1. Introduction
With the increasing difficulty of developing unconventional oil and gas resources, enhancing oil recovery (EOR) and extending the production life of mature reservoirs have become important challenges in the petroleum industry. CO
2 flooding has attracted wide attention due to its dual advantages of improving recovery efficiency and contributing to carbon sequestration [
1,
2,
3]. However, because of reservoir heterogeneity and the unfavorable mobility ratio between CO
2 and crude oil, CO
2 tends to finger through high-permeability channels during displacement, resulting in limited sweep efficiency and low displacement efficiency. These issues have to some extent restricted the practical effectiveness of CO
2 flooding [
4,
5,
6,
7,
8].
To improve both sweep efficiency and displacement efficiency in CO
2 flooding, various mobility-control strategies have been proposed, including water-alternating-gas (WAG) injection [
9,
10,
11,
12,
13], CO
2 foam flooding [
14,
15], polymer-assisted CO
2 flooding [
16], and CO
2 thickening techniques [
17,
18]. On this basis, several modified approaches have been developed in recent years. For example, chemically enhanced WAG introduces chemical agents into the water slug, which interact with subsequently injected CO
2 through chemical reactions or interfacial stabilization, thereby improving fluid transport characteristics [
19]. Another example is nanocomposite viscosity-enhancing systems, in which nanoparticles and polymers act synergistically to increase solution viscosity, delay CO
2 breakthrough in high-permeability channels, and demonstrate potential applicability in WAG or gas flooding processes [
20]. These methods not only alleviate CO
2 channeling and improve sweep efficiency but may also enhance displacement efficiency by reducing interfacial tension or increasing relative pressure gradients, thereby contributing to further improvements in oil recovery.
Among these methods, gel systems have been reported to show potential for mitigating CO
2 channeling in high-permeability zones due to their plugging capability and adaptability to reservoir temperature and pressure conditions. In recent years, CO
2-responsive gels, which undergo sol–gel transitions upon CO
2 stimulation, have been regarded as promising smart fluids for improving the effectiveness of CO
2 flooding [
21]. Such gels can undergo structural transformations under CO
2 exposure, enabling relatively selective plugging and flow redirection. In contrast, traditional gels or nanocomposite viscosity-enhancing systems mainly rely on high pre-injection viscosity to improve the mobility ratio, which may limit injectivity and selectivity. CO
2-responsive gels, however, can be injected as low-viscosity solutions and subsequently undergo in situ gelation under CO
2 stimulation, making them more suitable for blocking high-permeability channels.
Previous studies have reported promising results for CO
2-responsive gels under different conditions. For instance, a carrageenan–PEI–ethylenediamine system was reported to form a three-dimensional network structure under CO
2 exposure, exhibiting strong plugging capability and possible oil recovery enhancement [
22]. CO
2-responsive preformed particle gels demonstrated strong swelling capacity and plugging strength under acidic environments, achieving nearly complete plugging in core flooding experiments [
23]. Furthermore, a CO
2-responsive gel–wormlike micelle coupling system exhibited significant viscosity enhancement and improved displacement performance upon CO
2 stimulation [
24]. In addition, PEI–SDS gels containing nano-SiO
2 were reported to display favorable rheological reinforcement and plugging ability [
25]. More recently, Xin and co-workers investigated the application of CO
2-responsive gels in fractured and low-permeability reservoirs, suggesting their potential applicability under complex heterogeneous conditions [
26].
In this context, a CO2-responsive TMPDA–SDS–SiO2 gel system was developed in this study. The formulation, rheological properties, and CO2-triggered behavior of the gel were characterized, and parallel core flooding experiments were conducted under permeability contrasts of 10, 20, and 30 to examine its plugging and displacement features under heterogeneous conditions. Building on previous work, this study provides additional experimental observations regarding the applicability of the CO2-responsive TMPDA–SDS–SiO2 gel system under heterogeneous conditions.
4. Materials and Methods
4.1. Experimental Materials and Equipment
Experimental Materials: The low-molecular-weight amine used in this study was N,N,N′,N′-tetramethyl-1,3-propanediamine (TMPDA, analytical grade). The surfactant was sodium dodecyl sulfate (SDS, analytical grade). The nano-silica consisted of spherical particles with an average diameter of approximately 20 nm. Carbon dioxide (CO2, 99 mol%) was used as the triggering gas. Analytical-grade sodium chloride (NaCl), calcium chloride (CaCl2), and magnesium chloride (MgCl2) were used to prepare synthetic brines. Deionized water was used in all experiments. The oil sample was a mixture of crude oil from the Zhongyuan Oilfield and kerosene at a 1:1 mass ratio, with the viscosity of the simulated oil measured as 3.08 mPa·s at 40 °C.
