Organogels for the Preservation of Cultural Heritage
Abstract
1. Introduction
2. Traditional Polymeric “Solvent Gels” and First Organogel Formulations
3. New Sustainable Organogels for Cultural Heritage Preservation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajdzik, B.; Wolniak, R.; Nagaj, R.; Žuromskaitė-Nagaj, B.; Grebski, W.W. The Influence of the Global Energy Crisis on Energy Efficiency: A Comprehensive Analysis. Energies 2024, 17, 947. [Google Scholar] [CrossRef]
- Hultman, L.; Mazur, S.; Ankarcrona, C.; Palmqvist, A.; Abrahamsson, M.; Antti, M.-L.; Baltzar, M.; Bergström, L.; de Laval, P.; Edman, L.; et al. Advanced materials provide solutions towards a sustainable world. Nat. Mater. 2024, 23, 160–161. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, X.; Chen, X. Advanced Materials and Technologies toward Carbon Neutrality. Acc. Mater. Res. 2022, 3, 913–921. [Google Scholar] [CrossRef]
- Sesana, E.; Gagnon, A.S.; Ciantelli, C.; Cassar, J.; Hughes, J.J. Climate change impacts on cultural heritage: A literature review. WIREs Clim. Chang. 2021, 12, e710. [Google Scholar] [CrossRef]
- Bonazza, A.; Sardella, A. Climate Change and Cultural Heritage: Methods and Approaches for Damage and Risk Assessment Addressed to a Practical Application. Heritage 2023, 6, 3578–3589. [Google Scholar] [CrossRef]
- Kalman, H. Destruction, mitigation, and reconciliation of cultural heritage. Int. J. Herit. Stud. 2017, 23, 538–555. [Google Scholar] [CrossRef]
- Casini, A.; Chelazzi, D.; Baglioni, P. Advanced methodologies for the cleaning of works of art. Sci. China Technol. Sci. 2023, 66, 2162–2182. [Google Scholar] [CrossRef]
- Caruso, M.R.; D’Agostino, G.; Milioto, S.; Cavallaro, G.; Lazzara, G. A review on biopolymer-based treatments for consolidation and surface protection of cultural heritage materials. J. Mater. Sci. 2023, 58, 12954–12975. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Galvagno, E.; Tartaglia, E.; Stratigaki, M.; Tossi, C.; Marasco, L.; Menegazzo, F.; Zanardi, C.; Omenetto, F.; Coletti, C.; Traviglia, A.; et al. Present Status and Perspectives of Graphene and Graphene-related Materials in Cultural Heritage. Adv. Funct. Mater. 2024, 34, 2313043. [Google Scholar] [CrossRef]
- Gorgolis, G.; Kotsidi, M.; Messina, E.; Mazzurco Miritana, V.; Di Carlo, G.; Nhuch, E.L.; Martins Leal Schrekker, C.; Cuty, J.A.; Schrekker, H.S.; Paterakis, G.; et al. Antifungal Hybrid Graphene–Transition-Metal Dichalcogenides Aerogels with an Ionic Liquid Additive as Innovative Absorbers for Preventive Conservation of Cultural Heritage. Materials 2024, 17, 3174. [Google Scholar] [CrossRef]
- Gorgolis, G.; Ziemann, S.; Kotsidi, M.; Paterakis, G.; Koutroumanis, N.; Tsakonas, C.; Anders, M.; Galiotis, C. Novel Graphene-Based Materials as a Tool for Improving Long-Term Storage of Cultural Heritage. Materials 2023, 16, 3528. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Missori, M.; Fantoni, R. (Eds.) Advanced Technologies for Cultural Heritage Monitoring and Conservation; Digital Innovations in Architecture, Engineering and Construction; Springer Nature Switzerland: Cham, Switzerland, 2024; ISBN 978-3-031-52496-7. [Google Scholar]
- Talamo, M.; Valentini, F.; Dimitri, A.; Allegrini, I. Innovative Technologies for Cultural Heritage. Tattoo Sensors and AI: The New Life of Cultural Assets. Sensors 2020, 20, 1909. [Google Scholar] [CrossRef]
- Valentini, F.; Calcaterra, A.; Antonaroli, S.; Talamo, M. Smart Portable Devices Suitable for Cultural Heritage: A Review. Sensors 2018, 18, 2434. [Google Scholar] [CrossRef] [PubMed]
- Saber, D.; Abd El-Aziz, K. Advanced materials used in wearable health care devices and medical textiles in the battle against coronavirus (COVID-19): A review. J. Ind. Text. 2022, 51, 246S–271S. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Azeredo, H.M.C.; Mattos, B.D.; Beaumont, M.; Correa, D.S.; Rojas, O.J. The Food–Materials Nexus: Next Generation Bioplastics and Advanced Materials from Agri-Food Residues. Adv. Mater. 2021, 33. [Google Scholar] [CrossRef]
