Size-Controlled Fabrication of Alginate Hydrogel Microbeads Optimized for Lipase Entrapment
Abstract
1. Introduction
2. Results and Discussion
2.1. Entrapment of CRL into Size-Controlled Alginate Hydrogel Microbeads
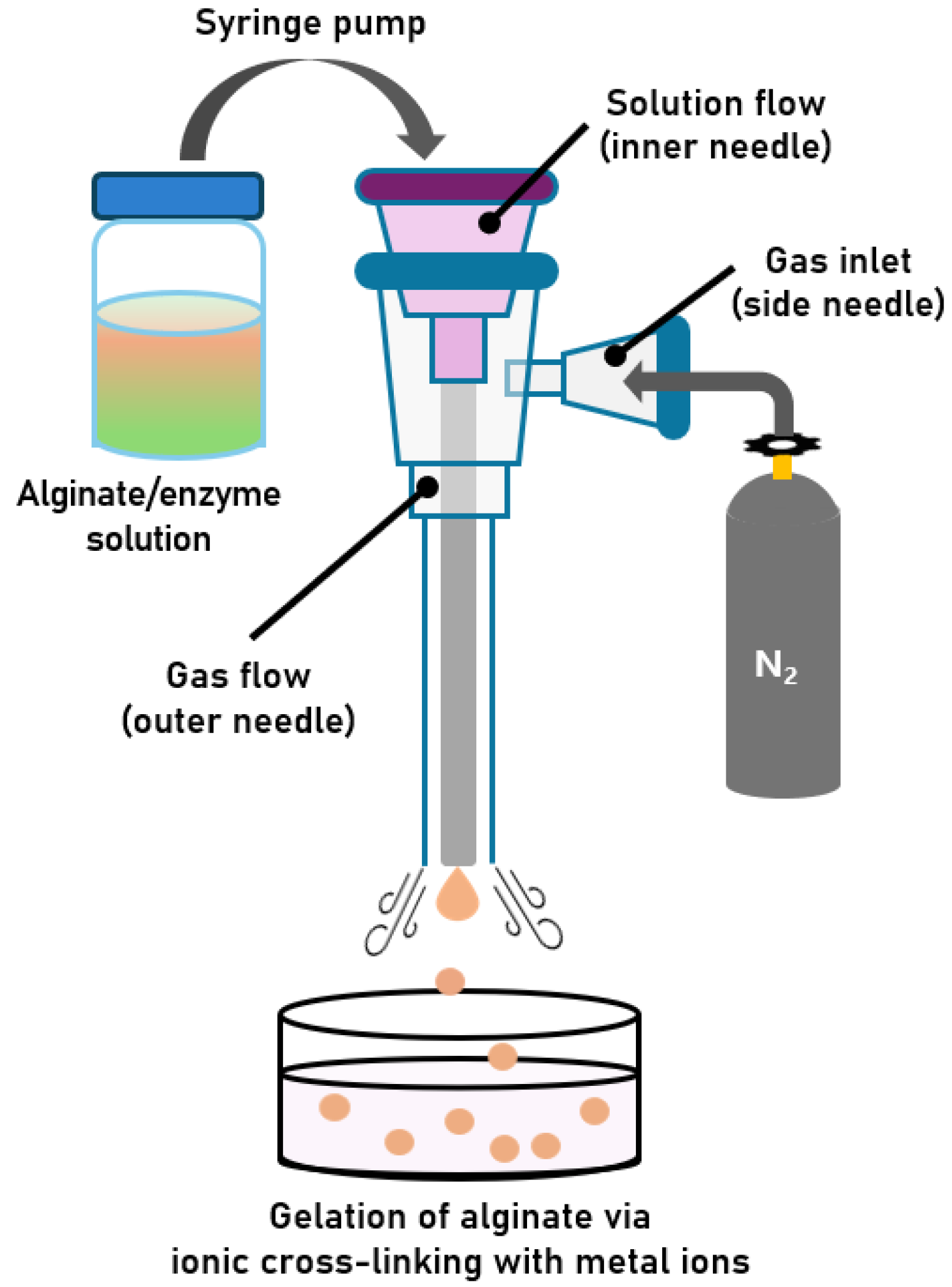
2.1.1. Development of a Gas-Shearing Apparatus for Preparing Alginate Hydrogel Microbeads
2.1.2. Effects of Gas Flow Rate on the Microbead Size and the Activity of Entrapped CRL
2.1.3. Effects of Alginate Concentration on the Activity and Reusability of Entrapped CRL
2.1.4. Effects of Cross-Linking Metal Ions on the Activity and Reusability of Entrapped CRL
2.2. Characteristics of CRL Entrapped in Alginate Hydrogel Microbeads
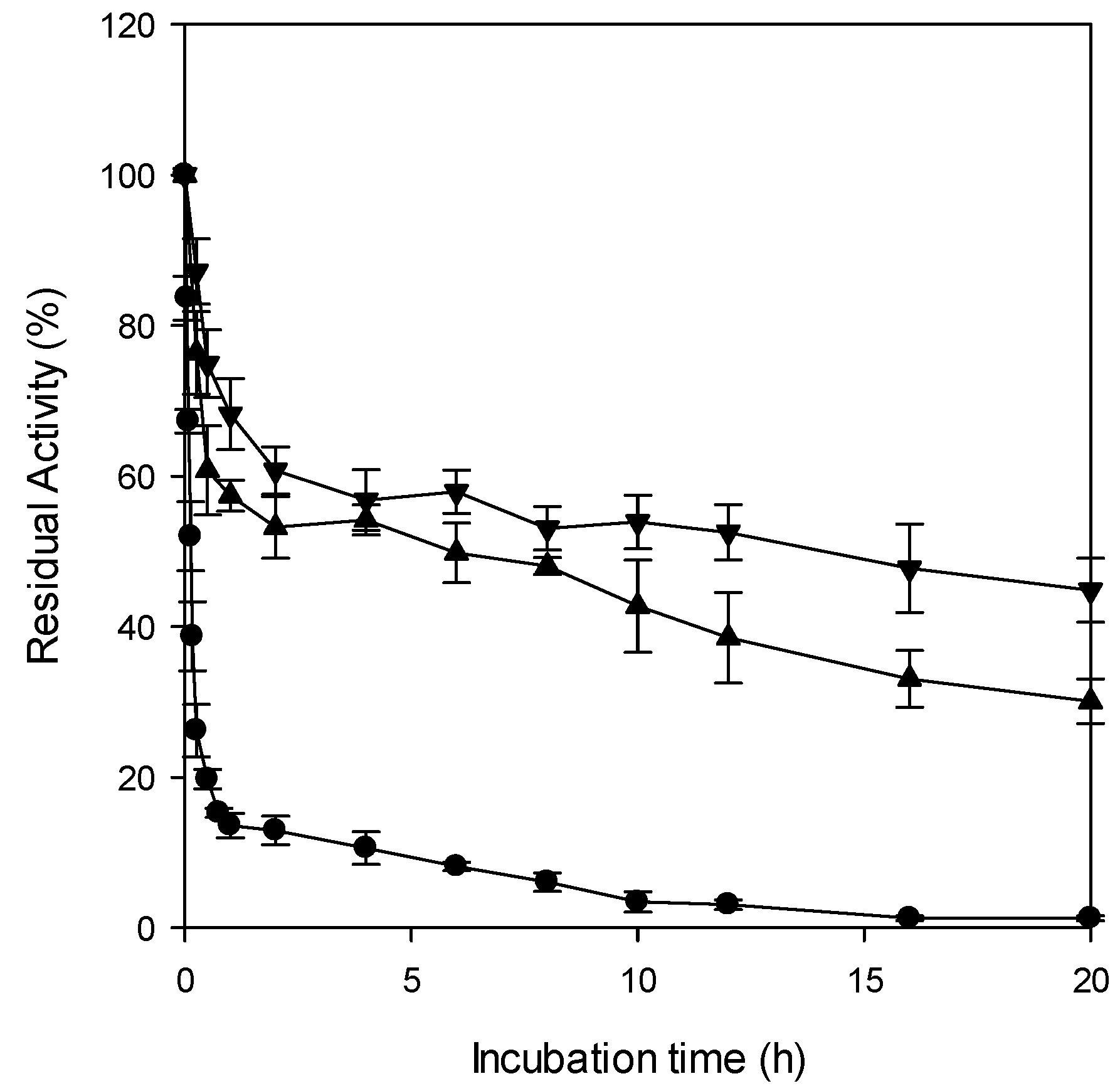
2.2.1. Thermal Stability of Entrapped CRL
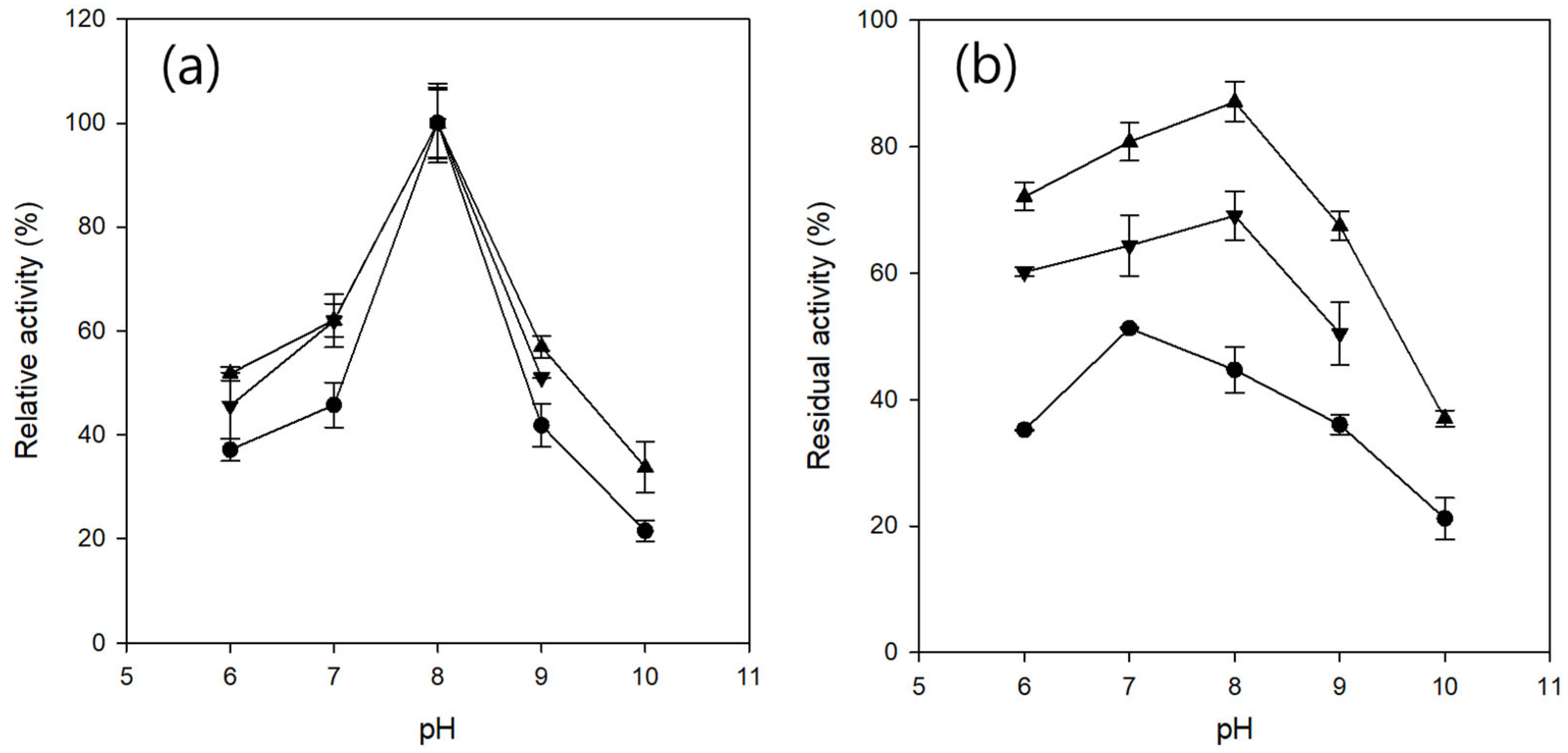
2.2.2. pH Profile and pH Stability of Entrapped CRL
2.2.3. Kinetic Study of Entrapped CRL
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Development of a Gas-Shearing Apparatus for Preparing Size-Controlled Alginate Hydrogel Microbeads
4.3. Entrapment of CRL in Alginate Microbeads via the Gas-Shearing Apparatus
4.4. Physical Properties of CRL-Entrapped Alginate Microbeads
4.4.1. Average Size
4.4.2. Microbead Morphology
4.4.3. Swelling Ratio
4.5. Characterization of CRL Entrapped in Alginate Microbeads
4.5.1. Determination of Lipase Activity and Loaded Protein Content
4.5.2. Reusability of Entrapped CRL
4.5.3. Determination of Thermal Stability of Entrapped CRL
4.5.4. Determination of pH Profile and pH Stability of Entrapped CRL
4.5.5. Kinetic Analysis of Entrapped CRL
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRL | lipase from Candida rugosa |
| G-block | guluronic acid residues |
| ND | not detected |
| SA | specific activity |
| pNB | p-nitrophenyl butyrate |
| Km | Michaelis constant |
| kcat | turnover number |
| kcat/Km | catalytic efficiency |
| t1/2 | half-life time |
| ACN | acetonitrile |
| IPA | isopropanol |
References
