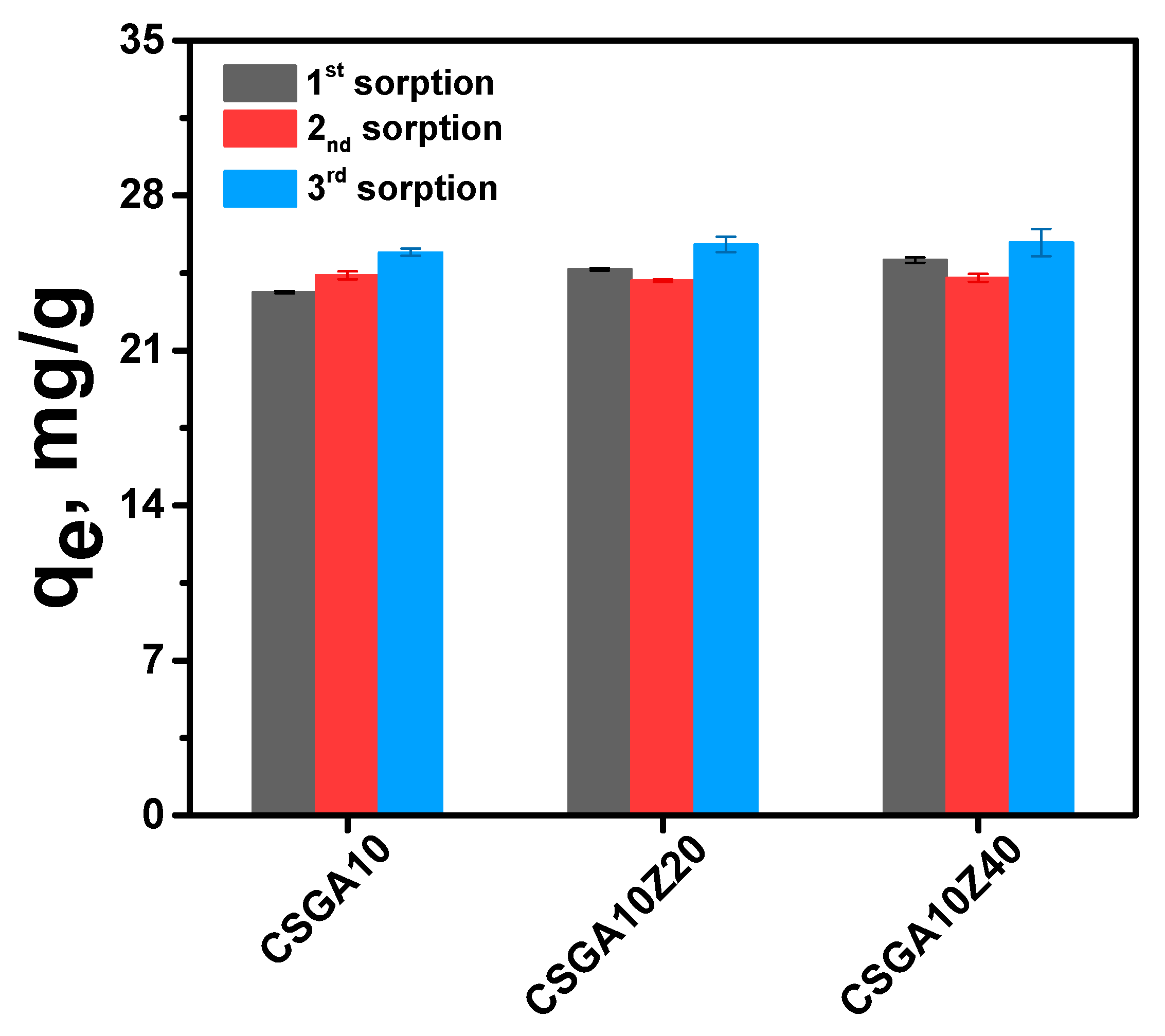
2.5.4. Desorption and Reusability Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. CS-Based Nanocomposite Cryogels
4.2.2. Gel Fraction Yield
4.2.3. FTIR Spectroscopy
4.2.4. SEM, EDX, and Pore-Size Analysis
4.2.5. Swelling Ratio, SR
4.2.6. Sorption Experiments
4.2.7. Sorption of CAS in the Presence of Competing Ions
4.2.8. Desorption of CAS Dye
4.2.9. Reusability Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M. Impact of textile dyes on human health and environment. In Impact of Textile Dyes on Public Health and the Environment, 3rd ed.; Wani, K.A., Jangid, N.K., Bhat, A.R., Eds.; IGI Global Scientific Publishing: Hershey, PA, USA, 2020; pp. 162–169. [Google Scholar]
- Garg, R.; Sabouni, R. Efficient removal of cationic dye using ZIF-8 based sodium alginate composite beads: Performance evaluation in batch and column systems. Chemosphere 2023, 342, 140163. [Google Scholar] [CrossRef]
- Vaid, V.; Yadav, K.; Kaur, K.; Bansal, A.; Panwar, A.; Devi, A.; Jindal, R. Removal of organic dyes from aqueous solutions by adsorption of chitosan-guar gum-based glyoxal crosslinked hydrogel. Fibers Polym. 2023, 24, 383–401. [Google Scholar] [CrossRef]
- Benhalima, T.; Sadi, A.; Dairi, N.; Ferfera-Harrar, H. Multifunctional carboxymethyl cellulose-dextran sulfate/AgNPs@zeolite hydrogel beads for basic red 46 and methylene blue dyes removal and water disinfection control. Sep. Purif. Technol. 2024, 342, 127001. [Google Scholar] [CrossRef]
- Sekar, N. Acid dyes. In Handbook of Textile and Industrial Dyeing Principles, Processes and Types of Dyes, 1st ed.; Clark, M., Ed.; Woodhead Publishing Series in Textiles: Cambridge, UK, 2011; Volume 1, pp. 486–514. [Google Scholar]
- Yao, T.; Gan, Y.; Li, Q.; Tan, M.; Shi, X. Removal and recovery of triphenylmethane dyes from wastewater with temperature-sensitive magnetic ionic liquid aqueous two-phase system. J. Clean. Prod. 2021, 328, 129648. [Google Scholar] [CrossRef]
- Qu, S.; Huang, F.; Yu, S.; Chen, G.; Kong, J. Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes fifilled with Fe2O3 particles. J. Hazard. Mater. 2008, 160, 643–647. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Yang, X.; Wu, Z.; Li, Y. Construction of novel 2D/1D g-C3N4/CaTiO3 heterojunction with face-toface contact for boosting photodegradation of triphenylmethane dyes under simulated sunlight. J. Taiwan Inst. Chem. Eng. 2020, 107, 98–109. [Google Scholar] [CrossRef]