- Ghazwani, K.; Beach, T.; Rezgui, Y. Energy retrofitting using advanced building envelope materials for sustainable housing: A review. Build. Environ. 2025, 267, 112243. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar Sharma, N. Advanced materials contribution towards sustainable development and its construction for green buildings. Mater. Today Proc. 2022, 68, 968–973. [Google Scholar] [CrossRef]
- Chelazzi, D.; Baglioni, P. From Nanoparticles to Gels: A Breakthrough in Art Conservation Science. Langmuir 2023, 39, 10744–10755. [Google Scholar] [CrossRef]
- Ormsby, B.; Keefe, M.; Phenix, A.; von Aderkas, E.; Learner, T.; Tucker, C.; Kozak, C. Mineral Spirits-Based Microemulsions: A Novel Cleaning System for Painted Surfaces. J. Am. Inst. Conserv. 2016, 55, 12–31. [Google Scholar] [CrossRef]
- Guilminot, E. The Use of Hydrogels in the Treatment of Metal Cultural Heritage Objects. Gels 2023, 9, 191. [Google Scholar] [CrossRef]
- Mazzuca, C.; Severini, L.; Domenici, F.; Toumia, Y.; Mazzotta, F.; Micheli, L.; Titubante, M.; Di Napoli, B.; Paradossi, G.; Palleschi, A. Polyvinyl alcohol based hydrogels as new tunable materials for application in the cultural heritage field. Colloids Surf. B Biointerfaces 2020, 188, 110777. [Google Scholar] [CrossRef]
- Mastrangelo, R.; Poggi, G.; Chelazzi, D.; Baglioni, P. Twin chain polymer networks: Recent developments in hydrogels for Cultural Heritage. Polymer 2025, 325, 128294. [Google Scholar] [CrossRef]
- Ulrich, K.; Centeno, S.A.; Arslanoglu, J.; Del Federico, E. Absorption and diffusion measurements of water in acrylic paint films by single-sided NMR. Prog. Org. Coat. 2011, 71, 283–289. [Google Scholar] [CrossRef]
- Banti, D.; La Nasa, J.; Tenorio, A.L.; Modugno, F.; Jan van den Berg, K.; Lee, J.; Ormsby, B.; Burnstock, A.; Bonaduce, I. A molecular study of modern oil paintings: Investigating the role of dicarboxylic acids in the water sensitivity of modern oil paints. RSC Adv. 2018, 8, 6001–6012. [Google Scholar] [CrossRef]
- La Nasa, J.; Lee, J.; Degano, I.; Burnstock, A.; van den Berg, K.J.; Ormsby, B.; Bonaduce, I. The role of the polymeric network in the water sensitivity of modern oil paints. Sci. Rep. 2019, 9, 3467. [Google Scholar] [CrossRef] [PubMed]
- Bonaduce, I.; Duce, C.; Lluveras-Tenorio, A.; Lee, J.; Ormsby, B.; Burnstock, A.; van den Berg, K.J. Conservation Issues of Modern Oil Paintings: A Molecular Model on Paint Curing. Acc. Chem. Res. 2019, 52, 3397–3406. [Google Scholar] [CrossRef]
- Melchiorre, C.; Melchiorre, M.; Marra, M.; Rizzo, E.; Fatigati, G.; Rossi, P.; Cerruti, P.; Improta, I.; Amoresano, A.; Marino, G.; et al. Green solvents and restoration: Application of biomass-derived solvents in cleaning procedures. J. Cult. Herit. 2023, 62, 3–12. [Google Scholar] [CrossRef]
- Macchia, A.; Strangis, R.; De Angelis, S.; Cersosimo, M.; Docci, A.; Ricca, M.; Gabriele, B.; Mancuso, R.; La Russa, M.F. Deep Eutectic Solvents (DESs): Preliminary Results for Their Use Such as Biocides in the Building Cultural Heritage. Materials 2022, 15, 4005. [Google Scholar] [CrossRef] [PubMed]
- Biribicchi, C.; Macchia, A.; Favero, G.; Strangis, R.; Gabriele, B.; Mancuso, R.; La Russa, M.F. Sustainable solutions for removing aged wax-based coatings from cultural heritage: Exploiting hydrophobic deep eutectic solvents (DESs). New J. Chem. 2023, 47, 5991–6000. [Google Scholar] [CrossRef]
- Vadrucci, M. Sustainable Cultural Heritage Conservation: A Challenge and an Opportunity for the Future. Sustainability 2025, 17, 584. [Google Scholar] [CrossRef]
- Hainsch, K.; Löffler, K.; Burandt, T.; Auer, H.; Crespo del Granado, P.; Pisciella, P.; Zwickl-Bernhard, S. Energy transition scenarios: What policies, societal attitudes, and technology developments will realize the EU Green Deal? Energy 2022, 239, 122067. [Google Scholar] [CrossRef]
- Semenzin, E.; Giubilato, E.; Badetti, E.; Picone, M.; Volpi Ghirardini, A.; Hristozov, D.; Brunelli, A.; Marcomini, A. Guiding the development of sustainable nano-enabled products for the conservation of works of art: Proposal for a framework implementing the Safe by Design concept. Environ. Sci. Pollut. Res. 2019, 26, 26146–26158. [Google Scholar] [CrossRef] [PubMed]
