3D-Bioprinting of Stromal Vascular Fraction for Gastrointestinal Regeneration
Abstract
1. Introduction
2. Results and Discussion
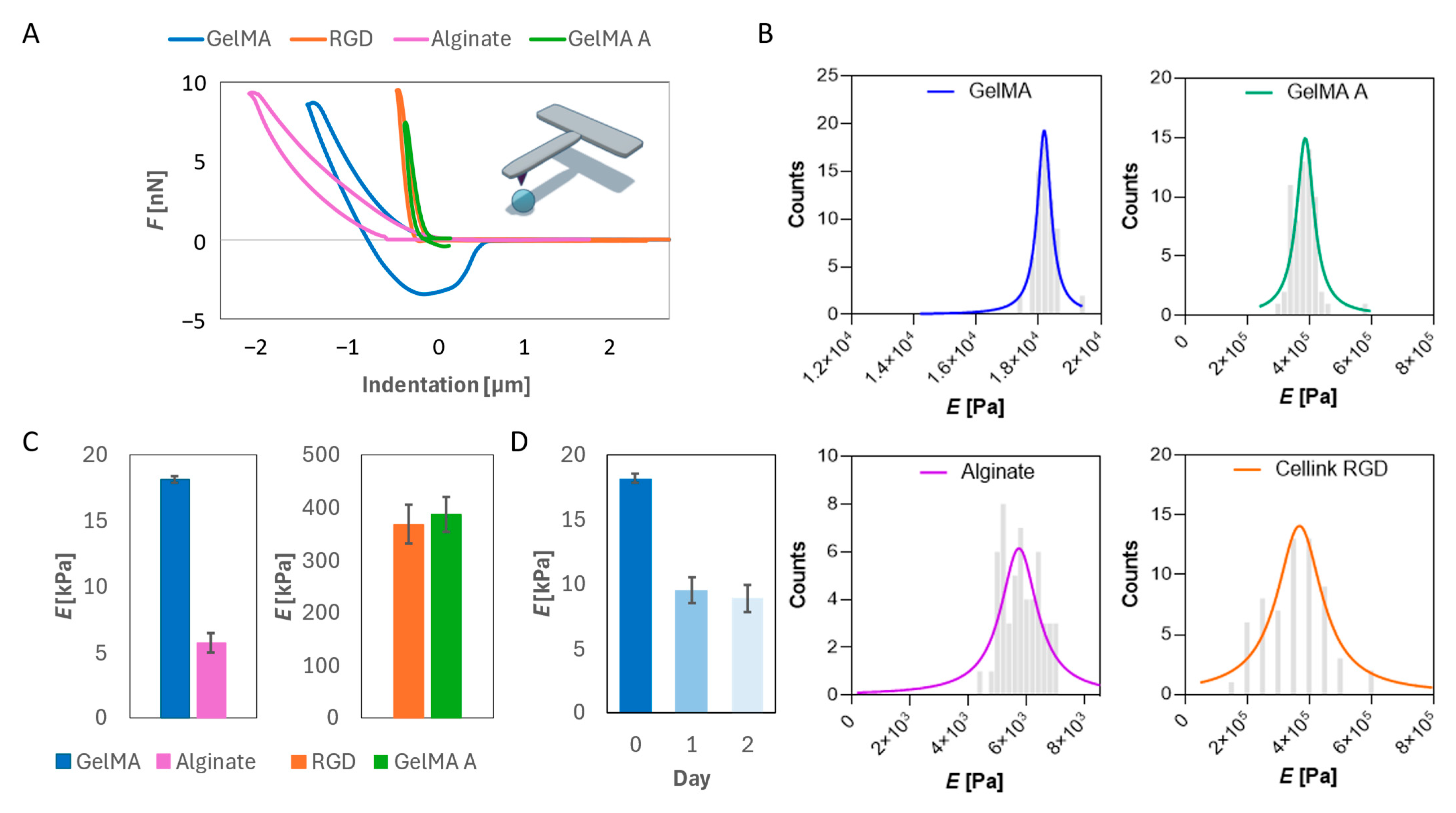
2.1. Mechanical Characterization of Hydrogels
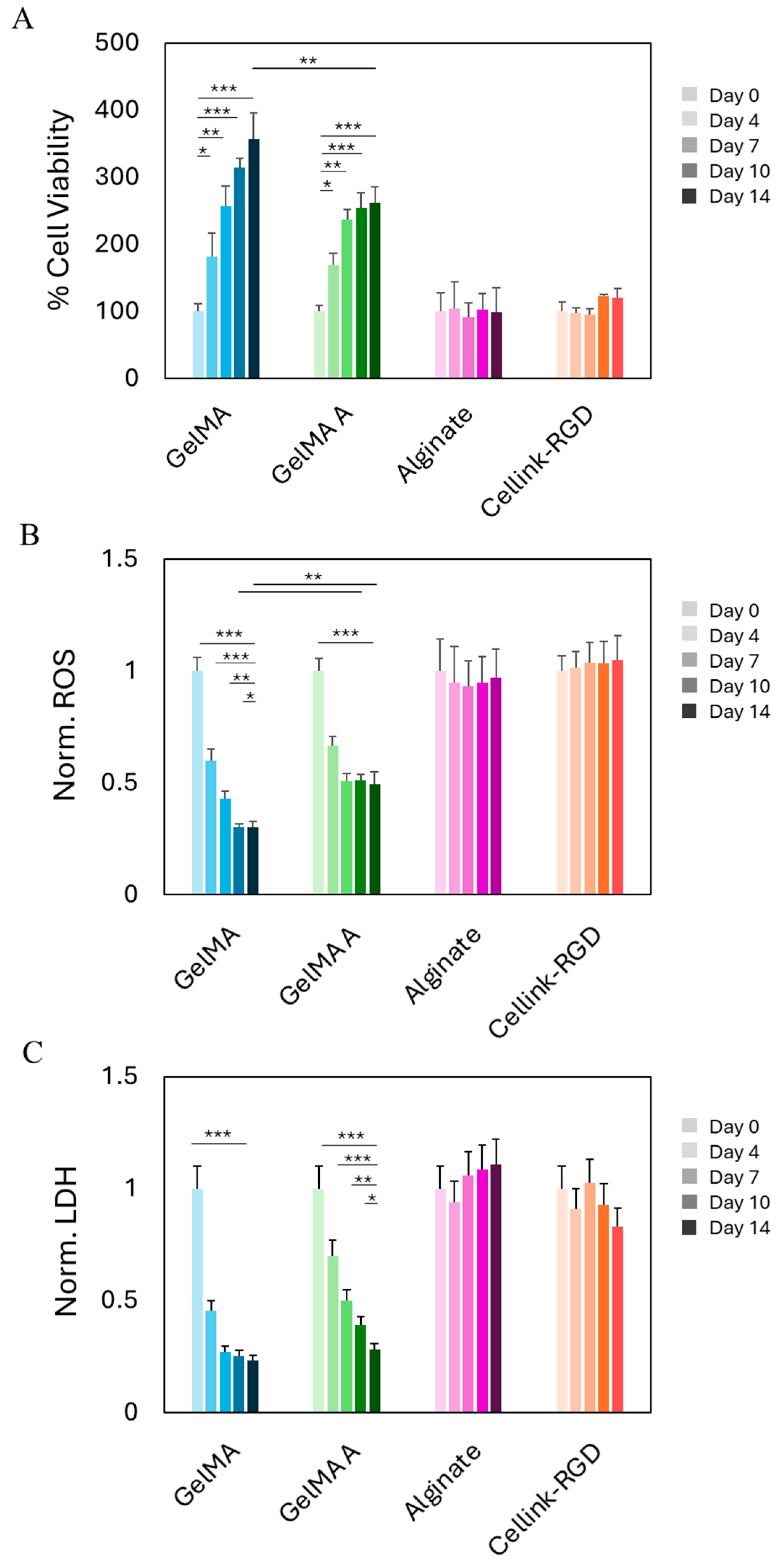
2.2. Biocompatibility of Hydrogels After Bioprinting
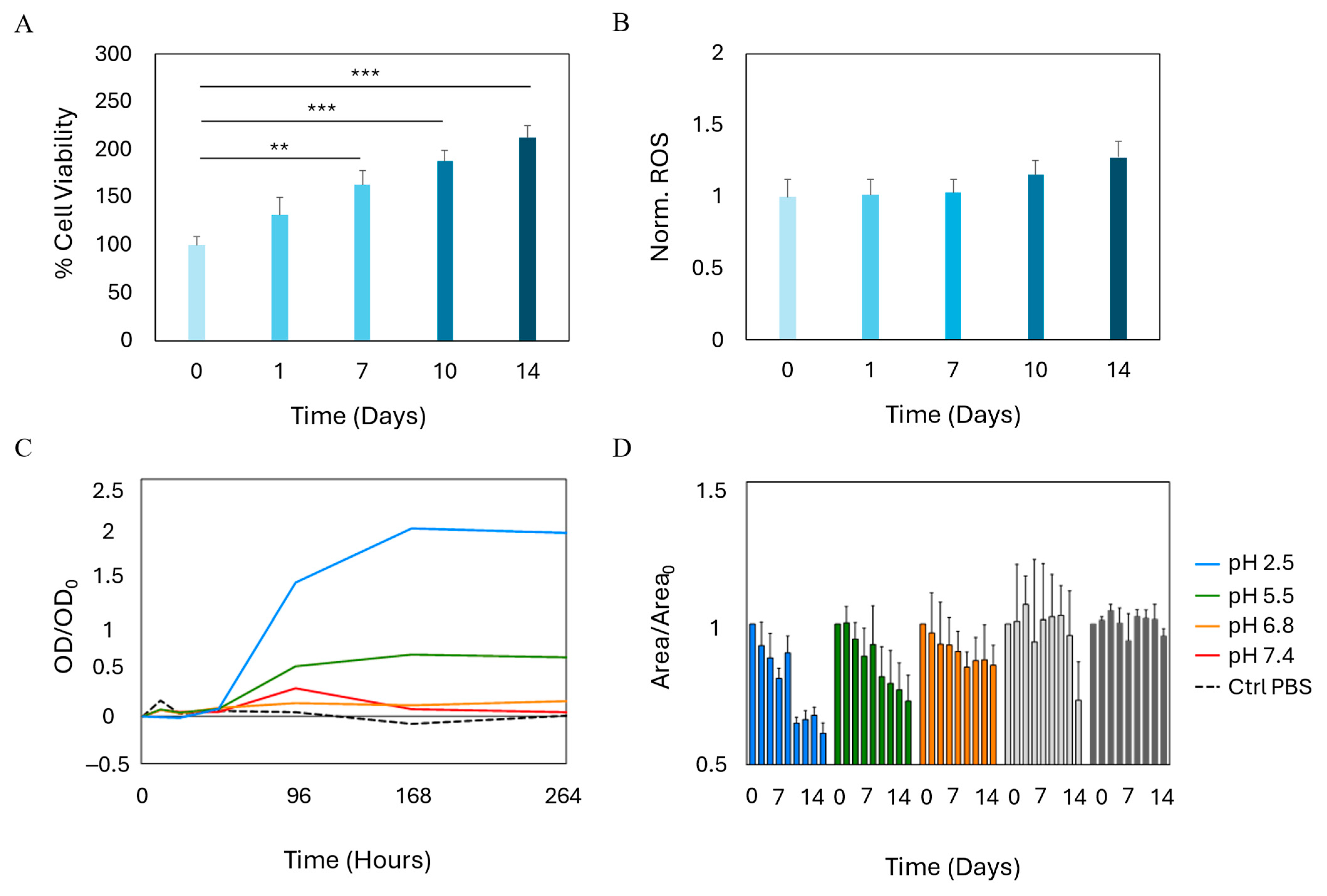
2.3. Growth and Stability of Bioprinted SVF
2.4. SVF-Enhanced Intestinal Epithelial Regeneration
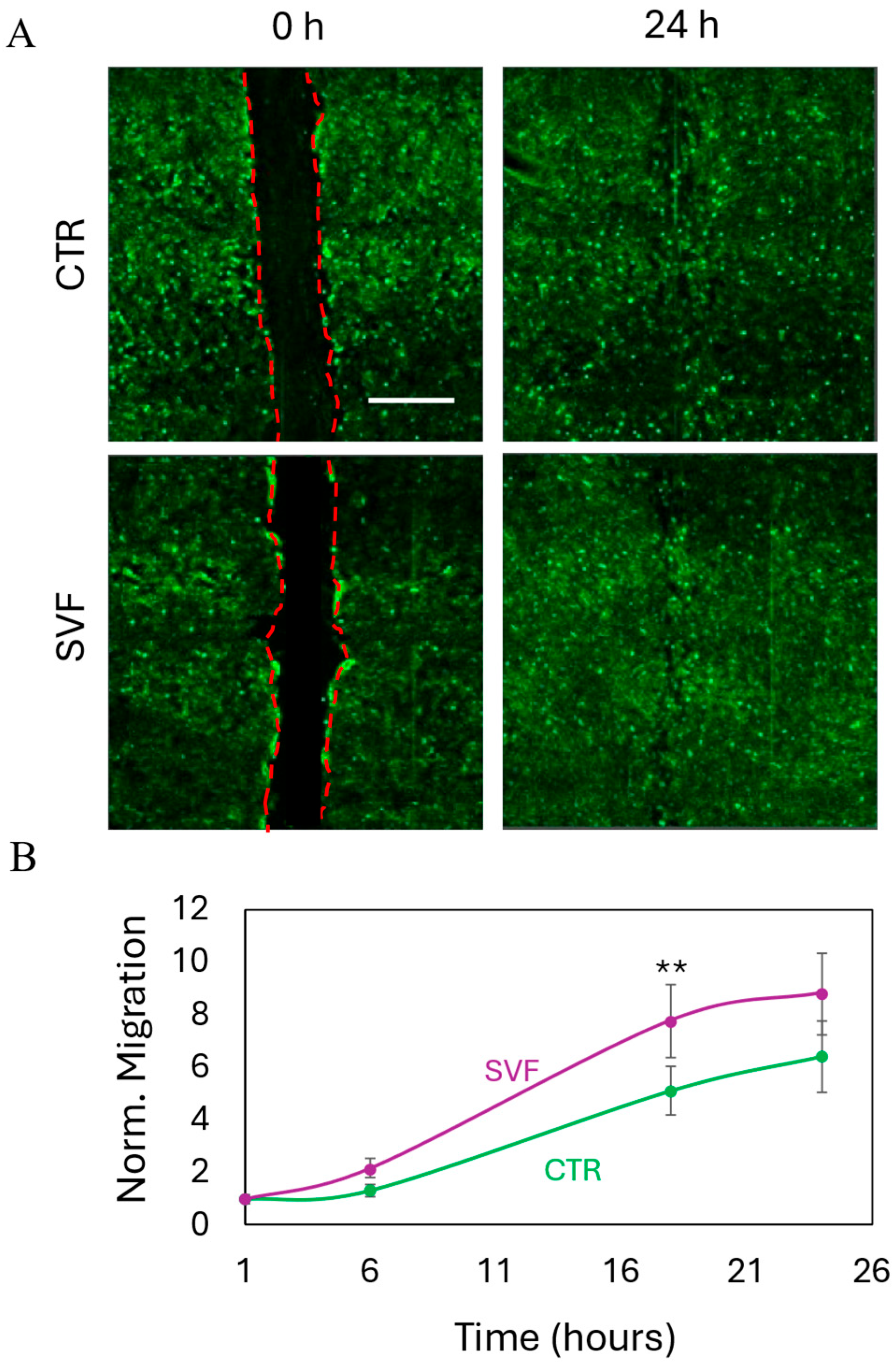
2.5. Wound Healing
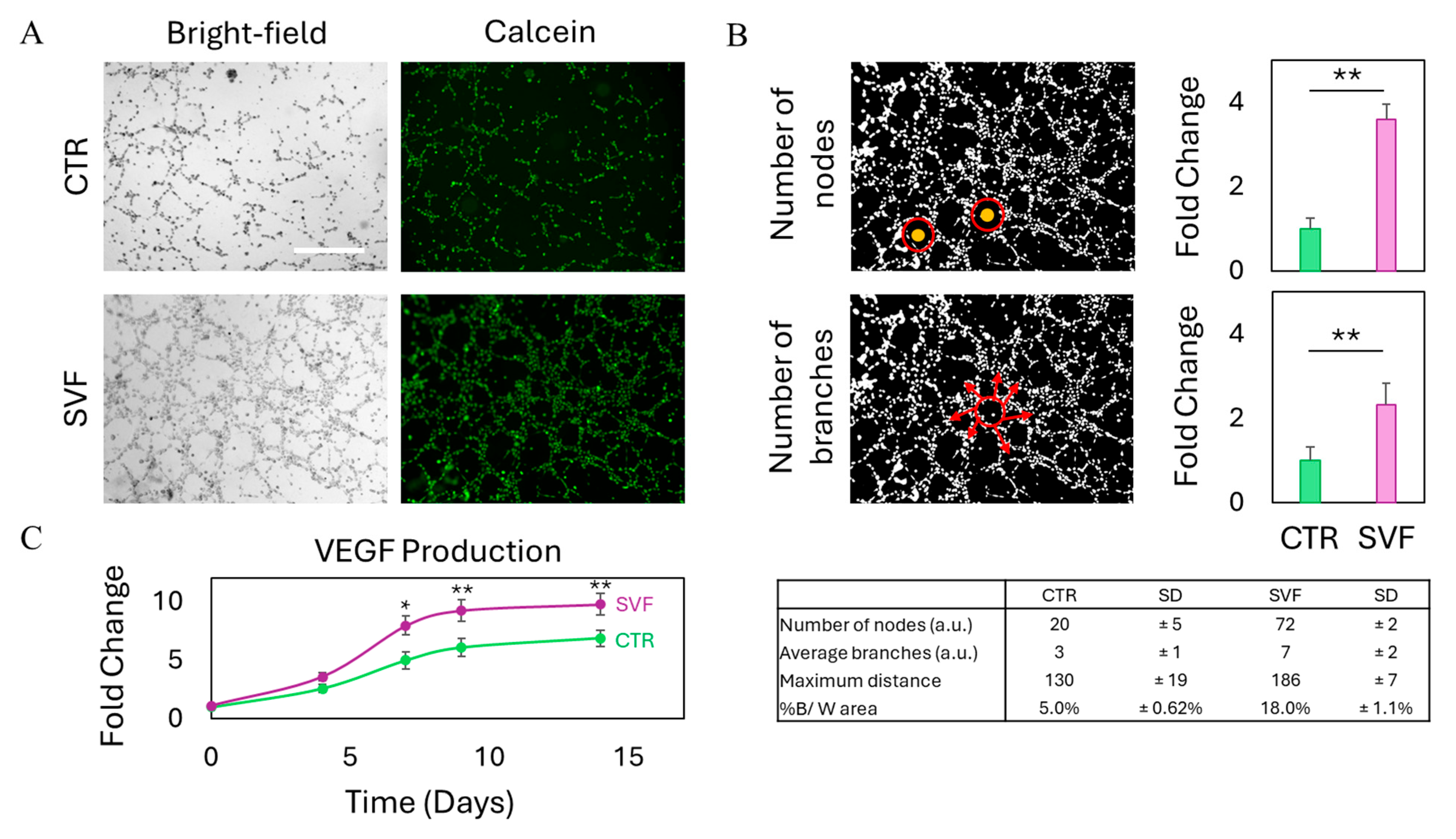
2.6. Tube Formation Assay
3. Conclusions
4. Materials and Methods
4.1. Hydrogel Preparation
4.2. Atomic Force Microscopy
4.3. Cell Culture
4.4. Isolation of SVF
4.5. Bioprinting of BM-MSCs and SVF
4.6. Degradation of Bioprinted SVF
4.7. Cell Viability
4.8. Trans-Epithelial Electrical Resistance
4.9. Reactive Oxygen Species Production
4.10. Lactate Dehydrogenase Release
4.11. Wound Healing Assay
4.12. Tube Formation Assay
4.13. ELISA Assay
4.14. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nachira, D.; Trivisonno, A.; Costamagna, G.; Toietta, G.; Margaritora, S.; Pontecorvi, V.; Punzo, G.; Porziella, V.; Boškoski, I. Successful Therapy of Esophageal Fistulas by Endoscopic Injection of Emulsified Adipose Tissue Stromal Vascular Fraction. Gastroenterology 2021, 160, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Boskoski, I.; Pontecorvi, V.; Caretto, A.A.; Nachira, D.; Bove, V.; Papi, M.; De Siena, M.; Matteo, M.V.; Gualtieri, L.; Margaritora, S.; et al. Endoscopic injection of autologous fat tissue for the treatment of chronic gastrointestinal fistulas. Gut 2025, 0, 1–4. [Google Scholar] [CrossRef]