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahum, A.H.; El-Said Azzazy, H.M. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Sharma, N.; Ahlawat, Y.K.; Stalin, N.; Mehmood, S.; Morya, S.; Malik, A.; Malathi, H.; Nellor, J.; Bhanot, D. Microbial enzymes in industrial biotechnology: Sources, production, and significant applications of lipases. J. Ind. Microbiol. Biotechnol. 2025, 52, kuaf010. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Basso, A.; Brady, D. New frontiers in enzyme immobilisation: Robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 2021, 50, 5850. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.E.; Yang, Q.; Xiao, Z.; Liu, L.; Wang, N.; Cui, X.; Yang, L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech 2019, 9, 440. [Google Scholar] [CrossRef]
- Santos, M.P.F.; de Souza Junior, E.C.; Villadóniga, C.; Vallés, D.; Castro-Sowinski, S.; Bonomo, R.C.F.; Veloso, C.M. Proteases: Importance, immobilization protocols, potential of activated carbon as support, and the importance of modifying supports for immobilization. BioTech 2024, 13, 13. [Google Scholar] [CrossRef]
- Ferraraccio, L.S.; Lisa, D.D.; Pastorino, L.; Bertoncello, P. Enzymes encapsulated within alginate hydrogels: Bioelectrocatalysis and electrochemiluminescence applications. Anal. Chem. 2022, 94, 16122–16131. [Google Scholar] [CrossRef]
- Arad, M.; Frey, C.; Balagtas, R.; Hare, R.; Ku, K.; Jereb, D.; Nestman, Z.; Sidhu, A.; Shi, Y.; Fordwour, O.; et al. Development of an automated, ultra-rapid bottom-up proteomics workflow utilizing alginate-based hydrogels. Anal. Chem. 2024, 96, 18880–18889. [Google Scholar] [CrossRef]
- Tverdokhlebova, A.; Sterin, I.; Jayaweera, T.M.; Darie, C.C.; Katz, E.; Smutok, O. pH-driven enzymatic breakdown and release of catalase from alginate hydrogel. ACS Appl. Mater. Interfaces 2024, 16, 68816–68824. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Voo, W.-P.; Ooi, C.-W.; Islam, A.; Tey, B.-T.; Chan, E.-S. Calcium alginate hydrogel beads with high stiffness and extended dissolution behaviour. Eur. Polym. J. 2016, 75, 343–353. [Google Scholar] [CrossRef]