- Sardella, A.; Palazzi, E.; von Hardenberg, J.; Del Grande, C.; De Nuntiis, P.; Sabbioni, C.; Bonazza, A. Risk Mapping for the Sustainable Protection of Cultural Heritage in Extreme Changing Environments. Atmosphere 2020, 11, 700. [Google Scholar] [CrossRef]
- Rajendran, S.; Al-Samydai, A.; Palani, G.; Trilaksana, H.; Sathish, T.; Giri, J.; Saravanan, R.; Lalvani, J.I.J.; Nasri, F. Replacement of Petroleum Based Products With Plant-Based Materials, Green and Sustainable Energy—A Review. Eng. Rep. 2025, 7, e70108. [Google Scholar] [CrossRef]
- Biswal, T.; BadJena, S.K.; Pradhan, D. Sustainable biomaterials and their applications: A short review. Mater. Today Proc. 2020, 30, 274–282. [Google Scholar] [CrossRef]
- Burke, J. Solubility Parameters: Theory and Application. AIC Book Pap. Group Annu. 1984, 3, 13–58. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849372483. [Google Scholar]
- Cremonesi, P. Un approccio più scientifico alla pulitura dei dipinti: Il test di solubilità di Feller. Progett. Restauro 1998, 38–42. [Google Scholar]
- Mollet, H.; Grubenmann, A. Formulation Technology; Wiley: Hoboken, NJ, USA, 2000; ISBN 9783527302017. [Google Scholar]
- Hansen, C.M.; Just, L. Prediction of Environmental Stress Cracking in Plastics with Hansen Solubility Parameters. Ind. Eng. Chem. Res. 2001, 40, 21–25. [Google Scholar] [CrossRef]
- Fardi, T.; Stefanis, E.; Panayiotou, C.; Abbott, S.; van Loon, S. Artwork conservation materials and Hansen solubility parameters: A novel methodology towards critical solvent selection. J. Cult. Herit. 2014, 15, 583–594. [Google Scholar] [CrossRef]
- Brouwer, T.; Schuur, B. Model Performances Evaluated for Infinite Dilution Activity Coefficients Prediction at 298.15 K. Ind. Eng. Chem. Res. 2019, 58, 8903–8914. [Google Scholar] [CrossRef]
- Lopez, C.G.; Richtering, W. Does Flory–Rehner theory quantitatively describe the swelling of thermoresponsive microgels? Soft Matter 2017, 13, 8271–8280. [Google Scholar] [CrossRef]
- Baij, L.; Hermans, J.J.; Keune, K.; Iedema, P.D. Time-Dependent ATR-FTIR Spectroscopic Studies on Solvent Diffusion and Film Swelling in Oil Paint Model Systems. Macromolecules 2018, 51, 7134–7144. [Google Scholar] [CrossRef]
- Wolbers, R.C. Workshop on New Methods in the Cleaning of Paintings and other Decorative Surfaces; 1988 Marina del Rey, CA; Canadian Conservation Institute: Ottawa, ON, Canada, 1990. [Google Scholar]
- Burnstock, A.; Kieslich, T. A study of the clearance of solvent gels used for varnish removal from paintings. In Proceedings of the ICOM Committee for Conservation, 11th Triennial Meeting, Edinburgh, UK, 1–6 September 1996. [Google Scholar]
- Chelazzi, D.; Fratini, E.; Giorgi, R.; Mastrangelo, R.; Rossi, M.; Baglioni, P. Gels for the Cleaning of Works of Art. In Gels and Other Soft Amorphous Solids-ACS Symposium Series Vol. 1296; Horkay, F., Douglas, J.F., Del Gado, E., Eds.; American Chemical Society: Washington, DC, USA, 2018; pp. 291–314. [Google Scholar]
- Casoli, A.; Di Diego, Z.; Isca, C. Cleaning painted surfaces: Evaluation of leaching phenomenon induced by solvents applied for the removal of gel residues. Environ. Sci. Pollut. Res. 2014, 21, 13252–13263. [Google Scholar] [CrossRef]
- Roy, A.; Smith, P.; Barclay, R.L. Tradition and Innovation: Advances in Conservation: Contributions to the Melbourne Congress 10–14 October 2000; The International Institute for Conservation of Historic and Artistic Works: London, UK, 2000. [Google Scholar]
- Mastrangelo, R.; Chelazzi, D.; Poggi, G.; Fratini, E.; Pensabene Buemi, L.; Petruzzellis, M.L.; Baglioni, P. Twin-chain polymer hydrogels based on poly(vinyl alcohol) as new advanced tool for the cleaning of modern and contemporary art. Proc. Natl. Acad. Sci. USA 2020, 117, 7011–7020. [Google Scholar] [CrossRef]
- Mastrangelo, R.; Bandelli, D.; Pensabene Buemi, L.; Baglioni, P. “Twin Chain” PVA Cryogels with Controlled Tortuosity as Advanced Materials for Cleaning of Works of Art. Adv. Funct. Mater. 2024. [Google Scholar] [CrossRef]
- Baglioni, P.; Bonelli, N.; Chelazzi, D.; Chevalier, A.; Dei, L.; Domingues, J.; Fratini, E.; Giorgi, R.; Martin, M. Organogel formulations for the cleaning of easel paintings. Appl. Phys. A 2015, 121, 857–868. [Google Scholar] [CrossRef]
