Comparative Analysis of Chemical Activators and Expansive Agents for Aeolian Sand Stabilization Using Industrial Solid Waste-Based Geopolymers
Abstract
1. Introduction
2. Results and Discussion
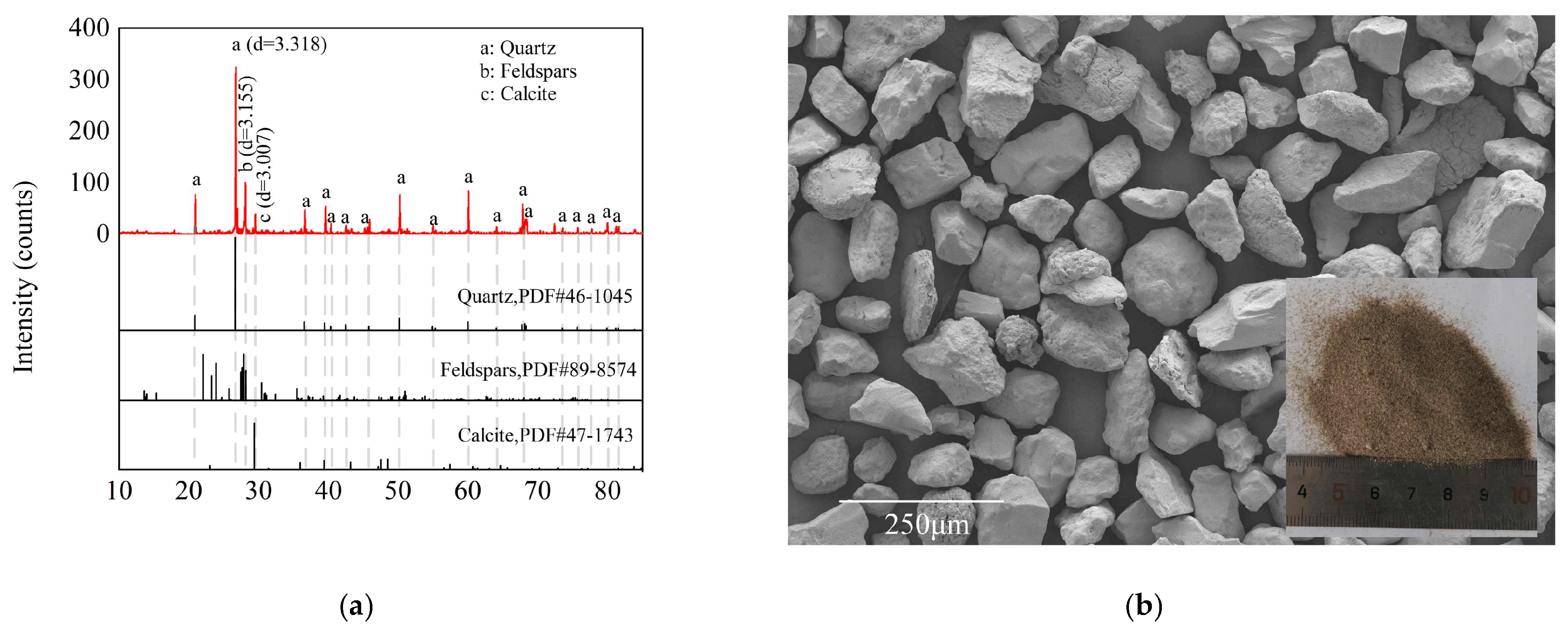
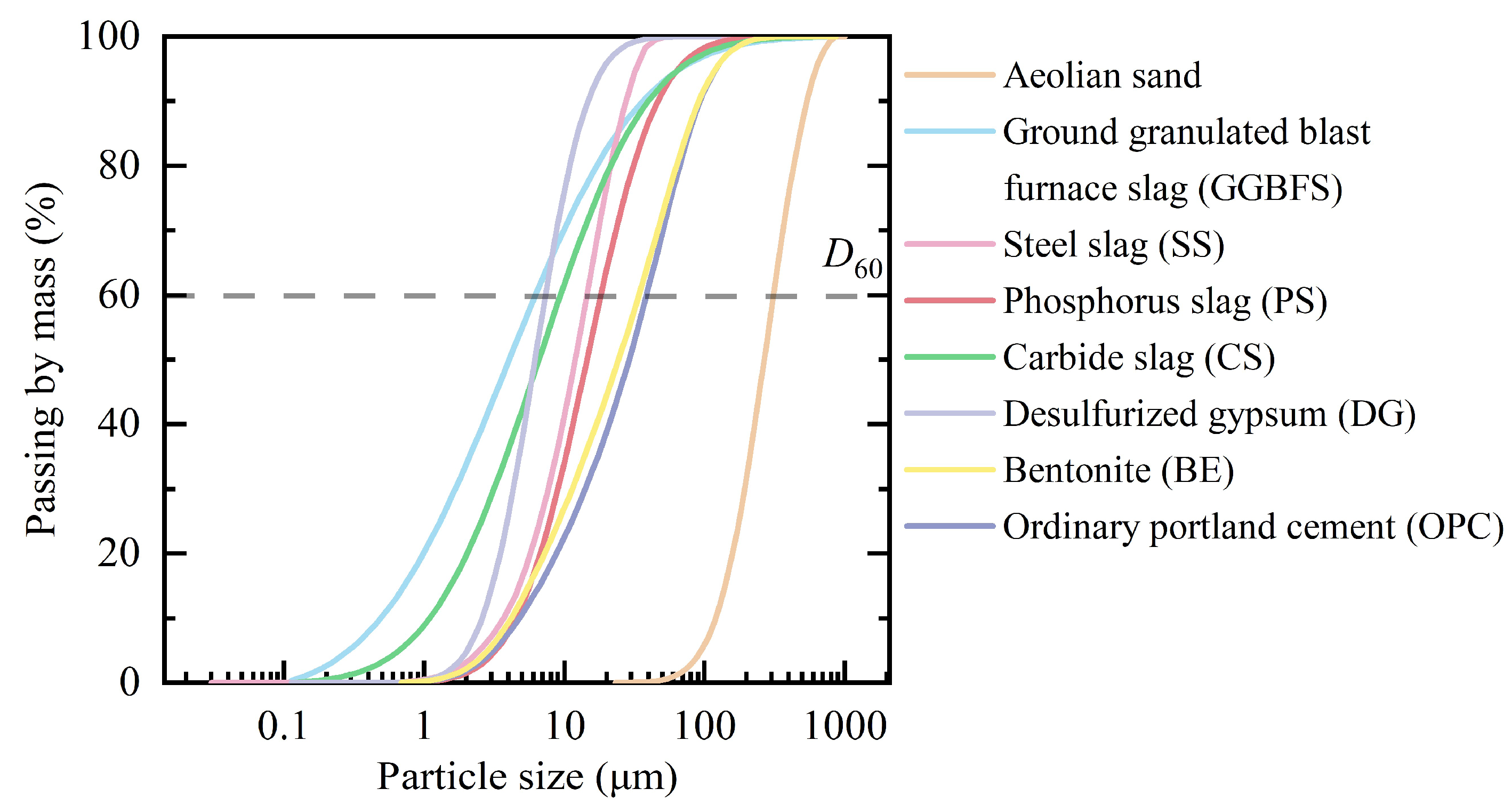
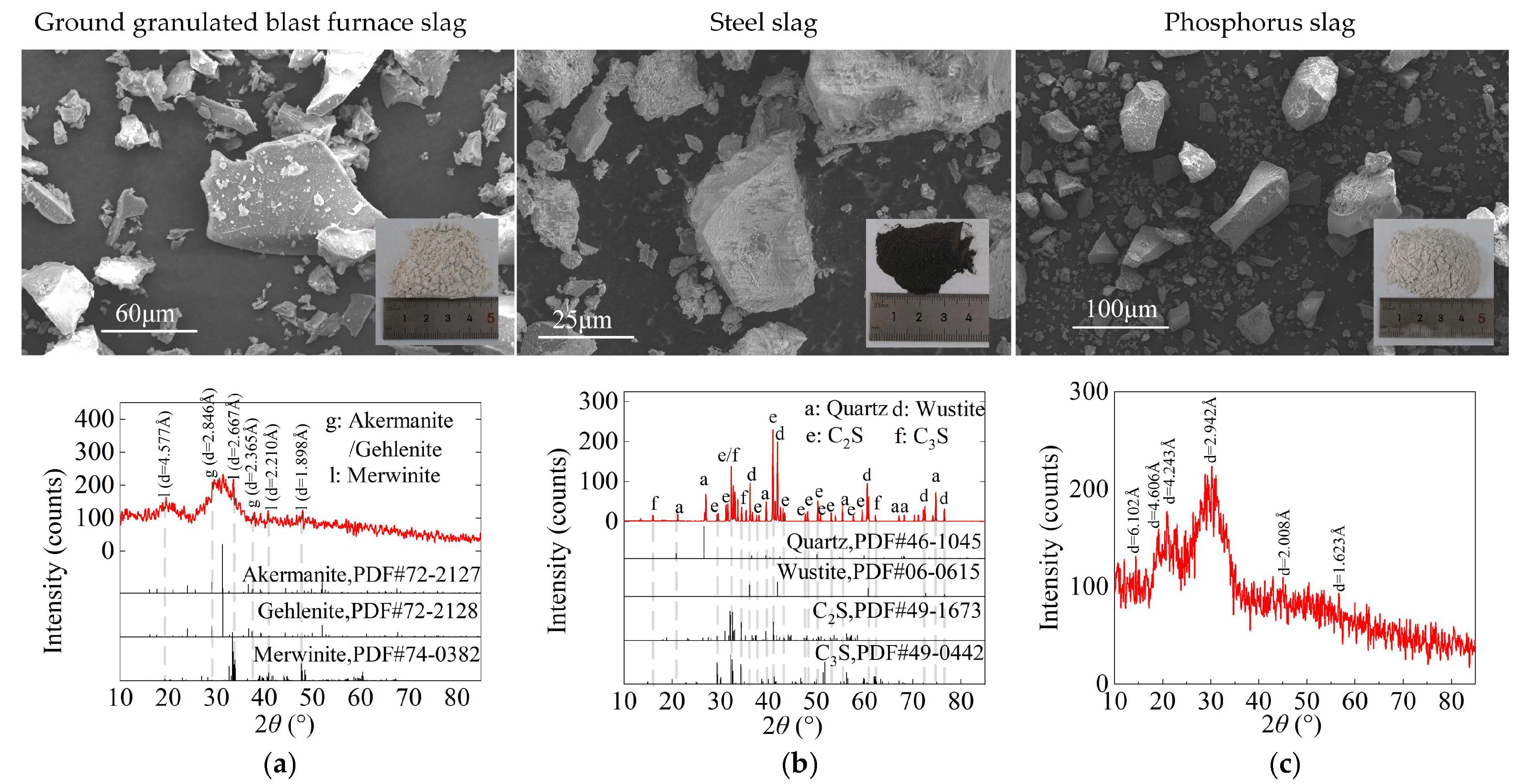
2.1. Results of Material Characterization
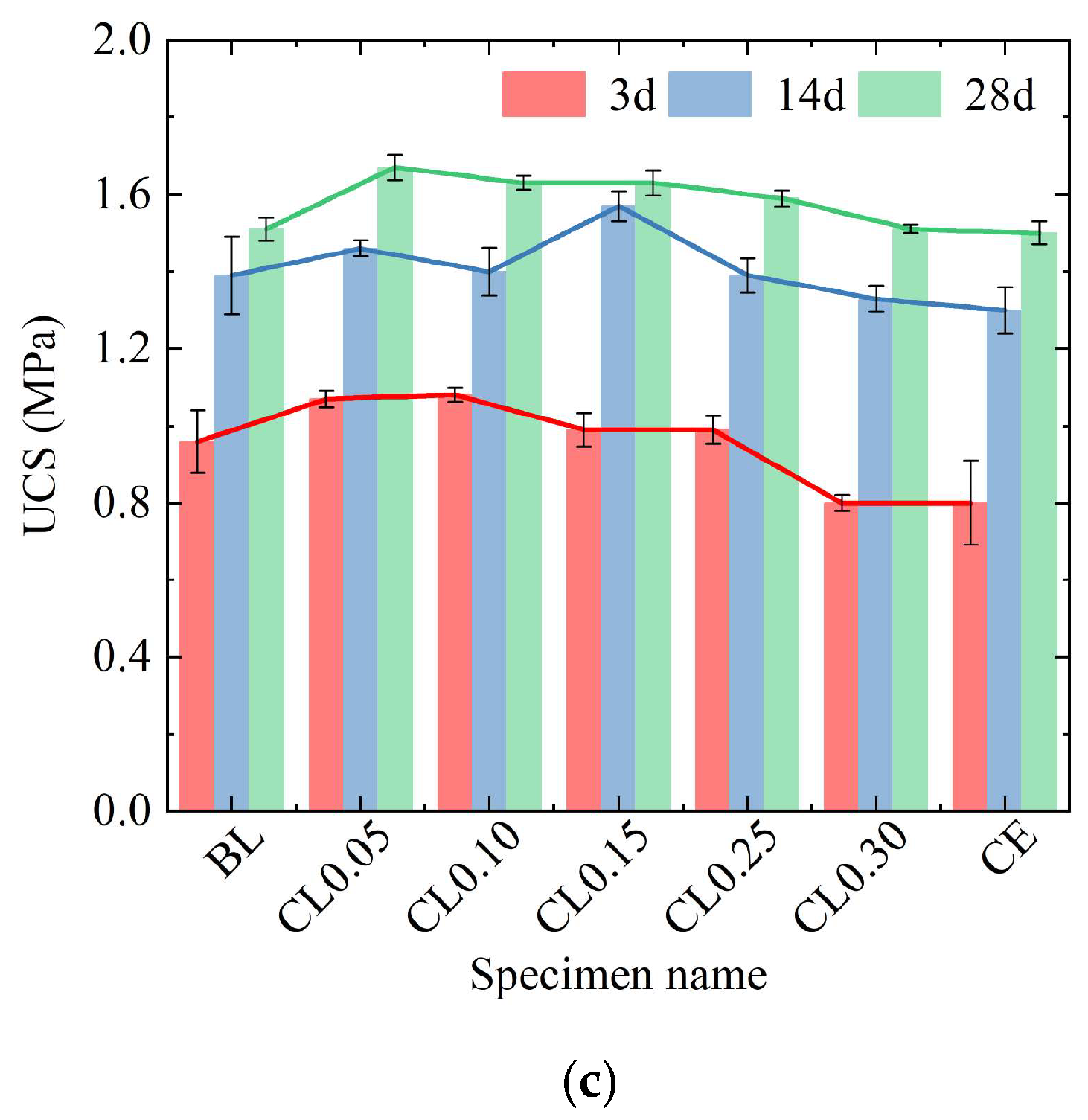
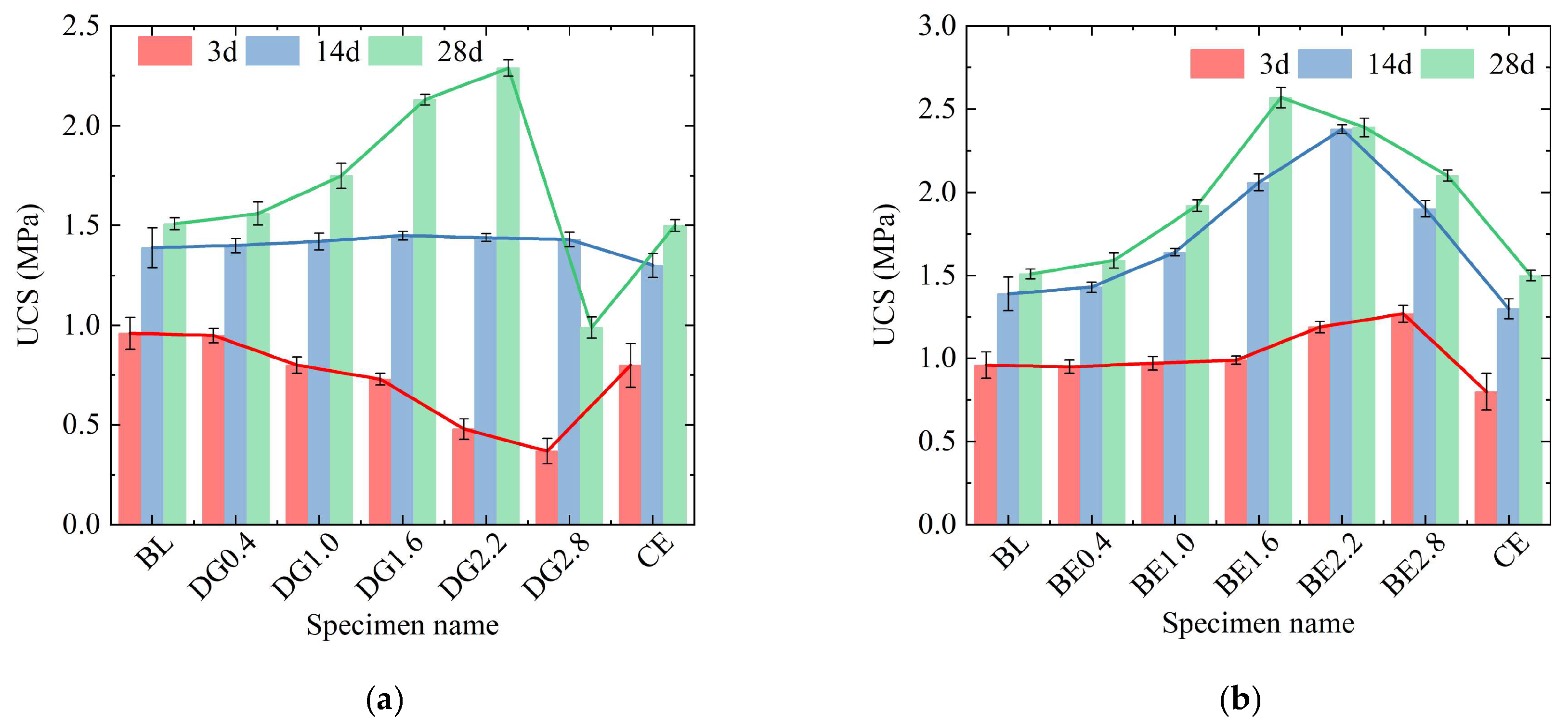
2.2. Effect of Additives on UCS of ASIG
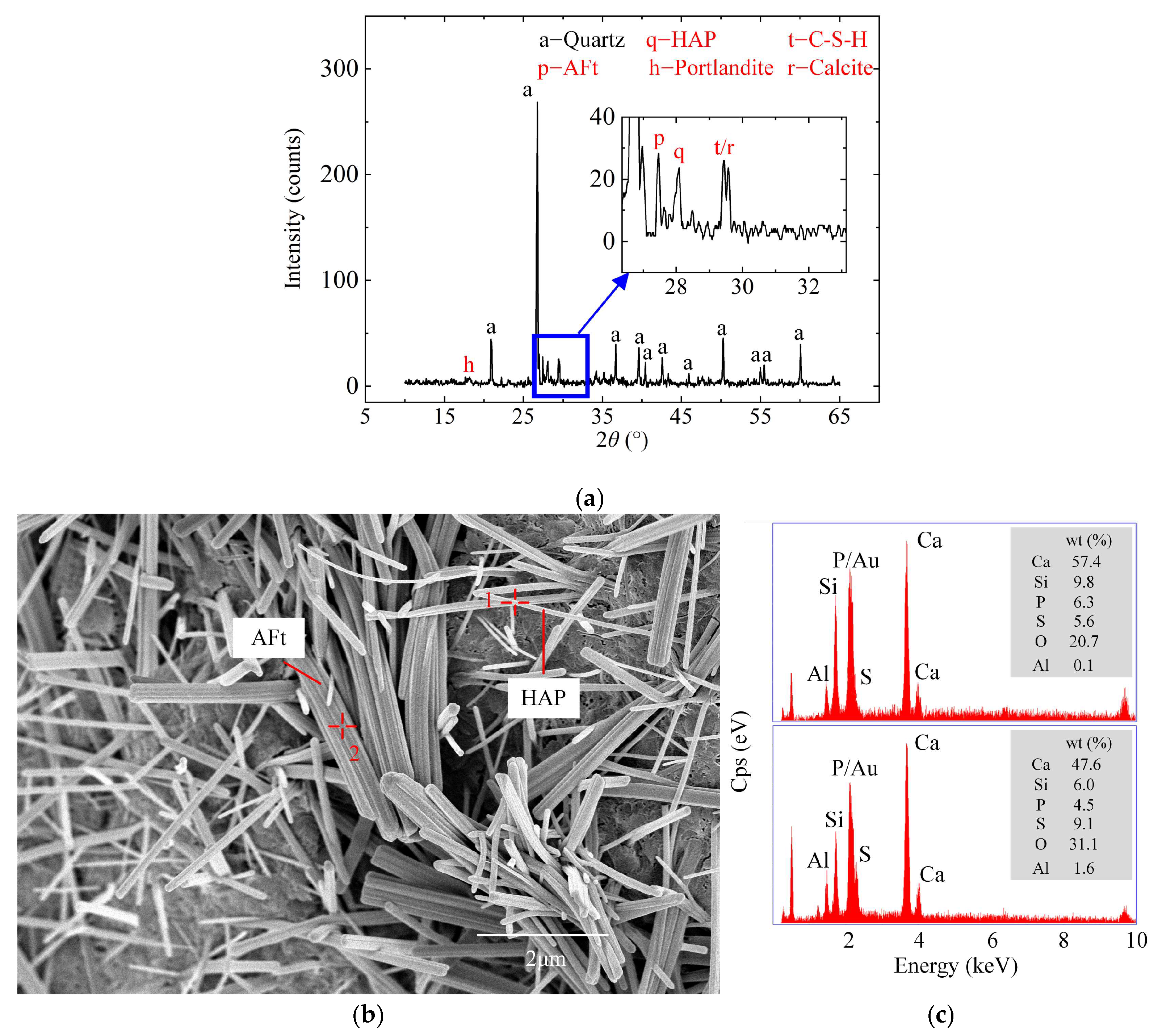
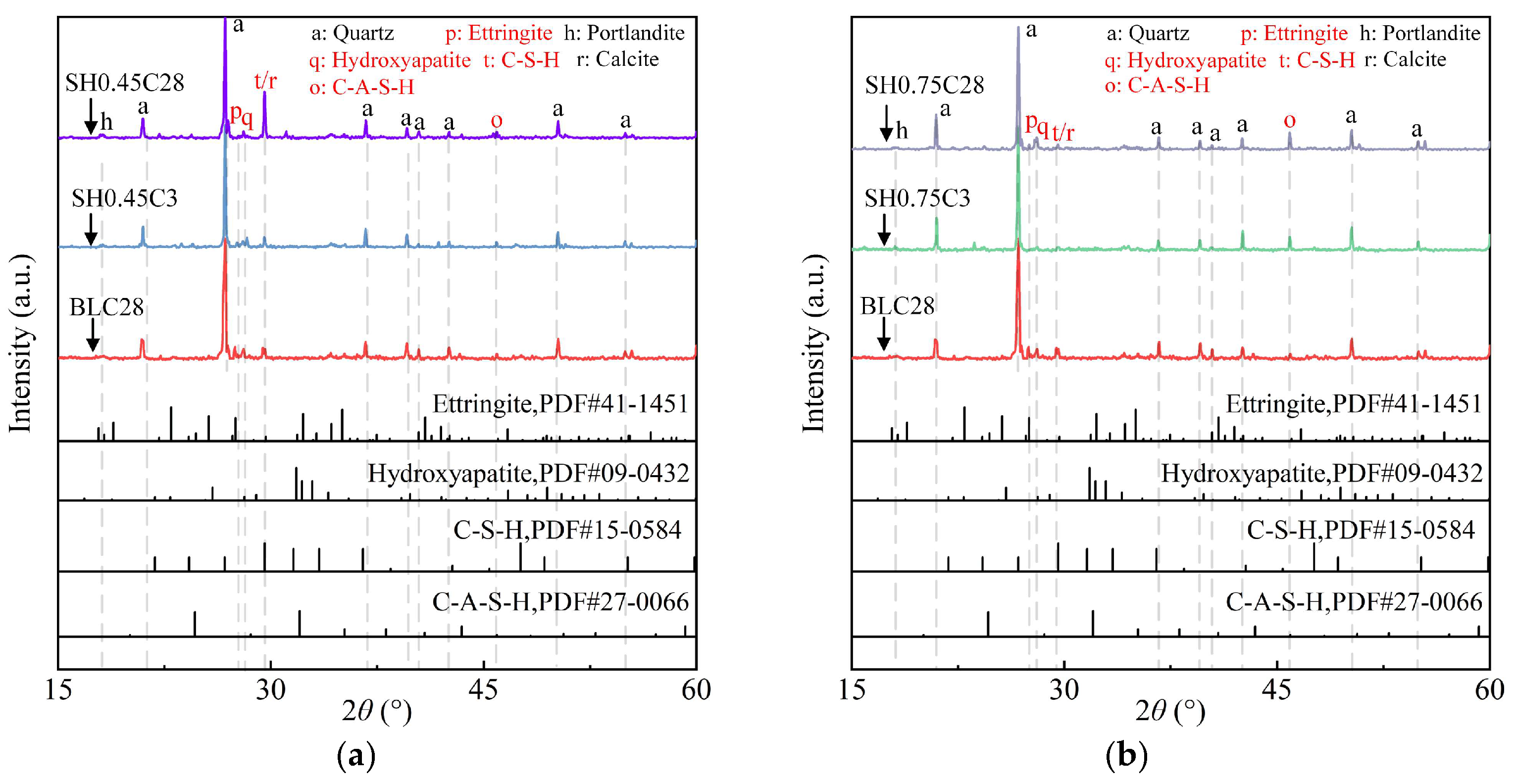