- Pontecorvi, V.; Nachira, D.; Margaritora, S.; Trivisonno, A.; Papi, M.; Matteo, M.V.; Vincenzo, B.; De Siena, M.; Gualtieri, L.; A Caretto, A.; et al. Regenerative Endoscopy for the treatment of difficult gastrointestinal defects. Endoscopy 2025, 57, OP117. [Google Scholar] [CrossRef]
- Parian, A.M.; Obi, M.; Fleshner, P.; Schwartz, D.A. Management of perianal Crohn’s disease. Off. J. Am. Coll. Gastroenterol. ACG 2023, 118, 1323–1331. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef]
- Agrawal, M.; Jess, T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United Eur. Gastroenterol. J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef]
- Coward, S.; Benchimol, E.I.; Kuenzig, M.E.; Windsor, J.W.; Bernstein, C.N.; Bitton, A.; Jones, J.L.; Lee, K.; Murthy, S.K.; E Targownik, L.; et al. The 2023 impact of inflammatory bowel disease in Canada: Epidemiology of IBD. J. Can. Assoc. Gastroenterol. 2023, 6 (Suppl. S2), S9–S15. [Google Scholar] [CrossRef]
- Matteo, M.V.; Birligea, M.M.; Bove, V.; Pontecorvi, V.; De Siena, M.; Gualtieri, L.; Barbaro, F.; Spada, C.; Boškoski, I. Management of fistulas in the upper gastrointestinal tract. Best. Pract. Res. Clin. Gastroenterol. 2024, 70, 101929. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Boskoski, I.; Vincenzoni, C.; Montanari, F.; Tinelli, G.; Donati, T.; Tshomba, Y. A new mini-invasive approach for a catastrophic disease: Staged endovascular and endoscopic treatment of aorto-esophageal fistulas. J. Pers. Med. 2022, 12, 1735. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Saha, K.; Nighot, P. Intestinal Epithelial Tight Junction Barrier Regulation by Novel Pathways. Inflamm. Bowel Dis. 2025, 31, 259–271. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Papavassiliou, A.G. Stabilizing the integrity of intestinal barrier to control arthritis. Arthritis Res. Ther. 2024, 26, 135. [Google Scholar] [CrossRef]
- Serek, P.; Oleksy-Wawrzyniak, M. The effect of bacterial infections, probiotics and zonulin on intestinal barrier integrity. Int. J. Mol. Sci. 2021, 22, 11359. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of inflammatory bowel disease: Innate immune system. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Kałużna, A.; Olczyk, P.; Komosińska-Vassev, K. The role of innate and adaptive immune cells in the pathogenesis and development of the inflammatory response in ulcerative colitis. J. Clin. Med. 2022, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- La Mendola, D.; Trincavelli, M.L.; Martini, C. Angiogenesis in disease. Int. J. Mol. Sci. 2022, 23, 10962. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Gene therapy to enhance angiogenesis in chronic wounds. Mol. Ther. Nucleic Acids 2022, 29, 871–899. [Google Scholar] [CrossRef]
- Papait, A.; Perini, G.; Palmieri, V.; Cargnoni, A.; Vertua, E.; Pasotti, A.; Rosa, E.; De Spirito, M.; Silini, A.R.; Papi, M.; et al. Defining the Immunological Compatibility of Graphene Oxide-Loaded PLGA Scaffolds For biomedical Applications. Biomater. Adv. 2024, 165, 214024. [Google Scholar] [CrossRef] [PubMed]
