Synthesis of Porous Materials on Hybrid Wormlike Micelles of Zwitterionic and Anionic Surfactants for Efficient Oilfield Wastewater Treatment
Abstract
1. Introduction
2. Result and Discussion
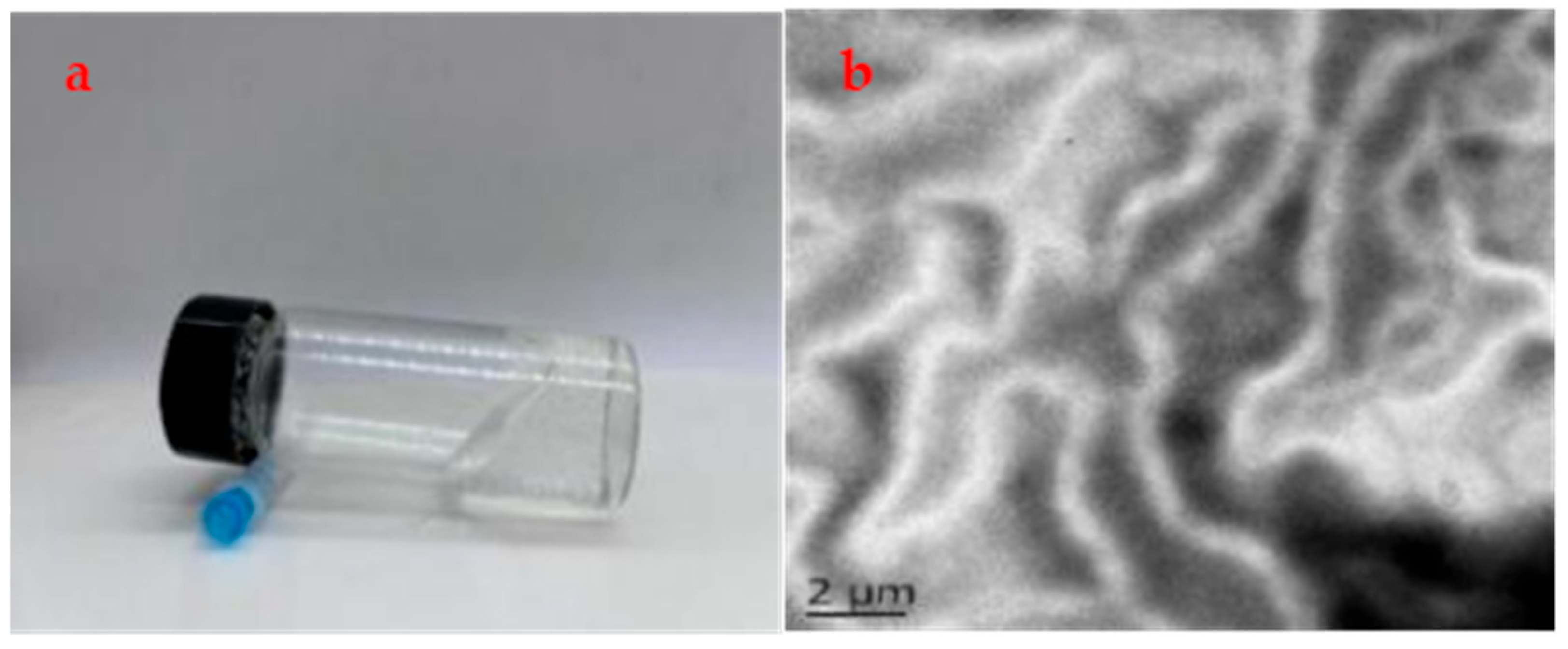
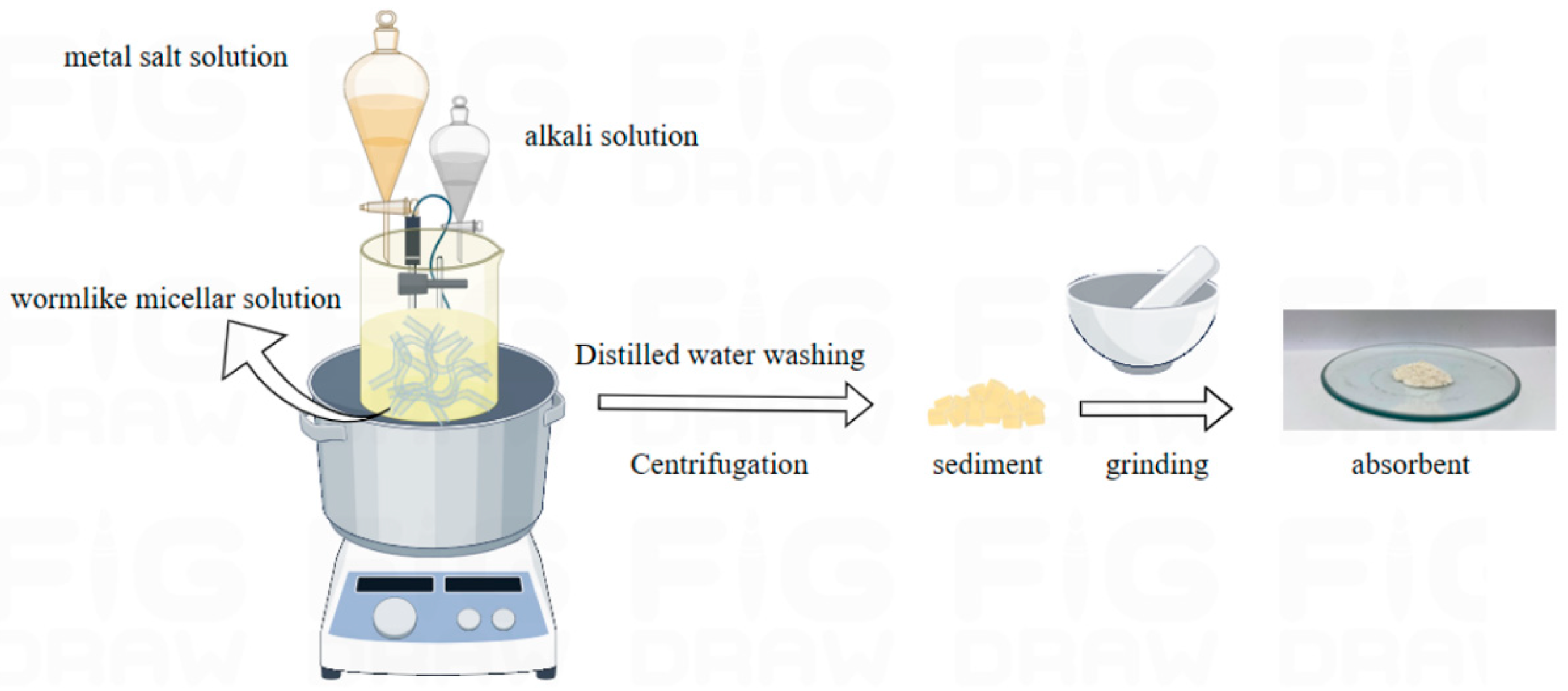
2.1. Preparation of Wormlike Micelles
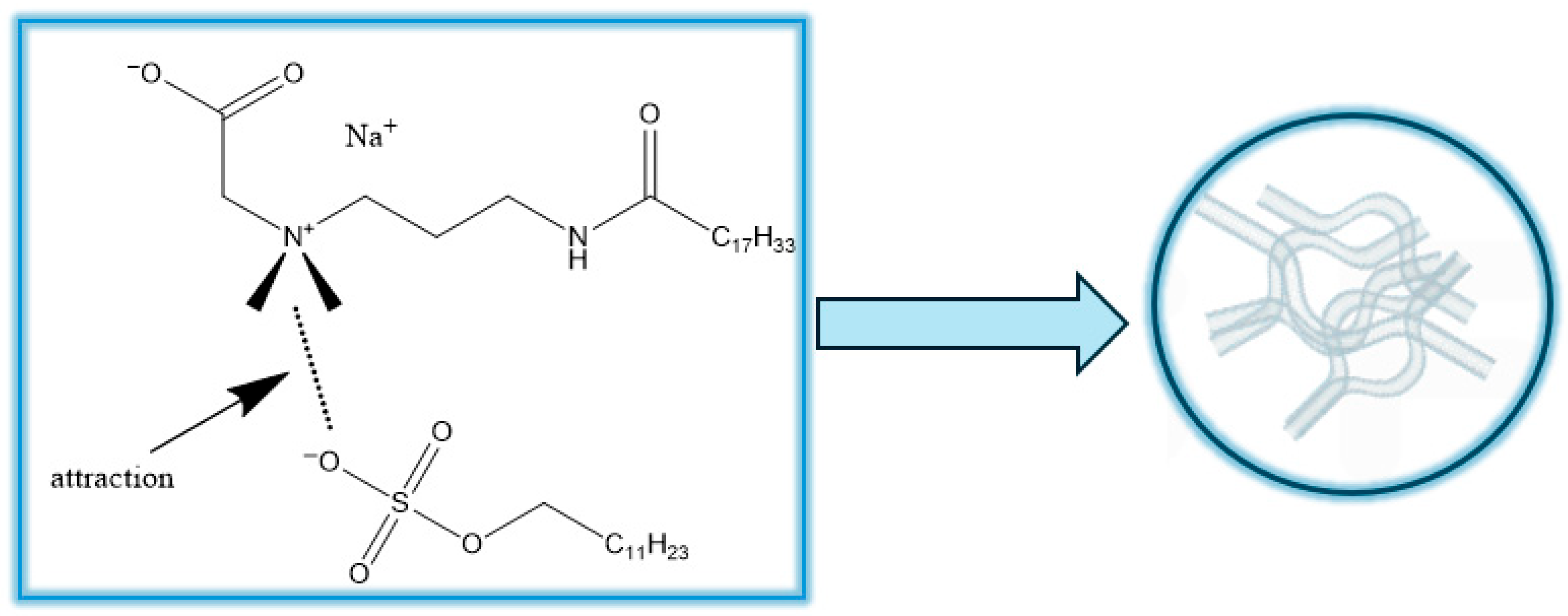
2.2. Formation of Wormlike Micelles
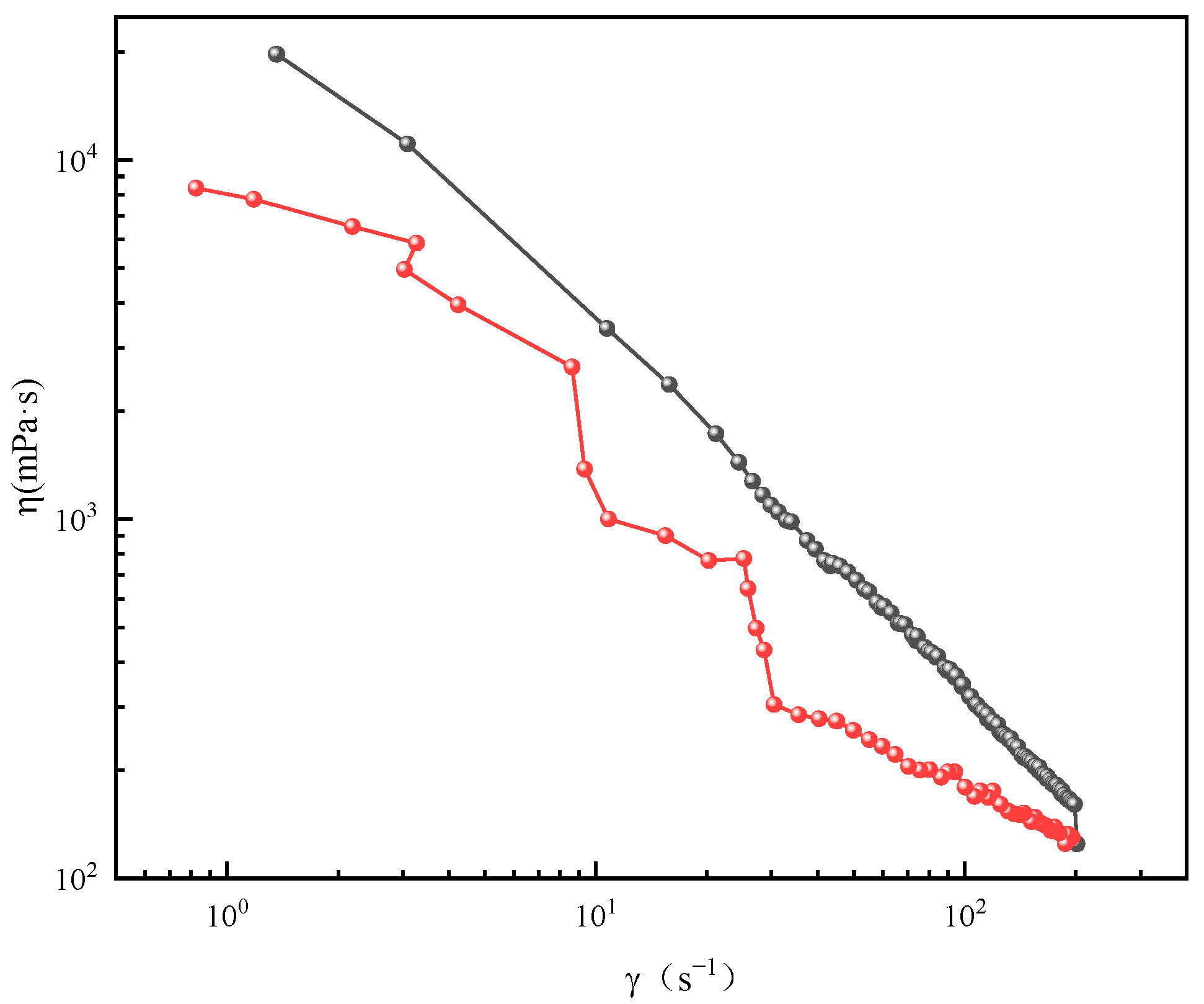
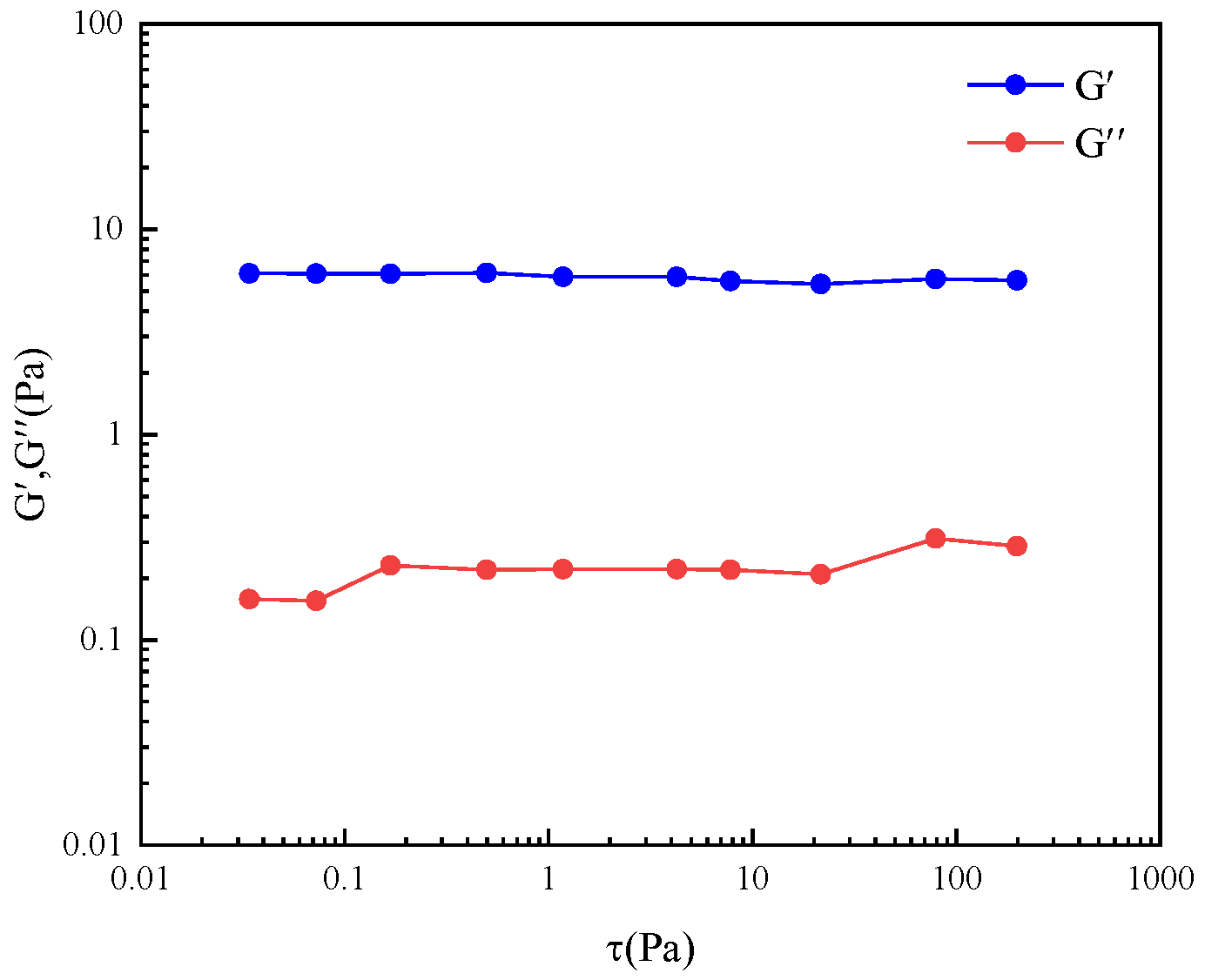
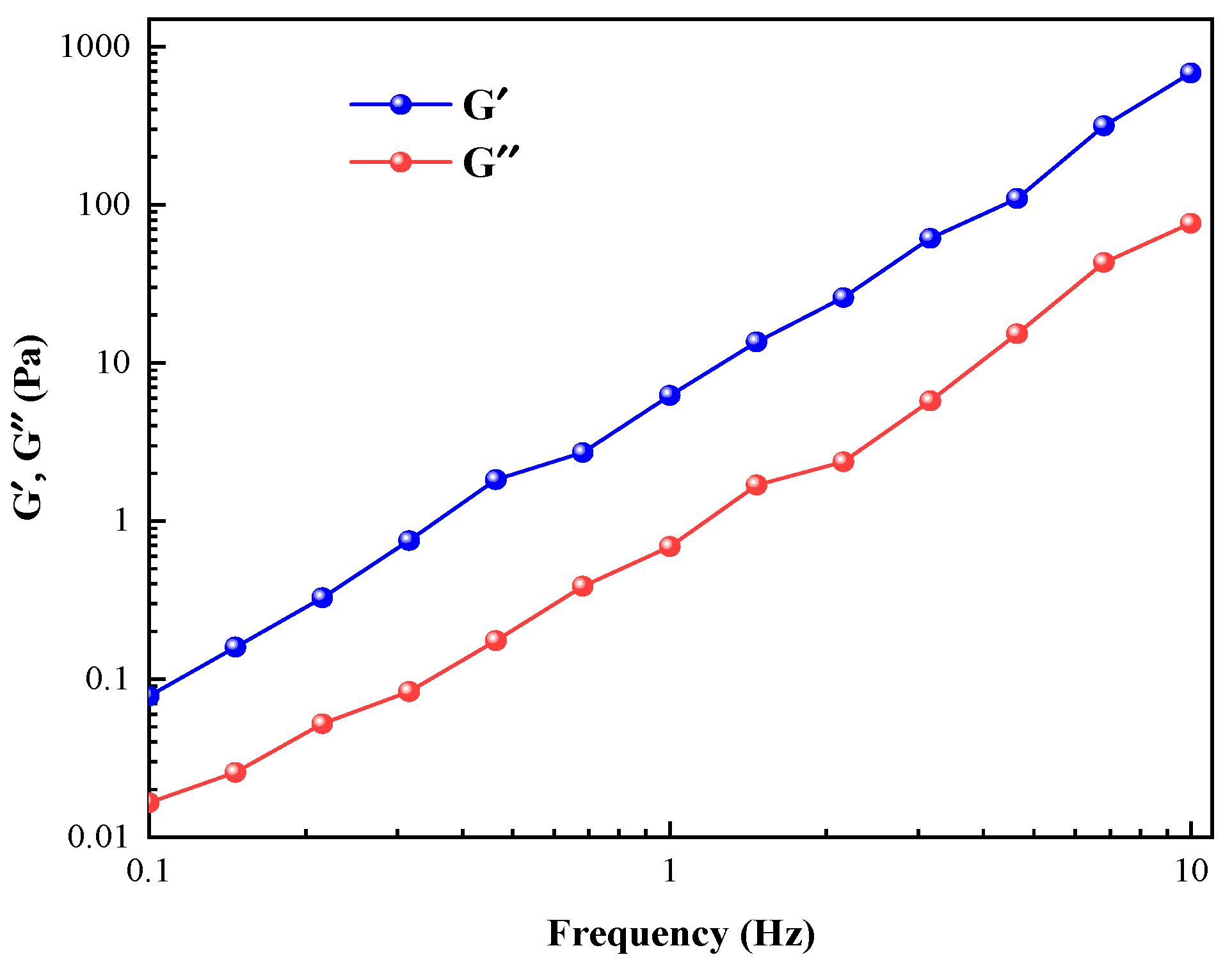
2.3. Rheological Behavior of Wormlike Micelles
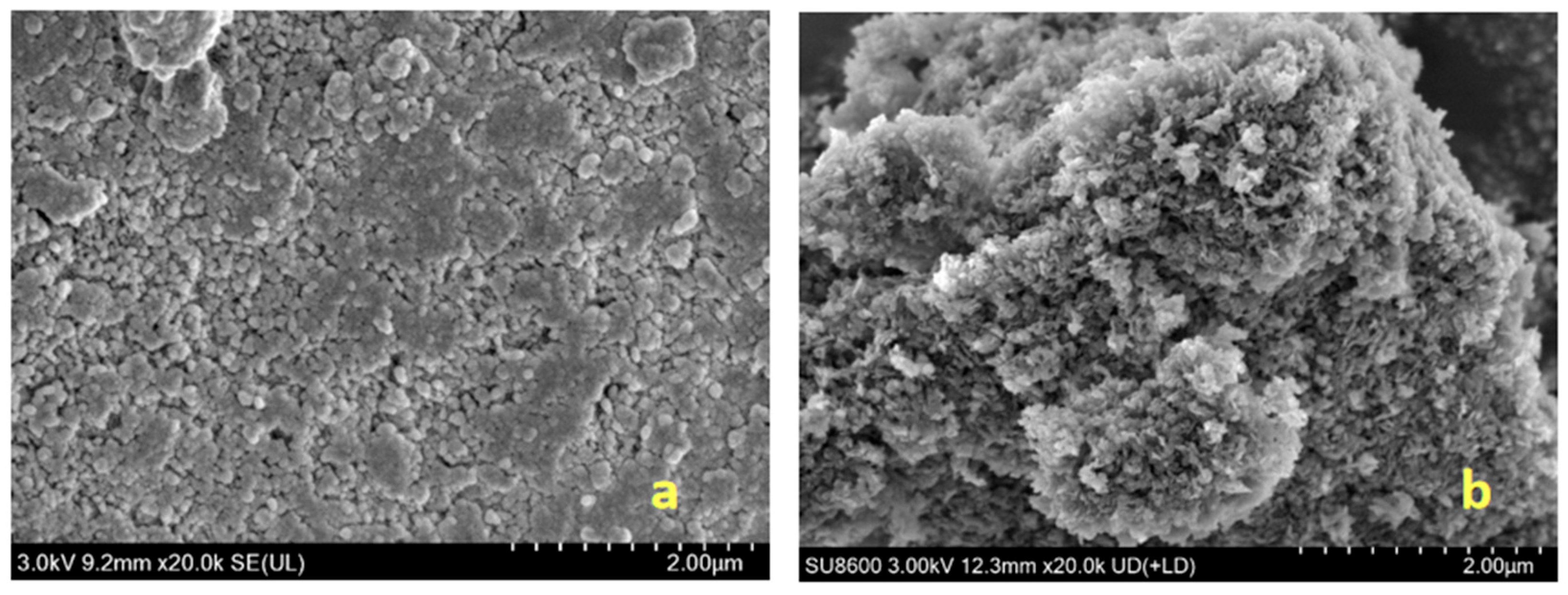
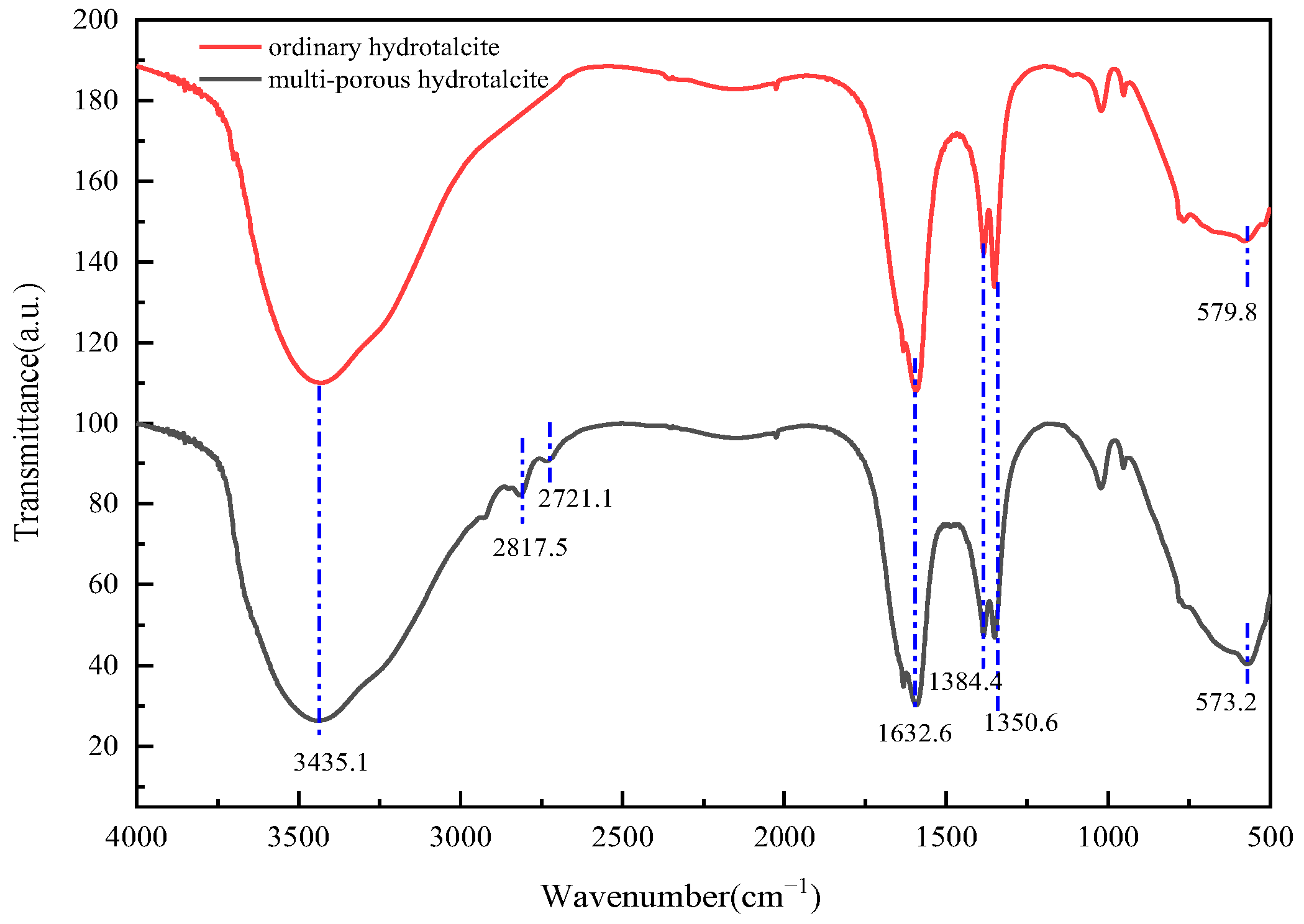
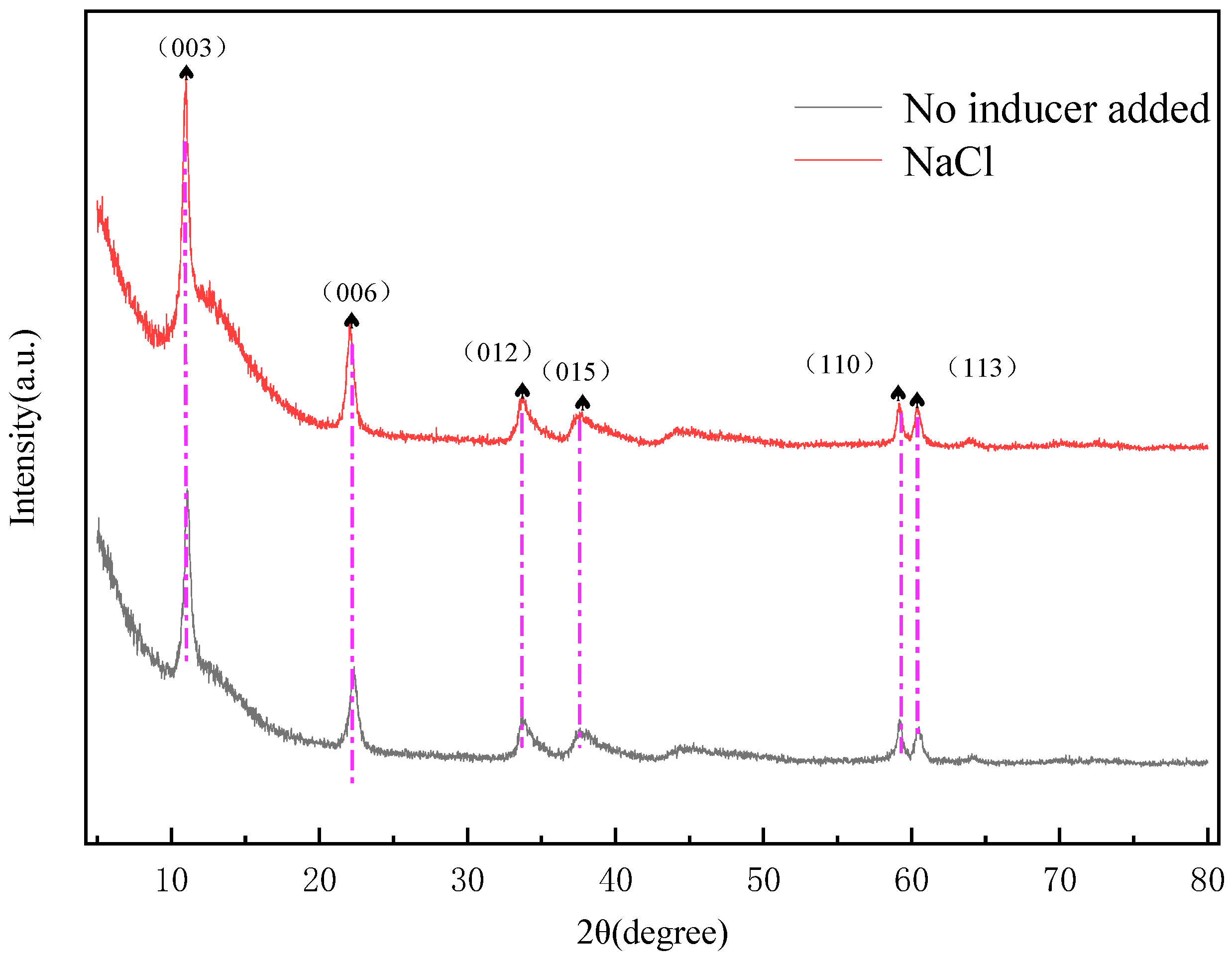
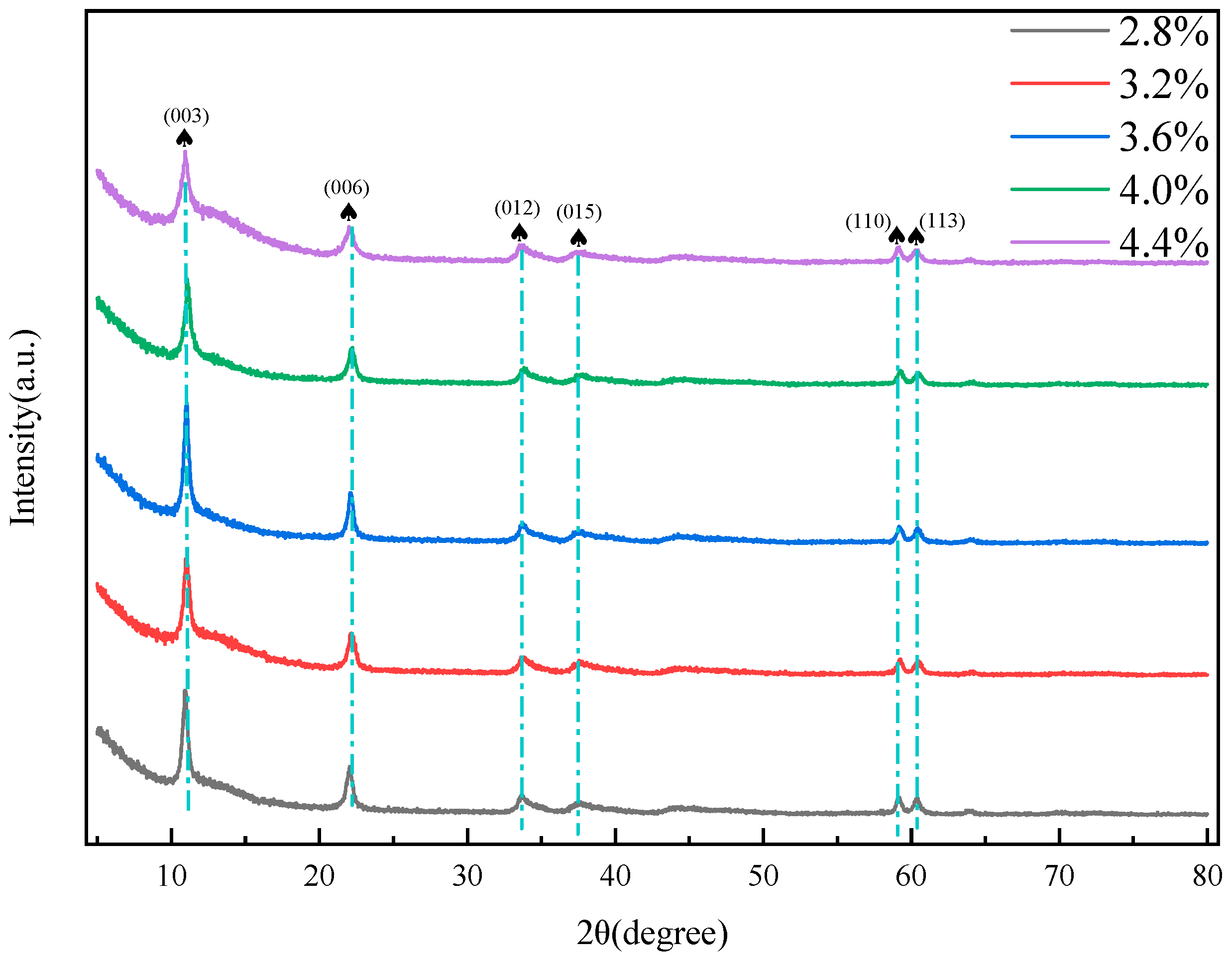
2.4. Characterization of MgFe-LDH Samples
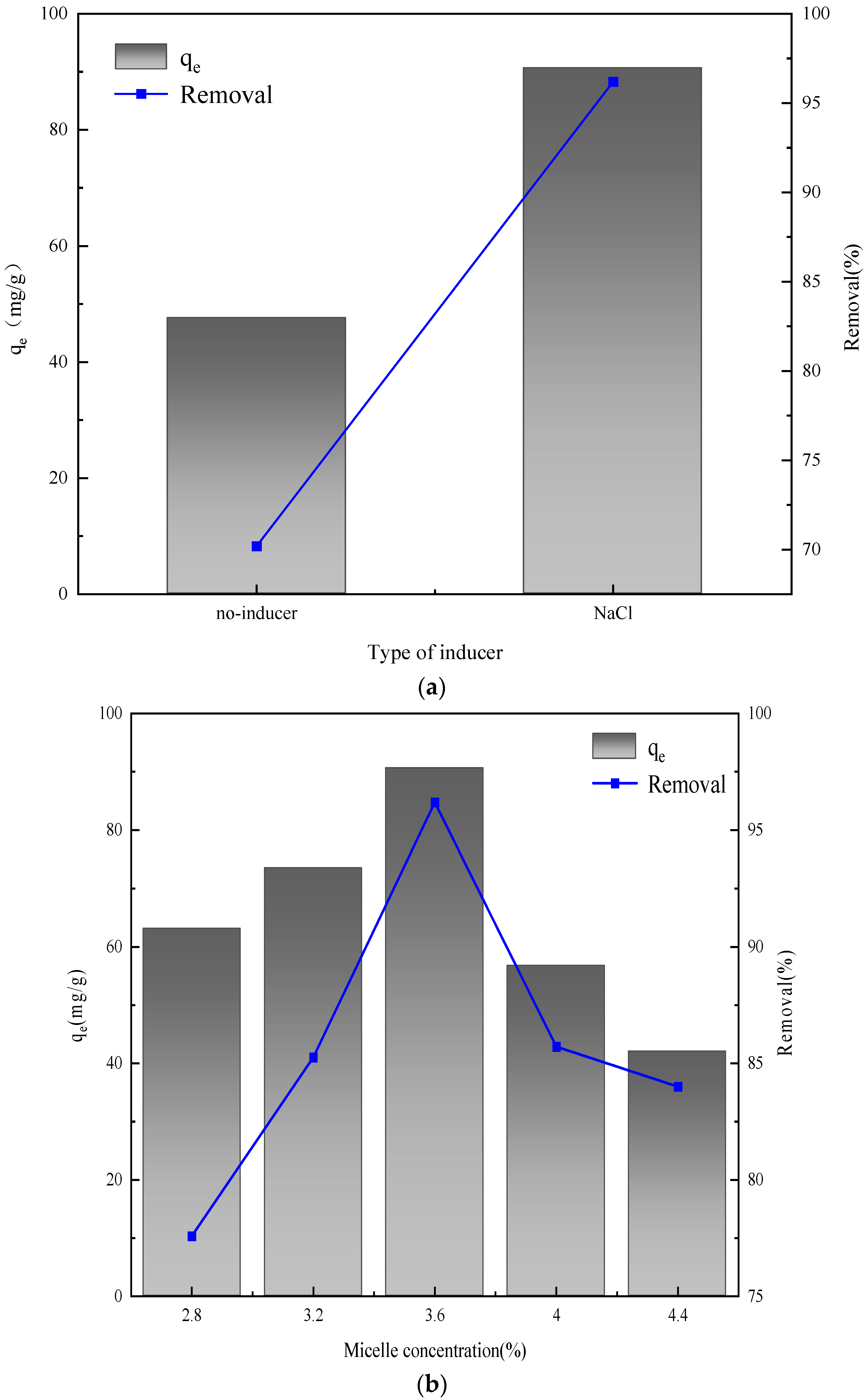
2.5. Preparation Parameters of Hydrotalcite
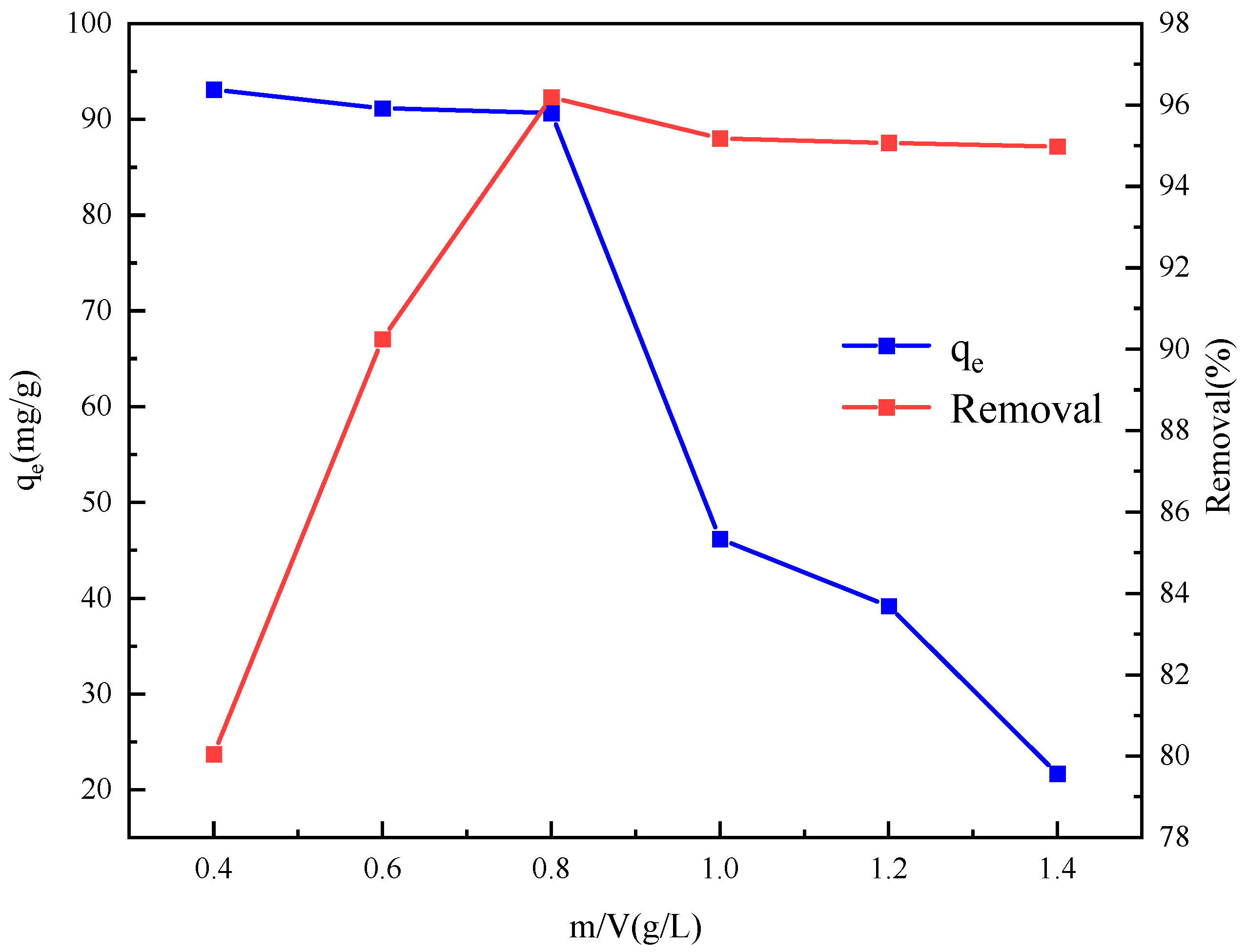
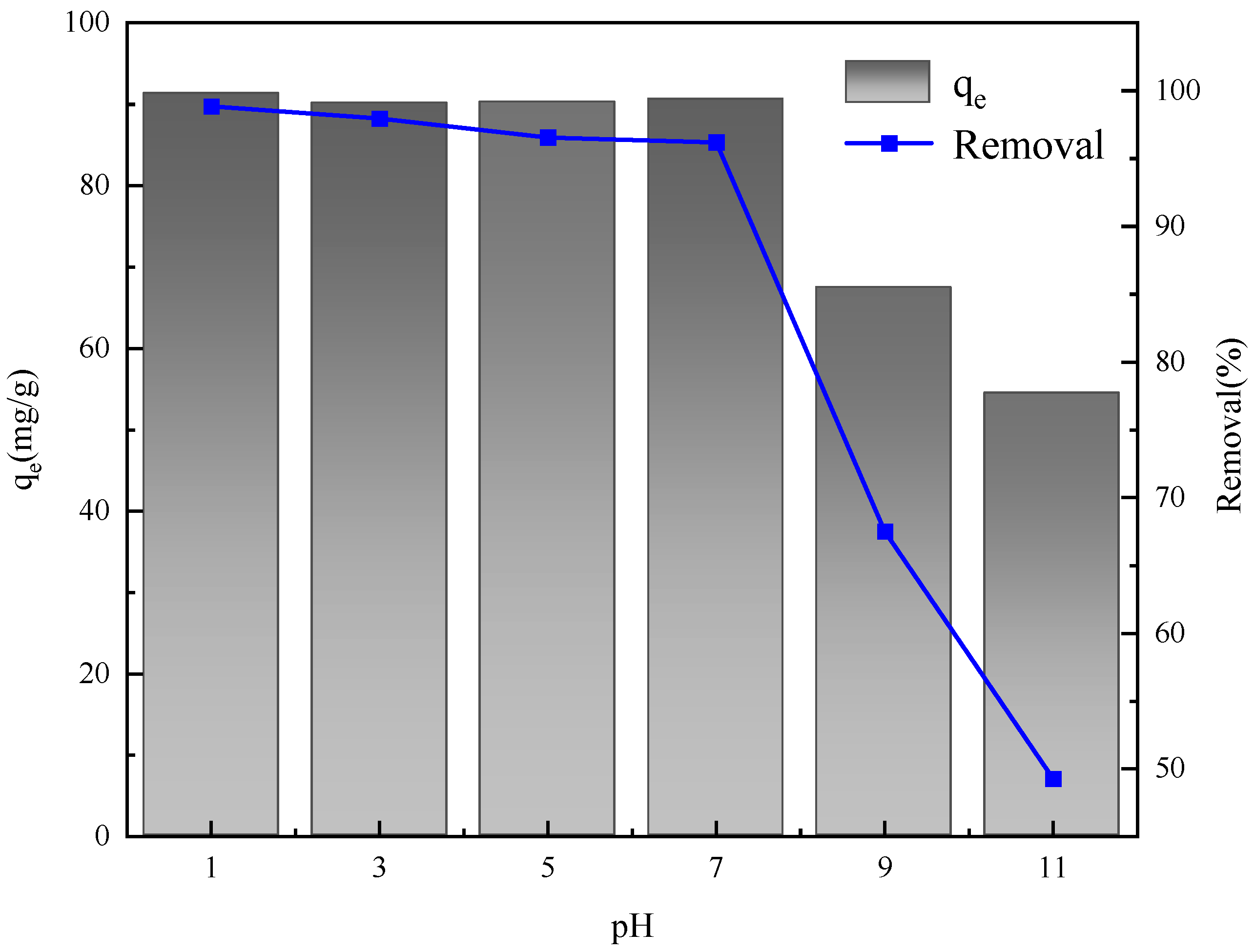
2.6. Absorption Experiment
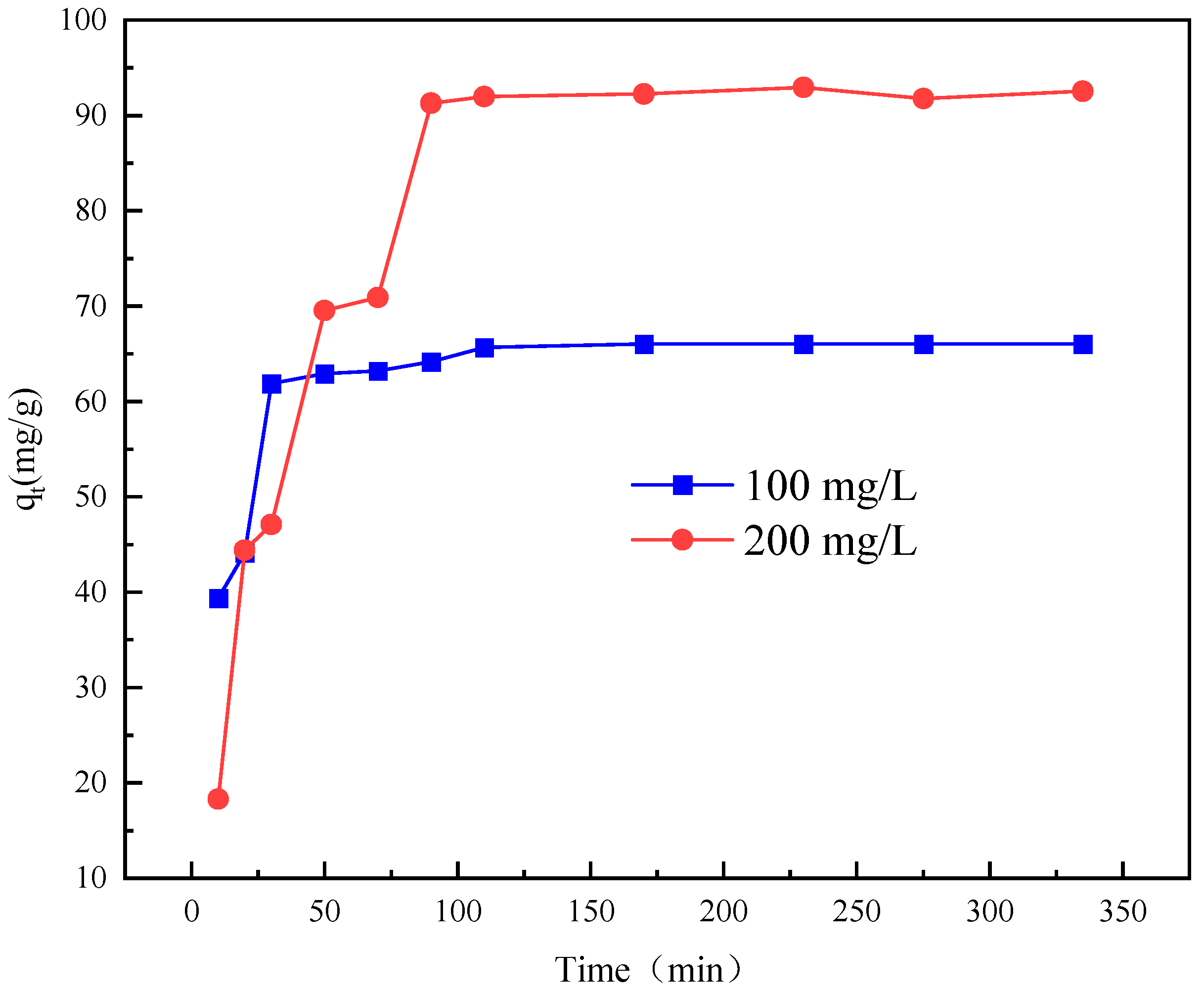
2.7. Adsorption Kinetics Analysis
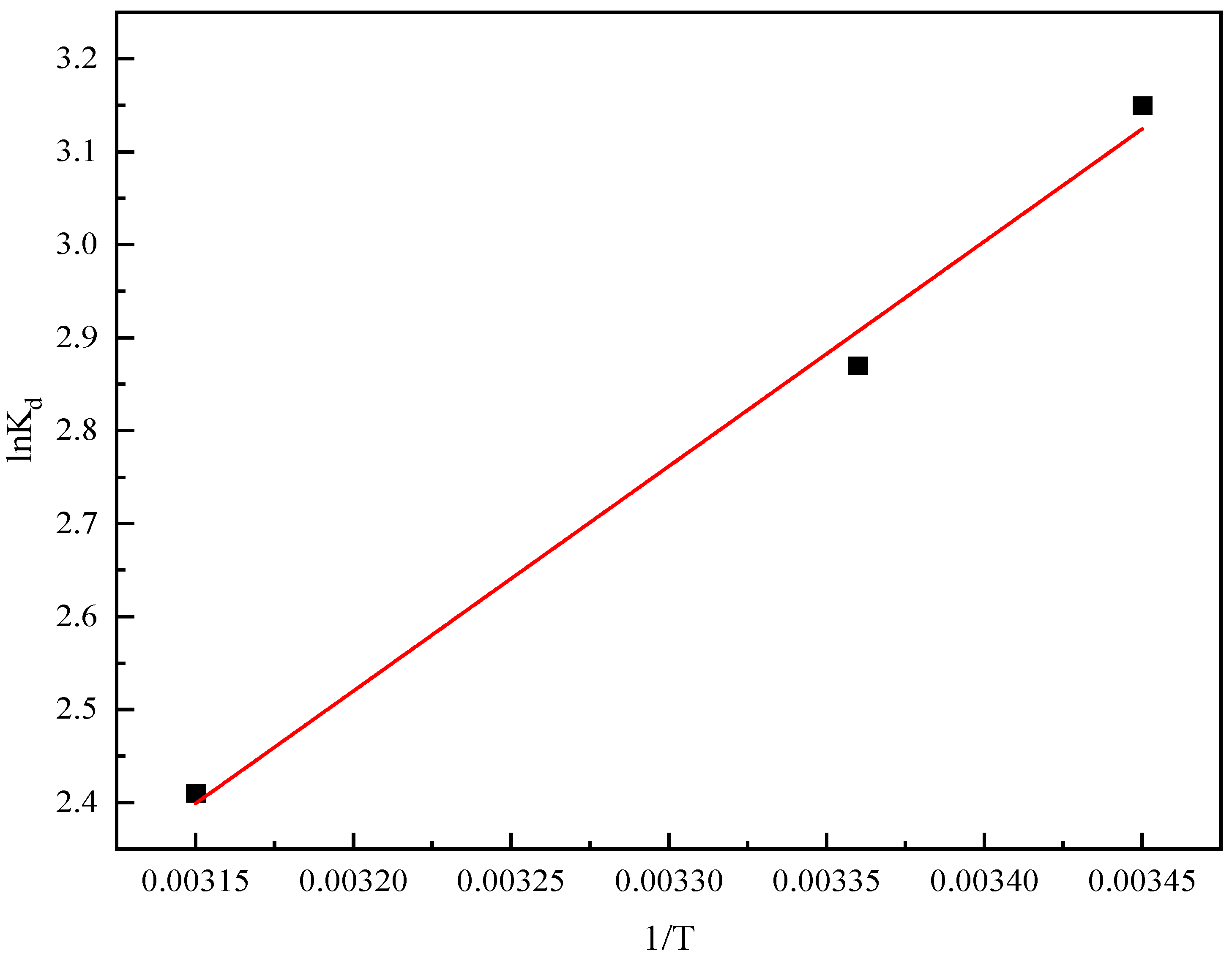
2.8. Analysis of Adsorption Thermodynamics
2.9. Adsorption Isotherm Model
2.10. Adsorption Competition Experiment
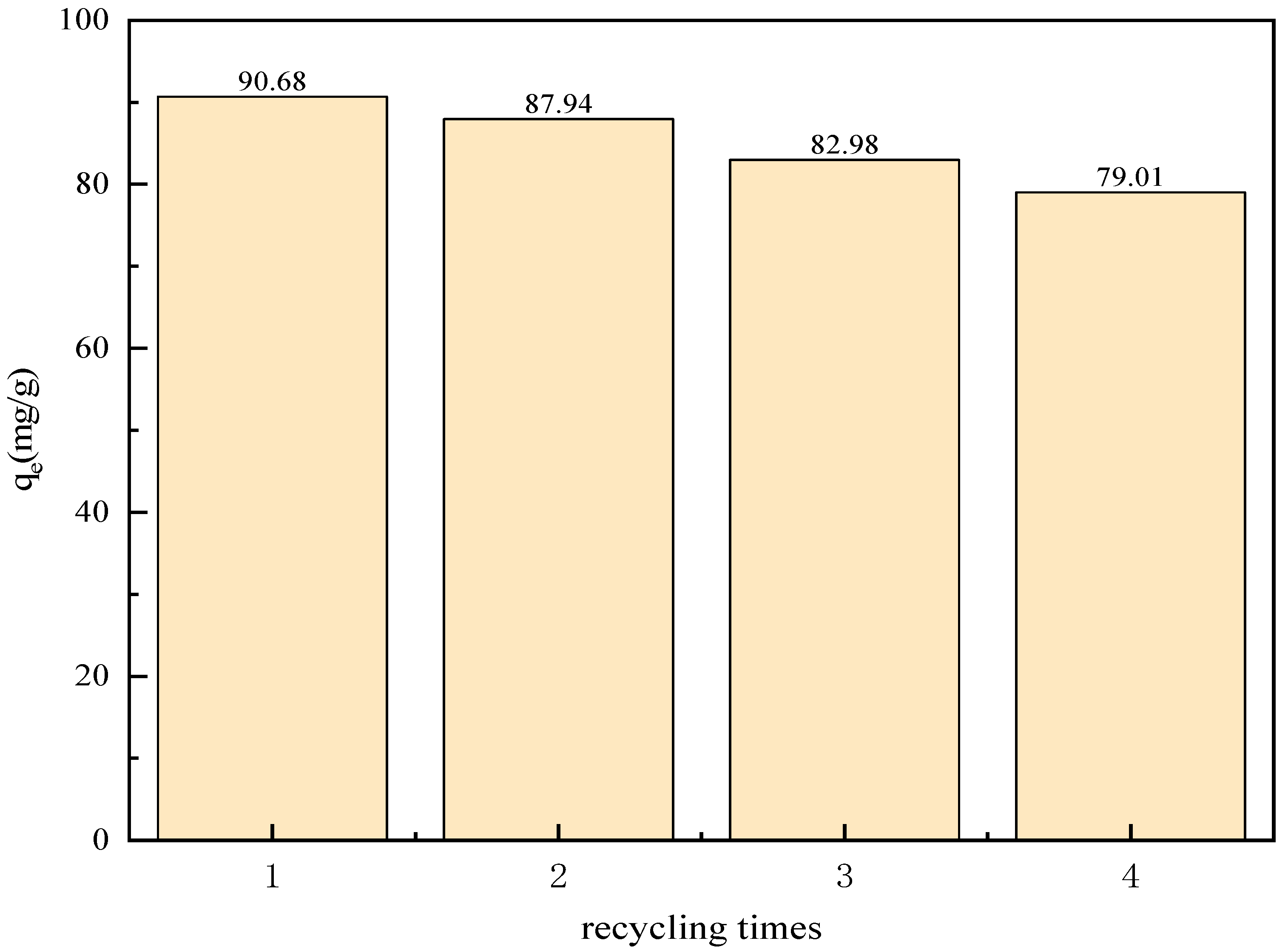
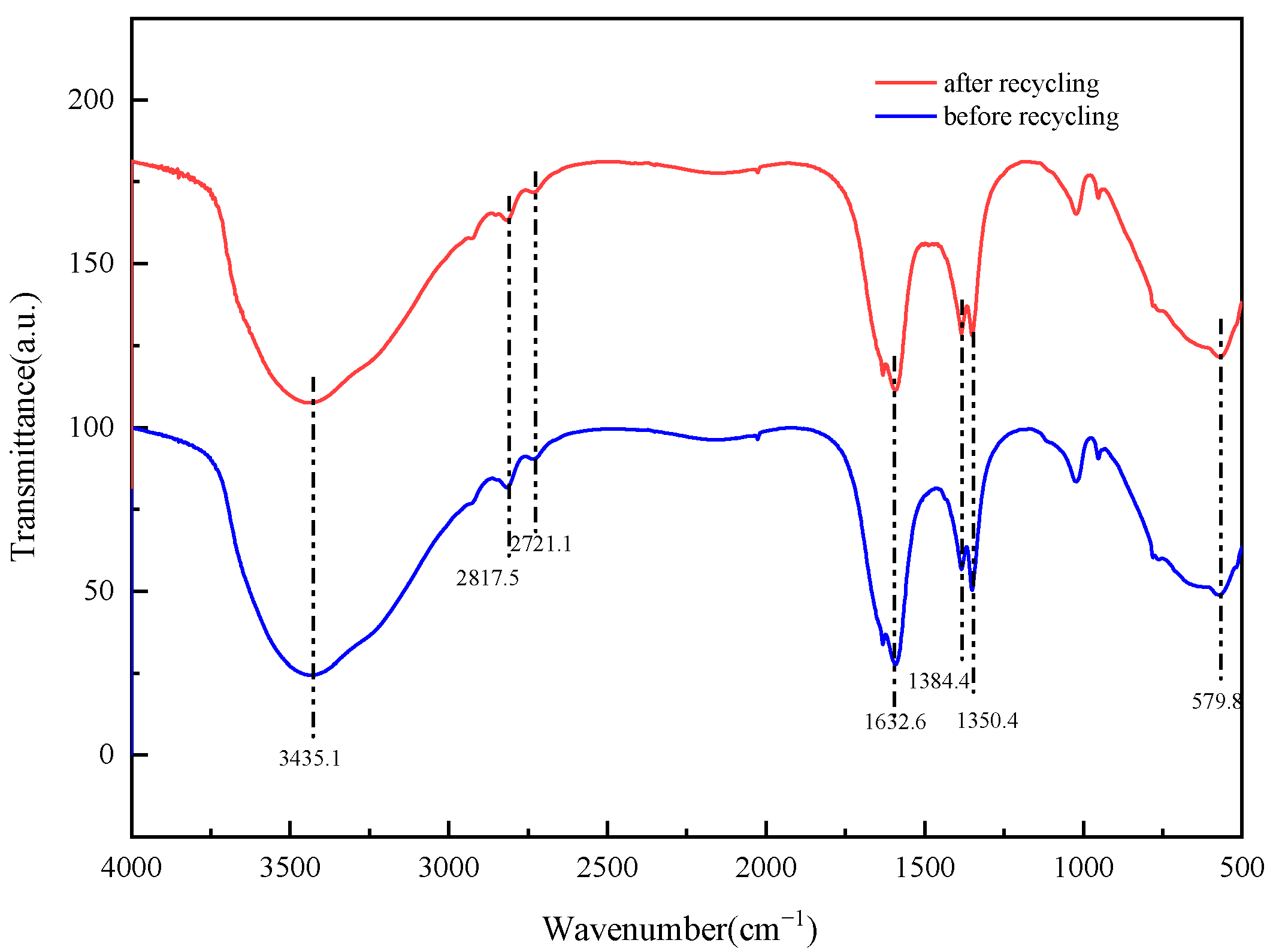
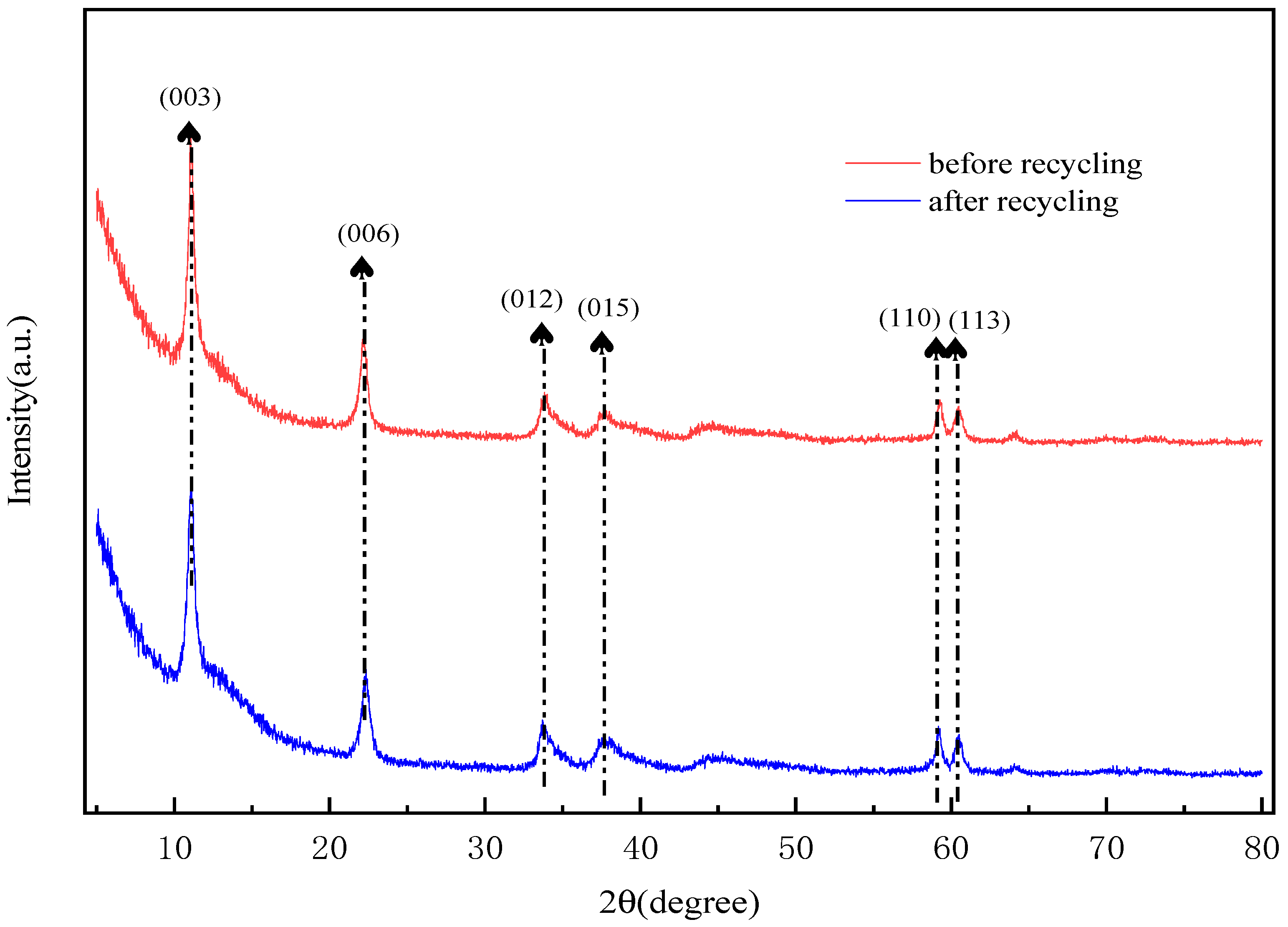
2.11. Analysis of Recycling
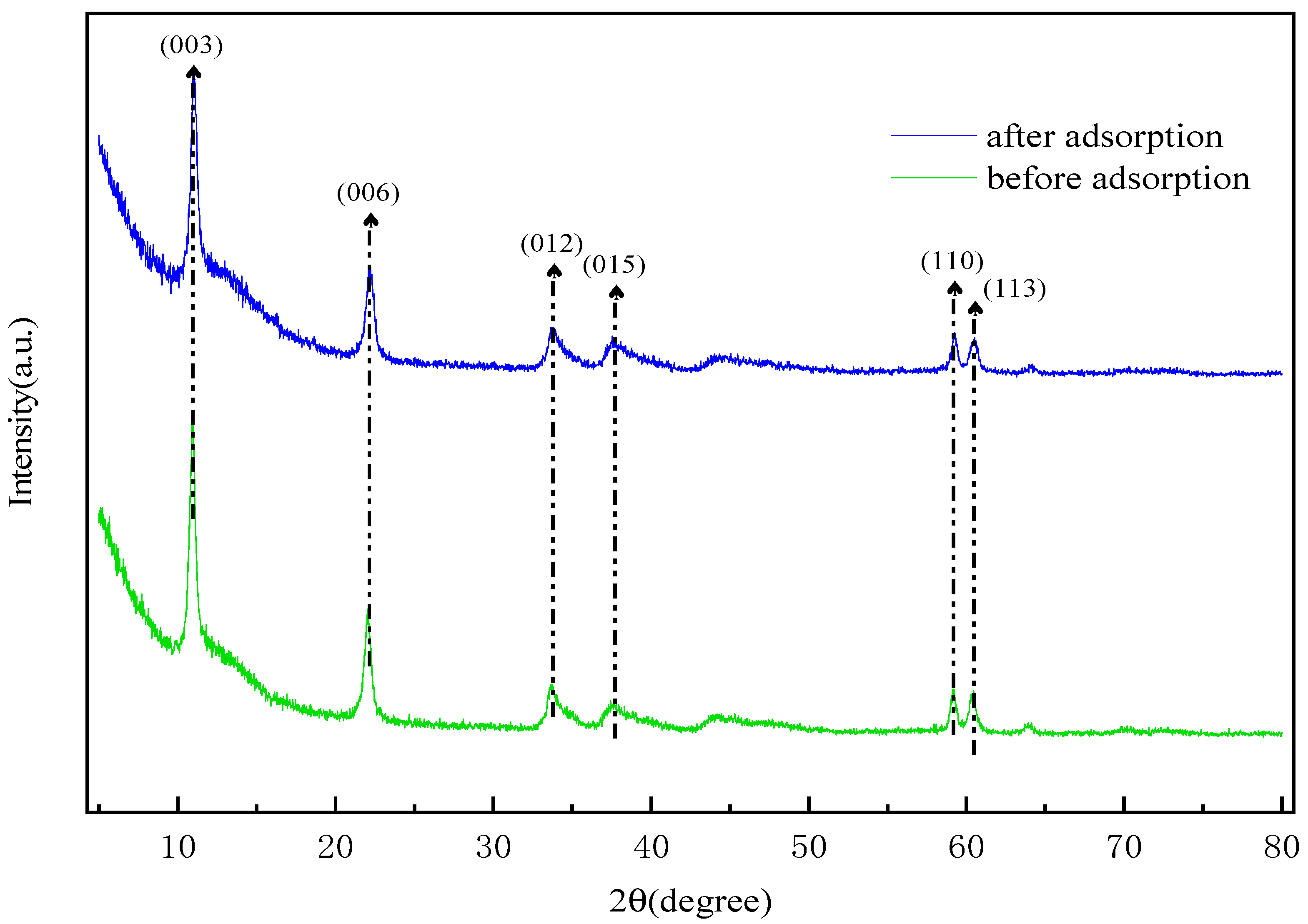
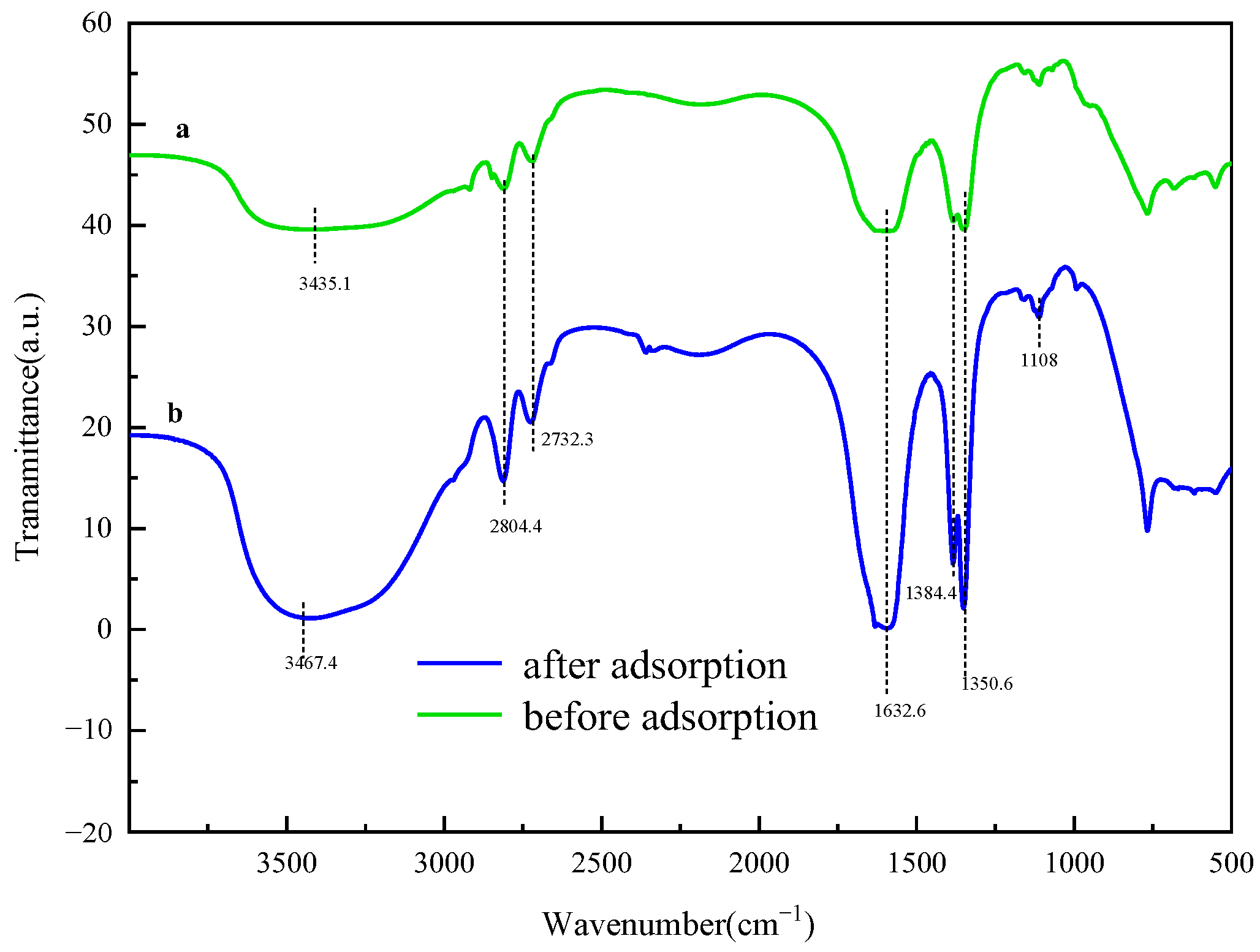
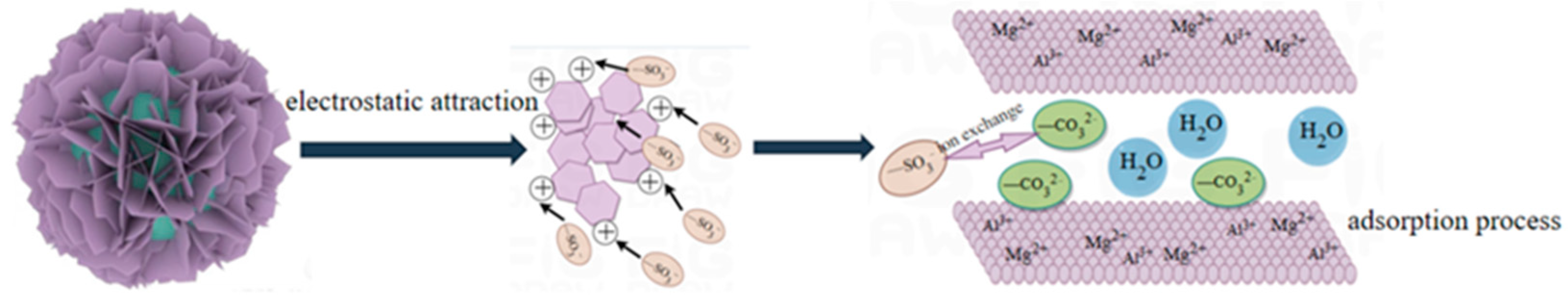
2.12. Adsorption Mechanism of Hierarchical MgFe-LDH
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Absorbents
4.3. Adsorption Experiments
4.4. Recycling Experiments
4.5. MgFe-LDH Characterization
4.6. Rheological Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, H.; Zhang, W.Y. Synthesis of Lignite Graft Polycondensate as Drilling Fluid Additive and its Influence on the Properties of Water-Bentonite Suspensions. Chem. Technol. Fuels Oils 2018, 53, 6. [Google Scholar] [CrossRef]