- George, M.; Luo, C.; Wang, C.; Carretti, E.; Dei, L.; Weiss, R.G. Chemically and physically induced (reversible) gelation of organic liquids by monomeric and polymeric gelators. In Proceedings of the Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2005; Volume 227, pp. 173–182. [Google Scholar]
- Carretti, E.; Dei, L.; Macherelli, A.; Weiss, R.G. Rheoreversible Polymeric Organogels: The Art of Science for Art Conservation. Langmuir 2004, 20, 8414–8418. [Google Scholar] [CrossRef] [PubMed]
- Carretti, E.; Dei, L.; Weiss, R.G.; Baglioni, P. A new class of gels for the conservation of painted surfaces. J. Cult. Herit. 2008, 9, 386–393. [Google Scholar] [CrossRef]
- Ferrari, P.; Chelazzi, D.; Bonelli, N.; Mirabile, A.; Giorgi, R.; Baglioni, P. Alkyl carbonate solvents confined in poly (ethyl methacrylate) organogels for the removal of pressure sensitive tapes (PSTs) from contemporary drawings. J. Cult. Herit. 2018, 34, 227–236. [Google Scholar] [CrossRef]
- Pianorsi, M.D.; Raudino, M.; Bonelli, N.; Chelazzi, D.; Giorgi, R.; Fratini, E.; Baglioni, P. Organogels for the cleaning of artifacts. In Proceedings of the Pure and Applied Chemistry; De Gruyter Brill: Berlin, Germany, 2017; Volume 89, pp. 3–17. [Google Scholar]
- Trabace, M.; Mirabile, A.; Montalbano, L.; Giorgi, R.; Ferrari, P. An Innovative Method To Remove Pressure-Sensitive Tape From Contemporary Felt-Tip Pen And Ballpoint Pen Drawings On Paper. The Case Studies Of Federico Fellini From Rimini Film Library. Le Nuove Front. Del Restauro. Trasferimenti, Contam. Ibridazioni. Sci. e Beni Cult. 2017, 33, 849–859. [Google Scholar]
- Duncan, T.T.; Berrie, B.H.; Weiss, R.G. Soft, Peelable Organogels from Partially Hydrolyzed Poly(vinyl acetate) and Benzene-1,4-diboronic Acid: Applications to Clean Works of Art. ACS Appl. Mater. Interfaces 2017, 9, 28069–28078. [Google Scholar] [CrossRef]
- Angelova, L.V.; Terech, P.; Natali, I.; Dei, L.; Carretti, E.; Weiss, R.G. Cosolvent Gel-like Materials from Partially Hydrolyzed Poly(vinyl acetate)s and Borax. Langmuir 2011, 27, 11671–11682. [Google Scholar] [CrossRef]
- Stagno, V.; Genova, C.; Zoratto, N.; Favero, G.; Capuani, S. Single-Sided Portable NMR Investigation to Assess and Monitor Cleaning Action of PVA-Borax Hydrogel in Travertine and Lecce Stone. Molecules 2021, 26, 3697. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, L.; Genova, C.; Stagno, V.; Paoletti, L.; Matulac, A.L.; Ciccola, A.; Di Fazio, M.; Capuani, S.; Favero, G. Multi-Technique Assessment of Chelators-Loaded PVA-Borax Gel-like Systems Performance in Cleaning of Stone Contaminated with Copper Corrosion Products. Gels 2024, 10, 455. [Google Scholar] [CrossRef]
- Duncan, T.T.; Weiss, R.G. Influence of length and structure of aryl boronic acid crosslinkers on organogels with partially hydrolyzed poly (vinyl acetate). Colloid Polym. Sci. 2018, 296, 1047–1056. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure–function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent progress in fabrication and application of polydimethylsiloxane sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Porpora, F.; Dei, L.; Duncan, T.T.; Olivadese, F.; London, S.; Berrie, B.H.; Weiss, R.G.; Carretti, E. Non-Aqueous Poly (dimethylsiloxane) Organogel Sponges for Controlled Solvent Release: Synthesis, Characterization, and Application in the Cleaning of Artworks. Gels 2023, 9, 985. [Google Scholar] [CrossRef]
- Stulik, D.; Miller, D.; Khanjian, H.; Khandekar, N.; Wolbers, R.; Carlson, J.; Petersen, W.C. Solvent Gels for the Cleaning of Works of Art: The Residue Question; Stulik, D., Dorge, V., Eds.; Research in conservation; The Getty Conservation Institute—J. Paul Getty Trust: Los Angeles, CA, USA, 2004; ISBN 9780892367597. [Google Scholar]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Green Chemistry, Biocatalysis, and the Chemical Industry of the Future. ChemSusChem 2022, 15, e202102628. [Google Scholar] [CrossRef]
