2.1. Structural Features of Gel-Derived Cu9S5/TiO2 Composites
The phase composition and crystallinity of the gel-derived CuS
x/TiO
2 composites, both with and without the inclusion of hydrazine hydrate (N
2H
4), were thoroughly examined using X-ray diffraction (XRD), as illustrated in
Figure 1. The XRD pattern of pure TiO
2 exhibits characteristic peaks at 25.3°, 37.8°, 48.1°, and 55.1°, corresponding to the anatase phase (PDF#78-2486). The XRD peaks of TiO
2 in the 0.36CuS
x/TiO
2 sample synthesized with hydrazine hydrate are sharper and more defined, indicating an enhancement in the crystallinity of TiO
2. This improvement in TiO
2 crystallinity is likely due to the better phase formation and reduced defects induced by the presence of hydrazine hydrate, which helps to stabilize the TiO
2 structure during the hydrothermal synthesis.
For the 0.36CuSx/TiO2 sample synthesized without hydrazine hydrate, additional diffraction peaks appear at 29.15°, which correspond to the Cu9S8 phase (PDF#36-0379). The main peak at 47.9° overlaps with those of TiO2, suggesting that the TiO2 structure remains largely intact while Cu9S8 is formed. This confirms that the Cu9S8 phase is the dominant copper sulfide species when hydrazine hydrate is not used in the synthesis. In contrast, the 0.36CuSx/TiO2 sample synthesized with hydrazine hydrate shows distinct peaks at 27.9°, 32.4°, and 46.4°, which can be indexed to Cu9S5 (PDF#26-0476). These peaks do not overlap with TiO2, indicating the formation of Cu9S5 and confirming that hydrazine hydrate influences the phase transition from Cu9S8 to Cu9S5. Importantly, no CuS species were observed in any of the samples, highlighting that the formation of Cu9S8 or Cu9S5 is strictly controlled by the presence of hydrazine hydrate under the hydrothermal conditions. These results emphasize the critical role of hydrazine hydrate in modulating the copper sulfide phase in CuSx/TiO2 composites. In the next sections, the samples synthesized with hydrazine hydrate are referred to as Cu9S5/TiO2, while those synthesized without hydrazine hydrate are referred to as Cu9S8/TiO2.
The morphology and microstructure of the gel-derived Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 composites were investigated using a combination of SEM, TEM, high-resolution TEM (HRTEM), and energy-dispersive X-ray spectroscopy (EDS) mapping, as illustrated in
Figure 2. For the Cu
9S
5/TiO
2 composite synthesized with hydrazine hydrate, the SEM image (
Figure 2a) reveals a relatively uniform distribution of Cu
9S
5 particles across the TiO
2 surface. The TEM image (
Figure 2b) further confirms that the Cu
9S
5 domains are evenly dispersed without significant agglomeration. High-resolution TEM analysis (
Figure 2c) displays distinct lattice fringes with an interplanar spacing of approximately 0.240 nm, which can be indexed to the (101) plane of Cu
9S
5, indicating the formation of a well-crystallized phase. The corresponding FFT pattern (
Figure 2d) shows sharp diffraction spots, consistent with a highly ordered crystalline structure. A low-magnification TEM overview (
Figure 2i) supports the homogeneous distribution of Cu
9S
5 on the TiO
2 support. In contrast, the Cu
9S
8/TiO
2 composite prepared without hydrazine hydrate exhibits less favorable morphology. As shown in the SEM image (
Figure 2e), Cu
9S
8 particles are more aggregated and less uniformly spread across the TiO
2 surface. TEM observation (
Figure 2f) also reveals uneven distribution and particle clustering. The HRTEM image (
Figure 2g) shows lattice fringes with a spacing of 0.190 nm, corresponding to the (111) plane of Cu
9S
8, confirming the formation of the Cu
9S
8 phase. However, the associated FFT pattern (
Figure 2h) is less distinct, suggesting relatively lower crystallinity compared to the Cu
9S
5 counterpart. The overall particle arrangement in the overview TEM image (
Figure 2n) further confirms the irregular distribution of Cu
9S
8 domains.