- Verdon, E.; Bessiral, M.; Chotard, M.P.; Couëdor, P.; Fourmond, M.P.; Fuselier, R.; Gaugain, M.; Gautier, S.; Hurtaud-Pessel, D.; Laurentie, M.; et al. The monitoring of triphenylmethane dyes in aquaculture products through the european union network of official control laboratories. J. AOAC Int. 2015, 98, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Oplatowska, M.; Donnelly, R.F.; Majithiya, R.J.; Kennedy, D.G.; Elliott, C.T. The potential for human exposure, direct and indirect, to the suspected carcinogenic triphenylmethane dye Brilliant Green from green paper towels. Food Chem. Toxicol. 2011, 49, 1870–1876. [Google Scholar] [CrossRef]
- Adenan, N.H.; Lim, Y.Y.; Su Yien Ting, A. Identification and optimization of triphenylmethane dyes removal by Streptomyces sp. from forest soil. Sustain. Environ. Res. 2021, 31, 8. [Google Scholar] [CrossRef]
- Tkaczyk-Wlizło, A.; Mitrowska, K.; Błądek, T. Quantification of twenty pharmacologically active dyes in water samples using UPLC-MS/MS. Heliyon 2022, 8, e09331. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Kumar, S.S.; Kumar, G.A. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Sadiq, A.C.; Olasupo, A.; Ngah, W.S.W.; Rahim, N.Y.; Suah, F.B.M. A decade development in the application of chitosan-based materials for dye adsorption: A short review. Int. J. Biol. Macromol. 2021, 191, 1151–1163. [Google Scholar] [CrossRef]
- Pandey, S.; Makhado, E.; Kim, S.; Kang, M. Recent developments of polysaccharide based superabsorbent nanocomposite for organic dye contamination removal from wastewater—A review. Environ. Res. 2023, 217, 114909. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef]
- Yang, T.; Gao, H.; Chen, H.; Xiao, X.; Zhao, C.; Gong, H.; Li, X.; Liu, L.; Liu, Y. Insights and perspectives of chitosan-based hydrogels for the removal of heavy metals and dyes from wastewater. Int. J. Biol. Macromol. 2025, 292, 139280. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Yahyaoui, S.; Hanafy, H.; Seliem, M.K.; Bonilla-Petriciolet, A.; Dotto, G.L.; Sellaoui, L.; Li, Q. Effective adsorption of dyes on an activated carbon prepared from carboxymethyl cellulose: Experiments, characterization and advanced modeling. Chem. Eng. J. 2021, 417, 128116. [Google Scholar] [CrossRef]
- Azama, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Polysaccharide-based composite hydrogels as sustainable materials for removal of pollutants from wastewater. Molecules 2022, 27, 8574. [Google Scholar] [CrossRef]