- Gao, H.; Khera, E.; Lee, J.-K.; Wen, F. Immobilization of multi-biocatalysts in alginate beads for cofactor regeneration and improved reusability. J. Vis. Exp. 2016, 110, e53944. [Google Scholar] [PubMed]
- Kirchner, C.N.; Träuble, M.; Wittstock, G. Diffusion and reaction in microbead agglomerates. Anal. Chem. 2010, 82, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Grøtli, M.; Meldal, M. Diffusion of reagents in macrobeads. J. Comb. Chem. 2001, 3, 461–468. [Google Scholar] [CrossRef]
- Chinh, N.T.; Hoang, T. Review: Emulsion techniques for producing polymer based drug delivery systems. Vietnam J. Sci. Technol. 2023, 61, 1–26. [Google Scholar]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef]
- Boda, S.K.; Li, X.; Xie, J. Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J. Aerosol Sci. 2018, 125, 164–181. [Google Scholar] [CrossRef]
- Wang, J.; Janzen, J.A.; Yang, F. Electrospraying: Possibilities and challenges of engineering carriers for biomedical applications—A mini review. Front. Chem. 2019, 7, 258. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Tang, G.; Xiong, R.; Lv, D.; Xu, R.X.; Braeckmans, K.; Huang, C.; De Smedt, S.C. Gas-shearing fabrication of multicompartmental microspheres: A one-step and oil-free approach. Adv. Sci. 2019, 6, 1802342. [Google Scholar] [CrossRef]
- Qu, Q.; Cheng, W.; Zhang, X.; Ravanbakhsh, H.; Tang, G.; Zhou, A.; Pei, D.; Xiong, R.; Huang, C. Glucose-responsive enzymatic cascade microreactors in gas-shearing microfluidics microcapsules. Adv. Mater. Technol. 2023, 8, 2201559. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Q.; Cheng, W.; Zhou, A.; Deng, Y.; Ma, W.; Zhu, M.; Xiong, R.; Huang, C. A Prussian blue alginate microparticles platform based on gas-shearing strategy for antitumor and antibacterial therapy. Int. J. Biol. Macromol. 2022, 209, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.-Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zhang, X.; Ravanbakhsh, H.; Tang, G.; Zhang, J.; Deng, Y.; Braeckmans, K.; De Smedt, S.C.; Xiong, R.; Huang, C. Gas-shearing synthesis of core–shell multicompartmental microparticles as cell-like system for enzymatic cascade reaction. Chem. Eng. J. 2022, 428, 132607. [Google Scholar] [CrossRef]