- Song, J.; Han, B. Green chemistry: A tool for the sustainable development of the chemical industry. Natl. Sci. Rev. 2015, 2, 255–256. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef] [PubMed]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical Applications of Methyl Methacrylate: A Review of Toxicity. Arch. Environ. Occup. Health 2009, 64, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhu, X.; Cao, Y.; Xu, X.; Wang, S.; Dong, X. Toward replacement of methyl methacrylate by sustainable bio-based isobornyl methacrylate in latex pressure sensitive adhesive. Int. J. Adhes. Adhes. 2020, 100, 102623. [Google Scholar] [CrossRef]
- Bandelli, D.; Mastrangelo, R.; Poggi, G.; Chelazzi, D.; Baglioni, P. New sustainable polymers and oligomers for Cultural Heritage conservation. Chem. Sci. 2024, 15, 2443–2455. [Google Scholar] [CrossRef]
- Li, X.; Saleh, A.S.M.; Wang, P.; Wang, Q.; Yang, S.; Zhu, M.; Duan, Y.; Xiao, Z. Characterization of Organogel Prepared from Rice Bran Oil with Cinnamic Acid. Food Biophys. 2017, 12, 356–364. [Google Scholar] [CrossRef]
- Aguilar-Zárate, M.; De la Peña-Gil, A.; Álvarez-Mitre, F.M.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Vegetable and Mineral Oil Organogels Based on Monoglyceride and Lecithin Mixtures. Food Biophys. 2019, 14, 326–345. [Google Scholar] [CrossRef]
- Samorì, C.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Prati, S.; Sciutto, G.; Volpi, F.; Tagliavini, E. The Green Attitude in Art Conservation: Polyhydroxybutyrate-based Gels for the Cleaning of Oil Paintings. ChemistrySelect 2016, 1, 4502–4508. [Google Scholar] [CrossRef]
- Prati, S.; Volpi, F.; Fontana, R.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Samorì, C.; Sciutto, G.; Tagliavini, E. Sustainability in art conservation: A novel bio-based organogel for the cleaning of water sensitive works of art. Pure Appl. Chem. 2018, 90, 239–251. [Google Scholar] [CrossRef]
- Yiming, J.; Sciutto, G.; Prati, S.; Catelli, E.; Galeotti, M.; Porcinai, S.; Mazzocchetti, L.; Samorì, C.; Galletti, P.; Giorgini, L.; et al. A new bio-based organogel for the removal of wax coating from indoor bronze surfaces. Herit. Sci. 2019, 7, 34. [Google Scholar] [CrossRef]
- Passaretti, A.; Cuvillier, L.; Sciutto, G.; Joseph, E. Innovative perspective for the cleaning of historical iron heritage: Novel bio-organogel for the combined removal of undesired organic coatings and corrosion. Herit. Sci. 2024, 12, 181. [Google Scholar] [CrossRef]
- Jia, Y.; Sciutto, G.; Mazzeo, R.; Samorì, C.; Focarete, M.L.; Prati, S.; Gualandi, C. Organogel Coupled with Microstructured Electrospun Polymeric Nonwovens for the Effective Cleaning of Sensitive Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 39620–39629. [Google Scholar] [CrossRef]
- Passaretti, A.; Cuvillier, L.; Guilminot, E.; Sciutto, G.; Joseph, E. Biocleaning of historical metal artworks: Innovative green gels amended with microbial derivatives. Front. Mater. 2023, 10, 1277972. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Tan, K.; Dong, S.; He, Y.; Ye, L.; Zhao, W.; Gu, L.; Prati, S.; Bai, J. A new deep eutectic solvent-based green gel for the removal of polymeric coating from mural painting. J. Cult. Herit. 2025, 71, 51–60. [Google Scholar] [CrossRef]
- Jia, Y.; Sciutto, G.; Botteon, A.; Conti, C.; Focarete, M.L.; Gualandi, C.; Samorì, C.; Prati, S.; Mazzeo, R. Deep eutectic solvent and agar: A new green gel to remove proteinaceous-based varnishes from paintings. J. Cult. Herit. 2021, 51, 138–144. [Google Scholar] [CrossRef]
- Macchia, A.; Zaratti, C.; Ciogli, D.; Rivici, G.; Pilati, S.; Sbiri, N.; de Caro, T.; Navarra, M.A. Developing Innovative Apolar Gels Based on Cellulose Derivatives for Cleaning Metal Artworks. Gels 2024, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Macchia, A.; Biribicchi, C.; Carnazza, P.; Montorsi, S.; Sangiorgi, N.; Demasi, G.; Prestileo, F.; Cerafogli, E.; Colasanti, I.A.; Aureli, H.; et al. Multi-Analytical Investigation of the Oil Painting “Il Venditore di Cerini” by Antonio Mancini and Definition of the Best Green Cleaning Treatment. Sustainability 2022, 14, 3972. [Google Scholar] [CrossRef]