- Qi, X.; Li, Z.; Shen, L.; Qin, T.; Qian, Y.; Zhao, S.; Liu, M.; Zeng, Q.; Shen, J. Highly efficient dye decontamination via microbial salecan polysaccharide based gels. Carbohydr. Polym. 2019, 219, 1–11. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Sabaa, M.W.; ElHafeez, E.A.; Mohamed, R.R. Crystal violet dye removal using crosslinked grafted xanthan gum. Int. J. Biol. Macromol. 2019, 137, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Ren, J.; Chen, Y.; Guo, S.; Wu, M.; You, L. Sargassum fusiforme polysaccharide-based hydrogel microspheres enhance crystal violet dye adsorption properties. Molecules 2022, 27, 4686. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.; Sayed, A.Z.; El-Wahab, H.A.; Sayah, M.M. Synthesis of a hydrogel by grafting of acrylamide-co-sodium methacrylate onto chitosan for effective adsorption of Fuchsin basic dye. Int. J. Biol. Macromol. 2020, 159, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Huang, W.; Li, K.; Yang, Y. Highly efficient removal of adsorbed cationic dyes by dual-network chitosan-based hydrogel. Environ. Res. 2024, 263, 120195. [Google Scholar] [CrossRef]
- Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Dual ionic cross-linked alginate/clinoptilolite composite microbeads with improved stability and enhanced sorption properties for methylene blue. React. Funct. Polym. 2017, 116, 31–40. [Google Scholar] [CrossRef]
- Rani, I.; Warkar, S.G.; Kumar, A. Removal of cationic crystal violet dye using zeolite- embedded carboxymethyl tamarind kernel gum (cmtkg) based hydrogel adsorbents. ChemistrySelect 2023, 8, e202301434. [Google Scholar] [CrossRef]
- Selkälä, T.; Suopajärvi, T.; Sirviö, J.A.; Luukkonen, T.; Kinnunen, P.; Kling, K.I.; Wagner, J.B.; Liimatainen, H. Efficient entrapment and separation of anionic pollutants from aqueous solutions by sequential combination of cellulose nanofibrils and halloysite nanotubes. Chem. Eng. J. 2019, 374, 1013–1024. [Google Scholar] [CrossRef]
- Qi, X.; Zeng, Q.; Tong, X.; Su, T.; Xie, L.; Yuan, K.; Xu, J.; Shen, J. Polydopamine/montmorillonite-embedded pullulan hydrogels as efficient adsorbents for removing crystal violet. J. Hazard. Mater. 2021, 402, 123359. [Google Scholar] [CrossRef]