Elemental mapping via EDS was employed to examine the spatial distribution of key elements in both composites. For Cu
9S
5/TiO
2, the EDS maps (
Figure 2j–m) clearly show the co-localization of Ti, Cu, and S, with Cu and S signals uniformly distributed across the TiO
2 substrate, consistent with the homogeneous dispersion of Cu
9S
5. The oxygen map further supports the presence of TiO
2 throughout the sample. In comparison, the EDS maps of Cu
9S
8/TiO
2 (
Figure 2o–r) show more localized and concentrated Cu and S signals, corresponding to the Cu
9S
8 aggregates, while Ti and O remain uniformly distributed.
Collectively, the X-ray diffraction and transmission electron microscopy analyses confirm the formation of Cu9S5 in the composites. The role of hydrazine hydrate in promoting the phase transition from Cu9S8 to Cu9S5 can be summarized in the following reaction scheme. In the first stage, Cu9S8 is initially formed during the synthesis process in the absence of hydrazine hydrate. In the second stage, hydrazine hydrate acts as a strong reducing agent, donating electrons to Cu9S8 and reducing Cu2+ to Cu+, which then combine with sulfur to form Cu9S5. The Cu9S5/TiO2 composite exhibits superior microstructural uniformity and phase clarity, which are expected to enhance interfacial contact and charge separation during photocatalysis, aligning with the enhanced performance discussed in later sections.
To gain deeper insight into the surface composition and electronic states of the gel-derived Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 composites, X-ray photoelectron spectroscopy (XPS) was performed on both materials as shown in
Figure 3. The XPS spectra of Ti 2p, O 1s, Cu 2p, and S 2p regions were analyzed to investigate the chemical environment of titanium, oxygen, copper, and sulfur species, which are crucial for understanding the photocatalytic behavior of these composites. The Ti 2p spectra of both composites exhibited a characteristic binding energy of 458.6 eV, which is typical for TiO
2. This confirms that the TiO
2 phase is preserved in both the Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 composites. The spectra also show a satellite peak at lower binding energies, which is a signature feature of TiO
2, indicating that the introduction of copper sulfides did not significantly alter the TiO
2 structure. There were no significant shifts in the Ti 2p binding energy, further suggesting the stability of the TiO
2 phase. In the O 1s region, two distinct peaks were observed corresponding to lattice oxygen (Lattice O) and vacancy oxygen (Vacancy O), with binding energies around 529 eV and 532 eV, respectively. These peaks are indicative of the oxygen species present in the TiO
2 lattice and the oxygen vacancies that are often present in metal oxides. Both Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 show similar peak positions and relative intensities, suggesting that the incorporation of Cu
9S
5 or Cu
9S
8 did not cause significant changes in the oxygen species or the oxygen vacancies within the TiO
2 lattice.
The Cu 2p spectra showed notable differences between the two composites. For Cu9S5/TiO2, the Cu 2p spectrum exhibited peaks corresponding to Cu0 at 932.4 eV and Cu+ at 932.7 eV, indicating the presence of both Cu(I) and Cu(0) species. This suggests that hydrazine hydrate, used during synthesis, played a key role in reducing Cu2+ to Cu0 and Cu+, both of which are important for enhancing photocatalytic activity. In contrast, the Cu9S8/TiO2 composite exhibited peaks corresponding to Cu+ at 932.73 eV and Cu2+ at 933.6 eV, indicating a higher proportion of Cu2+ species. This suggests that in the absence of hydrazine hydrate, Cu2+ remains more prevalent, which likely limits the photocatalytic activity compared to Cu9S5/TiO2. This further emphasizes the importance of hydrazine hydrate in reducing copper species and enhancing catalytic efficiency.