- De Spirito, M.; Palmieri, V.; Perini, G.; Papi, M. Bridging the gap: Integrating 3D bioprinting and microfluidics for advanced multi-organ models in biomedical research. Bioengineering 2024, 11, 664. [Google Scholar] [CrossRef]
- De Gregorio, M.; Tiang, T.; Lee, T.; Stellingwerf, M.E.; Singh, S.; Thompson, A.J.; D’Souza, B.; Ding, N.S.; Group, S.I.B.D.C. Autologous fat graft injections for the treatment of perianal fistulas in Crohn’s disease: A systematic review and single-arm meta-analysis. ANZ J. Surg. 2023, 93, 1162–1168. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Stevens, H.P.; Parvizi, M.; van der Lei, B.; Harmsen, M.C. The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair Regen. 2016, 24, 994–1003. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Boyd, N.L. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng. Part B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef]
- Svalgaard, J.D.; Juul, S.; Vester-Glovinski, P.V.; Haastrup, E.K.; Ballesteros, O.R.; Lynggaard, C.D.; Jensen, A.K.; Fischer-Nielsen, A.; Herly, M.; Munthe-Fog, L. Lipoaspirate storage time and temperature: Effects on stromal vascular fraction quality and cell composition. Cells Tissues Organs 2020, 209, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, J.; Vonk, L.A.; Stevens, H.P.; van Dongen, J.A. Mechanically derived tissue stromal vascular fraction acts anti-inflammatory on TNF alpha-stimulated chondrocytes in vitro. Bioengineering 2022, 9, 345. [Google Scholar] [CrossRef]