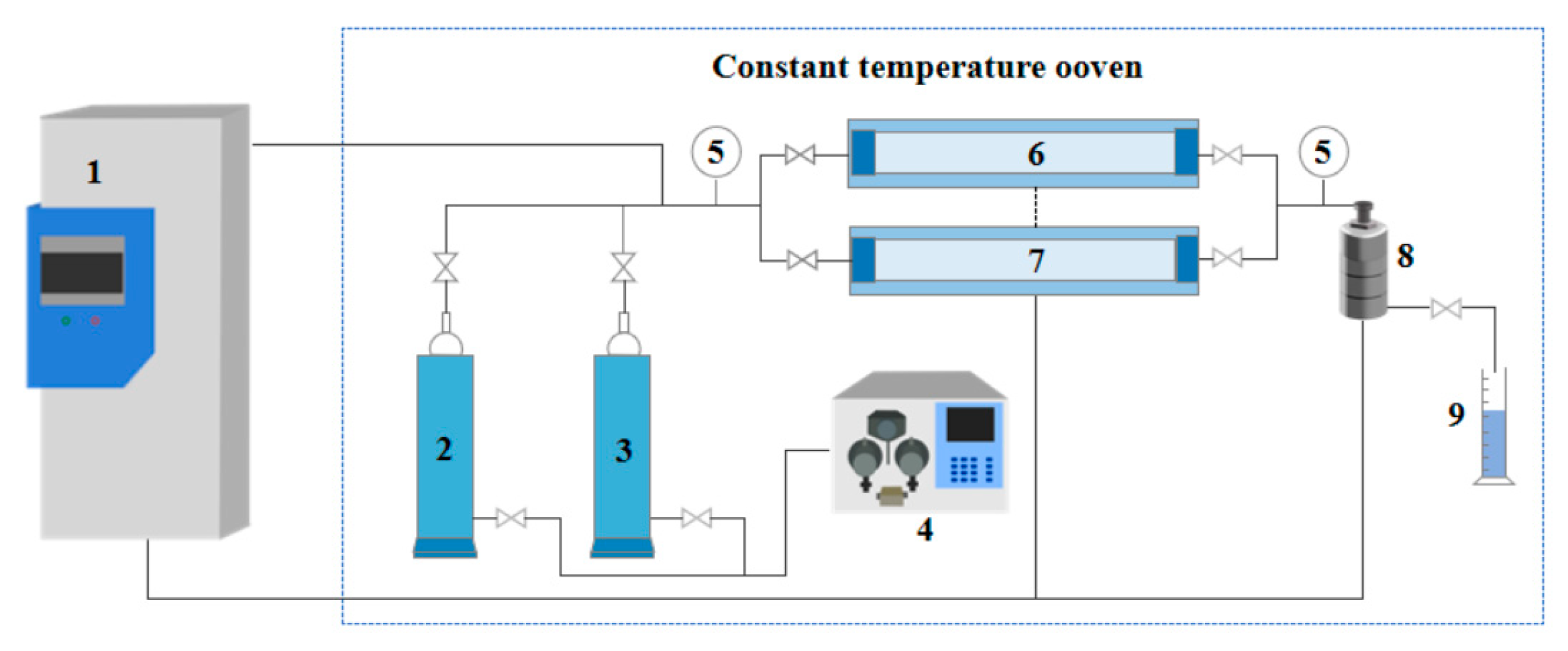
Experimental Equipment: The main instruments included a digital viscometer (DV-II, Brookfield, AMETEK, Middleboro, MA, USA), a flowmeter (G10-15F, Chengfeng Flowmeter Co., Ltd., Shandong, China), a nuclear magnetic resonance (NMR) spectrometer (Avance III 300 MHz, Bruker Corporation, Billerica, MA, USA), a rotational rheometer (Haake MARS 60, Thermo Fisher Scientific, Karlsruhe, Germany), a scanning electron microscope (SU3500, Hitachi High-Tech Corporation, Tokyo, Japan), and a Fourier transform infrared (FTIR) spectrometer (Tensor 27, Bruker Corporation, Billerica, MA, USA). A core flooding system (see
Figure 19) was employed to conduct heterogeneous dual-core parallel flooding experiments.
4.2. Preparation and Optimization of the CO2-Responsive TMPDA–SDS–SiO2 Gel System
Gel Preparation Method: SDS, TMPDA, and nano-silica were dissolved in deionized water in the designed proportions and placed in a three-necked flask. The mixture was stirred at room temperature for 5 min until a transparent solution was obtained, representing the pre-gelation state. After standing for 