- Yuan, J.; Kan, H.; Wang, H.; Wang, N.; Liu, Y.; Pei, D.; Qu, Q. Efficient, stable and sustainable alginate@chitosan enzymatic microreactors based on gas-shearing microfluidics. Compos. Commun. 2023, 44, 101765. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Knezevic, Z.; Bobic, S.; Milutinovic, A.; Obradovic, B.; Mojovic, L.; Bugarski, B. Alginate-immobilized lipase by electrostatic extrusion for the purpose of palm oil hydrolysis in lecithin/isooctane system. Process Biochem. 2002, 38, 313–318. [Google Scholar] [CrossRef]
- Won, K.; Kim, S.; Kim, K.-J.; Park, H.W.; Moon, S.-J. Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem. 2005, 40, 2149–2154. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Bahamondes, C.; Álvaro, G.; Wilson, L.; Illanes, A. Effect of enzyme load and catalyst particle size on the diffusional restrictions in reactions of synthesis and hydrolysis catalyzed by α-chymotrypsin immobilized into glyoxal-agarose. Process Biochem. 2017, 53, 172–179. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Paredes-Juarez, G.A.; Niclou, S.P.; de Vos, P. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J. Mech. Behav. Biomed. Mater. 2014, 37, 196–208. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, X.; Yan, H.; Lin, Q. Comparative study of physicochemical properties of alginate composite hydrogels prepared by the physical blending and electrostatic assembly methods. Gels 2022, 8, 799. [Google Scholar] [CrossRef] [PubMed]
- RAGHU, S.; Pennathur, G. Enhancing the stability of a carboxylesterase by entrapment in chitosan coated alginate beads. Turk. J. Biol. 2018, 42, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Xia, W.-J.; Ma, G.-P.; Chen, Y.-L.; Ma, Y.-Y. A study on the enzymatic properties and reuse of cellulase immobilized with carbon nanotubes and sodium alginate. AMB Express 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.