In the S 2p region, both Cu9S5/TiO2 and Cu9S8/TiO2 composites exhibited peaks corresponding to sulfate and sulfide species. The presence of sulfate at 168.6 eV suggests that sulfur is bonded to oxygen, possibly forming sulfate species due to surface oxidation or interaction with surface oxygen atoms from TiO2. The more intense sulfide peak at 161.4 eV indicates that sulfur primarily exists in its reduced state as sulfide. The higher intensity of the sulfide peak in both composites suggests that the sulfide species are more abundant and better dispersed on the surface. This finding is consistent with the energy dispersive spectroscopy (EDS) results, which showed a more homogeneous distribution of Cu9S5.
2.2. Optical and Photoelectrochemical Properties of Gel-Derived Cu9S5/TiO2 and Cu9S8/TiO2
Figure 4 presents the optical properties of the gel-derived Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 composites, shedding light on their potential for photocatalytic applications.
Figure 4a,b display the Mott–Schottky plots for Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 composites, respectively, used to determine the flat-band potential (E_fb) and semiconductor type. The plot for Cu
9S
5/TiO
2 (
Figure 4a) reveals a flat-band potential around −0.43 eV, indicative of n-type semiconductor characteristics, which is important for efficient electron transfer during photocatalytic reactions. Similarly, the Mott–Schottky plot for Cu
9S
8/TiO
2 (
Figure 4b) shows a flat-band potential of approximately −0.48 eV, confirming the n-type nature of this composite as well.
In
Figure 4c,d, the optical properties of the composites are examined through UV–vis absorption spectra and Tauc plots.
Figure 4c shows that TiO
2 has a sharp absorption edge around 380 nm, typical of its wide band gap, which limits its absorption to the UV region. Both Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 composites exhibit enhanced absorption in the visible light range compared to pure TiO
2. Cu
9S
5/TiO
2 demonstrates a more prominent absorption in the visible region, extending well beyond 600 nm and up to 800 nm, indicating significantly improved visible light absorption. On the other hand, Cu
9S
8/TiO
2 also absorbs in the visible region, but the absorption intensity is slightly lower than that of Cu
9S
5/TiO
2, which suggests that Cu
9S
5/TiO
2 has a better capacity for visible light utilization.
Figure 4d presents the Tauc plots, where the optical band gaps (Eg) of the composites are determined by plotting (αhν)
2 versus hν. The optical band gap of Cu
9S
8/TiO
2 is measured to be 3.28 eV, which is slightly smaller than that of TiO
2 (3.30 eV), indicating enhanced visible light absorption due to the Cu
9S
8 phase. In contrast, the optical band gap of Cu
9S
5/TiO
2 is even smaller, measured at 3.26 eV, confirming that Cu
9S
5 further reduces the band gap and enhances the ability of the composite to absorb visible light more efficiently.
Therefore, the results from the Mott–Schottky analysis and UV–vis absorption spectra show that the incorporation of Cu9S5 and Cu9S8 into TiO2 enhances their photocatalytic performance by improving visible light absorption. Cu9S5/TiO2 (3.26 eV) exhibits a smaller band gap than Cu9S8/TiO2 (3.28 eV), which enables better visible light absorption and higher photocatalytic efficiency, making both composites promising candidates for visible-light-driven photocatalysis.
Figure 5 demonstrates the structural and electrochemical properties of TiO
2 and Cu
9S
5/TiO
2 composites, providing insights into their photocatalytic performance. As shown in
Figure 5a, the Raman spectrum of TiO
2 shows the characteristic peaks around 143 cm
−1 and 445 cm
−1, which correspond to the Eg and A1g vibrational modes of the TiO
2 anatase phase. In contrast, the Cu
9S
5 spectrum exhibits an additional peak at approximately 200 cm
−1, corresponding to the Cu-S vibrational mode, which confirms the successful incorporation of Cu
9S
5 into the composite.