- Zhang, S.X.; Wang, Z.H.; Lü, N.C.; He, H.J.; Wang, Z.M.; Yang, Z.G. Physicochemical and microbial treatment technology for polysulfonate drilling fluid wastewater. Chin. J. Environ. Eng. 2015, 9, 3803–3808. [Google Scholar]
- Guo, D.W.; Wu, J.B.; Feng, D.D.; Zhang, Y.; Zhu, X.; Luo, Z.; Kang, Y.; Zhao, Y.; Sun, S. Mechanism of efficient magnetic biochar for typical aqueous organic contaminant combined-adsorption removal. Fuel Process. Technol. 2023, 247, 107795. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ba, M.B.; Oukhrib, R.; Dehbi, A.; Lamhasni, T.; Brahmi, Y.; El-Kordy, A.; Franco, D.S.; Georgin, J.; Lima, E.C.; et al. Adsorption of various inorganic and organic pollutants by natural and synthetic zeolites: A critical review. Arab. J. Chem. 2024, 17, 105474. [Google Scholar] [CrossRef]
- Chen, C.L.; Ma, J.Y.; Wang, Y.; Yi, Z.; Wang, S.; Gao, H.; Wu, X.; Liu, G.; Yang, H. CTAB-assisted synthesis of Bi2MoO6 hierarchical microsphere and its application as a novel efficient and recyclable adsorbent in removing organic pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130441. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Smreczak, B.; Podlasiński, M. Sorption of Organic Contaminants by Stable Organic Matter Fraction in Soil. Molecules 2023, 28, 429. [Google Scholar] [CrossRef]
- Wu, H.F.; Chao, Y.H.; Jin, Y.; Tao, D.; Li, X.; Luo, J.; Xia, G.; Zhu, L.; Zhu, W. Sustainable preparation of graphene-analogue boron nitride by ball-milling for adsorption of organic pollutants. Chin. J. Chem. Eng. 2022, 42, 73–81. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Morales, D.S.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 adsorption by organohydrotalcites at low temperatures and high pressure. Chem. Eng. J. 2022, 431, 134324. [Google Scholar] [CrossRef]