- Çakmak, Y.; Çakmakçi, E.; Apohan, N.K.; Karadag, R. Isosorbide, pyrogallol, and limonene-containing thiol-ene photocured bio-based organogels for the cleaning of artworks. J. Cult. Herit. 2022, 55, 391–398. [Google Scholar] [CrossRef]
- Zuliani, A.; Rapisarda, M.; Chelazzi, D.; Baglioni, P.; Rizzarelli, P. Synthesis, Characterization, and Soil Burial Degradation of Biobased Polyurethanes. Polymers 2022, 14, 4948. [Google Scholar] [CrossRef]
- Poggi, G.; Santan, H.D.; Smets, J.; Chelazzi, D.; Noferini, D.; Petruzzellis, M.L.; Pensabene Buemi, L.; Fratini, E.; Baglioni, P. Nanostructured bio-based castor oil organogels for the cleaning of artworks. J. Colloid Interface Sci. 2023, 638, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bandelli, D.; Mastrangelo, R.; Poggi, G.; Chelazzi, D.; Baglioni, P. Tailoring the properties of castor oil polyurethanes organogels with green oligoesters. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134528. [Google Scholar] [CrossRef]
- Impemba, S.; Bandelli, D.; Mastrangelo, R.; Poggi, G.; Chelazzi, D.; Baglioni, P. Development of biobased poly(urethanes- co -oxazolidones) organogels. Soft Matter 2025, 21, 2623–2632. [Google Scholar] [CrossRef]
- Khaksar-Baghan, N.; Koochakzaei, A.; Hamzavi, Y. An overview of gel-based cleaning approaches for art conservation. Herit. Sci. 2024, 12, 248. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Li, P.; Liu, F.-Q.; Guo, H.; Li, W.-H. Recent advances of organogels: From fabrications and functions to applications. Prog. Org. Coat. 2021, 159, 106417. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A. Organogels versus Hydrogels: Advantages, Challenges, and Applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Albano, M.; Grassi, S.; Fiocco, G.; Invernizzi, C.; Rovetta, T.; Licchelli, M.; Marotti, R.; Merlo, C.; Comelli, D.; Malagodi, M. A Preliminary Spectroscopic Approach to Evaluate the Effectiveness of Water- and Silicone-Based Cleaning Methods on Historical Varnished Brass. Appl. Sci. 2020, 10, 3982. [Google Scholar] [CrossRef]
- Passaretti, A.; Cuvillier, L.; Sciutto, G.; Guilminot, E.; Joseph, E. Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage. Appl. Sci. 2021, 11, 3405. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s Solvent Selection Guide?application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy 2004, 7, 42–50. [Google Scholar] [CrossRef]
- Biribicchi, C.; Doutre, M.; Favero, G. Characterization and assessment of cleaning systems based on fatty acid methyl esters (FAMEs) for the removal of wax-based coatings from cultural heritage objects. Mater. Adv. 2024, 5, 9359–9375. [Google Scholar] [CrossRef]
- Biribicchi, C.; Doutre, M.; Favero, G. Assessing the swelling behavior of oil paint in fatty acid methyl esters (FAMEs). RSC Adv. 2024, 14, 39692–39699. [Google Scholar] [CrossRef]
- Biribicchi, C.; Capuani, S.; Ciarleglio, G.; Santonicola, M.G.; Stagno, V.; Piancastelli, A.; Favero, G. Retention and capillary flow dynamics assessment of Fatty Acid Methyl Esters (FAMEs) in Carrara marble. Mater. Chem. Phys. 2025, 333, 130400. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Tabone, M.D.; Cregg, J.J.; Beckman, E.J.; Landis, A.E. Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers. Environ. Sci. Technol. 2010, 44, 8264–8269. [Google Scholar] [CrossRef]
- Kaplan, D.L. Introduction to Biopolymers from Renewable Resources. In Biopolymers from Renewable Resources; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1998; pp. 1–29. [Google Scholar]
- Knutson, C.M.; Schneiderman, D.K.; Yu, M.; Javner, C.H.; Distefano, M.D.; Wissinger, J.E. Polymeric Medical Sutures: An Exploration of Polymers and Green Chemistry. J. Chem. Educ. 2017, 94, 1761–1765. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Grosso, M. Bioplastics and waste management. Waste Manag. 2018, 78, 800–801. [Google Scholar] [CrossRef]
- Tariq, Z.; Iqbal, D.N.; Rizwan, M.; Ahmad, M.; Faheem, M.; Ahmed, M. Significance of biopolymer-based hydrogels and their applications in agriculture: A review in perspective of synthesis and their degree of swelling for water holding. RSC Adv. 2023, 13, 24731–24754. [Google Scholar] [CrossRef]