- Su, T.; Wu, L.; Pan, X.; Zhang, C.; Shi, M.; Gao, R.; Qi, X.; Dong, W. Pullulan-derived nanocomposite hydrogels for wastewater remediation: Synthesis and characterization. J. Colloid Interface Sci. 2019, 542, 253–262. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, G.; Hou, F.; Zhu, J. Highly effective removal of basic fuchsin dye using carboxymethyl konjac glucomannan grafted acrylic acid acrylamide/ montmorillonite composite hydrogel. Int. J. Biol. Macromol. 2024, 277, 134163. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, L.-P.; Zhang, H.-Y.; Ren, J.-J.; Liang, T.; Lv, X.-B.; Cheng, C.-J.; Yu, H.-R. Efficient adsorption of cationic dyes by a novel honeycomb-like porous hydrogel with ultrahigh mechanical property. Int. J. Biol. Macromol. 2024, 278, 134457. [Google Scholar] [CrossRef]
- Moharrami, P.; Motamedi, E. Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: Efficient and selective nanoadsorbent for removal of cationic dyes from water. Bioresour. Technol. 2020, 313, 123661. [Google Scholar] [CrossRef]
- Mittal, H.; Kumar, V.; Saruchi Ray, S.S. Adsorption of methyl violet from aqueous solution using gum xanthan/Fe3O4 based nanocomposite hydrogel. Int. J. Biol. Macromol. 2016, 89, 1–11. [Google Scholar] [CrossRef]
- Lei, C.; Xiao, Q.; Zhou, S.; Zu, W.; Li, J.; Zeng, J.; Yan, L.; Huang, Y.; Wang, B. Synthesis and characterization of magnetic carboxymethyl chitosan-poly(acrylic acid-itaconic acid) hydrogel for the efficient adsorption of malachite green. J. Appl. Polym. Sci. 2022, 139, e52347. [Google Scholar] [CrossRef]
- Benhalima, T.; Mokhtari, M.; Ferfera-Harrar, H. Synergistic adsorption/photodegradation effect for effective removal of crystal violet dye and acetamiprid pesticide using Fe3+ cross-linked ternary carboxymethyl cellulose/polyaniline/TiO2 photocomposites. J. Water Process Eng. 2024, 57, 104670. [Google Scholar] [CrossRef]
- Hussain, S.; Salman, M.; Al-Ahmary, K.M.; Ahmed, M. Synthesis of potential adsorbent for removal of malachite green dye using alginate hydrogel nanocomposites. Int. J. Biol. Macromol. 2025, 289, 138816. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Zhou, J.; Bao, L.; Zhang, L.; Yang, L.; Wu, J.; Hao, C. Novel carboxymethyl cellulose-based hydrogel embedded with metal organic framework for efficient cationic dye removal from water. Int. J. Biol. Macromol. 2024, 282, 137387. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Freeze-thaw as a route to build manageable polysaccharide cryogel for deep cleaning of crystal violet. Chem. Eng. J. 2020, 396, 125354. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, R.; Norton, I.T. Study of cryostructuration of polymer systems. XXI. Cryotropic gel formation of the water–maltodextrin systems. J. Appl. Polym. Sci. 2002, 83, 1658–1667. [Google Scholar] [CrossRef]