- Shu, P.; Zhang, Y.N.; Deng, J.; Duan, Z.; Zhai, F. Characteristics and mechanism of modified hydrotalcite for coal spontaneous combustion preventing. Energy 2023, 265, 126353. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Kishida, M.; Nakamura, T.; Kawasaki, N. Interaction between phosphate ions and Fe-Mg type hydrotalcite for purification of wastewater. J. Environ. Chem. Eng. 2019, 7, 102897. [Google Scholar] [CrossRef]
- Avendaño, R.; Fals, J.; Bocanegra, S.; Dieuzeide, M.L.; Amadeo, N. Sorption enhanced steam reforming of ethanol for hydrogen production, over Mg/Al hydrotalcites modified with K. Int. J. Hydrogen Energy 2023, 48, 20889–20900. [Google Scholar] [CrossRef]
- López-Santiago, R.F.; Delgado, J.; Castillo, R. Micellar entanglement and its relation to the elastic behavior of wormlike micelle fluids. J. Colloid Interface Sci. 2022, 626, 1015–1027. [Google Scholar] [CrossRef]
- Stancheva, T.N.; Georgiev, M.T.; Radulova, G.M.; Danov, K.D.; Marinova, K.G. Rheology of saturated micellar networks: Wormlike micellar solutions vs. bicontinuous micellar phases. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129927. [Google Scholar] [CrossRef]
- Mushi, S.J.; Kang, W.; Yang, H.; Li, Z.; Ibrashev, K.; Issakhov, M.; Mabeyo, P.E. Effect of aromatic acid on the rheological behaviors and microstructural mechanism of wormlike micelles in betaine surfactant. J. Mol. Liq. 2021, 332, 115908. [Google Scholar] [CrossRef]
- Wu, A.L.; Gao, Y.N.; Zheng, L.Q. Zwitterionic amphiphiles: Their aggregation behavior and applications. Green Chem. 2019, 21, 4290–4312. [Google Scholar] [CrossRef]