- Patel, D.K.; Jung, E.; Priya, S.; Won, S.-Y.; Han, S.S. Recent advances in biopolymer-based hydrogels and their potential biomedical applications. Carbohydr. Polym. 2024, 323, 121408. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Zhang, D.; Ren, P.; Li, L.; Li, X. Preparation and characterization of a chitosan-based low-pH-sensitive intelligent corrosion inhibitor. Int. J. Miner. Metall. Mater. 2015, 22, 998–1004. [Google Scholar] [CrossRef]
- Lai, H.; Liu, S.; Yan, J.; Xing, F.; Xiao, P. Facile Fabrication of Biobased Hydrogel from Natural Resources: L-Cysteine, Itaconic Anhydride, and Chitosan. ACS Sustain. Chem. Eng. 2020, 8, 4941–4947. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar]
- Murakami, S.; Aoki, N.; Matsumura, S. Bio-based biodegradable hydrogels prepared by crosslinking of microbial poly(γ-glutamic acid) with L-lysine in aqueous solution. Polym. J. 2011, 43, 414–420. [Google Scholar] [CrossRef]
- Rahman, L.; Goswami, J. Poly(Vinyl Alcohol) as Sustainable and Eco-Friendly Packaging: A Review. J. Packag. Technol. Res. 2023, 7, 1–10. [Google Scholar] [CrossRef]
- Bandelli, D.; Casini, A.; Guaragnone, T.; Baglioni, M.; Mastrangelo, R.; Pensabene Buemi, L.; Chelazzi, D.; Baglioni, P. Tailoring the properties of poly(vinyl alcohol) “twin-chain” gels via sebacic acid decoration. J. Colloid Interface Sci. 2024, 657, 178–192. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Licchelli, M. Heritage Conservation and Restoration: Surface Characterization, Cleaning and Treatments. Coatings 2023, 13, 457. [Google Scholar] [CrossRef]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Bandelli, D.; Alex, J.; Helbing, C.; Ueberschaar, N.; Görls, H.; Bellstedt, P.; Weber, C.; Jandt, K.D.; Schubert, U.S. Poly(3-ethylglycolide): A well-defined polyester matching the hydrophilic hydrophobic balance of PLA. Polym. Chem. 2019, 10, 5440–5451. [Google Scholar] [CrossRef]
- Bandelli, D.; Alex, J.; Weber, C.; Schubert, U.S. Polyester Stereocomplexes Beyond PLA: Could Synthetic Opportunities Revolutionize Established Material Blending? Macromol. Rapid Commun. 2020, 41, 1900560. [Google Scholar] [CrossRef]
- Wan Osman, W.N.A.; Rosli, M.H.; Mazli, W.N.A.; Samsuri, S. Comparative review of biodiesel production and purification. Carbon Capture Sci. Technol. 2024, 13, 100264. [Google Scholar] [CrossRef]
- Porcu, D.; Innocenti, S.; Galeotti, M.; Striova, J.; Dei, L.; Carretti, E.; Fontana, R. Spectroscopic and Morphologic Investigation of Bronze Disease: Performance Evaluation of Portable Devices. Heritage 2022, 5, 3548–3561. [Google Scholar] [CrossRef]
- Kwon, H. Corrosion Behaviors of Artificial Chloride Patina for Studying Bronze Sculpture Corrosion in Marine Environments. Coatings 2023, 13, 1630. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D. Emerging Ionic Soft Materials Based on Deep Eutectic Solvents. J. Phys. Chem. B 2020, 124, 8465–8478. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Sarma, S.J.; Brar, S.K. A comprehensive review of castor oil-derived renewable and sustainable industrial products. Environ. Prog. Sustain. Energy 2023, 42, e14008. [Google Scholar] [CrossRef]
- Chakraborty, I.; Chatterjee, K. Polymers and Composites Derived from Castor Oil as Sustainable Materials and Degradable Biomaterials: Current Status and Emerging Trends. Biomacromolecules 2020, 21, 4639–4662. [Google Scholar] [CrossRef]
- Zuliani, A.; Bandelli, D.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Environmentally friendly ZnO/Castor oil polyurethane composites for the gas-phase adsorption of acetic acid. J. Colloid Interface Sci. 2022, 614, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, A.; Chelazzi, D.; Mastrangelo, R.; Giorgi, R.; Baglioni, P. Adsorption kinetics of acetic acid into ZnO/castor oil-derived polyurethanes. J. Colloid Interface Sci. 2023, 632, 74–86. [Google Scholar] [CrossRef]
- Khattab, M.M.; Dahman, Y. Production and recovery of poly-3-hydroxybutyrate bioplastics using agro-industrial residues of hemp hurd biomass. Bioprocess Biosyst. Eng. 2019, 42, 1115–1127. [Google Scholar] [CrossRef]
- Biribicchi, C.; Giuliani, L.; Macchia, A.; Favero, G. Organogels for Low-Polar Organic Solvents: Potential Applications on Cultural Heritage Materials. Sustainability 2023, 15, 16305. [Google Scholar] [CrossRef]