In
Figure 5b, the electrochemical impedance spectroscopy (EIS) results are shown for Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 composites. The Nyquist plots display the charge transfer resistance (Rct) of both composites. The Cu
9S
5/TiO
2 composite shows a significantly lower Rct compared to Cu
9S
8/TiO
2, suggesting enhanced charge carrier mobility and more efficient charge transfer in Cu
9S
5/TiO
2. This lower impedance indicates better photocatalytic performance, as reduced resistance facilitates more effective electron transport during the photocatalytic process.
Figure 5c presents the photocurrent responses of Cu
9S
5/TiO
2 and Cu
9S
8/TiO
2 under periodic light illumination. The Cu
9S
5/TiO
2 composite shows a stronger and more stable photocurrent response compared to Cu
9S
8/TiO
2, which demonstrates the superior photoelectrochemical performance of Cu
9S
5/TiO
2. This is due to the enhanced light absorption and efficient charge separation promoted by Cu
9S
5, which contributes to higher photocurrent generation under visible light irradiation.
Figure 5d shows the photoluminescence (PL) spectra of TiO
2, Cu
9S
5/TiO
2, and Cu
9S
8/TiO
2 composites. The PL intensity of Cu
9S
5/TiO
2 is significantly lower than that of TiO
2 and Cu
9S
8/TiO
2, indicating a reduced recombination of photogenerated electron–hole pairs. This reduced recombination enhances the photocatalytic efficiency of Cu
9S
5/TiO
2, as more charge carriers are available for photocatalytic reactions.
Obviously, the incorporation of Cu9S5 improves charge transfer, reduces charge recombination, and enhances light absorption, making Cu9S5/TiO2 a promising composite for efficient photocatalytic applications.
2.3. Photocatalytic Performance and Selectivity Toward Methane
Figure 6 presents a comprehensive analysis of the photocatalytic CO
2 reduction performance of various Cu
9S
5/TiO
2 composites under different experimental conditions, highlighting their efficiency and stability.
Figure 6a shows the production rates of CH
4 and CO from CO
2 reduction by Cu
9S
5/TiO
2 composites with varying Cu
9S
5 content (0.12Cu
9S
5/TiO
2, 0.24Cu
9S
5/TiO
2, 0.36Cu
9S
5/TiO
2, and 0.48Cu
9S
5/TiO
2). The results indicate that the 0.36Cu
9S
5/TiO
2 composite exhibits the highest CH
4 production rate, reaching approximately 34 μmol/g·h, followed by the 0.48Cu
9S
5/TiO
2 composite, which produces 28 μmol/g·h of CH
4. The 0.12Cu
9S
5/TiO
2 and 0.24Cu
9S
5/TiO
2 composites show lower CH
4 production rates, around 20 μmol/g·h and 22 μmol/g·h, respectively. On the other hand, the CO production rates remain relatively consistent across all composites, with 0.36Cu
9S
5/TiO
2 showing 15 μmol/g·h, the highest CO production rate among the composites. This suggests that a moderate amount of Cu
9S
5 (0.36 mmol) enhances the photocatalytic activity for CH
4 production, while maintaining a relatively balanced CO generation.
Figure 6b compares the CO
2 reduction performance of the 0.36Cu
9S
5/TiO
2 composite under various experimental conditions. In Condition 1, under standard experimental conditions with light irradiation, the 0.36Cu
9S
5/TiO
2 composite shows significant production of both CH
4 (around 34 μmol·g
−1·h
−1) and CO (approximately 15 μmol·g
−1·h
−1). In Condition 2, when the reaction is conducted in the dark, the production of both CH
4 and CO is almost negligible, demonstrating the essential role of light in driving the photocatalytic process. Condition 3, where no catalyst is used, results in zero CH
4 and CO production, highlighting the need for an efficient catalyst to facilitate the CO
2 reduction. Finally, in Condition 4, where CO
2 is replaced by Ar, the reaction effectively ceases, further confirming that CO
2 is crucial for the reaction to occur.