2.3. Evolution of Reaction Products
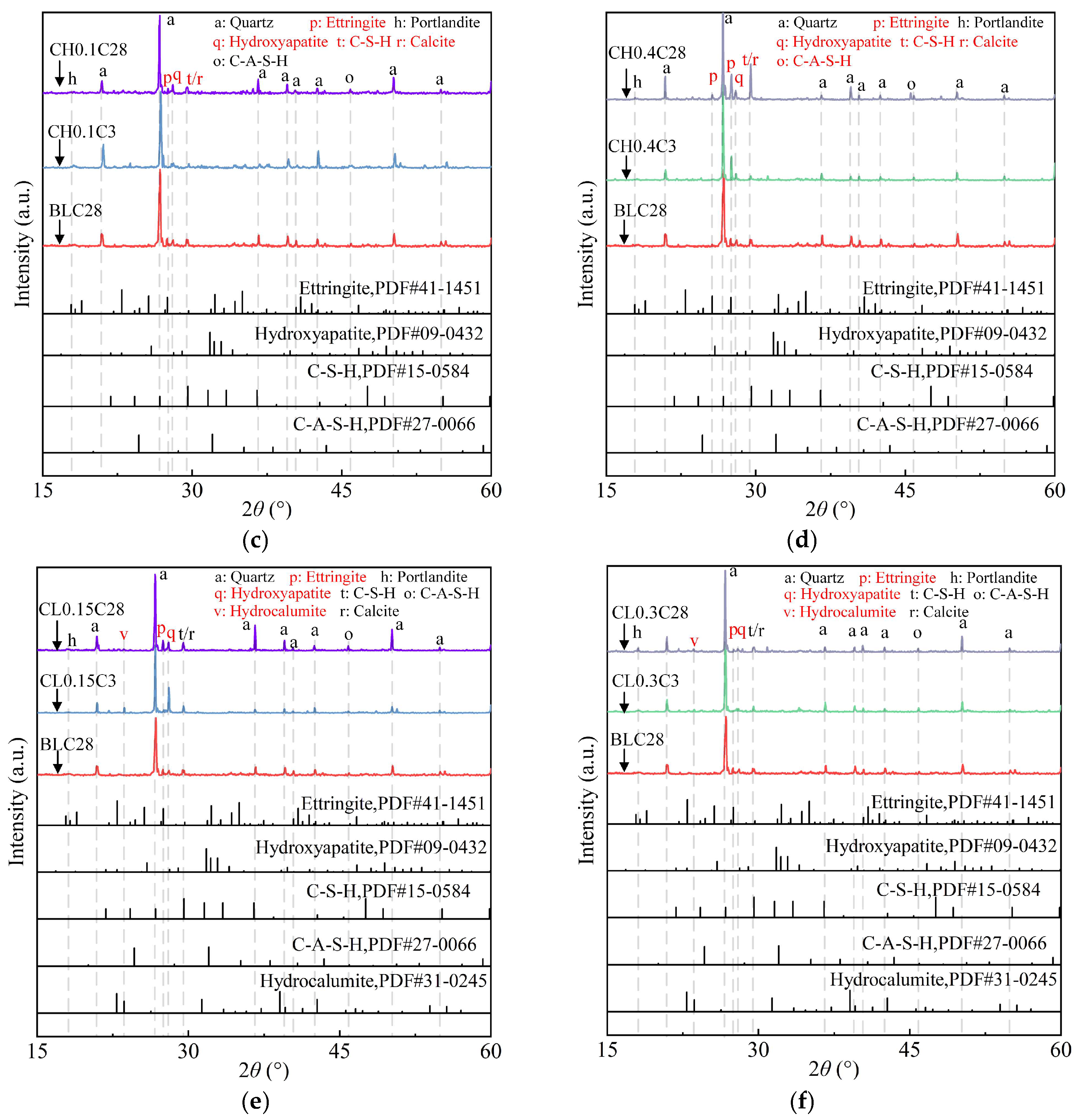
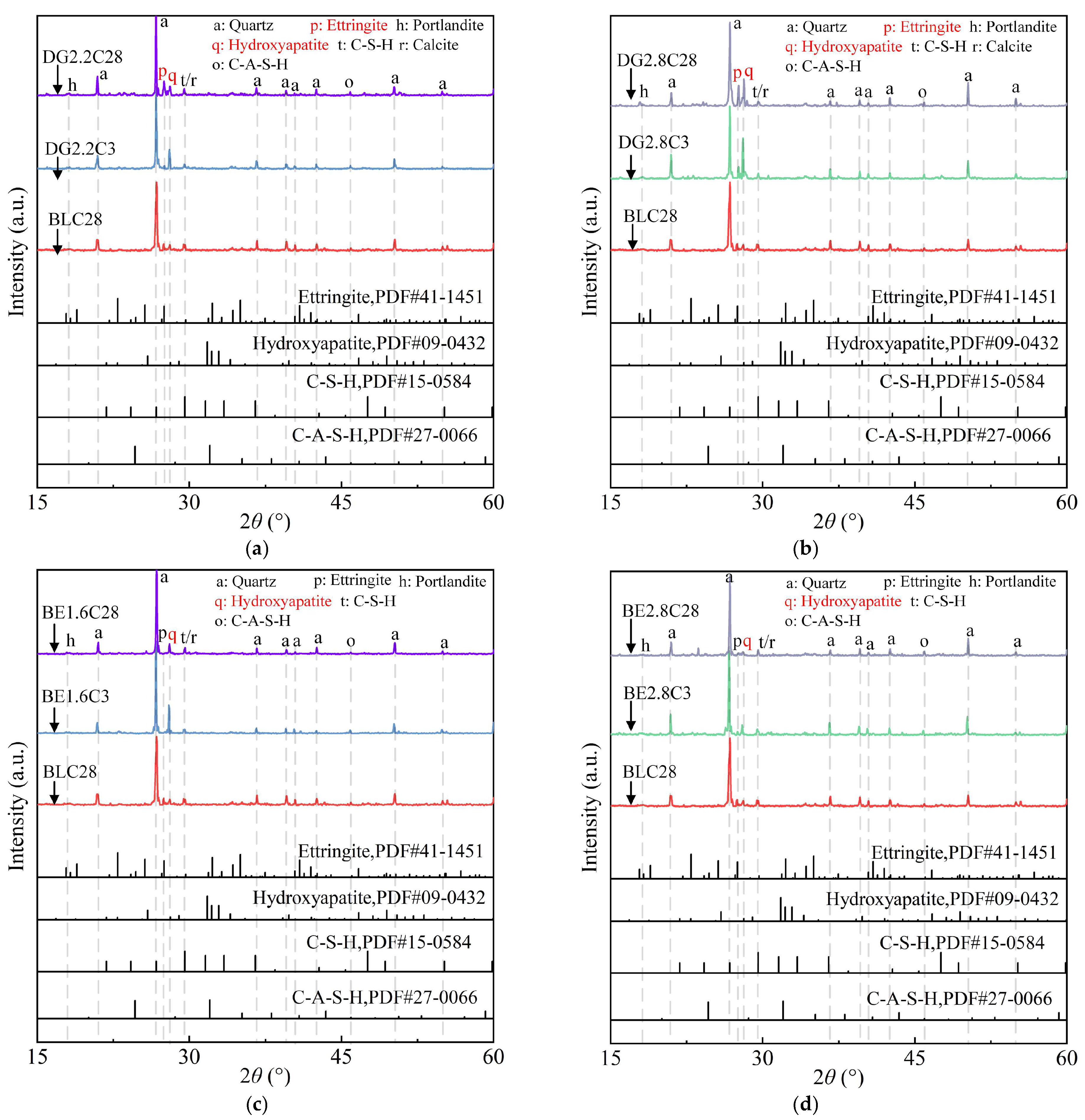
2.3.1. XRD Pattern
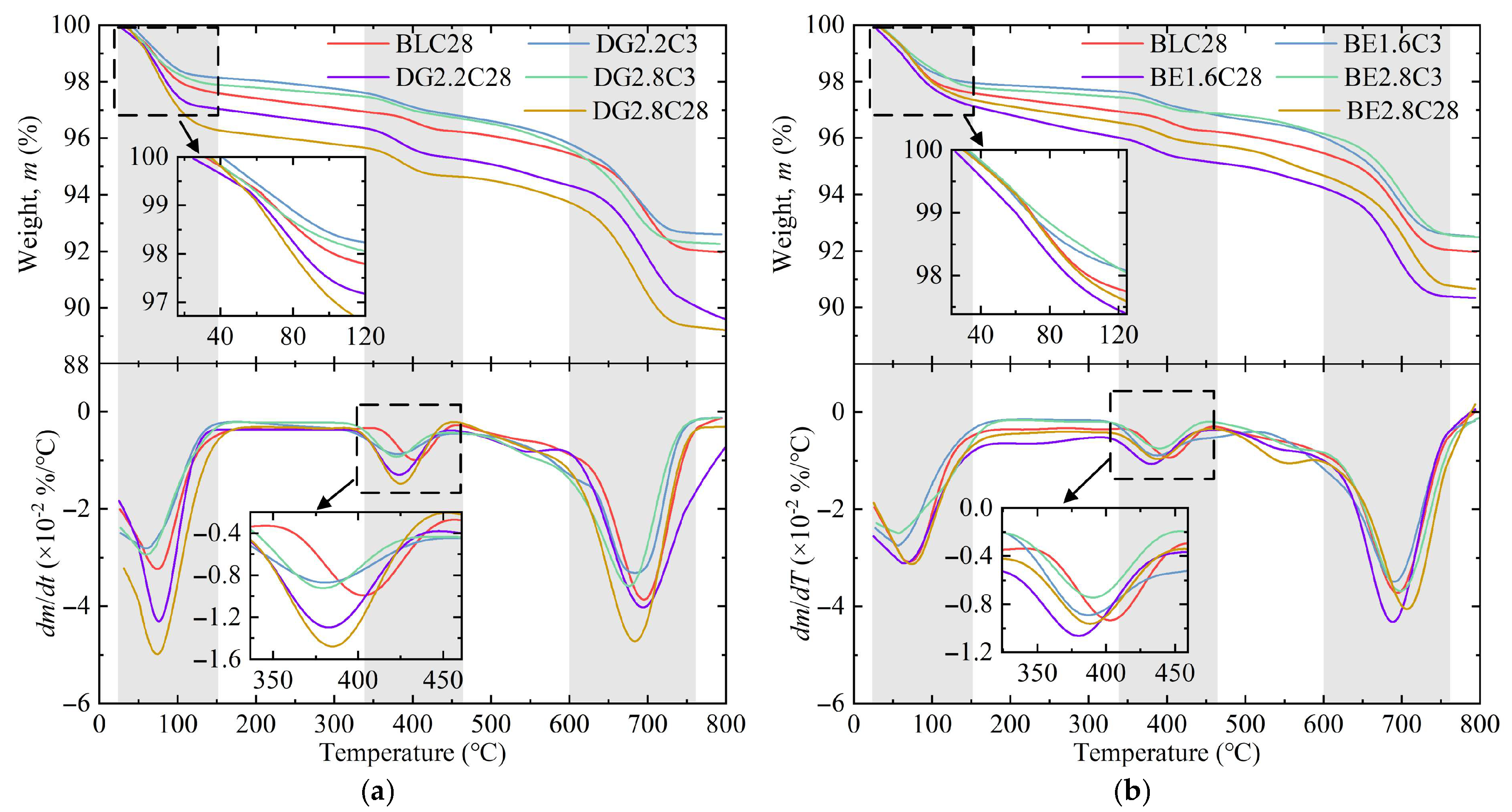
2.3.2. TG/DTG
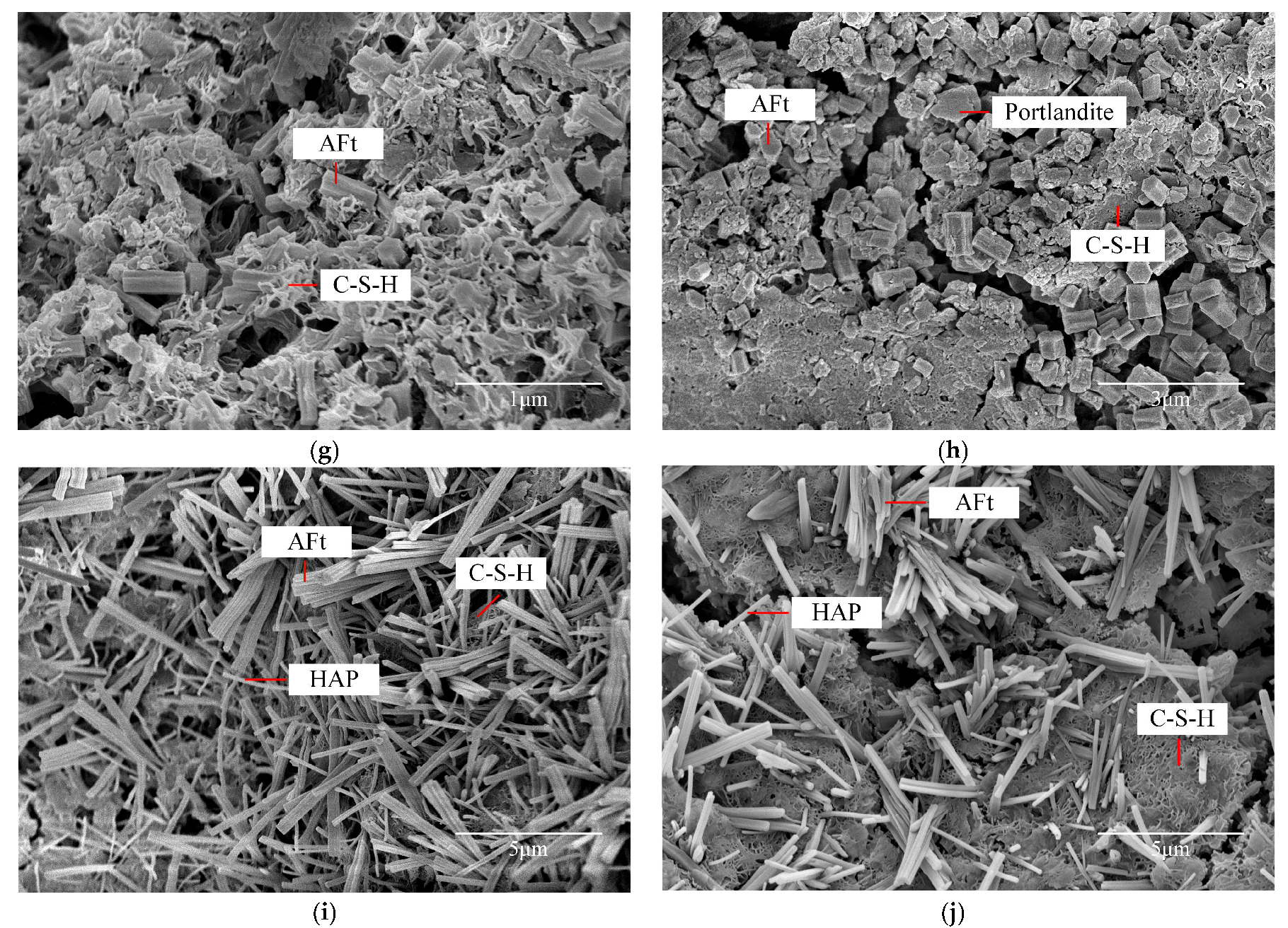
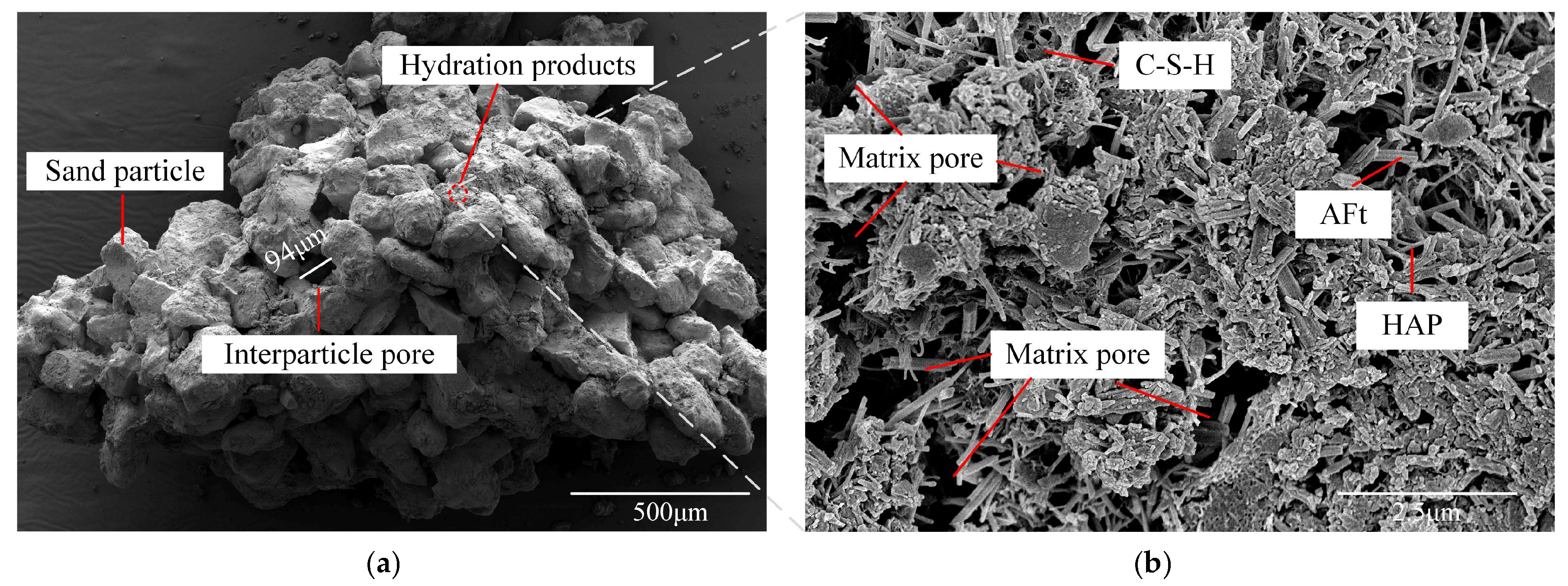
2.4. Characteristic of Microscopic Morphology and Structure
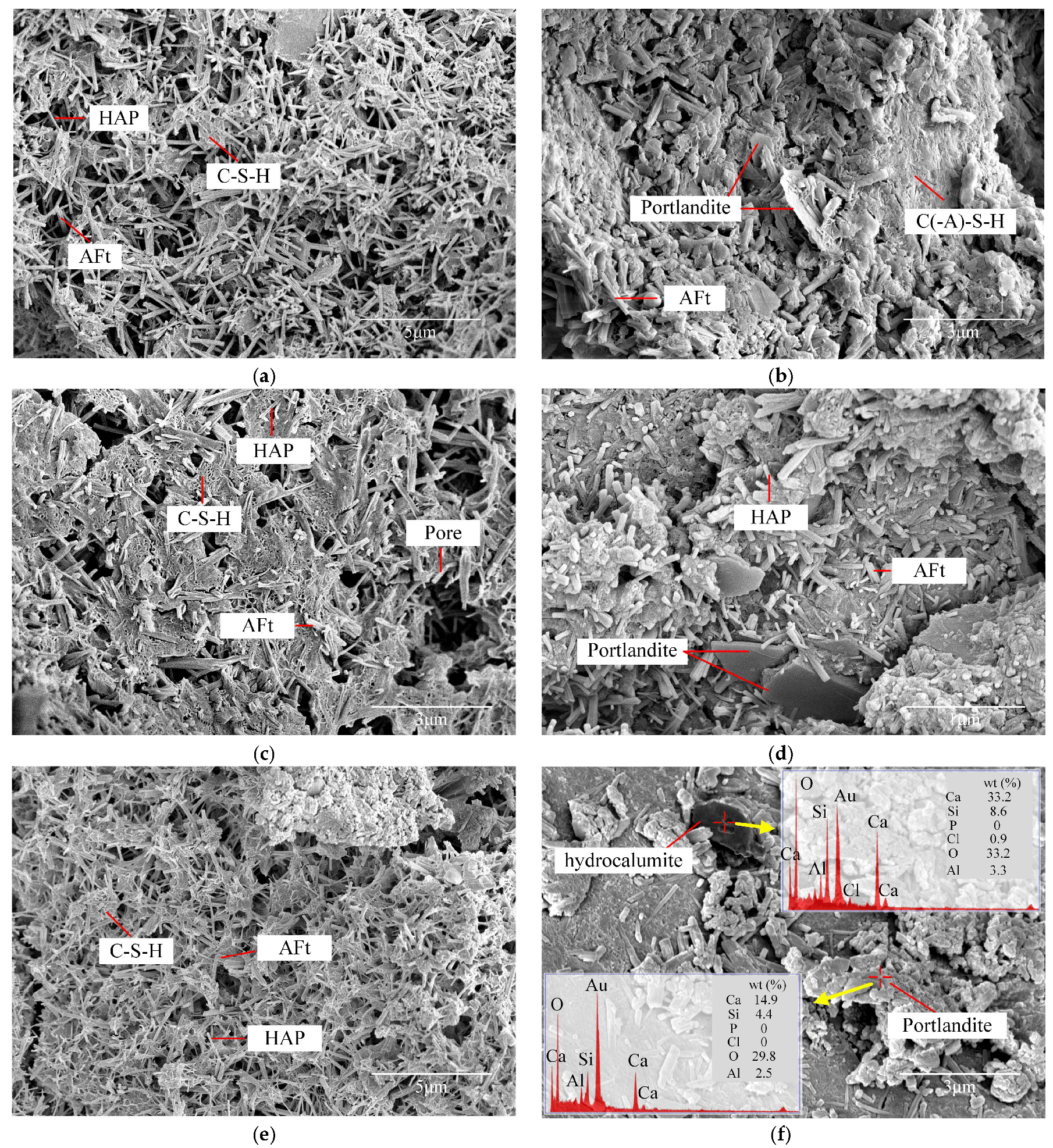
2.4.1. Morphology of Hydration Products
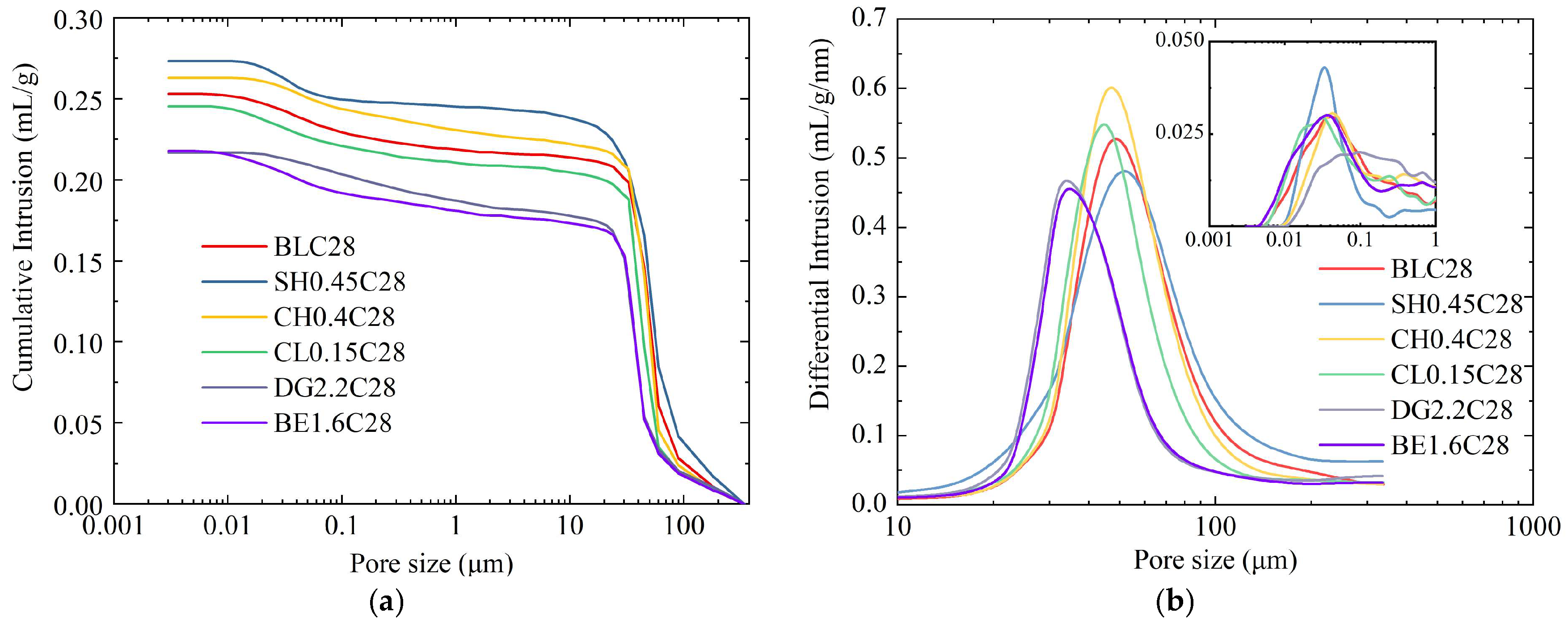
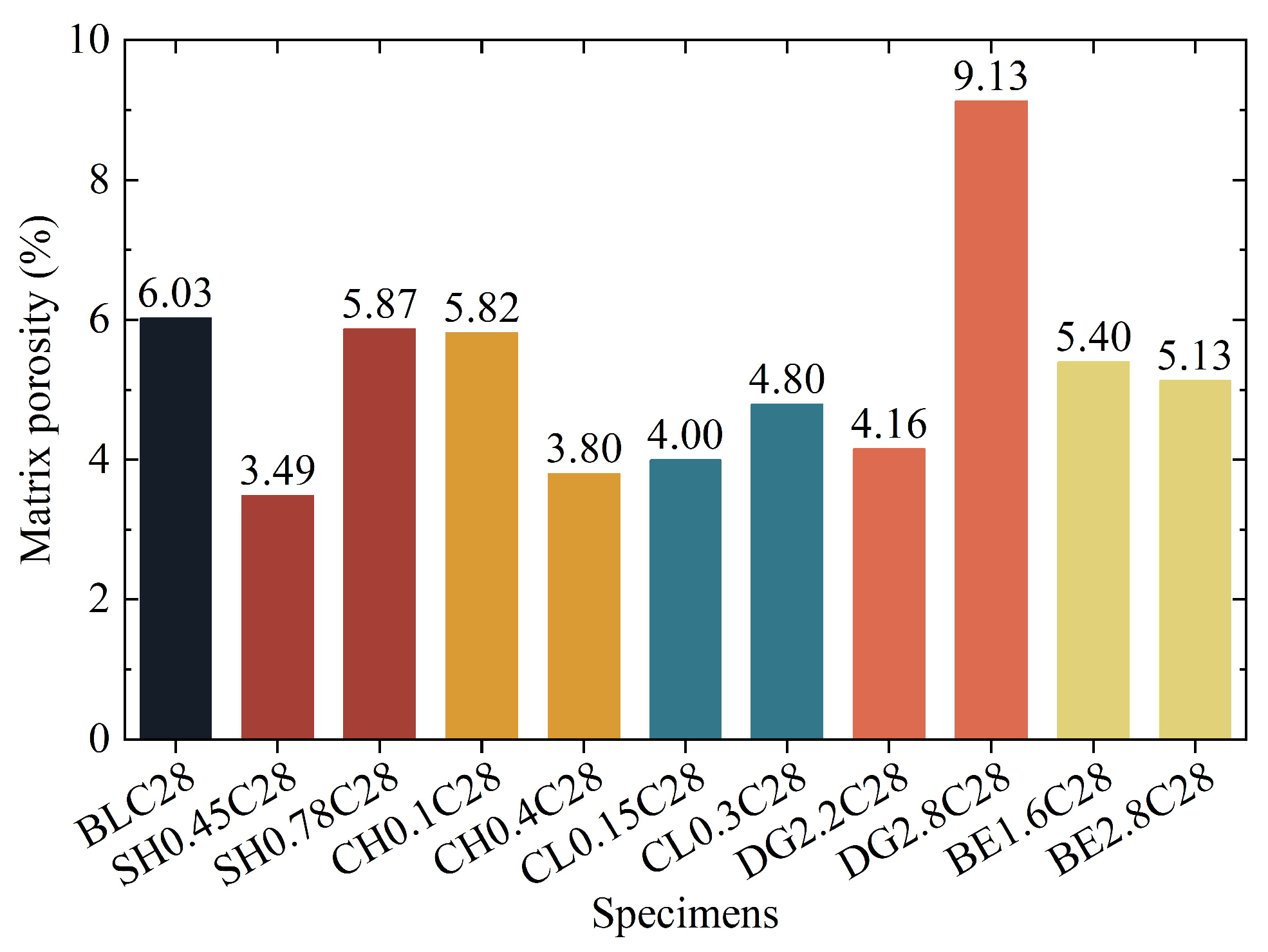
2.4.2. Microstructures and Pore