- Lazar, M.M.; Damaschin, R.P.; Volf, I.; Dinu, M.V. Deep cleaning of crystal violet and methylene blue dyes from aqueous solution by dextran-based cryogel adsorbents. Gels 2024, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbohydr. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Dinu, I.A.; Lazar, M.M.; Dragan, E.S. Chitosan-based ion-imprinted cryo-composites with excellent selectivity for copper ions. Carbohydr. Polym. 2018, 186, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sáez, P.; Dinu, I.A.; Rodríguez, A.; Gómez, J.M.; Lazar, M.M.; Rossini, D.; Dinu, M.V. Composite cryo-beads of chitosan reinforced with natural zeolites with remarkable elasticity and switching on/off selectivity for heavy metal ions. Int. J. Biol. Macromol. 2020, 164, 2432–2449. [Google Scholar] [CrossRef]
- Dinu, M.V.; Humelnicu, I.; Ghiorghita, C.A.; Humelnicu, D. Aminopolycarboxylic acids-functionalized chitosan-based composite cryogels as valuable heavy metal ions sorbents: Fixed-bed column studies and theoretical analysis. Gels 2022, 8, 221. [Google Scholar] [CrossRef]
- Dinu, M.V.; Humelnicu, D.; Lazar, M.M. Analysis of copper(II), cobalt(II) and iron(III) sorption in binary and ternary systems by chitosan-based composite sponges obtained by ice-segregation approach. Gels 2021, 7, 103. [Google Scholar] [CrossRef]
- Humelnicu, D.; Lazar, M.M.; Ignat, M.; Dinu, I.A.; Dragan, E.S.; Dinu, M.V. Removal of heavy metal ions from multi-component aqueous solutions by eco-friendly and low-cost composite sorbents with anisotropic pores. J. Hazard. Mater. 2020, 381, 120980. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Lazar, M.M.; Platon, I.-V.; Humelnicu, D.; Doroftei, F.; Dinu, M.V. Feather-weight cryostructured thiourea-chitosan aerogels for highly efficient removal of heavy metal ions and bacterial pathogens. Int. J. Biol. Macromol. 2023, 235, 123910. [Google Scholar] [CrossRef]
- Platon, I.-V.; Ghiorghita, C.-A.; Lazar, M.M.; Raschip, I.E.; Dinu, M.V. Chitosan sponges with instantaneous shape recovery and multistrain antibacterial activity for controlled release of plant-derived polyphenols. Int. J. Mol. Sci. 2023, 24, 4452. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Borchert, K.B.L.; Vasiliu, A.-L.; Zaharia, M.-M.; Schwarz, D.; Mihai, M. Porous thiourea-grafted-chitosan hydrogels: Synthesis and sorption of toxic metal ions from contaminated waters. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 607, 125504. [Google Scholar] [CrossRef]