- Lu, S.; Dong, J.F.; Li, X.F. Gradual transformation of anionic/zwitterionic wormlike micelles from viscous to elastic domains: Unravelling the effect of anionic surfactant chain length. J. Colloid Interface Sci. 2023, 641, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Molchanov, V.S.; Kuklin, A.I.; Orekhov, A.S.; Arkharova, N.; Philippova, O. Temporally persistent networks of long-lived mixed wormlike micelles of zwitterionic and anionic surfactants. J. Mol. Liq. 2021, 342, 116955. [Google Scholar] [CrossRef]
- Qiao, Y.; Lin, Y.Y.; Wang, Y.J.; Li, Z.; Huang, J. Metal-Driven Viscoelastic Wormlike Micelle in Anionic/Zwitterionic Surfactant Systems and Template-Directed Synthesis of Dendritic Silver Nanostructures. Langmuir 2011, 27, 1718–1723. [Google Scholar] [CrossRef]
- Lu, S.; Mei, Q.L.; Chen, J.Y.; Wang, Z.; Li, W.; Feng, C.; Li, X.; Dong, J. Cryo-TEM and rheological study on shear-thickening wormlike micelles of zwitterionic/anionic (AHSB/SDS) surfactants. J. Colloid Interface Sci. 2022, 608, 513–524. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wang, R.T.; Zheng, Y.; Sun, L.; Yu, L.; Jiao, J.; Wang, R. Interaction between Zwitterionic Surface Activity Ionic Liquid and Anionic Surfactant: Na+-Driven Wormlike Micelles. J. Phys. Chem. B 2013, 117, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Q.Y. Novel self-assembly nano OSA starch micelles controlled by protonation in aqueous media. Carbohydr. Polym. 2023, 299, 120146. [Google Scholar] [CrossRef]
- Yang, J.W.; Wu, T.J.; Liu, Q.N.; Huang, H.; Chen, S.; Chen, G. Research of a fracturing-oil displacement integrated working fluid based on betaine surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133371. [Google Scholar] [CrossRef]
- Rothstein, J.P.; Mohammadigoushki, H. Complex flows of viscoelastic wormlike micelle solutions. J. Non-Newton. Fluid Mech. 2020, 285, 104382. [Google Scholar] [CrossRef]