10 min, the solution was transferred to a gas-washing bottle. Under ambient temperature and pressure, CO2 was continuously introduced at a flow rate of 0.1 L·min−1 using a glass rotameter, while stirring was maintained to ensure adequate gas–liquid contact until the solution transformed into a viscoelastic gel.
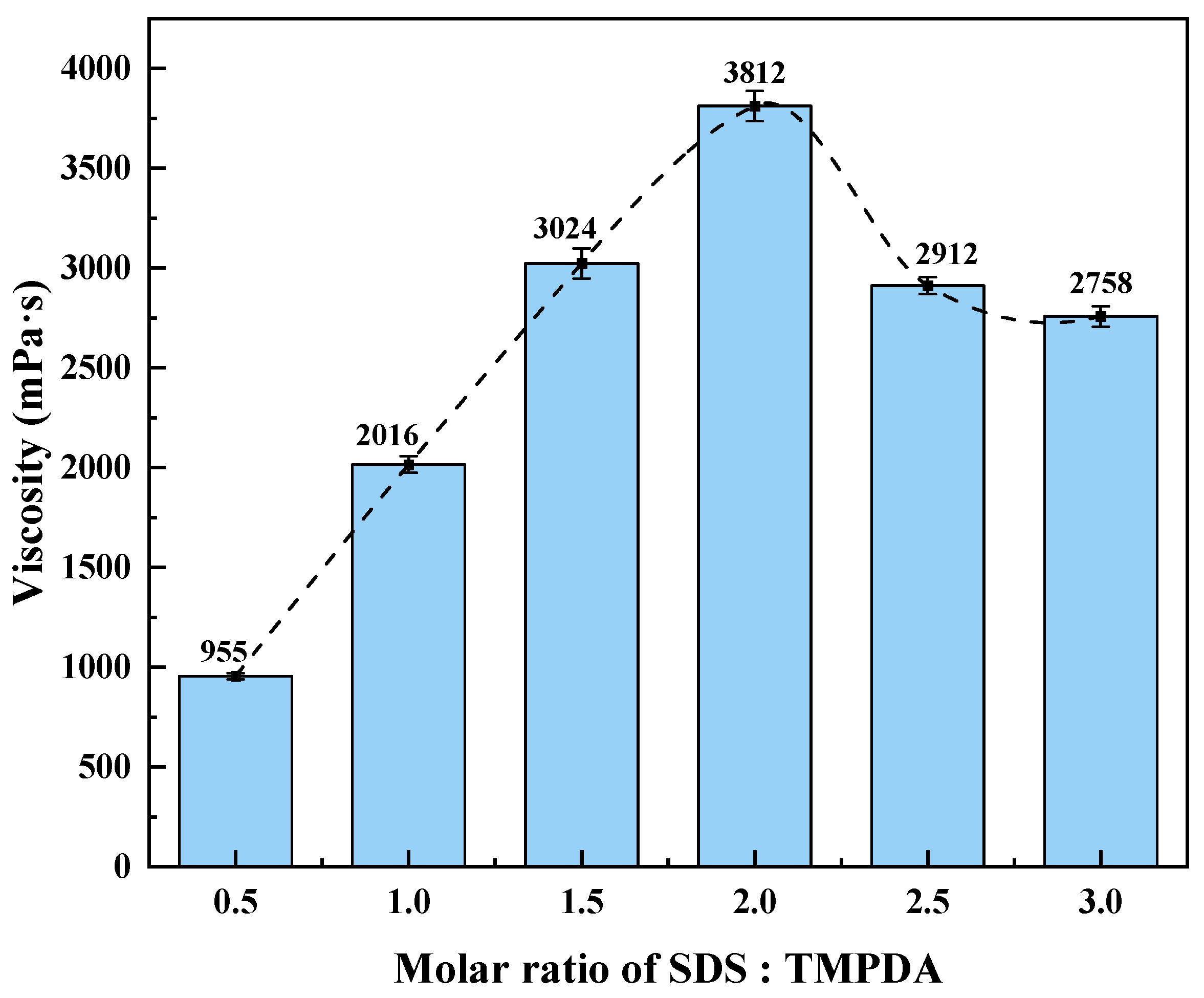
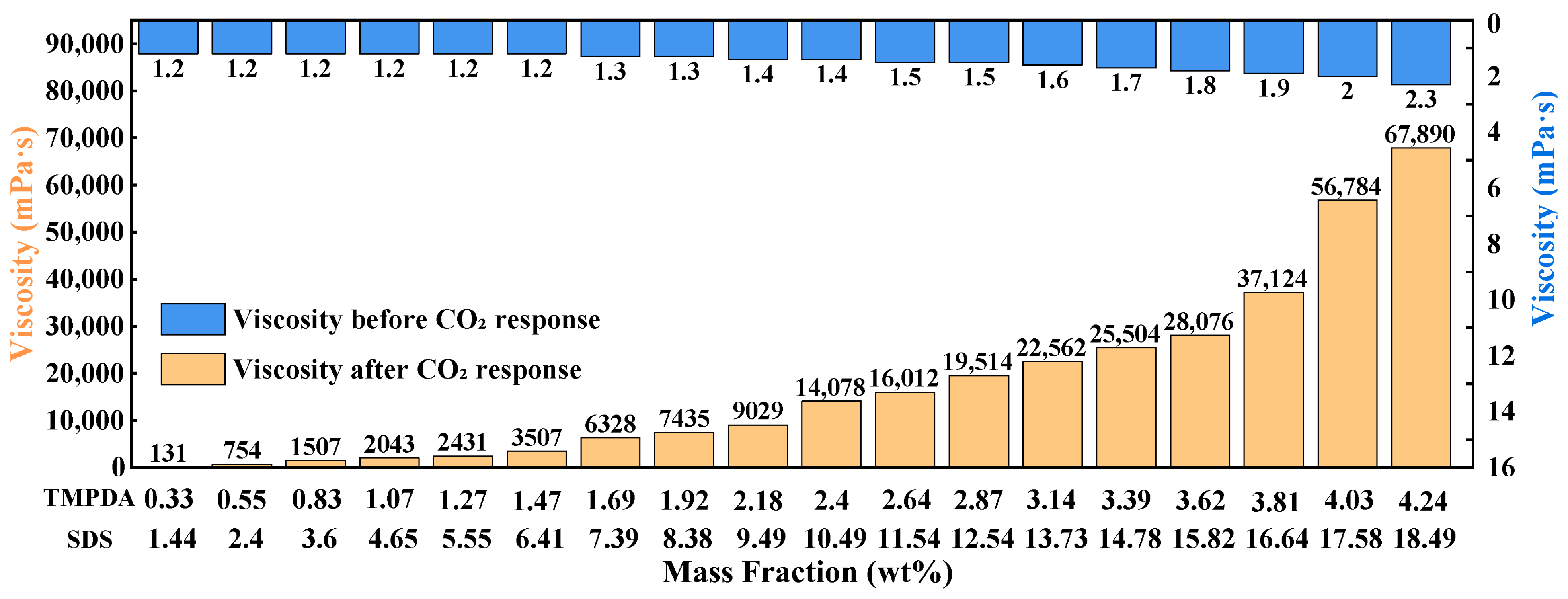
Formulation Optimization Method: A single-factor method was used to evaluate the effects of the SDS:TMPDA molar ratio, the total mass fraction of SDS + TMPDA, and the nano-silica content on the viscosity of the CO2-responsive TMPDA–SDS–SiO2 gel system, with the goal of determining the optimal formulation. The experimental design included the following: (1) SDS:TMPDA molar ratios of 0.5, 1.0, 1.5, 2.0, 2.5, and 6.0; (2) 18 groups of total SDS + TMPDA mass fractions, ranging from 1.44% SDS + 0.33% TMPDA to 18.49% SDS + 4.24% TMPDA; and (3) nano-silica concentrations of 0.02%, 0.04%, 0.06%, 0.08%, 0.09%, 0.1%, 0.3%, 0.5%, 0.7%, and 0.9%. The viscosity of the gel after CO2 stimulation was measured using a digital viscometer.