- Demir, D.; Ceylan, S.; Bolgen, N. Composite cryogels for drug delivery applications: A preliminary study with dye as a model drug. J. Turk. Chem. Soc. Sec. B: Chem. Eng. 2023, 6, 17–26. [Google Scholar]
- Hajizadeh, S. Application of composite cryogels in downstream processing—a review. React. Funct. Polym. 2023, 191, 105693. [Google Scholar] [CrossRef]
- Miranda, R.; Nicu, R.; Latour, I.; Lupei, M.; Bobu, E.; Blanco, A. Efficiency of chitosans for the treatment of papermaking process water by dissolved air flotation. Chem. Eng. J. 2013, 231, 304–313. [Google Scholar] [CrossRef]
- Xu, X.; Bai, B.; Ding, C.; Wang, H.; Suo, Y. Synthesis and properties of an ecofriendly superabsorbent composite by grafting the poly(acrylic acid) onto the surface of dopamine-coated sea buckthorn branches. Ind. Eng. Chem. Res. 2015, 54, 3268–3278. [Google Scholar] [CrossRef]
- Jayanudin Lestari, R.S.D.; Barleany, D.R.; Pitaloka, A.B.; Yulvianti, M.; Rahmayetty Kanani, N.; Prasetyo, D. Ion-responsive chitosan-graft-poly(acrylic acid) hydrogels via saponification: Structure, swelling kinetics, and water retention. Polymer 2025, 336, 128930. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated carbon for dyes removal: Modeling and understanding the adsorption process. J. Chem. 2020, 2020, 9. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: Optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef]
- Ren, J.; Wang, X.; Zhao, L.; Li, M.; Yang, W. Effective Removal of Dyes from Aqueous Solutions by a Gelatin Hydrogel. J. Polym. Environ. 2021, 29, 3497–3508. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, W.; Zhang, Y.; Yang, Y.; Guo, D.; Liu, W.; Liu, L. Influence of multivalent background ions competition adsorption on the adsorption behavior of azo dye molecules and removal mechanism: Based on machine learning, DFT and experiments. Sep. Purif. Technol. 2024, 341, 126810. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye adsorption by resins: Effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201–202, 68–93. [Google Scholar] [CrossRef]
- Gamzazade, A.I.; Shimac, V.M.; Skljar, A.M.; Stykova, E.V.; Pavlova, S.A.; Rogozin, S.V. Investigation of the hydrodynamic properties of chitosan solutions. Acta Polym. 1985, 36, 420–424. [Google Scholar] [CrossRef]
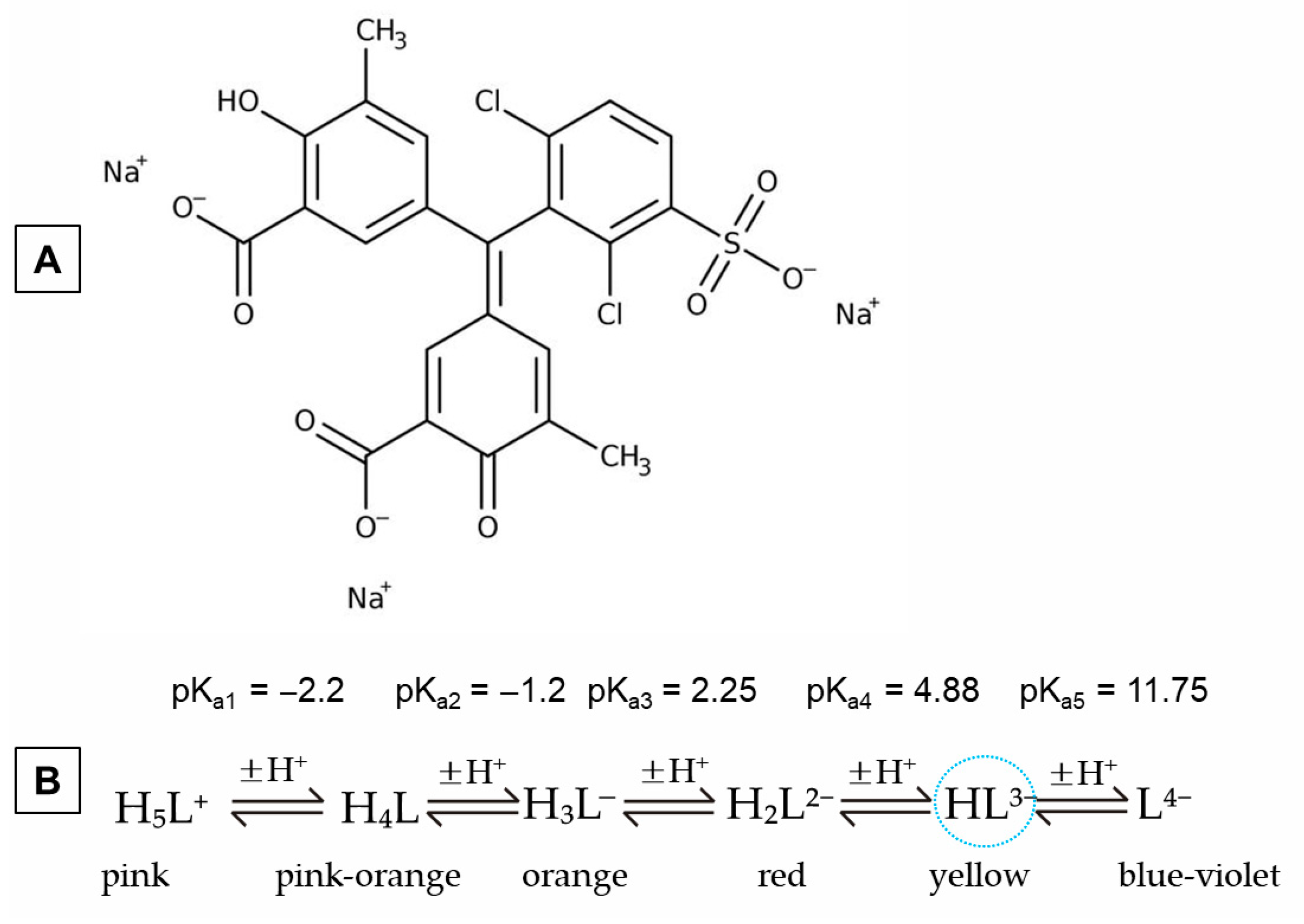
- Zerzucha, P.; Pytlakowska, K.; Kocot, K. Spectroscopic and electric properties of C.I. Mordant Blue 29: A theoretical and experimental study. New J. Chem. 2013, 37, 2810. [Google Scholar] [CrossRef]