2.5. Enhancement Mechanism of Chemical Activators
2.6. Enhancement Mechanism of Expensive Agents
2.7. Limits and Future Perspectives
3. Conclusions
- In terms of strength enhancement, expansive agent including BE and DG proved significantly more effective than traditional alkaline activators (NaOH, Ca(OH)2, CaCl2) in improving the UCS of aeolian sand-based geopolymer. While chemical activators enhanced 28-day strength by approximately 10–15% at optimal concentrations, DG and BE led to increases of 52.6% and 71.3%, respectively, primarily by effectively filling interparticle pores. However, excessive dosage of all additives ultimately leads to a reduction in strength.
- Regarding the strengthening mechanism, the granular and low-water-content nature of aeolian sand necessitates reliance on physical pore filling rather than interparticle bonding. Chemical activators promoted gel and crystalline phase formation (e.g., AFt, HAP), but excessive use coarsened the pore structure or consumed reactive components. In contrast, DG facilitated dense AFt formation and morphology refinement, while BE compacted the matrix through expansion—both effectively reducing harmful pores and optimizing pore size distribution.
- The findings of this study highlight that employing expansion-based additives offers a more effective strategy for aeolian sand stabilization than reliance on chemical activation alone. BE compensates for the insufficient pore-filling capability of gels, resulting in a denser microstructure and higher ultimate strength, thereby providing a new material selection and methodological direction for stabilization of aeolian sand.