- Tamate, R.; Hashimoto, K.; Li, X.; Shibayama, M.; Watanabe, M. Effect of ionic liquid structure on viscoelastic behavior of hydrogen-bonded micellar ion gels. Polymer 2019, 178, 121694. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M.; Hussain, C.M. Recent innovations in functionalized layered double hydroxides: Fabrication, characterization, and industrial applications. Adv. Colloid Interface Sci. 2020, 283, 102216. [Google Scholar] [CrossRef]
- Balbino, T.A.C.; Bellato, C.R.; da Silva, A.D.; Neto, J.d.O.M.; Ferreira, S.O. Preparation and evaluation of iron oxide/hydrotalcite intercalated with dodecylsulfate/β-cyclodextrin magnetic organocomposite for phenolic compounds removal. Appl. Clay Sci. 2020, 193, 105659. [Google Scholar] [CrossRef]
- Gao, H.M.; Yao, A.; Shi, Y.H.; Noor, N.; Zeb, A.; Li, M.; Li, H. Preparation and properties of hierarchical Al–Mg layered double hydroxides as UV resistant hydrotalcite. Mater. Chem. Phys. 2020, 256, 123630. [Google Scholar] [CrossRef]
- Alaei, R.; Javanshir, S.; Behnamfard, A. Treatment of gold ore cyanidation wastewater by adsorption onto a Hydrotalcite-type anionic clay as a novel adsorbent. J. Environ. Health Sci. Eng. 2020, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- DVelázquez-Herrera, F.; Fetter, G.; Rosato, V.; Pereyra, A.M.; Basaldella, E.I. Effect of structure, morphology and chemical composition of Zn-Al, Mg/Zn-Al and Cu/Zn-Al hydrotalcites on their antifungal activity against A. niger. J. Environ. Chem. Eng. 2018, 6, 3376–3383. [Google Scholar] [CrossRef]
- Comelli, N.A.; Ruiz, M.L.; Leguizamón Aparicio, M.S.; Merino, N.A.; Cecilia, J.A.; Rodríguez-Castellón, E.; Lick, I.D.; Ponzi, M.I. Influence of the synthetic conditions on the composition, morphology of CuMgAl hydrotalcites and their use as catalytic precursor in diesel soot combustion reactions. Appl. Clay Sci. 2018, 157, 148–157. [Google Scholar] [CrossRef]
- Vu, T.; Weaver, M.R.; Kasting, G.B.; Koenig, P. Effect of pH on the Structure and Dynamics of Wormlike Micelles in an Amino Acid-Derived Surfactant Composition. Langmuir 2021, 37, 4112–4120. [Google Scholar] [CrossRef]