-S.; Lim, T.-K.; Voo, W.-P.; Pogaku, R.; Tey, B.T.; Zhang, Z. Effect of formulation of alginate beads on their mechanical behavior and stiffness. Particuology 2011, 9, 228–234. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Idota, Y.; Kogure, Y.; Kato, T.; Yano, K.; Arakawa, H.; Miyajima, C.; Kasahara, F.; Ogihara, T. Relationship between physical parameters of various metal ions and binding affinity for alginate. Biol. Pharm. Bull. 2016, 39, 1893–1896. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Khair, T.; Kazantseva, M.A.; Zuev, Y.F.; Zueva, O.S. Ion-induced polysaccharide gelation: Peculiarities of alginate egg-box association with different divalent cations. Polymers 2023, 15, 1243. [Google Scholar] [CrossRef]
- Donati, I.; Asaro, F.; Paoletti, S. Experimental evidence of counterion affinity in alginates: The case of nongelling ion Mg2+. J. Phys. Chem. B 2009, 113, 12877–12886. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; Christiansen, J.d. Mechanical properties of alginate hydrogels cross-linked with multivalent cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef] [PubMed]
- Al-Musa, S.; Abu Fara, D.; Badwan, A.A. Evaluation of parameters involved in preparation and release of drug loaded in crosslinked matrices of alginate. J. Control. Release 1999, 57, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Roquero, D.M.; Othman, A.; Melman, A.; Katz, E. Iron(III)-cross-linked alginate hydrogels: A critical review. Mater. Adv. 2022, 3, 1849–1873. [Google Scholar] [CrossRef]
- Nengsih, N.Z.; Jetsrisuparb, K.; Knijnenburg, J.T.N. Development of iron alginate beads by ionotropic gelation for nutritional applications. In Proceedings of the 7th TICC International Conference 2023, Toward Sustainable Development Goals: Transformation and Beyond, Chiang Mai, Thailand, 4–5 February 2023. [Google Scholar]
- Lee, L.-C.; Yen, C.-C.; Malmis, C.C.; Chen, L.-F.; Chen, J.-C.; Lee, G.-C.; Shaw, J.-F. Characterization of codon-optimized recombinant Candida rugosa lipase 5 (LIP5). J. Agric. Food Chem. 2011, 59, 10693–10698. [Google Scholar] [CrossRef]