4.3. Structural Characterization
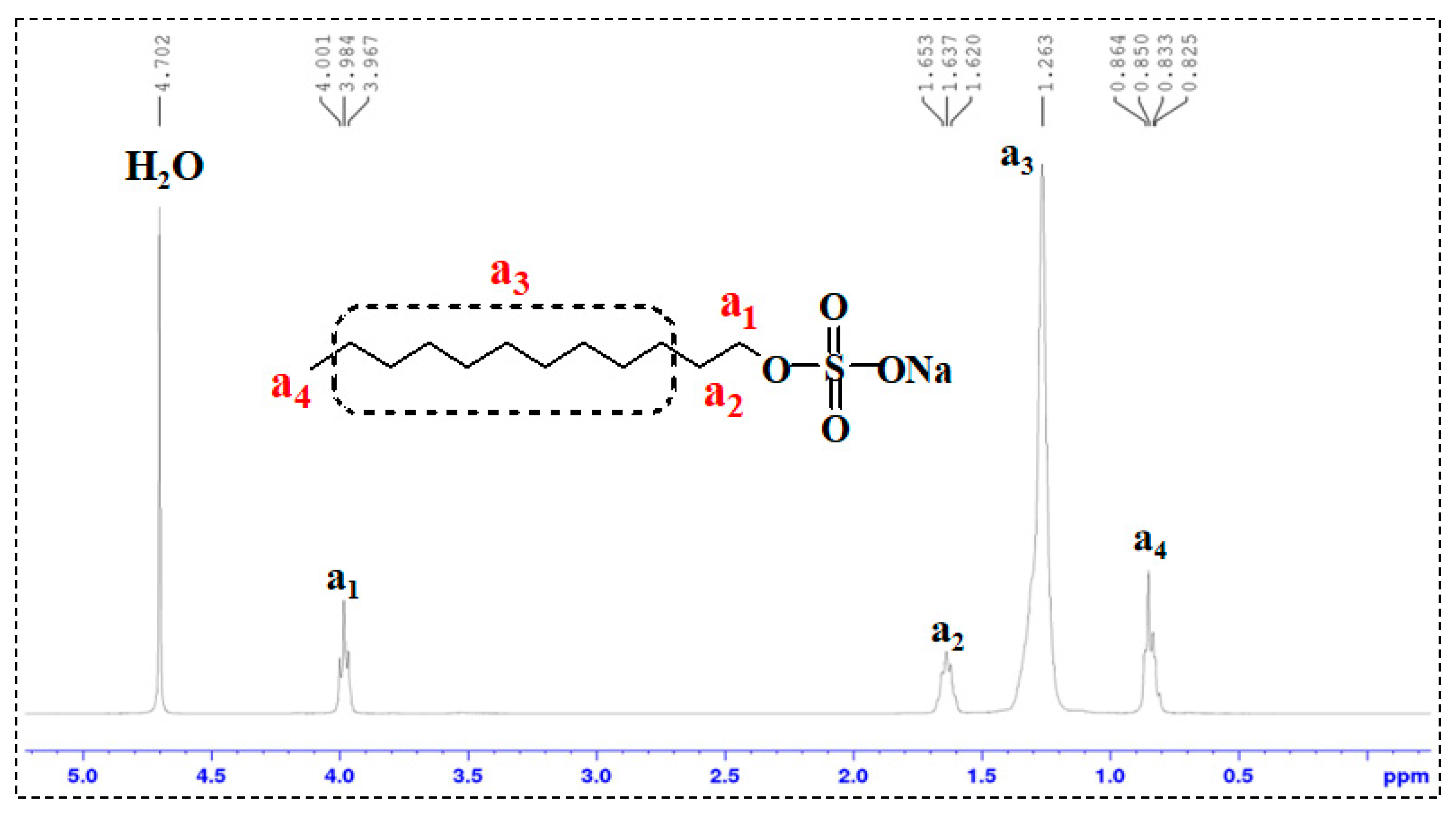
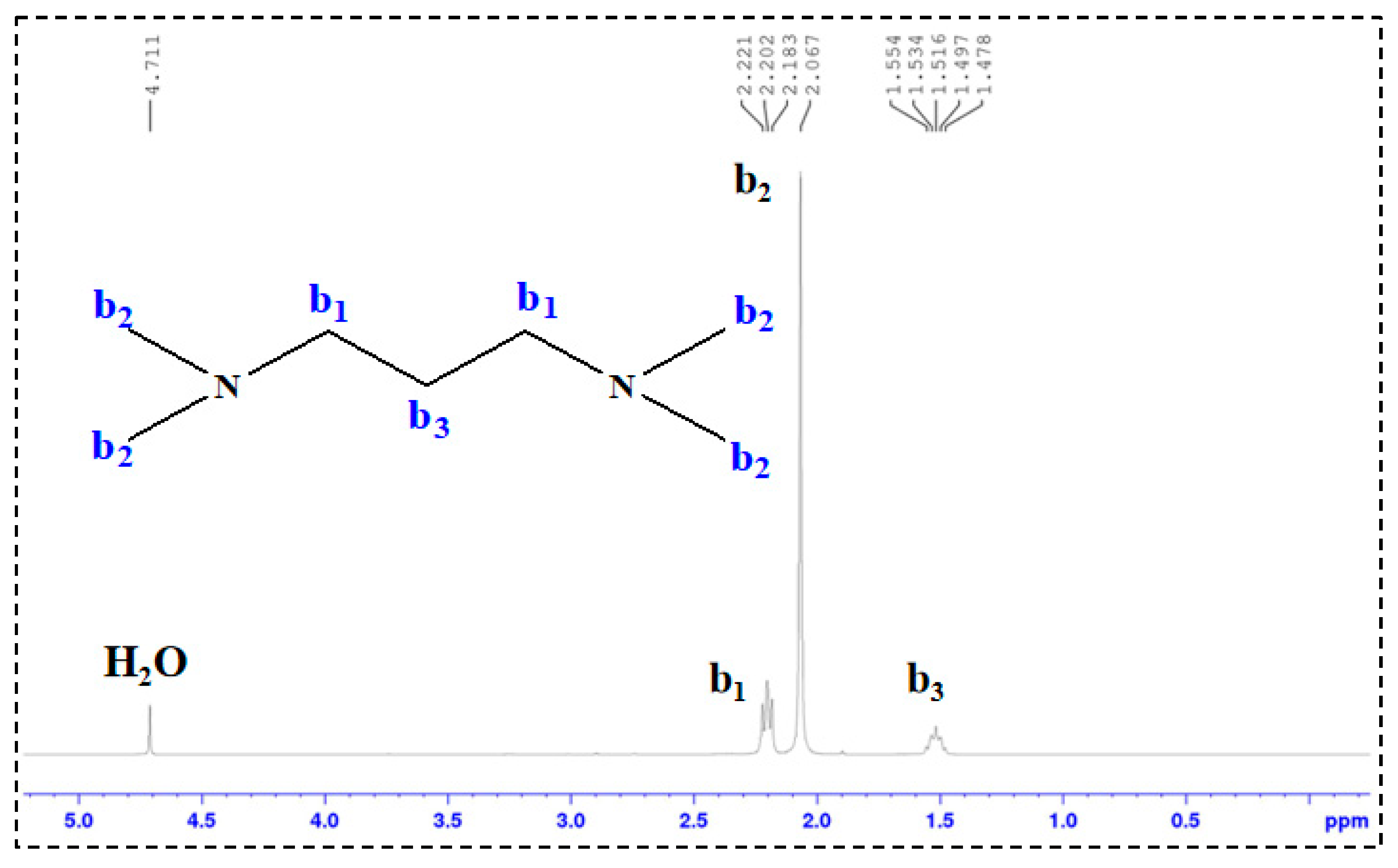
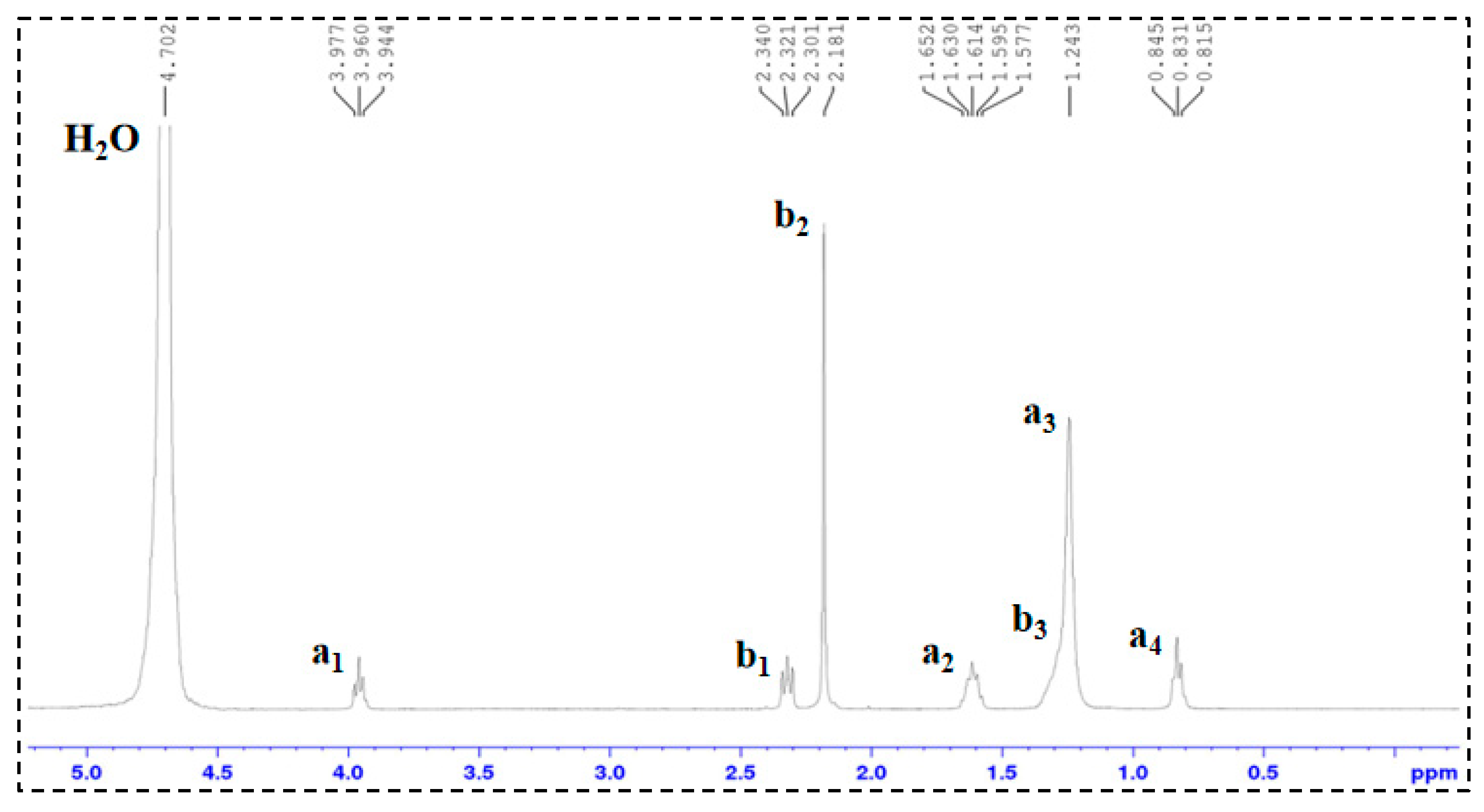
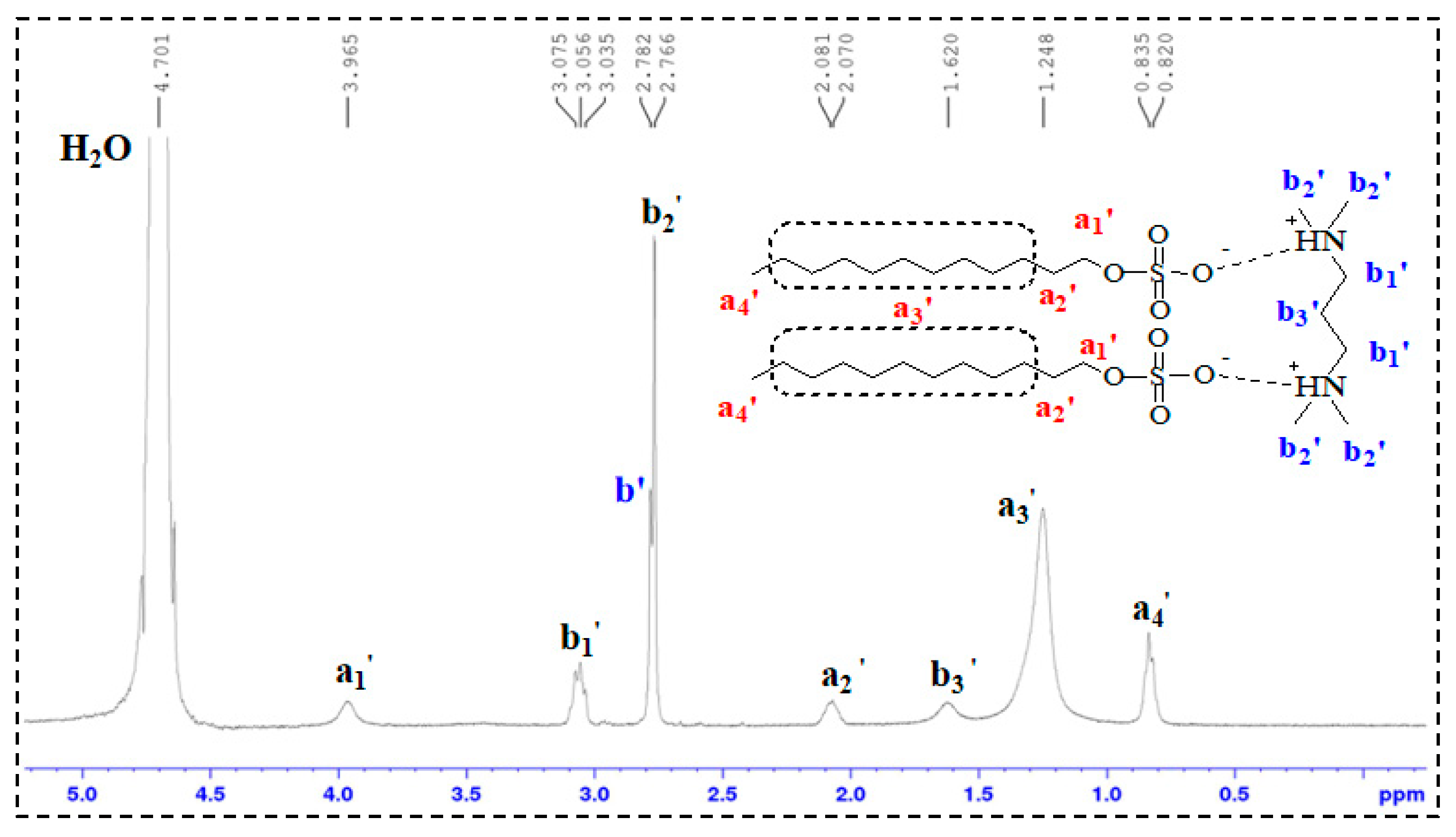
1H-NMR Analysis: 1H nuclear magnetic resonance (1H-NMR) spectroscopy was performed on TMPDA, SDS, a TMPDA–SDS mixture, and the CO2-responsive TMPDA–SDS mixture. The procedure was as follows: (1) each sample was dissolved in D2O and transferred to an NMR tube, with the measurement temperature set at 25 °C; (2) the NMR probe was tuned to match the magnetic field and radio frequency source; (3) the instrument was calibrated and parameters were set; and (4) data were acquired, and free induction decay (FID) signals were collected.