- Klingenberg, M.; Dineva, A.; Hoyer, A.; Kaltschmidt, B.; Leimkühler, P.; Vordemvenne, T.; Elsner, A.; Wähnert, D. Injection of autologous adipose stromal vascular fraction in combination with autologous conditioned plasma for the treatment of advanced knee osteoarthritis significantly improves clinical symptoms. J. Clin. Med. 2024, 13, 3031. [Google Scholar] [CrossRef]
- Han, X.; Ji, D.; Liu, Y.; Hu, S. Efficacy and safety of transplantation of autologous fat, platelet-rich plasma (PRP) and stromal vascular fraction (SVF) in the treatment of acne scar: Systematic review and meta-analysis. Aesthetic Plast. Surg. 2023, 47, 1623–1632. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.J.; Lee, Y.J.; Lee, J.C.; Kim, J.H.; Kim, D.H.; Do, Y.H.; Choi, J.W.; Chung, S.I.; Do, B.-R. Innovative method of alopecia treatment by autologous adipose-derived SVF. Stem Cell Res. Ther. 2021, 12, 486. [Google Scholar] [CrossRef]
- Daumas, A.; Magalon, J.; Jouve, E.; Casanova, D.; Philandrianos, C.; Abellan Lopez, M.; Mallet, S.; Veran, J.; Auquit-Auckbur, I.; Farge, D.; et al. Adipose tissue-derived stromal vascular fraction for treating hands of patients with systemic sclerosis: A multicentre randomized trial Autologous AD-SVF versus placebo in systemic sclerosis. Rheumatology 2022, 61, 1936–1947. [Google Scholar] [CrossRef]
- Perini, G.; Minopoli, A.; Zambrano, D.; Cui, L.; Ferrara, V.; Perfili, C.; Artemi, G.; De Spirito, M.; Palmieri, V.; Rosenkranz, A.; et al. Impact of different 2D materials on the efficacy of photothermal and photodynamic therapy in 3D-bioprinted breast cancer. Nanoscale 2025, 17, 3221–3235. [Google Scholar] [CrossRef]