- Ghofrani, S.; Allameh, A.; Yaghmaei, P.; Norouzian, D. Immobilization of Candida rugosa lipase for resolution of racemic ibuprofen. DARU J. Pharm. Sci. 2021, 29, 117–123. [Google Scholar] [CrossRef]
- Mazzocato, M.C.; Jacquier, J.-C. Recent advances and perspectives on food-grade immobilisation systems for enzymes. Foods 2024, 13, 2127. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Lin, C.-C. A diffusion-reaction model for predicting enzyme-mediated dynamic hydrogel stiffening. Gels 2019, 5, 17. [Google Scholar] [CrossRef]
- Anobom, C.D.; Pinheiro, A.S.; De-Andrade, R.A.; Aguieiras, E.C.G.; Andrade, G.C.; Moura, M.V.; Almeida, R.V.; Freire, D.M. From structure to catalysis: Recent developments in the biotechnological applications of lipases. BioMed Res. Int. 2014, 2014, 684506. [Google Scholar] [CrossRef]
- Hertzberg, S.; Kvittingen, L.; Anthonsen, T.; Skjåk-Bræk, T. Alginate as immobilization matrix and stabilizing agent in a two-phase liquid system: Application in lipase-catalysed reactions. Enzyme Microb. Technol. 1992, 14, 42–47. [Google Scholar] [CrossRef]
- Saxena, A.; Sharda, S.; Kumar, S.; Kumar, B.; Shirodkar, S.; Dahiya, P.; Sahney, R. Synthesis of alginate nanogels with polyvalent 3D transition metal cations: Applications in urease immobilization. Polymers 2022, 14, 1277. [Google Scholar] [CrossRef]

| Gas Flow Rate (L/min) a | Mean Diameter of Microbeads (μm) | Number of Microbeads | Immobilization Yield (%) | Activity Retention (%) | Activity Recovery (%) | Residual Activity After Last Reuse (%) | Maximum No. of Reuse Cycles |
|---|---|---|---|---|---|---|---|
| 0.5 | 893 b | 122 | 44.9 ± 3.5 | 5.8 ± 0.2 | 2.6 ± 0.1 | 54.3 ± 3.9 | 2 |
| 0.8 | 576 | 681 | 36.2 ± 1.0 | 14.8 ± 1.1 | 5.3 ± 0.2 | 136.4 ± 3.0 | 1 |
| 1.0 | 482 | 2281 | 31.9 ± 2.4 | 17.7 ± 1.0 | 5.7 ± 0.7 | ND c | 0 |
| 1.5 | 282 | 10,273 | 25.6 ± 3.7 | 21.5 ± 5.1 | 5.4 ± 0.5 | ND | 0 |
| 2.0 | 247 | 14,500 | 20.3 ± 2.6 | 29.6 ± 2.7 | 6.0 ± 1.3 | ND | 0 |