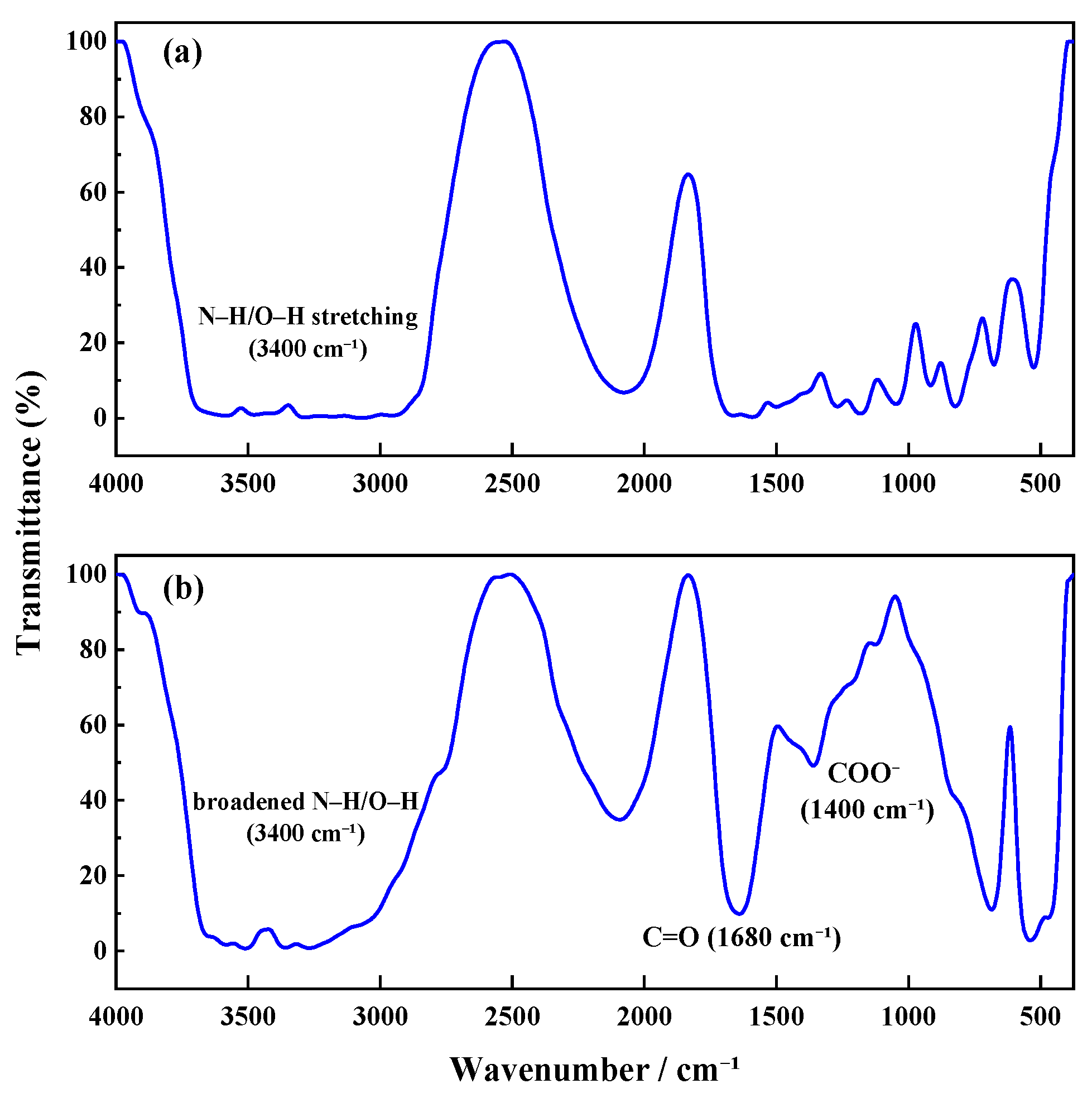
FTIR Analysis: Fourier-transform infrared (FTIR) spectroscopy was used to characterize TMPDA–SDS solutions before and after CO2 stimulation. Samples were tested using the smear method, and spectra were recorded at 25 °C.
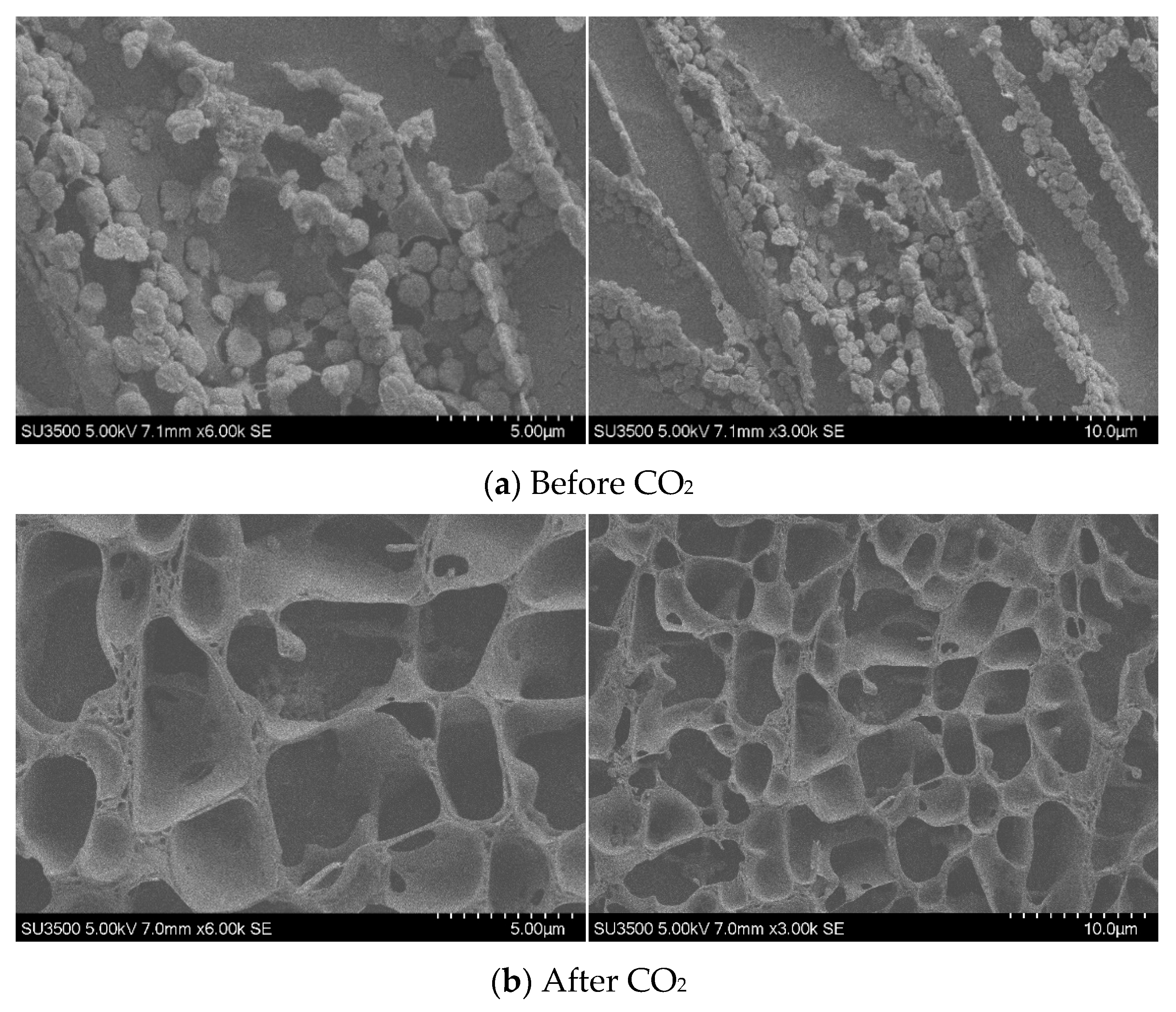
SEM Analysis: Scanning electron microscopy (SEM) was employed to observe TMPDA–SDS mixtures before and after CO2 stimulation. The procedure included the following: (1) ultra-low-temperature freezing of the samples; (2) fracturing of the frozen samples under cryogenic conditions, followed by sputter-coating with a thin metal layer; and (3) low-temperature SEM imaging.