In
Figure 6c, the photocatalytic CO
2 reduction performance of various composites is shown: TiO
2, 0.36Cu
9S
5/TiO
2 with hydrazine hydrate (N
2H
4), 0.36Cu
9S
8/TiO
2 without hydrazine hydrate, and 0.36Cu
9S
5/TiO
2 without TiO
2. The 0.36Cu
9S
5/TiO
2 composite with hydrazine hydrate achieves the highest CH
4 production rate of 34 μmol·g
−1·h
−1 and CO production rate of 15 μmol/g·h, significantly outperforming TiO
2 (5 μmol·g
−1·h
−1 CO) and the 0.36Cu
9S
8/TiO
2 without hydrazine hydrate (46 μmol·g
−1·h
−1 CO). The addition of hydrazine hydrate improves the separation of photogenerated charge carriers and increases the photocatalytic efficiency, leading to a higher CH
4 production rate.
In addition, in order to evaluate the activity of our Cu
9S
5/TiO
2 composites, we compared the results with representative copper sulfide–TiO
2 systems reported in the literature. For example, a 0D/1D Cu
2-xS/TiO
2 S-scheme heterojunction exhibited a CH
4 formation rate of 14.1 μmol·h
−1, which was nearly 3.9 times higher than that of pristine TiO
2 under similar conditions [
32]. Another study on an in situ constructed Cu
2S/TiO
2 Schottky junction achieved CO and CH
4 production rates of 6.71 μmol·g
−1·h
−1 and 1.20 μmol·g
−1·h
−1, corresponding to 1.41- and 5.71-fold enhancements relative to bare TiO
2 [
33]. For CuS/TiO
2 composites, a photothermal–photocatalytic system utilizing 2 wt% CuS/TiO
2 under full-spectrum irradiation demonstrated enhanced CO
2 conversion efficiency due to the synergistic effect of CuS-induced photothermal heating [
34]. To the best of our knowledge, no prior studies have directly investigated Cu
9S
8- or Cu
9S
5-based composites for CO
2 adsorption or reduction. The only related work involving Cu
9S
5 is its combination with S,C,N-doped TiO
2 for photocatalytic N
2 fixation rather than CO
2 conversion. Therefore, the present study provides a novel strategy by employing hydrazine-assisted phase transformation (Cu
9S
8 → Cu
9S
5) within a gel-derived TiO
2 framework, enabling both enhanced CO
2 adsorption and selective CH
4 photoreduction.
Figure 6d demonstrates the stability of the 0.36Cu
9S
5/TiO
2 composite in four consecutive cycles of CO
2 reduction. The production of CH
4 and CO remains relatively consistent across the cycles, with CH
4 production at 34 μmol·g
−1·h
−1 and CO production at 15 μmol·g
−1·h
−1 in the first cycle, and only a slight decrease observed in subsequent cycles. This shows that the 0.36Cu
9S
5/TiO
2 composite exhibits excellent stability and can be used for multiple cycles without significant degradation in photocatalytic performance. The minimal decrease in CH
4 and CO production over the cycles suggests that the composite is highly durable, making it suitable for real-world applications in photocatalytic CO
2 reduction.
In a word,
Figure 6 demonstrates that the 0.36Cu
9S
5/TiO
2 composite, especially with hydrazine hydrate treatment, is an efficient and stable photocatalyst for CO
2 reduction, producing substantial amounts of CH
4 and CO. The results also underscore the critical role of light, CO
2, and catalyst in achieving efficient photocatalytic CO
2 reduction. The excellent stability over four cycles further highlights the composite’s potential for practical applications in renewable energy and environmental sustainability.