- Zhou, L.; Slan, M.; Bai, B.B.; Du, W.; Qu, C.; Zhang, J.; Tang, Y. Enhanced Removal of Sulfonated Lignite from Oil Wastewater with Multidimensional MgAl-LDH Nanoparticles. Nanomaterials 2021, 11, 861. [Google Scholar] [CrossRef]
- Wang, W.H.; Hu, B.B.; Wang, C.; Liang, Z.; Cui, F.; Zhao, Z.; Yang, C. Cr(VI) removal by micron-scale iron-carbon composite induced by ball milling: The role of activated carbon. Chem. Eng. J. 2020, 389, 122633. [Google Scholar] [CrossRef]
- Motta, F.L.; Melo, B.A.G.; Santana, M.H.A. Deprotonation and protonation of humic acids as a strategy for the technological development of pH-responsive nanoparticles with fungicidal potential. New Biotechnol. 2016, 33, 773–780. [Google Scholar] [CrossRef]
- Bashiri, H.; Hassani Javanmardi, A.; Soltani, Z. A theoretical description for competitive adsorption at the Solid/Solution interface. Comput. Theor. Chem. 2024, 1237, 114652. [Google Scholar] [CrossRef]
- Wu, Z.S.; Sun, Z.H.; Liu, P.Y.; Li, Q.; Yang, R.; Yang, X. Competitive adsorption of naphthalene and phenanthrene on walnut shell based activated carbon and the verification via theoretical calculation. RSC Adv. 2020, 10, 10703–10714. [Google Scholar] [CrossRef]
- Ren, N.; Wang, C.H.; Zhao, Z.; Liang, Y.; Wei, W.; Qin, G. Recovery of ferulic acid from corn bran by adsorption on mesoporous carbon. J. Food Process Eng. 2021, 44, e13817. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, K.; Zhang, Y.P.; Peng, C.; Robledo-Cabrera, A.; López-Valdivieso, A. Highly surface activated carbon to remove Cr(VI) from aqueous solution with adsorbent recycling. Environ. Res. 2021, 197, 111151. [Google Scholar] [CrossRef] [PubMed]
- Legrand, U.; Castillo Sánchez, J.R.; Boudreault, R.; Meunier, J.-L.; Lauriault, P.-L.G.; Tavares, J.R. Fundamental thermodynamic properties of sorbents for atmospheric water capture. Chem. Eng. J. 2022, 431, 134058. [Google Scholar] [CrossRef]