4. Materials and Methods
4.1. Materials
4.2. Specimens Design
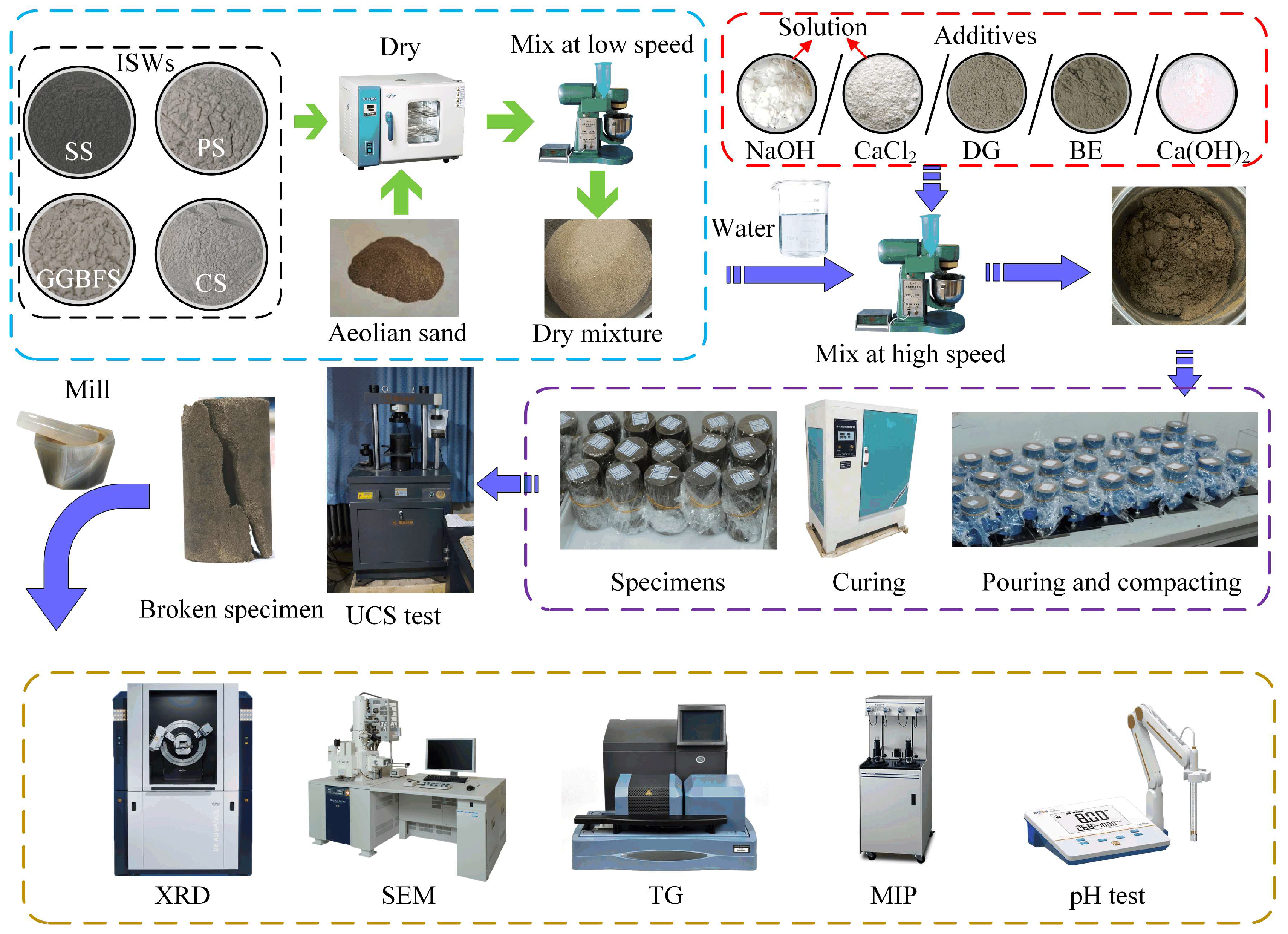
4.3. Specimens Preparation
4.4. Test Method
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
SEM Images Utilized for Pore Identification and Corresponding Results
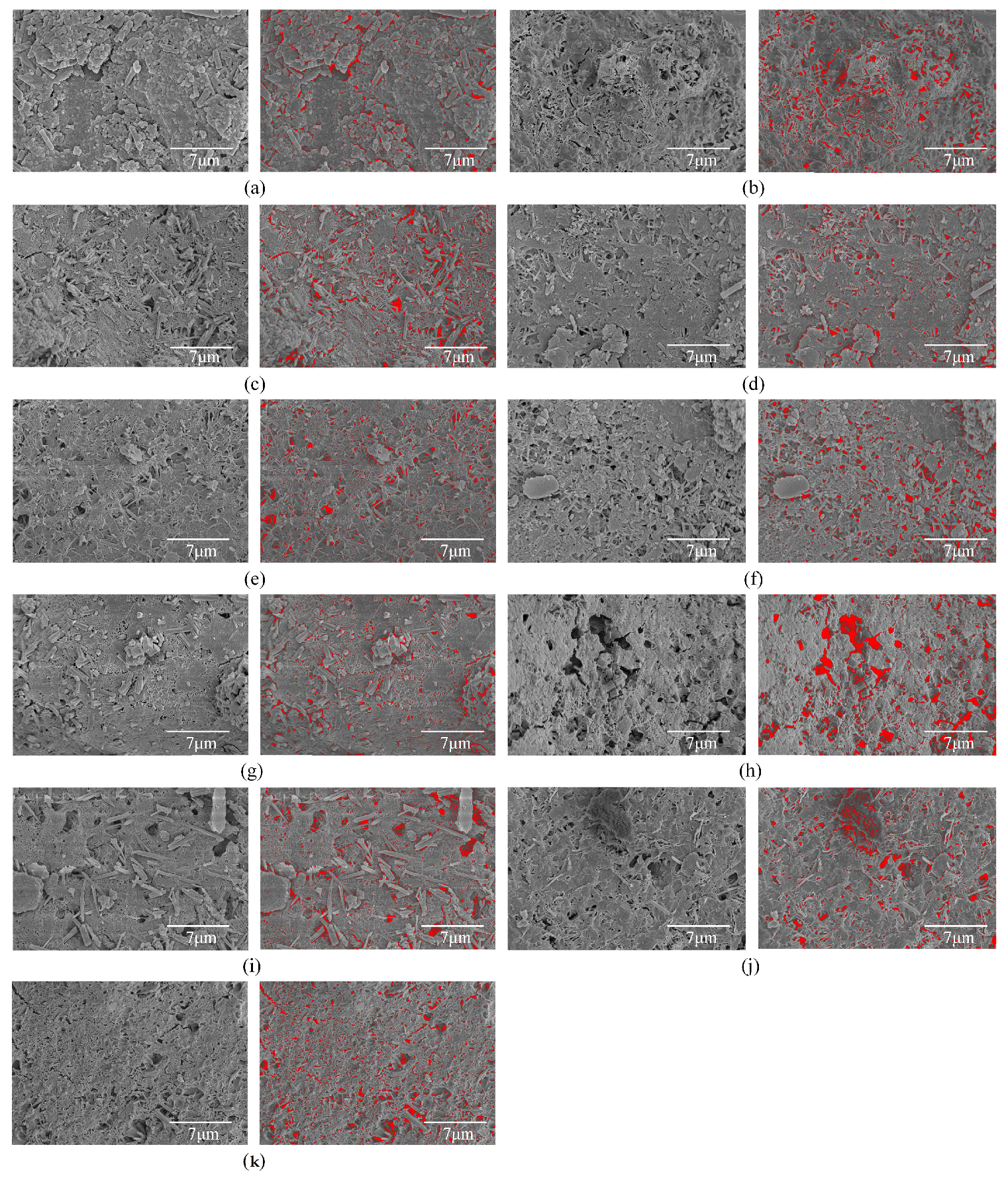
References
- Chen, Y.; Lu, H.Y.; Wu, H.J.; Wang, J.J.; Lyu, N. Global desert variation under climatic impact during 1982–2020. Sci. China-Earth Sci. 2023, 66, 1062–1071. [Google Scholar] [CrossRef]
- Rodenbiker, J. Green silk roads, partner state development, and environmental governance: Belt and road infrastructure on the Sino-East African frontier. Crit. Asian Stud. 2023, 55, 169–192. [Google Scholar] [CrossRef]
- Sayed, O.H.; Masrahi, Y.S. Climatology and phytogeography of Saudi Arabia. A review. Arid. Land Res. Manag. 2023, 37, 311–368. [Google Scholar] [CrossRef]
- Elipe, M.G.M.; López-Querol, S. Aeolian sands: Characterization, options of improvement and possible employment in construction—The State-of-the-art. Constr. Build. Mater. 2014, 73, 728–739. [Google Scholar] [CrossRef]
- Tian, K.L.; Wang, X.D.; Zhang, S.C.; Zhang, H.L.; Zhang, F.; Yang, A.Q. Effect of Reactant Injection Rate on Solidifying Aeolian Sand via Microbially Induced Calcite Precipitation. J. Mater. Civ. Eng. 2020, 32, 04020291. [Google Scholar] [CrossRef]
- Yin, Y.Z.; Wang, Y.L. Determine the Shear Strength of Aeolian Sand and Bearing Capacity. In Proceedings of the 4th International Conference on Civil Engineering, Architecture and Building Materials (CEABM), Haikou, China, 24–25 May 2014; pp. 165–168. [Google Scholar]
- Liang, C.; Xing, Y.M.; Hou, X.H. Multi-scale study on mechanical performance optimization mechanisms of aeolian sand cement-based materials incorporating discarded mask fibres and metakaolin. Constr. Build. Mater. 2025, 470, 140653. [Google Scholar] [CrossRef]
- Liu, W.Z.; Huang, X.J.; Yin, W.H.; Liu, G.Y. Static and dynamic characteristics of cement-treated and untreated aeolian sand from the Tengger desert hinterland: Laboratory tests and prediction models. Constr. Build. Mater. 2025, 458, 139733. [Google Scholar] [CrossRef]
- Ren, Z.Q.; Dong, W.; Dong, X.R. Analysis of fractal and erosion characteristics of aeolian sand concrete pore structure under capillary absorption. J. Build. Eng. 2025, 104, 112258. [Google Scholar] [CrossRef]
- Xie, D.T.; Liu, H.D.; Liang, X. Effect of curing regimes on mechanical properties and microstructure of ultra-high performance concrete with full aeolian sand. Constr. Build. Mater. 2025, 472, 140812. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, T.Y.; Guo, T.; Ding, W. Durability of Basalt fiber-reinforced aeolian sand concrete in extreme environments: Resistance to wind-sand erosion and salt freeze-thaw cycles. Constr. Build. Mater. 2024, 457, 139264. [Google Scholar] [CrossRef]
- Fathali, M.; Nasrabad, M.M.K.; Abbasi, H.R.; Amrollahi, A.; Soleymani, M. Aeolian sand challenges in desert rail infrastructures, overview of Iran’s experience and advancement. Constr. Build. Mater. 2024, 438, 136953. [Google Scholar] [CrossRef]
- He, Y.; Yuan, X.Z. Experimental Study on Solidifying Aeolian Sand Filled in the Narrow Space of Distress Culverts. Arab. J. Sci. Eng. 2020, 45, 3559–3568. [Google Scholar] [CrossRef]
- Yang, H.; Qian, Z.Z.; Yue, B.; Xie, Z.L. Effects of Cement Dosage, Curing Time, and Water Dosage on the Strength of Cement-Stabilized Aeolian Sand Based on Macroscopic and Microscopic Tests. Materials 2024, 17, 3946. [Google Scholar] [CrossRef]
- Ostovari, H.; Müller, L.; Skocek, J.; Bardow, A. From Unavoidable CO2 Source to CO2 Sink? A Cement Industry Based on CO2 Mineralization. Environ. Sci. Technol. 2021, 55, 5212–5223. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, P.Y.; Dong, W.T.; Sun, Q.; Yao, G.; Lv, R.C. Research on the preparation and properties of GBFS-based mud solidification materials. Constr. Build. Mater. 2024, 423, 135900. [Google Scholar] [CrossRef]
- Mahvash, S.; Lopez-Querol, S.; Bahadori-Jahromi, A. Effect of class F fly ash on fine sand compaction through soil stabilization. Heliyon 2017, 3, e00274. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, H.; Aziz, T.; Farooq, K.; Sivakugan, N.; Das, B.M. Improvement in Engineering Properties of Expansive Soils using Ground Granulated Blast Furnace Slag. J. Geol. Soc. India 2018, 92, 357–362. [Google Scholar] [CrossRef]
- Wang, J.Z.; Chen, G.; Chen, Y.H.; Ye, Z.; Lin, M.G.; Su, R.B.; Hu, N. Intelligent mixture optimization for stabilized soil containing solid waste based on machine learning and evolutionary algorithms. Constr. Build. Mater. 2024, 445, 137794. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, J.J.; Chang, R.Q. The design of ternary all-solid-waste binder for solidified soil and the mechanical properties, mechanism and environmental benefits of CGF solidified soil. J. Clean. Prod. 2023, 429, 139439. [Google Scholar] [CrossRef]
- Li, G.P.; Liu, P.Y.; Chao, S.; Zhang, X.; Li, J.G.; Zhang, Y.L.; Duan, Y.M. The mineral phase evolution characteristics and hydration activity enhancement mechanism of steel slag under NaOH alkaline excitation. J. Alloys Compd. 2024, 978, 173524. [Google Scholar] [CrossRef]
- Zhang, S.F.; Niu, D.T. Hydration and mechanical properties of cement-steel slag system incorporating different activators. Constr. Build. Mater. 2023, 363, 129981. [Google Scholar] [CrossRef]
- Bahadori, H.; Hasheminezhad, A.; Alizadeh, S. The Influence of Natural Pozzolans Structure on Marl Soil Stabilization. Transp. Infrastruct. Geotechnol. 2020, 7, 46–54. [Google Scholar] [CrossRef]
- Behnood, A. Soil and clay stabilization with calcium- and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Hanjitsuwan, S.; Li, L.Y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Adhesion characterisation of Portland cement concrete and alkali-activated binders. Adv. Cem. Res. 2019, 31, 69–79. [Google Scholar] [CrossRef]
- Wu, J.; Deng, Y.F.; Zhang, G.P.; Zhou, A.N.; Tan, Y.Z.; Xiao, H.L.; Zheng, Q.S. A Generic Framework of Unifying Industrial By-products for Soil Stabilization. J. Clean. Prod. 2021, 321, 128920. [Google Scholar] [CrossRef]
- Balczár, I.; Korim, T.; Hullár, H.; Boros, A.; Makó, É. Manufacture of air-cooled slag-based alkali-activated cements using mechanochemical activation. Constr. Build. Mater. 2017, 137, 216–223. [Google Scholar] [CrossRef]
- Wu, H.X.; Xu, J.G.; Yang, D.Y.; Ma, Z.M. Utilizing thermal activation treatment to improve the properties of waste cementitious powder and its newmade cementitious materials. J. Clean. Prod. 2021, 322, 129074. [Google Scholar] [CrossRef]
- Mahmood, A.H.; Babaee, M.; Foster, S.J.; Castel, A. Continuous Monitoring of the Early-Age Properties of Activated GGBFS with Alkaline Solutions of Different Concentrations. J. Mater. Civ. Eng. 2021, 33, 04021374. [Google Scholar] [CrossRef]
- Muñoz-Castillo, A.; Andrés-Castro, F.; Gómez-Casero, M.A.; Eliche-Quesada, D. Olive Pomace Fly Ash as an Alternative Alkaline Activator for Electric Arc Furnace Slag for Sustainable Cementitious Materials. Materials 2025, 18, 601. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, C.H.; Yan, Y.; Yi, S.Y.; Teng, K.; Li, C.J. Study on Mechanism of Mechanical Property Enhancement of Expansive Soil by Alkali-Activated Slag. Materials 2025, 18, 800. [Google Scholar] [CrossRef]
- Guo, S.Y.; Wu, Y.L.; Jia, Z.Q.; Qi, X.Q.; Wang, W.R. Sodium-based activators in alkali- activated materials: Classification and comparison. J. Build. Eng. 2023, 70, 106397. [Google Scholar] [CrossRef]
- Xu, F.; Wei, H.; Qian, W.X.; Cai, Y.B. Composite alkaline activator on cemented soil: Multiple tests and mechanism analyses. Constr. Build. Mater. 2018, 188, 433–443. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.X.; Dong, B.Q.; Yang, X.J. Chlorine immobilization and performances of cement paste/mortar with C-S-Hs-PCE and calcium chloride. Constr. Build. Mater. 2020, 262, 120694. [Google Scholar] [CrossRef]
- Yum, W.S.; Jeong, Y.; Yoon, S.; Jeon, D.; Jun, Y.; Oh, J.E. Effects of CaCl2 on hydration and properties of lime(CaO)-activated slag/fly ash binder. Cem. Concr. Compos. 2017, 84, 111–123. [Google Scholar] [CrossRef]
- Jeon, D.; Yum, W.S.; Jeong, Y.; Oh, J.E. Properties of quicklime(CaO)-activated Class F fly ash with the use of CaCl2. Cem. Concr. Res. 2018, 111, 147–156. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, G.Z.; Ding, Q.J.; Yang, J.; Zhu, J.H.; Qi, D.P. Effect of expansive agent on lightweight micro-expansive ultra-high performance concrete: Properties, microstructure, and interfacial bonding in concrete-filled steel tube. Constr. Build. Mater. 2025, 462, 139996. [Google Scholar] [CrossRef]