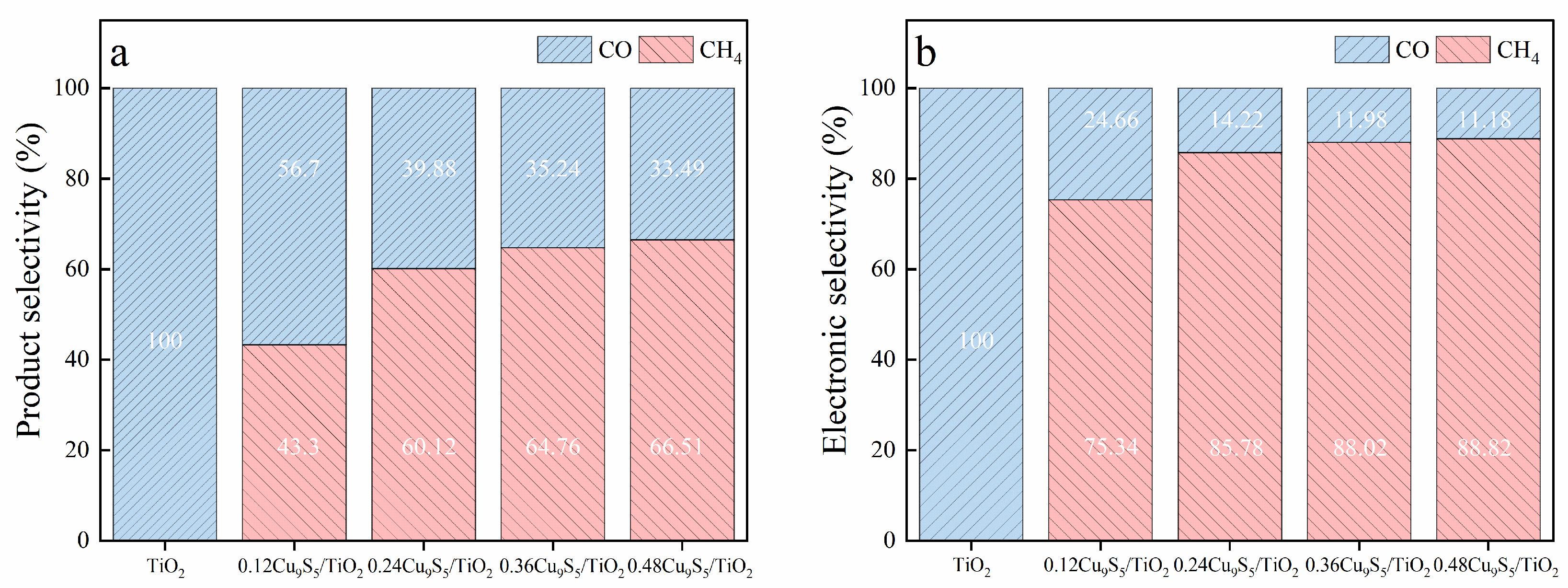
Figure 7a illustrates the product selectivity for CH
4 and CO over the TiO
2 and Cu
9S
5/TiO
2 composites. For TiO
2, no CH
4 is produced (0%), and the product selectivity is entirely CO (100%). As the Cu
9S
5 content in the composites increases, the selectivity for CH
4 significantly improves. Specifically, the 0.48Cu
9S
5/TiO
2 composite shows the highest CH
4 selectivity at 66.51%, accompanied by a CO selectivity of 33.49%. The 0.36Cu
9S
5/TiO
2 composite also shows a notable CH
4 selectivity of 64.76%, with CO selectivity at 35.24%, indicating that the addition of Cu
9S
5 enhances CH
4 formation. The 0.24Cu
9S
5/TiO
2 composite displays a balanced selectivity of 60.12% for CH
4 and 39.88% for CO, while the 0.12Cu
9S
5/TiO
2 composite exhibits 43.3% CH
4 selectivity and 56.7% CO selectivity. These results demonstrate the progressive role of Cu
9S
5 in shifting the product distribution towards CH
4.
Figure 7b presents the electronic selectivity based on electron consumption for CH
4 and CO. The 0.36Cu
9S
5/TiO
2 composite exhibits the highest electronic selectivity for CH
4 at 88.02%, confirming its role in promoting efficient electron utilization for CH
4 formation. The 0.48Cu
9S
5/TiO
2 composite follows with an electronic selectivity for CH
4 of 88.82% and 11.18% for CO. In contrast, TiO
2 and the other Cu
9S
5/TiO
2 composites demonstrate significantly higher electronic selectivity for CO, reflecting the greater electron demand for CO formation. Specifically, the 0.12Cu
9S
5/TiO
2 composite shows an electronic selectivity for CH
4 of 75.34%, and 0.24Cu
9S
5/TiO
2 shows 85.77% for CH
4, indicating an improvement in electron efficiency with increasing Cu
9S
5 content.