| Sample Code | GA, wt.% | CPL, wt.% | a GFY, % ± SD | b Diameter of Beads, mm |
|---|---|---|---|---|
| CSGA5 | 5 | 0 | 86.00 ± 0.61 | 3.00 ± 0.09 |
| CSGA5Z10 | 5 | 10 | 85.04 ± 0.80 | 2.90 ± 0.12 |
| CSGA5Z20 | 5 | 20 | 84.74 ± 0.60 | 3.03 ± 0.03 |
| CSGA5Z40 | 5 | 40 | 85.30 ± 0.87 | 2.93 ± 0.12 |
| CSGA7.5 | 7.5 | 0 | 82.55 ± 0.49 | 2.88 ± 0.13 |
| CSGA7.5Z10 | 7.5 | 10 | 86.61 ± 0.43 | 2.57 ± 0.11 |
| CSGA7.5Z20 | 7.5 | 20 | 88.17 ± 1.42 | 2.94 ± 0.11 |
| CSGA7.5Z40 | 7.5 | 40 | 83.99 ± 0.25 | 2.8 ± 0.09 |
| CSGA10 | 10 | 0 | 81.28 ± 0.97 | 2.69 ± 0.06 |
| CSGA10Z10 | 10 | 10 | 82.91 ± 0.65 | 2.77 ± 0.09 |
| CSGA10Z20 | 10 | 20 | 81.21 ± 1.41 | 2.88 ± 0.06 |
| CSGA10Z40 | 10 | 40 | 82.19 ± 0.08 | 2.68 ± 0.03 |
| Sample | PFO Kinetic Model | PSO Kinetic Model | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (g/g) | R2 | k2 (g/g·min) | qe (g/g) | R2 | |
| CSGA5 | 0.569 | 60.120 | 0.999 | 0.059 | 60.565 | 0.999 |
| CSGA5Z10 | 0.757 | 47.664 | 0.996 | 0.101 | 48.014 | 0.997 |
| CSGA5Z20 | 0.669 | 42.924 | 0.996 | 0.074 | 43.391 | 0.998 |
| CSGA5Z40 | 0.734 | 39.961 | 0.997 | 0.104 | 40.304 | 0.999 |
| CSGA7.5 | 0.897 | 51.747 | 0.999 | 0.204 | 51.920 | 0.999 |
| CSGA7.5Z10 | 0.889 | 47.277 | 0.999 | 0.224 | 47.433 | 0.999 |
| CSGA7.5Z20 | 0.545 | 43.807 | 0.996 | 0.055 | 44.354 | 0.997 |
| CSGA7.5Z40 | 0.578 | 38.340 | 0.999 | 0.078 | 38.719 | 0.999 |
| CSGA10 | 0.653 | 49.567 | 0.999 | 0.116 | 49.790 | 0.999 |
| CSGA10Z10 | 0.631 | 46.308 | 0.999 | 0.085 | 46.661 | 0.999 |
| CSGA10Z20 | 0.645 | 41.790 | 0.998 | 0.092 | 42.132 | 0.999 |
| CSGA10Z40 | 0.612 | 36.601 | 0.998 | 0.129 | 36.799 | 0.998 |
| Isotherm Model | Parameters | CSGA5 | CSGA5Z10 | CSGA5Z20 | CSGA5Z40 |
|---|---|---|---|---|---|
| Langmuir | qm,exp, mg/g | 117.948 | 182.811 | 213.017 | 250.811 |
| qm, mg/g | 109.516 | 164.438 | 188.6 | 249.607 | |
| KL, L/mg | 0.561 | 0.41 | 0.29 | 0.012 | |
| R2 | 0.946 | 0.936 | 0.905 | 0.815 | |
| χ2 | 91.666 | 275.571 | 553.38 | 1431.725 | |
| Freundlich | KF, L/mg | 45.192 | 59.789 | 55.578 | 52.954 |
| 1/n | 0.144 | 0.161 | 0.192 | 0.219 | |
| R2 | 0.964 | 0.935 | 0.955 | 0.964 | |
| χ2 | 60.74 | 281.204 | 261.301 | 274.186 | |
| D-R | qDR, mg/g | 107.638 | 157.241 | 180.301 | 199.643 |
| β | 0.147 | 0.13 | 0.246 | 0.238 | |
| E, kJ/mol | 1.844 | 1.961 | 1.426 | 1.449 | |
| R2 | 0.917 | 0.867 | 0.839 | 0.799 | |
| χ2 | 141.431 | 571.677 | 942.823 | 1552.938 |
| Isotherm Model | Parameters | CSGA7.5 | CSGA7.5Z10 | CSGA7.5Z20 | CSGA7.5Z40 |
|---|---|---|---|---|---|