| Alginate Concentration (%) a | Mean Diameter of Microbeads (μm) | Activity Recovery (%) | Residual Activity After Last Reuse (%) | Maximum No. of Reuse Cycles |
|---|---|---|---|---|
| 3.0 | 282 | 5.3 ± 0.5 | ND b | 0 |
| 4.0 | 334 | 4.3 ± 0.6 | ND | 0 |
| 5.0 | 399 | 3.4 ± 0.1 | 299.5 ± 32.5 | 1 |
| Crosslinking Metal Ion a | Mean Diameter of Microbeads (μm) | Immobilization Yield (%) | Activity Retention (%) | Activity Recovery (%) | Residual Activity After 4th Reuse (%) | Maximum No. of Reuse Cycles | Swelling Ratio |
|---|---|---|---|---|---|---|---|
| Ca2+ | 399 | 44.8 ± 3.2 | 7.7 ± 0.3 | 3.4 ± 0.1 | ND b | 1 | 16.4 ± 0.0 |
| Sr2+ | 345 | 53.6 ± 4.2 | 8.9 ± 0.2 | 4.8 ± 0.3 | 77.7 ± 14.5 | 4 | 14.2 ± 0.0 |
| Ba2+ | 341 | 57.8 ± 4.6 | 9.6 ± 0.7 | 5.5 ± 0.4 | 63.1 ± 11.8 | >4 | 12.6 ± 0.0 |
| Fe3+ | 383 | 67.1 ± 4.8 | 12.2 ± 0.7 | 8.2 ± 0.4 | 50.4 ± 0.8 | >4 | 20.9 ± 0.0 |
| Kinetic Constant | Free CRL | CRL Entrapped in Ba-Alginate | CRL Entrapped in Fe-Alginate |
|---|---|---|---|
| Km (mM) | 0.726 | 0.884 | 2.296 |
| kcat (s−1) | 1.01 × 104 | 1.07 × 103 | 2.53 × 103 |
| kcat/Km (s−1 M−1) | 1.39 × 107 | 1.21 × 106 | 1.10 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Cha, J.E.; Kim, D.; Lee, S.H. Size-Controlled Fabrication of Alginate Hydrogel Microbeads Optimized for Lipase Entrapment. Gels 2025, 11, 710. https://doi.org/10.3390/gels11090710
Kim DH, Cha JE, Kim D, Lee SH. Size-Controlled Fabrication of Alginate Hydrogel Microbeads Optimized for Lipase Entrapment. Gels. 2025; 11(9):710. https://doi.org/10.3390/gels11090710
Chicago/Turabian StyleKim, Dong Han, Jeong Eun Cha, Dojin Kim, and Sang Hyun Lee. 2025. "Size-Controlled Fabrication of Alginate Hydrogel Microbeads Optimized for Lipase Entrapment" Gels 11, no. 9: 710. https://doi.org/10.3390/gels11090710
APA StyleKim, D. H., Cha, J. E., Kim, D., & Lee, S. H. (2025). Size-Controlled Fabrication of Alginate Hydrogel Microbeads Optimized for Lipase Entrapment. Gels, 11(9), 710. https://doi.org/10.3390/gels11090710