These findings highlight the significant effect of Cu9S5 content on both product selectivity and electronic efficiency in CO2 photoreduction. The 0.36Cu9S5/TiO2 composite achieves the best balance of CH4 and CO selectivity with 64.76% CH4 selectivity and 35.24% CO selectivity, while demonstrating the highest electronic selectivity for CH4 at 88.02%. This makes the 0.36Cu9S5/TiO2 composite a promising photocatalyst for selective CO2 reduction towards methane, offering an efficient electron utilization and favorable product distribution.
2.4. DFT Calculations and Heterojunction Analysis
To clarify the origin of the enhanced CH
4 selectivity in Cu
9S
5/TiO
2 composites, we combined DFT calculations and band structure characterization to reveal how interfacial energetics and charge dynamics modulate CO
2 reduction pathways.
Figure 8 presents the DFT-calculated reaction free energy profiles and band structure diagrams for TiO
2 and Cu
9S
5/TiO
2 composites, offering a detailed insight into the photocatalytic CO
2 reduction mechanism, particularly focusing on the intermediates COOH, CO, and CHO, which are critical to methane (CH
4) formation.
Figure 8a illustrates the reaction free energy profiles for CO
2 photoreduction over TiO
2 and Cu
9S
5/TiO
2 composites, highlighting the key intermediates that drive the CO
2 reduction process. The energy profiles for COOH, CO, and CHO intermediates play a significant role in determining the overall efficiency of the reaction, particularly in methane generation. For TiO
2 (red line), the energy barrier for the COOH intermediate is relatively high at 0.8 eV, indicating a less favorable formation of CO and CHO, which are essential intermediates for methane production. The CO intermediate has a high energy value of 0.78 eV, while the CHO intermediate is similarly high, further limiting the pathway toward efficient methane formation.
In contrast, the Cu9S5/TiO2 composite (blue line) significantly reduces the energy barriers for the critical intermediates. The COOH intermediate’s energy is lowered to 0.3 eV, facilitating easier conversion to CO and CHO. This reduced barrier encourages the formation of CO (with an energy value of 0.32 eV) and CHO (with an energy value of 0.12 eV), both of which are key precursors to methane formation. The CHO intermediate is particularly important, as its strong adsorption is a key step in methane production. Additionally, the strong adsorption of CO and CHO intermediates at the active sites on the Cu9S5/TiO2 composite provides an ideal pathway for efficient methane production. This is evident in the lower energy profile for CH4 formation on Cu9S5/TiO2 compared to TiO2. The lower energy for CH4 formation on Cu9S5/TiO2 directly supports its higher photocatalytic activity for CO2 reduction to methane.
Figure 8b shows the band structure of TiO
2 and Cu
9S
5/TiO
2 composites, further supporting the enhanced photocatalytic performance. TiO
2 exhibits a conduction band edge at 0.54 eV, while the Cu
9S
5/TiO
2 composite has a lower conduction band edge at 0.43 eV, which facilitates more efficient electron transfer. This lowering of the conduction band edge enhances electron mobility, promoting better separation of charge carriers and leading to more efficient reduction of CO
2 to CH
4.
Together, these results demonstrate that the Cu9S5/TiO2 composite not only facilitates the efficient formation of key intermediates such as CO and CHO but also significantly lowers the energy barriers for CH4 production. This makes Cu9S5/TiO2 a superior photocatalyst for CO2 reduction, especially for selective methane production. The strong adsorption of CO and CHO, coupled with the improved electronic structure, is crucial for its enhanced photocatalytic activity.