- Niknezhad, M.; Lakouraj, M.M. Development of pH-sensitive hydrogel nanocomposite based on acrylic acid/graphene oxide/acryloyl tetra ammonium thiacalix[4]arene for separation of cationic dyes. J. Polym. Res. 2021, 28, 209. [Google Scholar] [CrossRef]
- Koyuncu, H.; Kul, A.R. Removal of aniline from aqueous solution by activated kaolinite: Kinetic, equilibrium and thermodynamic studies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 59–66. [Google Scholar] [CrossRef]
- Chadha, N.; Sharma, R.; Saini, P. A new insight into the structural modulation of graphene oxide upon chemical reduction probed by Raman spectroscopy and X-ray diffraction. Carbon Lett. 2021, 31, 1125–1131. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Structural transition to well-ordered phases of NaOH-activated slag-metakaolin cements aged by 6 years. Cem. Concr. Res. 2022, 156, 106791. [Google Scholar] [CrossRef]
- Liu, H.Z.; Huang, P.J.; Liang, Z.J.; Zhao, Z.; Cui, F. Selective adsorption of anions on hydrotalcite-like compounds derived from drinking water treatment residuals. Chemosphere 2022, 300, 134508. [Google Scholar] [CrossRef] [PubMed]
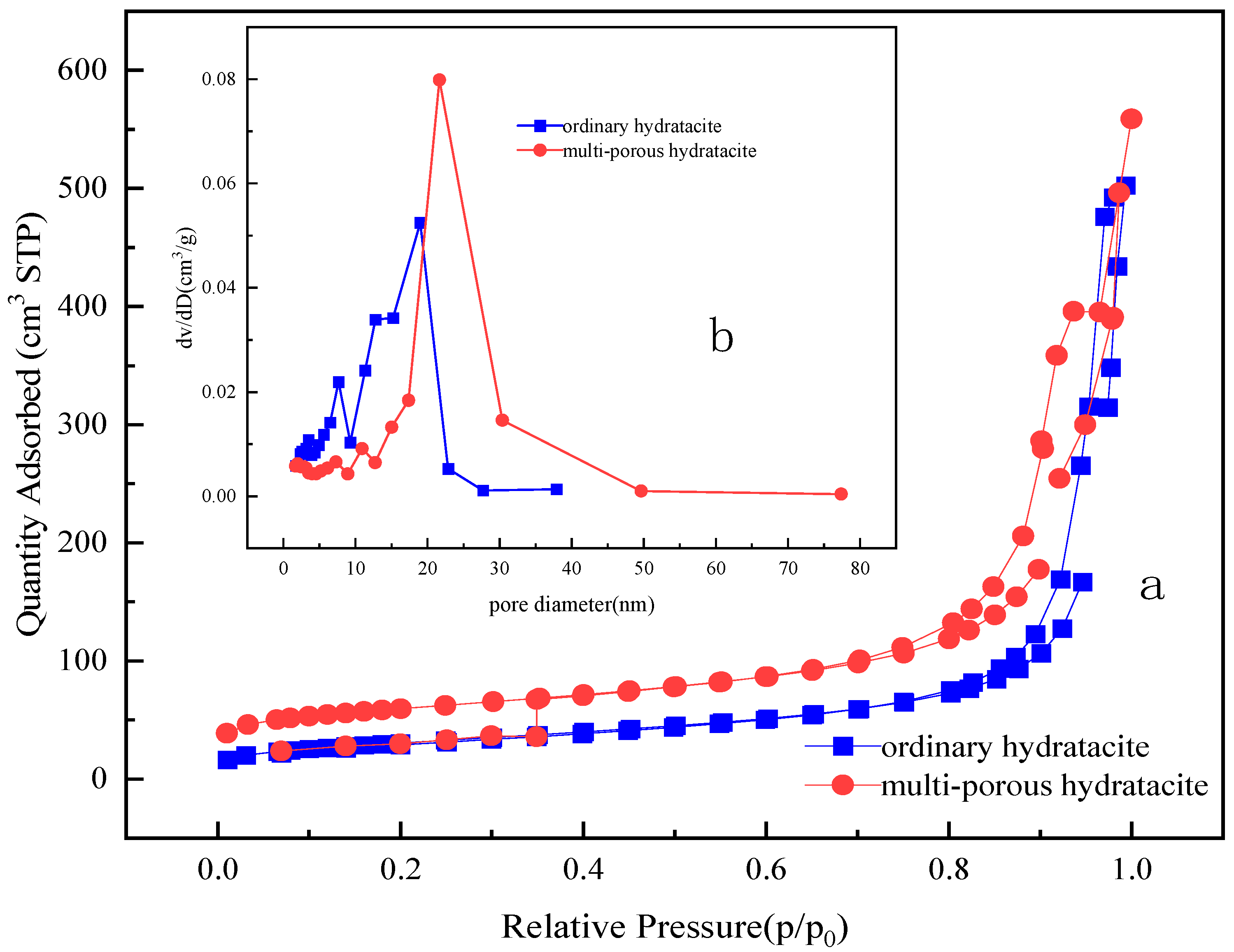


| Sample | Specific Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| ordinary hydrotalcite | 110.23 | 0.63 | 28.2 |
| multi-porous hydrotalcite | 199.82 | 0.78 | 12.7 |
| Kinetic Model | Parameter | Concentration (mg/L) | |
|---|---|---|---|
| 100 | 200 | ||
| pseudo-first-order | qe (mg/g) model | 29.37 | 156.02 |
| K1 (h−1) | 0.037 | 0.044 | |
| R2 | 0.85 | 0.87 | |
| pseudo-second-order | qe (mg/g) model | 66.67 | 102.66 |
| qe (mg/g) experiment | 66.02 | 92.95 | |
| K2 (g/m gh) | 0.0029 | 0.00036 | |
| R2 | 0.99 | 0.99 | |