- Murdan, S.; Gregoriadis, G.; Florence, A. Non-ionic surfactant based organogels incorporating niosomes. S.T.P. Pharma Sci. 1996, 6, 44–48. [Google Scholar]
- Prathap, A.; Sureshan, K.M. Sugar-Based Organogelators for Various Applications. Langmuir 2019, 35, 6005–6014. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, D.; Bordes, R.; Casini, A.; Mastrangelo, R.; Holmberg, K.; Baglioni, P. New perspectives on green and sustainable wet cleaning systems for art conservation. Soft Matter 2025, 21, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
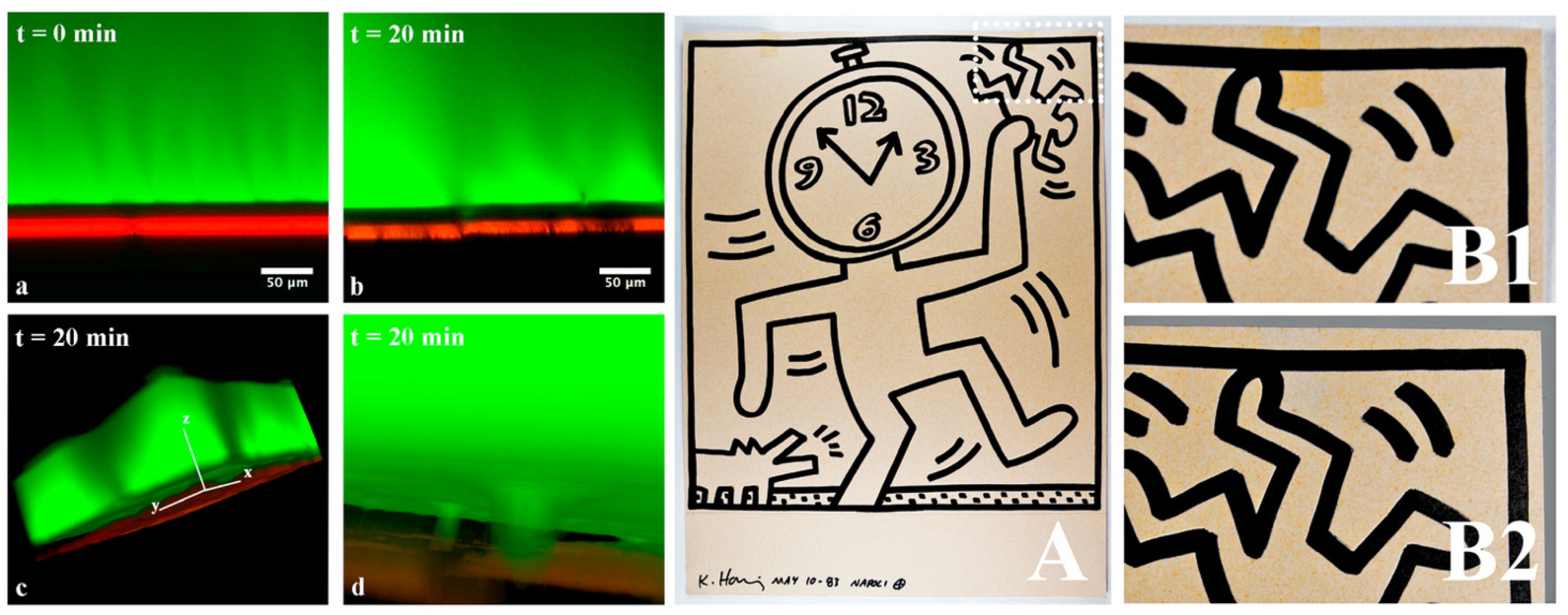
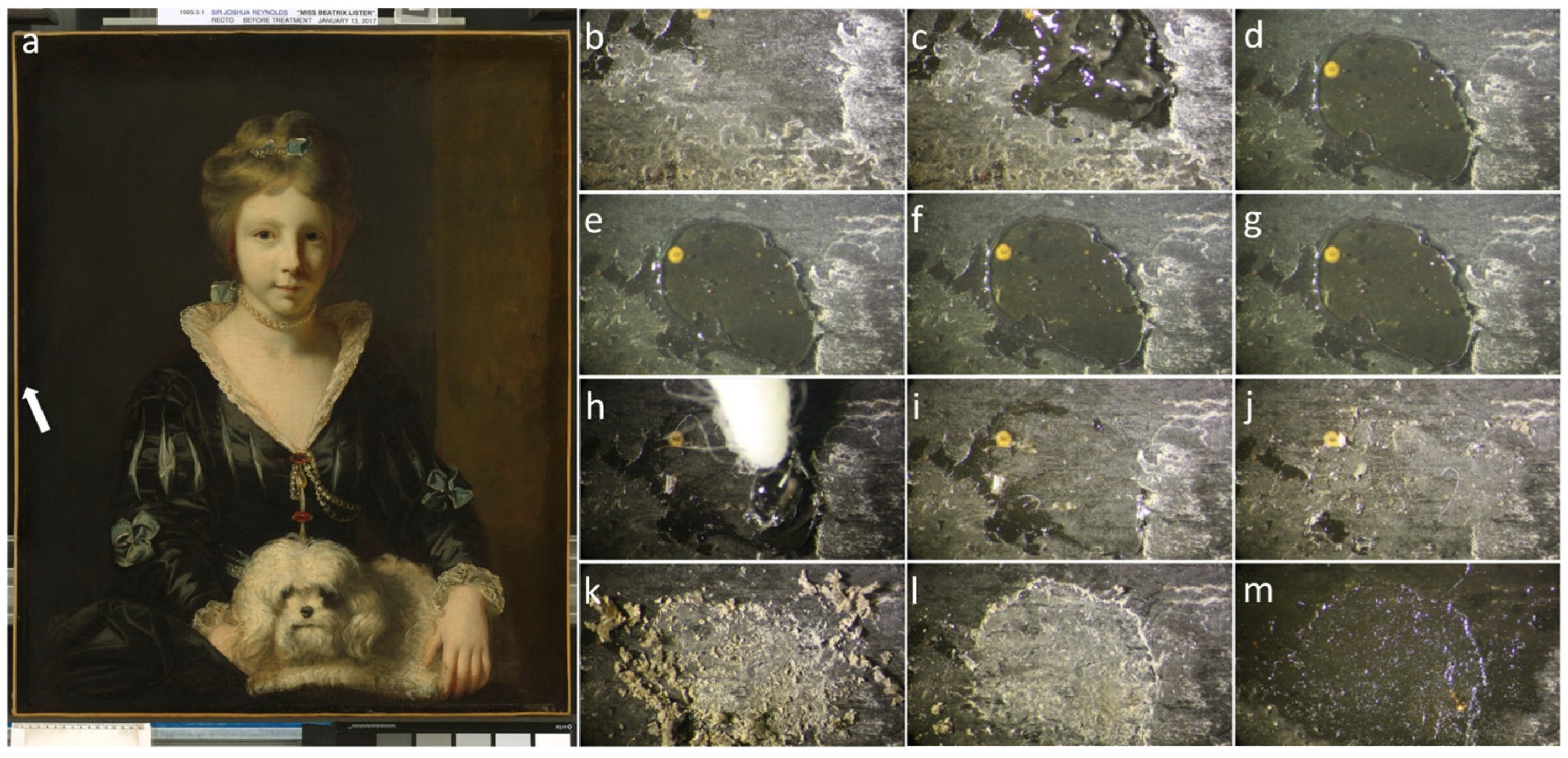
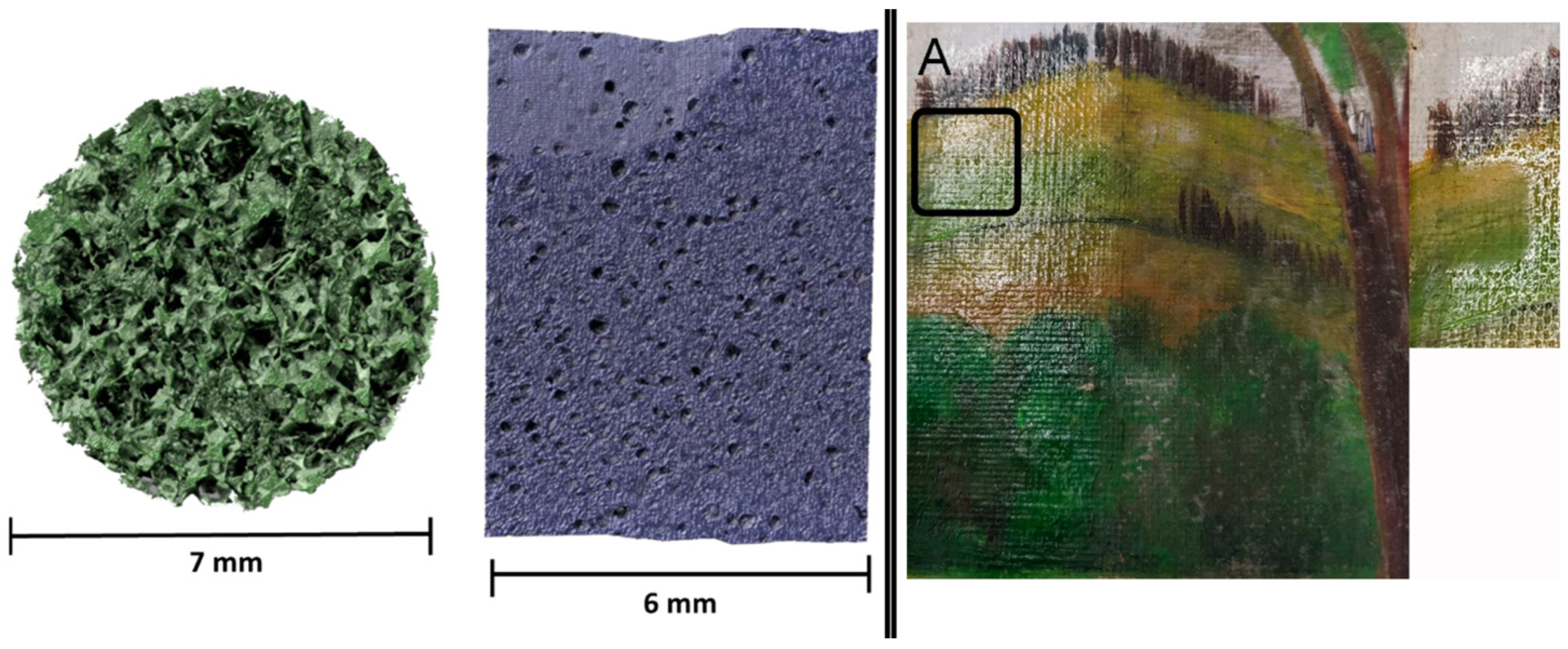
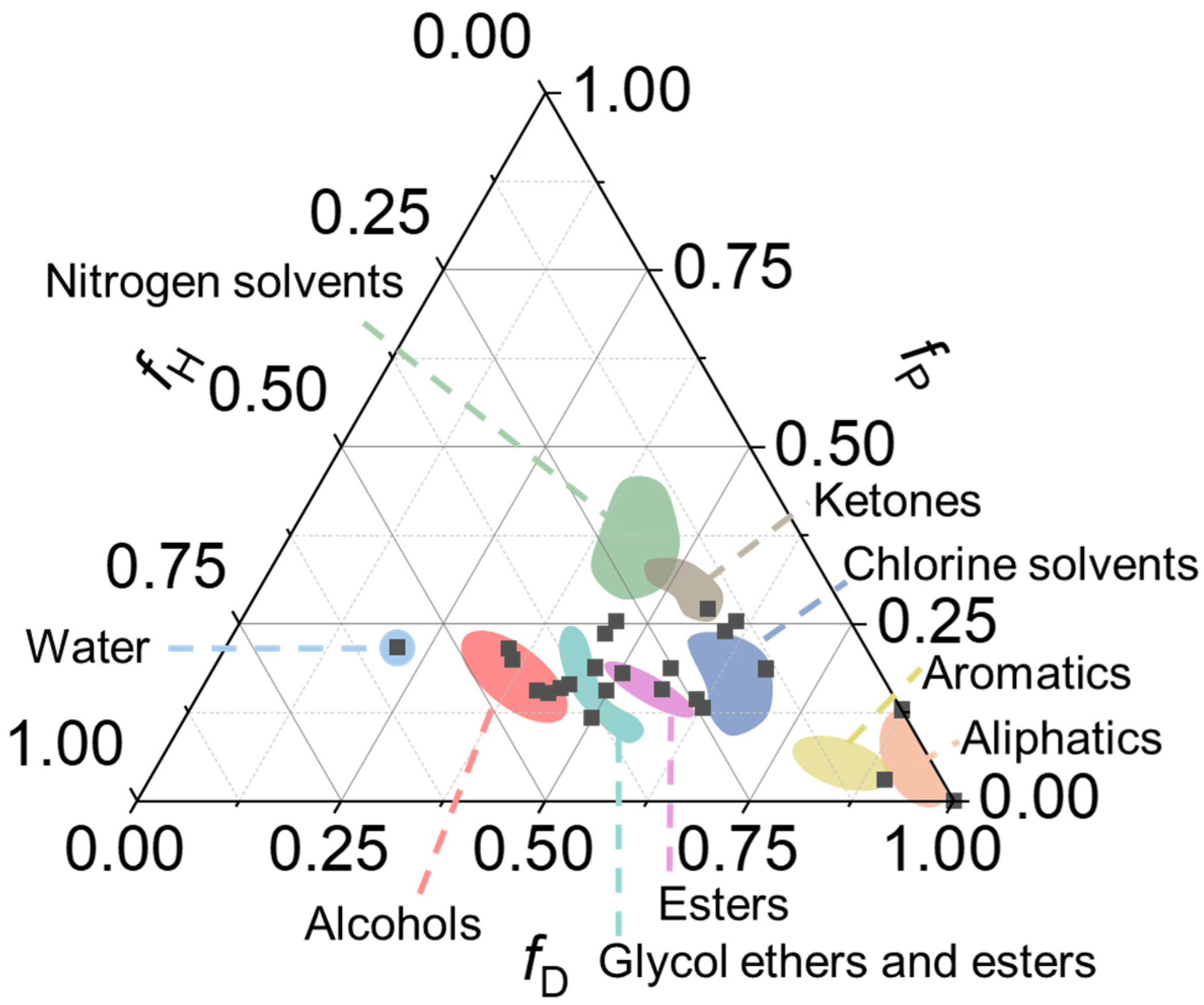
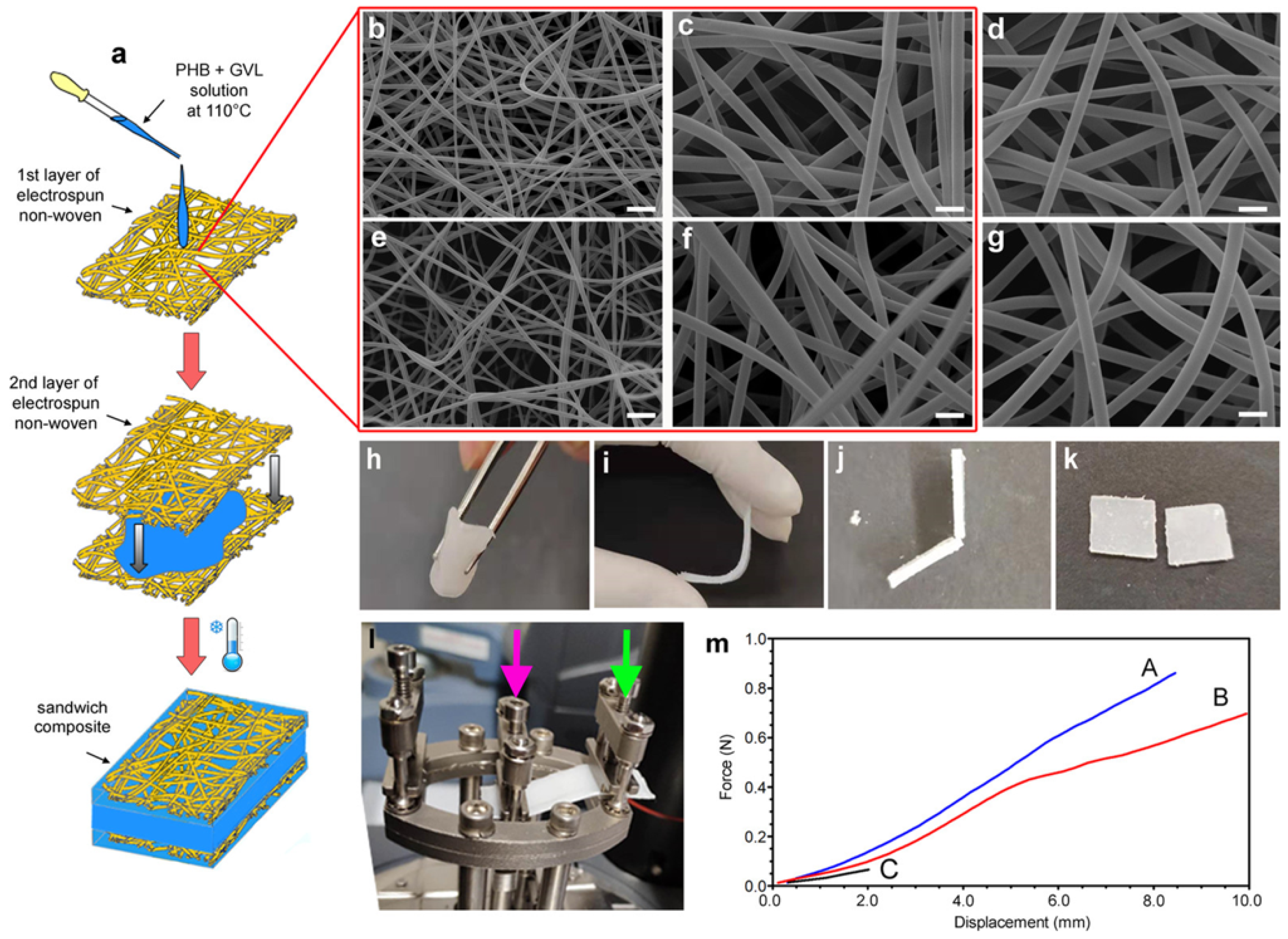

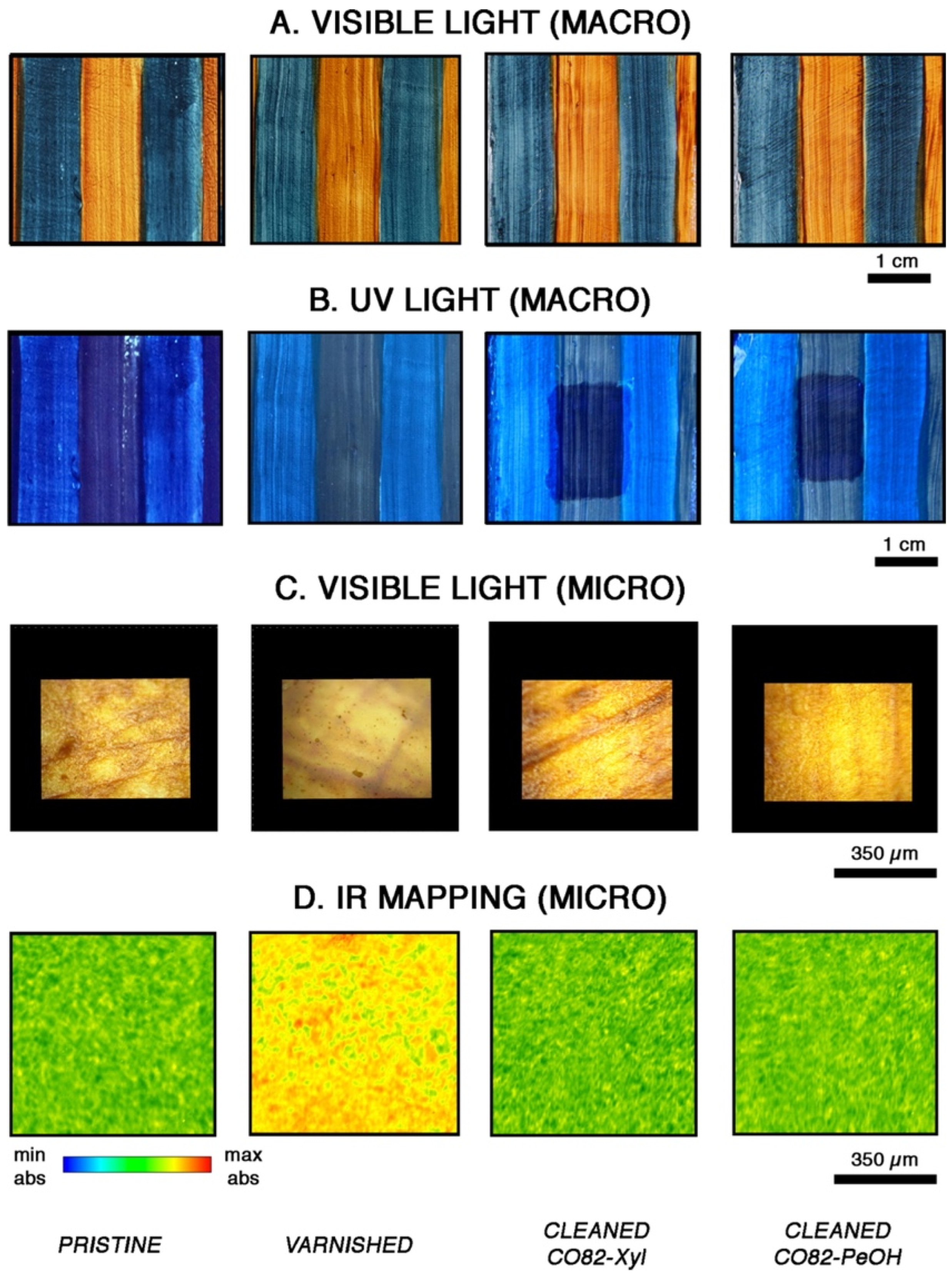
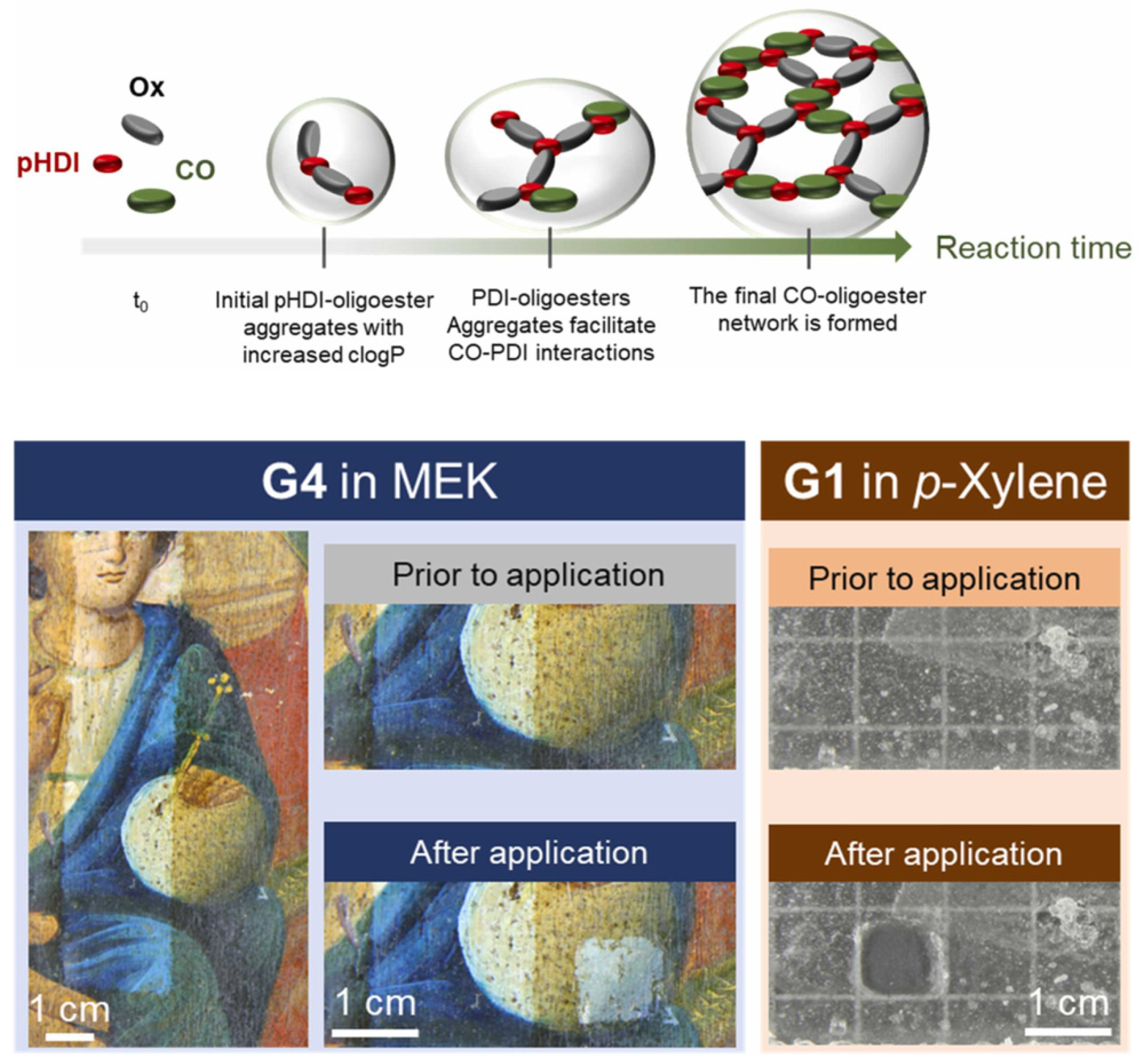
| Code | Type | Mechanical Properties (Rheology, G’) | Solvents | Cleaning Efficiency | Case Studies | Ref. |
|---|---|---|---|---|---|---|
| Solvent gel | Thickened polymer dispersion | 100–120 Pa | Alcohols, ketones, esters, aliphatic and aromatic hydrocarbons | FTIR, SEM, chromatography techniques | Widely spread in traditional practice | [7,46,47,48,49,50] |
| Traditional organogel formulations | ||||||
| PMMA | Chemical gel | Hard gels | MEK, ethyl acetate, cyclohexanone, butyl acetate, DEC | FTIR | Paper artworks, canvas paintings | [54,58,59,60] |
| PAA-CO2 | HVPD | >100 kPa | Acetic acid with methanol, ethanol-octanol | FTIR | Canvas painting, wood | [56,57] |
| PVA-borax | HVPD | Max ca. 1 kPa | Dimethyl sulfoxide, 2-ethoxyethanol, dimethylformamide, methanol, 1-methyl-2-pyrrolidinone, tetrahydrafuran, 95:5 ethanol/water | NMR, FTIR | Gilded wood, canvas paintings, marble | [61,62,64,65] |
| PDMS organogels | Chemical gel | >100 kPa | Acetone, DEC, DMSO, ethyl acetate, ethanol, water, isopropanol, cyclohexane | FTIR | Frescos, canvas paintings | [68] |
| Bio-derived organogel formulations | ||||||
| COPU-Ox | Chemical gel | >80 kPa | Acetone, MEK, p-xylene | N/A | Glass | [76] |
| Rice bran oil and cinnamic acid | Physical gel | 1–100 kPa | N/A | N/A | N/A | [77] |
| Vegetable and mineral oil | Physical gels | Vegetable max 10 kPa, mineral max 3000–6000 Pa | N/A | N/A | N/A | [78] |
| PHB | Physical gels | 20–200 kPa, or 1–300 kPa | G-valerolactone, biodiesel/dimethyl carbonate, ethyl lactate | ATR, GC-MS | Bronze, canvas paintings | [79,80,81,82,83,84] |
| PVA, ethylene glycol and choline chloride | Physical gels | 80–200 kPa | Ethylene glycol | ATR | Tempera, mural paintings | [85] |
| Choline chloride, urea and agar | Physical gels | Ca. 300 kPa | 1:1 water ethanol mixture | ATR | Oil and tempera | [86] |
| Cellulose ethers | Physical gels | N/A | Methyl myristate, isopropyl palmitate, Ligroin, ethanol | FTIR | Copper, canvas paintings | [87,88] |
| Isosorbide, pyrogallol, and limonene | Chemical gels | N/A | Acetone, ethyl acetate, DMC, hexane, limonene | FTIR | Canvas paintings | [89] |
| COPUs | Chemical gels | >20 kPa | p-xylene, pentanol | FTIR 2D Imaging | Canvas paintings | [90,91] |
| COPU-Ox | Chemical gel | >80 kPa | Acetone, MEK, p-xylene | N/A | Canvas paintings | [92] |
| COPUOxazolidones | Chemical gels | >20 kPa | DEC, MEK, Acetone | N/A | N/A | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandelli, D.; Adamo, C.; Poggi, G.; Chelazzi, D.; Baglioni, P. Organogels for the Preservation of Cultural Heritage. Gels 2025, 11, 715. https://doi.org/10.3390/gels11090715
Bandelli D, Adamo C, Poggi G, Chelazzi D, Baglioni P. Organogels for the Preservation of Cultural Heritage. Gels. 2025; 11(9):715. https://doi.org/10.3390/gels11090715
Chicago/Turabian StyleBandelli, Damiano, Céline Adamo, Giovanna Poggi, David Chelazzi, and Piero Baglioni. 2025. "Organogels for the Preservation of Cultural Heritage" Gels 11, no. 9: 715. https://doi.org/10.3390/gels11090715
APA StyleBandelli, D., Adamo, C., Poggi, G., Chelazzi, D., & Baglioni, P. (2025). Organogels for the Preservation of Cultural Heritage. Gels, 11(9), 715. https://doi.org/10.3390/gels11090715