- Balko, S.; Kerr, E.; Buchel, E.; Logsetty, S.; Raouf, A. Paracrine signalling between keratinocytes and SVF cells results in a new secreted cytokine profile during wound closure. Stem Cell Res. Ther. 2023, 14, 258. [Google Scholar] [CrossRef]
- Papi, M.; Sylla, L.; Parasassi, T.; Brunelli, R.; Monaci, M.; Maulucci, G.; Missori, M.; Arcovito, G.; Ursini, F.; De Spirito, M. Evidence of elastic to plastic transition in the zona pellucida of oocytes using atomic force spectroscopy. Appl. Phys. Lett. 2009, 94, 153902. [Google Scholar] [CrossRef]
- Deng, W.; Tang, Y.; Mao, J.; Zhou, Y.; Chen, T.; Zhu, X. Cellulose Nanofibril as a Crosslinker to Reinforce the Sodium Alginate/Chitosan Hydrogels. Int. J. Biol. Macromol. 2021, 189, 890–899. [Google Scholar] [CrossRef]
- Amato, F.; Fazi, M.; Giaccari, L.; Colecchia, S.; Perini, G.; Palmieri, V.; Papi, M.; Altimari, P.; Motta, A.; Giustini, M.; et al. Isolation by dialysis and characterization of luminescent oxidized carbon nanoparticles from graphene oxide dispersions: A facile novel route towards a more controlled and homogeneous substrate with a wider applicability. Nanotechnology 2025, 36, 185602. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, C.; Xu, Y.; Huang, K.; Wang, Y.; Wang, X.; Zhou, X.; Pang, W.; Yang, G.; Yu, T. Adipose-specific BMP and activin membrane-bound inhibitor (BAMBI) deletion promotes adipogenesis by accelerating ROS production. J. Biol. Chem. 2021, 296, 100037. [Google Scholar] [CrossRef]
- Oh, J.M.; Chun, S. Ginsenoside CK inhibits the early stage of adipogenesis via the AMPK, MAPK, and AKT signaling pathways. Antioxidants 2022, 11, 1890. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair. Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Kanaganti, S.; Banerjee, R.; Ramchandani, M.; Nabi, Z.; Reddy, D.N.; Tandan, M. Systematic review of endoscopic management of stricture, fistula and abscess in inflammatory bowel disease. Gastroenterol. Insights 2023, 14, 45–63. [Google Scholar] [CrossRef]
- Ganguly, S.; Tang, X. 3D Printing of High Strength Thermally Stable Sustainable Lightweight Corrosion-Resistant Nanocomposite by Solvent Exchange Postprocessing. ACS Sustain. Chem. Eng. 2024, 13, 423–435. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y.; Ruan, K.; Guo, H.; He, M.; Shi, X.; Guo, Y.; Kong, J.; Gu, J. Advances in 3D Printing for Polymer Composites: A Review; InfoMat; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2024. [Google Scholar]
- Villablanca, E.J.; Selin, K.; Hedin, C.R.H. Mechanisms of mucosal healing: Treating inflammatory bowel disease without immunosuppression? Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The impact of prolonged inflammation on wound healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota–gut–brain axis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 222–247. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.J.; Poling, H.M.; Chen, K.; Tsai, Y.H.; Wu, A.; Vallie, A.; Eiken, M.K.; Huang, S.; Sweet, C.W.; Schreiner, R.; et al. Coordinated differentiation of human intestinal organoids with functional enteric neurons and vasculature. Cell Stem Cell 2025, 32, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Orduno, M.; Liang, Z.; Gong, X.; Mak, M. Optimization of vascularized intestinal organoid model. Adv. Healthc. Mater. 2024, 13, 2400977. [Google Scholar] [CrossRef]