| Intraparticle diffusion model | Ki1 (mg/gh1/2) | 9.42 | 10.04 |
| R12 | 0.70 | 0.92 | |
| Ki2 (mg/gh1/2) | 0.70 | 1.23 | |
| R22 | 0.89 | 0.34 | |
| Liquid film diffusion model | Kfd (h−1) | 0.037 | 0.044 |
| R2 | 0.85 | 0.87 | |
| T (K) | ∆S [J/(mol·k)] | ∆H [kJ/mol] | ∆G (kJ/mol) | R2 |
|---|---|---|---|---|
| 298.15 | −43.53 | −20.09 | −7.11 | 0.992 |
| 303.15 | −6.89 | |||
| 313.15 | −6.29 |
| Isothermal Adsorption Model | Parameter | Temperature | |
|---|---|---|---|
| 298.15 K | 303.15 K | ||
| Langmuir | qm (mg/g) | 73.48 | 70.32 |
| b (mg/L) | 3333.33 | 2777.78 | |
| R2 | 0.99 | 0.99 | |
| Freundlich | Kf (L/g) | 33,884.42 | 40,738.03 |
| n | −18.18 | −13.33 | |
| R2 | 0.96 | 0.48 | |
| D-R | qm (mg/g) | 403.97 | 396.47 |
| β (mol2/kJ2) | 0.42 | 0.21 | |
| R2 | 0.89 | 0.29 | |
| E (kJ/mol) | 1.09 | 1.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Li, Z.; Yang, C.; Wu, Y.; Tang, Y. Synthesis of Porous Materials on Hybrid Wormlike Micelles of Zwitterionic and Anionic Surfactants for Efficient Oilfield Wastewater Treatment. Gels 2025, 11, 714. https://doi.org/10.3390/gels11090714
Liu F, Li Z, Yang C, Wu Y, Tang Y. Synthesis of Porous Materials on Hybrid Wormlike Micelles of Zwitterionic and Anionic Surfactants for Efficient Oilfield Wastewater Treatment. Gels. 2025; 11(9):714. https://doi.org/10.3390/gels11090714
Chicago/Turabian StyleLiu, Fei, Zhenzhen Li, Chenye Yang, Ya Wu, and Ying Tang. 2025. "Synthesis of Porous Materials on Hybrid Wormlike Micelles of Zwitterionic and Anionic Surfactants for Efficient Oilfield Wastewater Treatment" Gels 11, no. 9: 714. https://doi.org/10.3390/gels11090714
APA StyleLiu, F., Li, Z., Yang, C., Wu, Y., & Tang, Y. (2025). Synthesis of Porous Materials on Hybrid Wormlike Micelles of Zwitterionic and Anionic Surfactants for Efficient Oilfield Wastewater Treatment. Gels, 11(9), 714. https://doi.org/10.3390/gels11090714