- Cuevas, K.; Lopez, M. The effect of expansive agent and cooling rate in the performance of expanded glass lightweight aggregate as an internal curing agent. Constr. Build. Mater. 2021, 271, 121505. [Google Scholar] [CrossRef]
- Du, J.; Tan, X.; Wang, Y.H.; Bao, Y.; Meng, W.N. Reducing the cracking potential of ultra-high-performance concrete (UHPC) with the prewet expansive agent. Constr. Build. Mater. 2024, 431, 136597. [Google Scholar] [CrossRef]
- Liu, C.J.; Zhang, X.; Cheng, S.K.; Li, S.K.; Bao, M.; Qiao, L.G. Mechanical properties, hydration behavior and shrinkage deformation of ultra-high performance concrete (UHPC) incorporating MgO expansive agent. J. Build. Eng. 2024, 96, 110529. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.H.; Cao, Y.Y.Y.; Yu, Q.L. Enhancing the interfacial bond of steel tube confined ultra-high performance concrete: Synergistic effects of coarse aggregate and expansive agent. J. Build. Eng. 2025, 104, 112228. [Google Scholar] [CrossRef]
- Borah, D.; Nath, H.; Saikia, H. Modification of bentonite clay & its applications: A review. Rev. Inorg. Chem. 2022, 42, 265–282. [Google Scholar] [CrossRef]
- Li, W.T.; Ting, M.Z.Y.; Qin, J.D.; Yi, Y.L. Seawater effects on properties of bentonite cut-off walls with/without cement addition. Proc. Inst. Civ. Eng.-Geotech. Eng. 2025, 178, 393–404. [Google Scholar] [CrossRef]
- Zeng, Z.X.; Cui, Y.J.; Conil, N.; Talandier, J. Experimental Investigation and Modeling of the Hydraulic Conductivity of Saturated Bentonite-Claystone Mixture. Int. J. Geomech. 2020, 20, 04020184. [Google Scholar] [CrossRef]
- Liu, L.C.; Zuo, H.B.; Liu, W.G.; Xu, Z.Q. Preparation of calcium ferrite by flue gas desulfurization gypsum. J. Iron Steel Res. Int. 2021, 28, 1357–1365. [Google Scholar] [CrossRef]
- Cui, G.Y.; Kong, D.W.; Huang, Y.Y.; Qiu, W.; Cheng, L.L.; Wang, L.L. Effects of Different Admixtures on the Mechanical and Thermal Insulation Properties of Desulfurization Gypsum-Based Composites. Coatings 2023, 13, 1089. [Google Scholar] [CrossRef]
- Jiang, L.H.; Li, C.Z.; Wang, C.; Xu, N.; Chu, H.Q. Utilization of flue gas desulfurization gypsum as an activation agent for high-volume slag concrete. J. Clean. Prod. 2018, 205, 589–598. [Google Scholar] [CrossRef]
- Gu, X.W.; Ge, X.W.; Liu, J.P.; Song, G.; Wang, S.Y.; Hu, Z.Y.; Wang, H. Study on the synergistic effect of calcium carbide residue-fly ash enhanced desulphurisation gypsum under high temperature maintenance condition. Constr. Build. Mater. 2024, 412, 134706. [Google Scholar] [CrossRef]
- Zhonglin, L.; Ye, X.; Cheng, L.; Biao, P.; Yibing, L.; Weiguang, Z.; Chen, Y. Investigation on the compressive strength of desulfurization gypsum binary cementitious materials with low energy consumption: The utilization enhancement of industrial wastes. Case Stud. Constr. Mater. 2024, 21, e03582. [Google Scholar] [CrossRef]
- Xie, Z.; Qian, Z.; Wang, H.; Qi, Y.; Yue, B. Synergistic Preparation and Mechanistic Investigation of Full Industrial Solid Waste-Based Cementitious Materials for Aeolian Sand Stabilization. Appl. Sci. 2025, 15, 3858. [Google Scholar] [CrossRef]
- Yu, P.; Wang, Z.; Lai, P.F.; Zhang, P.; Wang, J.P. Evaluation of mechanic damping properties of montmorillonite/organo-modified montmorillonite-reinforced cement paste. Constr. Build. Mater. 2019, 203, 356–365. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.W.; Deng, Y.F.; Yu, X.B.; Feng, Q.; Yan, C. Expansive soil modified by waste steel slag and its application in subbase layer of highways. Soils Found. 2019, 59, 955–965. [Google Scholar] [CrossRef]
- Luo, Z.D.; Luo, B.; Zhao, Y.F.; Li, X.Y.; Su, Y.H.; Huang, H.; Wang, Q. Experimental Investigation of Unconfined Compression Strength and Microstructure Characteristics of Slag and Fly Ash-Based Geopolymer Stabilized Riverside Soft Soil. Polymers 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Z.; Zhang, Z.L.; Chen, K.Y.; Xia, L.L. Experimental Investigation and Mechanism of Fly Ash/Slag-Based Geopolymer-Stabilized Soft Soil. Appl. Sci. 2022, 12, 7438. [Google Scholar] [CrossRef]
- Kim, E.H.; Yim, S.B.; Jung, H.C.; Lee, E.J. Hydroxyapatite crystallization from a highly concentrated phosphate solution using powdered converter slag as a seed material. J. Hazard. Mater. 2006, 136, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Barca, C.; Gerente, C.; Meyer, D.; Cliazarenc, F.; Andrès, Y. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Res. 2012, 46, 2376–2384. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, S.; Wu, Z.; Huang, Y.H.; Peng, D.P.; Huang, T. Mechanically activated steel slag induces Ca-P crystallization to recover phosphates from phosphogypsum leachate: Adsorption and crystallization coupled processes. Colloids Surf. A-Physicochem. Eng. Asp. 2025, 713, 136535. [Google Scholar] [CrossRef]
- Ibrahim, A.R.; Zhou, Y.L.; Li, X.Y.; Chen, L.; Hong, Y.Z.; Su, Y.Z.; Wang, H.T.; Li, J. Synthesis of rod-like hydroxyapatite with high surface area and pore volume from eggshells for effective adsorption of aqueous Pb(II). Mater. Res. Bull. 2015, 62, 132–141. [Google Scholar] [CrossRef]
- Jiao, H.; Zhao, K.; Ma, L.N.; Bian, T.R.; Ma, Y.M.; Tang, Y.F. Preparation, electrical property and periodic DFT calculation of barium titanate/hydroxyapatite rod-like nanocomposite materials. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2018, 229, 184–192. [Google Scholar] [CrossRef]
- Verma, G.; Barick, K.C.; Manoj, N.; Sahu, A.K.; Hassan, P.A. Rod-like micelle templated synthesis of porous hydroxyapatite. Ceram. Int. 2013, 39, 8995–9002. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash-portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- L’Hôpital, E.; Lothenbach, B.; Le Saout, G.; Kulik, D.; Scrivener, K. Incorporation of aluminium in calcium-silicate-hydrates. Cem. Concr. Res. 2015, 75, 91–103. [Google Scholar] [CrossRef]
- Hiraga, Y.; Shigemoto, N. Performance of Ca(OH)2-Al2(SO4)3 Addition Method in Removing Boron from Wastewater Discharged from Flue-Gas Desulfurizer. J. Chem. Eng. Jpn. 2011, 44, 286–293. [Google Scholar] [CrossRef]
- Yu, X.; Qian, C.; Wang, X. Cementing mechanism of bio-phosphate cement. Sci. China-Technol. Sci. 2015, 58, 1112–1117. [Google Scholar] [CrossRef]
- Goetz-Neunhoeffer, F.; Neubauer, J.; Schwesig, P. Mineralogical characteristics of Ettringites synthesized from solutions and suspensions. Cem. Concr. Res. 2006, 36, 65–70. [Google Scholar] [CrossRef]
- Fasihnikoutalab, M.H.; Pourakbar, S.; Ball, R.J.; Unluer, C.; Cristelo, N. Sustainable soil stabilisation with ground granulated blast-furnace slag activated by olivine and sodium hydroxide. Acta Geotech. 2020, 15, 1981–1991. [Google Scholar] [CrossRef]
- Kasehchi, E.; Arjomand, M.A.; Elizei, M.H.A. Experimental investigation of the feasibility of stabilizing inshore silty sand soil using geopolymer based on ceramic waste powder: An approach to upcycling waste material for sustainable construction. Case Stud. Constr. Mater. 2024, 20, e02979. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, S.P. Strength and durability properties of expansive soil treated with geopolymer and conventional stabilizers. Constr. Build. Mater. 2022, 328, 127078. [Google Scholar] [CrossRef]
- Afrakoti, M.T.P.; Choobbasti, A.J.; Ghadakpour, M.; Kutanaei, S.S. Investigation of the effect of the coal wastes on the mechanical properties of the cement-treated sandy soil. Constr. Build. Mater. 2020, 239, 117848. [Google Scholar] [CrossRef]
- Feng, S.Y.; Zhang, G.F.; Ren, Y.L. Properties and microstructure of soil solidified by titanium slag-flue gas desulfurized gypsum-Portland cement composites as solidifiers. Constr. Build. Mater. 2024, 438, 137061. [Google Scholar] [CrossRef]
- Saride, S.; Puppala, A.J.; Chikyala, S.R. Swell-shrink and strength behaviors of lime and cement stabilized expansive organic clays. Appl. Clay Sci. 2013, 85, 39–45. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Using calcium carbide residue as an alkaline activator for glass powder-clay geopolymer. Constr. Build. Mater. 2018, 183, 417–428. [Google Scholar] [CrossRef]
- Chen, S.; Xiong, G.Q.; Su, Y.; He, X.Y.; Xie, Y.X.; Wang, Y.F.; Gong, M.H. Laboratory Evaluation for Utilization of Phosphogypsum through Carbide Slag Highly-Effective Activating Anhydrous Phosphogypsum. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2021, 36, 392–399. [Google Scholar] [CrossRef]