- Liu, M.; Charek, J.G.; Vicetti Miguel, R.D.; Cherpes, T.L. Ephrin-Eph signaling: An important regulator of epithelial integrity and barrier function. Tissue Barriers 2025, 2462855. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perini, G.; Montescagli, M.; Di Giulio, G.; Augello, A.; Ferrara, V.; Minopoli, A.; Evangelista, D.; Marras, M.; Artemi, G.; Caretto, A.A.; et al. 3D-Bioprinting of Stromal Vascular Fraction for Gastrointestinal Regeneration. Gels 2025, 11, 712. https://doi.org/10.3390/gels11090712
Perini G, Montescagli M, Di Giulio G, Augello A, Ferrara V, Minopoli A, Evangelista D, Marras M, Artemi G, Caretto AA, et al. 3D-Bioprinting of Stromal Vascular Fraction for Gastrointestinal Regeneration. Gels. 2025; 11(9):712. https://doi.org/10.3390/gels11090712
Chicago/Turabian StylePerini, Giordano, Margherita Montescagli, Giada Di Giulio, Alberto Augello, Valeria Ferrara, Antonio Minopoli, Davide Evangelista, Matteo Marras, Giulia Artemi, Anna Amelia Caretto, and et al. 2025. "3D-Bioprinting of Stromal Vascular Fraction for Gastrointestinal Regeneration" Gels 11, no. 9: 712. https://doi.org/10.3390/gels11090712
APA StylePerini, G., Montescagli, M., Di Giulio, G., Augello, A., Ferrara, V., Minopoli, A., Evangelista, D., Marras, M., Artemi, G., Caretto, A. A., Gentileschi, S., Nachira, D., Pontecorvi, V., Spada, C., Gualtieri, L., Palmieri, V., Boskoski, I., De Spirito, M., & Papi, M. (2025). 3D-Bioprinting of Stromal Vascular Fraction for Gastrointestinal Regeneration. Gels, 11(9), 712. https://doi.org/10.3390/gels11090712