| Langmuir | qm,exp, mg/g | 199.934 | 220.483 | 208.851 | 196.26 |
| qm, mg/g | 188.933 | 221.704 | 204.294 | 178.926 | |
| KL, L/mg | 0.804 | 0.052 | 0.134 | 1.725 | |
| R2 | 0.85 | 0.731 | 0.744 | 0.901 | |
| χ2 | 957.013 | 2088.811 | 1857.328 | 601.459 | |
| Freundlich | KF, L/mg | 103.775 | 106.695 | 130.416 | 103.481 |
| 1/n | 0.108 | 0.115 | 0.073 | 0.098 | |
| R2 | 0.855 | 0.732 | 0.746 | 0.914 | |
| χ2 | 922.727 | 2076.453 | 1844.613 | 521.128 | |
| D-R | qDR, mg/g | 187.238 | 200.643 | 193.191 | 178.214 |
| β | 0.209 | 2.938 | 0.046 | 0.047 | |
| E, kJ/mol | 1.547 | 0.413 | 3.297 | 3.262 | |
| R2 | 0.847 | 0.236 | 0.234 | 0.899 | |
| χ2 | 973.926 | 348.922 | 159.877 | 608.907 |
| Isotherm Model | Parameters | CSGA10 | CSGA10Z10 | CSGA10Z20 | CSGA10Z40 |
|---|---|---|---|---|---|
| Langmuir | qm,exp, mg/g | 185.074 | 182.053 | 180.889 | 164.844 |
| qm, mg/g | 169.276 | 174.773 | 165.536 | 146.274 | |
| KL, L/mg | 1.27 | 1.041 | 1.36 | 0.276 | |
| R2 | 0.894 | 0.9 | 0.881 | 0.833 | |
| χ2 | 576.069 | 564.914 | 603.454 | 624.377 | |
| Freundlich | KF, L/mg | 91.919 | 99.374 | 95.409 | 74.519 |
| 1/n | 0.107 | 0.099 | 0.097 | 0.114 | |
| R2 | 0.913 | 0.902 | 0.898 | 0.854 | |
| χ2 | 472.95 | 551.749 | 516.242 | 545.899 | |
| D-R | qDR, mg/g | 168.633 | 173.841 | 164.901 | 144.16 |
| β | 0.087 | 0.133 | 0.092 | 2.829 | |
| E, kJ/mol | 2.397 | 1.939 | 2.331 | 0.420 | |
| R2 | 0.893 | 0.899 | 0.88 | 0.83 | |
| χ2 | 582.045 | 571.226 | 609.082 | 639.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, M.M.; Ghiorghita, C.-A.; Rusu, D.; Dinu, M.V. Nanocomposite Cryogels Based on Chitosan for Efficient Removal of a Triphenylmethane Dye from Aqueous Systems. Gels 2025, 11, 729. https://doi.org/10.3390/gels11090729
Lazar MM, Ghiorghita C-A, Rusu D, Dinu MV. Nanocomposite Cryogels Based on Chitosan for Efficient Removal of a Triphenylmethane Dye from Aqueous Systems. Gels. 2025; 11(9):729. https://doi.org/10.3390/gels11090729
Chicago/Turabian StyleLazar, Maria Marinela, Claudiu-Augustin Ghiorghita, Daniela Rusu, and Maria Valentina Dinu. 2025. "Nanocomposite Cryogels Based on Chitosan for Efficient Removal of a Triphenylmethane Dye from Aqueous Systems" Gels 11, no. 9: 729. https://doi.org/10.3390/gels11090729
APA StyleLazar, M. M., Ghiorghita, C.-A., Rusu, D., & Dinu, M. V. (2025). Nanocomposite Cryogels Based on Chitosan for Efficient Removal of a Triphenylmethane Dye from Aqueous Systems. Gels, 11(9), 729. https://doi.org/10.3390/gels11090729