- Wang, Y.L.; Dong, S.J.; Liu, L.L.; Cui, S.P. Using Calcium Carbide Slag as one of Calcium-containing Raw Materials to Produce Cement Clinker. In Proceedings of the Chinese Materials Congress (CMC 2012), Taiyuan, China, 13–18 July 2012; pp. 171–174. [Google Scholar]
- Çomak, B. Effects of use of alkaline mixing waters on engineering properties of cement mortars. Eur. J. Environ. Civ. Eng. 2018, 22, 736–754. [Google Scholar] [CrossRef]
- Haridi, S.S.H.; El-Hosiny, F.I.; El Gamal, S.M.A.; Radwan, M.A.; Mohammed, M.S.; Abdel-Gawwad, H.A. Effect of Sodium Hydroxide and Magnesium Chloride on Magnesium Silicate Cement-Based Glass Waste. J. Mater. Civ. Eng. 2023, 35, 04023228. [Google Scholar] [CrossRef]
- Yan, Y.R.; Yang, S.Y.; Miron, G.D.; Collings, I.E.; L’Hôpital, E.; Skibsted, J.; Winnefeld, F.; Scrivener, K.; Lothenbach, B. Effect of alkali hydroxide on calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2022, 151, 106636. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Structural transition to well-ordered phases of NaOH-activated slag-metakaolin cements aged by 6 years. Cem. Concr. Res. 2022, 156, 106791. [Google Scholar] [CrossRef]
- Park, S.; Lothenbach, B.; Jang, J.G.; Kim, H.K.; Lee, N.M. Thermodynamic Modeling and Experimental Study of Carbonation of Alkali-Activated Slag Cements. ACS Sustain. Chem. Eng. 2023, 11, 4049–4063. [Google Scholar] [CrossRef]
- Willson, C.S.; Lu, N.; Likos, W.J. Quantification of Grain, Pore, and Fluid Microstructure of Unsaturated Sand from X-Ray Computed Tomography Images. Geotech. Test. J. 2012, 35, 911–923. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.J.; Huang, H.L.; Yin, S.H. Difference in the reaction process of slag activated by waterglass solution and NaOH solution. Struct. Concr. 2019, 20, 1528–1540. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Medepalli, S.; Wang, T.; Dai, J.G.; Zheng, Y.Q.; Ishida, T. Effect of alkali-hydroxide on hydration kinetics and microstructure of high-volume fly ash blended cement pastes. Cem. Concr. Res. 2024, 185, 107641. [Google Scholar] [CrossRef]
- Turan, C.; Javadi, A.A.; Vinai, R.; Russo, G. Effects of Fly Ash Inclusion and Alkali Activation on Physical, Mechanical, and Chemical Properties of Clay. Materials 2022, 15, 4628. [Google Scholar] [CrossRef]
- Wan, X.; Ding, J.W.; Wang, J.H.; Gao, P.J.; Wei, X. Exploring alkali-source, pore-filling and cementation damage effects in cemented clay with typical industrial waste binders. J. Rock Mech. Geotech. Eng. 2024, 16, 5209–5220. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, P.; Jiang, Q.A.; Liu, Q.; Wang, Q.; Qiu, J.; Hu, S.G.; Lyu, X. The competitive hydration of SO4−2 and Cl− in alkali-activated slag cementitious materials. Constr. Build. Mater. 2023, 396, 132267. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Zhang, Z.B.; Tang, Q.; Zhou, Y. Mechanical properties and microscopic research of different types of bentonite in conjunction with cement and fine sand. Soils Found. 2025, 65, 101573. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, L.; Xie, R.S.; Song, L.X.; Jiang, J. Effect of bentonite on the stability of fresh cement slurry. J. Sustain. Cem.-Based Mater. 2022, 11, 345–352. [Google Scholar] [CrossRef]
- Xue, L.L.; Ni, Z.K.; Zhou, Z.N.; Zhang, Z.H.; Xiong, H.R.; Wang, H.; Zhuge, X.F.; Liu, H.F. Effects of desulfurized gypsum on shrinkage behavior of alkali-activated slag (AAS) and hybrid alkali-activated cement (HAC). Case Stud. Constr. Mater. 2025, 22, e04320. [Google Scholar] [CrossRef]
- Khatib, J.M.; Wright, L.; Mangat, P.S. Effect of fly ash-gypsum blend on porosity and pore size distribution of cement pastes. Adv. Appl. Ceram. 2013, 112, 197–201. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for Geotechnical Testing Method. China Planning Press: Beijing, China, 2019.
- JTG/T F20-2015; Technical Guidelines for Construction of Highway Roadbases. People’s Communications Publishing House Co., Ltd.: Beijing, China, 2015.
- Cui, Q.; Liu, G.; Zhang, Z.H.; Fang, Y.Q.; Gu, X.D. Experimental Investigation on the Strength and Microscopic Properties of Cement-Stabilized Aeolian Sand. Buildings 2023, 13, 395. [Google Scholar] [CrossRef]
- Huang, T.J.; Zhang, K.; Kang, K.G.; Yuan, Q. The effect of NaOH addition on the physico-chemical phenomena of cement setting acceleration. Constr. Build. Mater. 2025, 458, 139761. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.S. Effect of Sodium Hydroxide on the Properties of Phosphogypsum based Cement. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010, 25, 342–345. [Google Scholar] [CrossRef]
- Ma, C.; Chen, L.Z.; Chen, B. Analysis of strength development in soft clay stabilized with cement-based stabilizer. Constr. Build. Mater. 2014, 71, 354–362. [Google Scholar] [CrossRef]
- Ma, C.; Qin, Z.H.; Zhuang, Y.C.; Chen, L.Z.; Chen, B. Influence of sodium silicate and promoters on unconfined compressive strength of Portland cement-stabilized clay. Soils Found. 2015, 55, 1222–1232. [Google Scholar] [CrossRef]
- ASTM 5102; Standard Test Methods for Unconfined Compressive Strength of Compacted Soil-Lime Mixtures. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM 4792; Standard Test Method for Potential Expansion of Aggregates from Hydration Reactions. ASTM International: West Conshohocken, PA, USA, 2022.





| Materials | SiO2 | CaO | Fe2O3 | Al2O3 | MgO | SO3 | Na2O | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Steel slag (SS) | 17.18 | 30.01 | 29.1 | 8.43 | 4.41 | 0.62 | 0 | 0.01 | 0.87 |
| Ground granulated blast furnace slag (GGBFS) | 30.07 | 38.22 | 0.88 | 16.50 | 8.33 | 2.08 | 0.51 | 0.86 | 0.02 |
| Phosphorus slag (PS) | 44.89 | 43.97 | 0.01 | 3.88 | 3.72 | 0 | 0.05 | 0.84 | 1.28 |
| Carbide slag (CS) | 2.72 | 70.35 | 1.03 | 0.89 | 0.62 | 0 | 0 | 0 | 0.02 |
| Desulfurized gypsum (DG) | 0.60 | 48.25 | 0.57 | 0.60 | 1.17 | 38.51 | 0.52 | 1.72 | 0.02 |
| Bentonite (BE) | 66.58 | 3.35 | 2.42 | 14.57 | 2.56 | 0 | 2.37 | 1.39 | 0.11 |
| Properties | Characteristics | Method |
|---|---|---|
| Specific gravity, Gs | 2.64 | Pycnometer |
| Natural water content, w0 (%) | 2.50 | Oven drying |
| Natural density, ρ (g/cm3) | 1.56 | Cutting ring |
| Initial void ratio, e0 | 0.81 | - |
| Saturation, Sr (%) | 8.14 | - |
| Cohesion, c (kPa) | 0.26 | Direct shear |
| Internal friction angle φ (°) | 33.70 | Direct shear |
| Sand fraction (>0.075 mm) (%) | 98.01 | laser size analysis |
| Silt fraction (0.002–0.075 mm) (%) | 1.99 | laser size analysis |
| Clay fraction (<0.002 mm) (%) | - | laser size analysis |
| Series | Specimen Name | Additives | Dosage | Mass of Additives (g) | Curing Ages (Days) |
|---|---|---|---|---|---|
| Activators | SH0.15Ci | NaOH | 0.15 M | 0.165 | 3, 14, 28 |
| SH0.30Ci | 0.30 M | 0.331 | |||
| SH0.45Ci | 0.45 M | 0.496 | |||
| SH0.60Ci | 0.60 M | 0.661 | |||
| SH0.75Ci | 0.75 M | 0.827 | |||
| CH0.10Ci | Ca(OH)2 | 0.10 M | 0.204 | ||
| CH0.20Ci | 0.20 M | 0.408 | |||
| CH0.30Ci | 0.30 M | 0.613 | |||
| CH0.40Ci | 0.40 M | 0.817 | |||
| CH0.50Ci | 0.50 M | 1.021 | |||
| CL0.05Ci | CaCl2 | 0.05 M | 0.153 | ||
| CL0.10Ci | 0.10 M | 0.306 | |||
| CL0.15Ci | 0.15 M | 0.459 | |||
| CL0.20Ci | 0.20 M | 0.612 | |||
| CL0.25Ci | 0.25 M | 0.765 | |||
| Expansive agents | DG0.4Ci | Desulfurized gypsum | 0.40% | 1.225 | |
| DG1.0Ci | 1.00% | 3.063 | |||
| DG1.6Ci | 1.60% | 4.901 | |||
| DG2.2Ci | 2.20% | 6.739 | |||
| DG2.8Ci | 2.80% | 8.576 | |||
| BE0.4Ci | Bentonite | 0.40% | 1.225 | ||
| BE1.0Ci | 1.00% | 3.063 | |||
| BE1.6Ci | 1.60% | 4.901 | |||
| BE2.2Ci | 2.20% | 6.739 | |||
| BE2.8Ci | 2.80% | 8.576 | |||
| Control | BLCi | - | - | - | |
| CECi | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Qian, Z.; Lu, X.; Wang, H.; Lai, P. Comparative Analysis of Chemical Activators and Expansive Agents for Aeolian Sand Stabilization Using Industrial Solid Waste-Based Geopolymers. Gels 2025, 11, 713. https://doi.org/10.3390/gels11090713
Xie Z, Qian Z, Lu X, Wang H, Lai P. Comparative Analysis of Chemical Activators and Expansive Agents for Aeolian Sand Stabilization Using Industrial Solid Waste-Based Geopolymers. Gels. 2025; 11(9):713. https://doi.org/10.3390/gels11090713
Chicago/Turabian StyleXie, Zilu, Zengzhen Qian, Xianlong Lu, Hao Wang, and Phatyoufy Lai. 2025. "Comparative Analysis of Chemical Activators and Expansive Agents for Aeolian Sand Stabilization Using Industrial Solid Waste-Based Geopolymers" Gels 11, no. 9: 713. https://doi.org/10.3390/gels11090713
APA StyleXie, Z., Qian, Z., Lu, X., Wang, H., & Lai, P. (2025). Comparative Analysis of Chemical Activators and Expansive Agents for Aeolian Sand Stabilization Using Industrial Solid Waste-Based Geopolymers. Gels, 11(9), 713. https://doi.org/10.3390/gels11090713