All structural and spectroscopic characterizations in this section were conducted on the TMPDA–SDS system with a composition of 7.39 wt% SDS and 1.69 wt% TMPDA, without the addition of nano-silica.
4.4. Rheological Behavior Evaluation
Steady Shear Behavior: A gel sample prepared with the optimized TMPDA–SDS–SiO2 formulation (7.39 wt% SDS, 1.69 wt% TMPDA, and 0.1 wt% SiO2) was tested for viscosity under varying shear rates (0.1–500 s−1) at 25 °C using a rotational rheometer.
Shear Recovery Property: The shear recovery performance was evaluated by conducting a shear–rest–shear cycle test at a constant shear rate of 100 s−1.
Alternating CO2/N2 Injection Test: To examine gas responsiveness, CO2 and N2 were alternately injected into the gel solution at a flow rate of 0.1 L·min−1 at 25 °C. Viscosity changes during gas alternation were monitored using a digital viscometer.
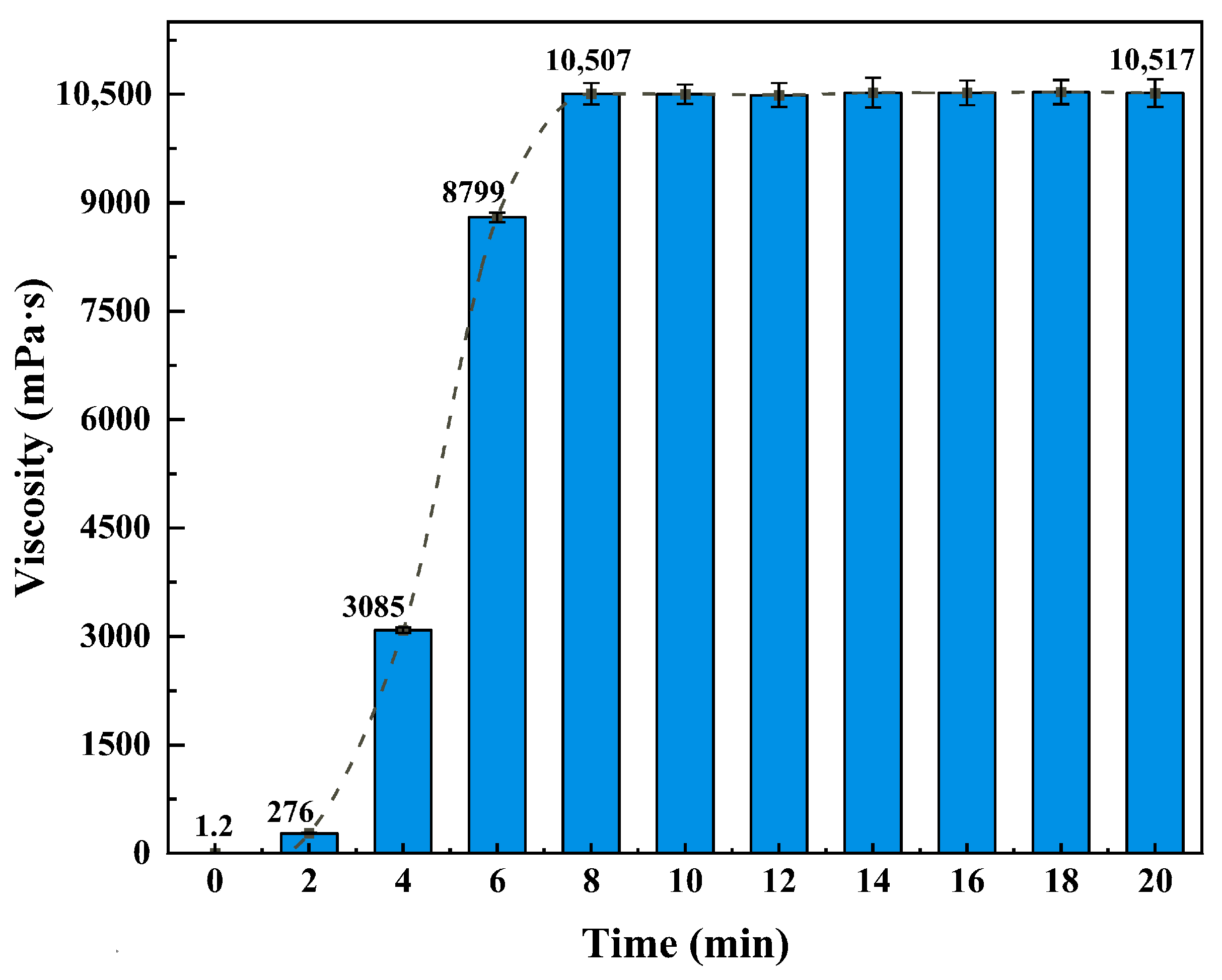
Effect of CO2 Injection Time: The influence of CO2 injection duration on system viscosity was investigated at 25 °C using a digital viscometer. CO2 was continuously injected at 0.1 L·min−1 for up to 20 min, and viscosity was recorded at 2 min intervals.
4.5. Thermal and Salt Resistance
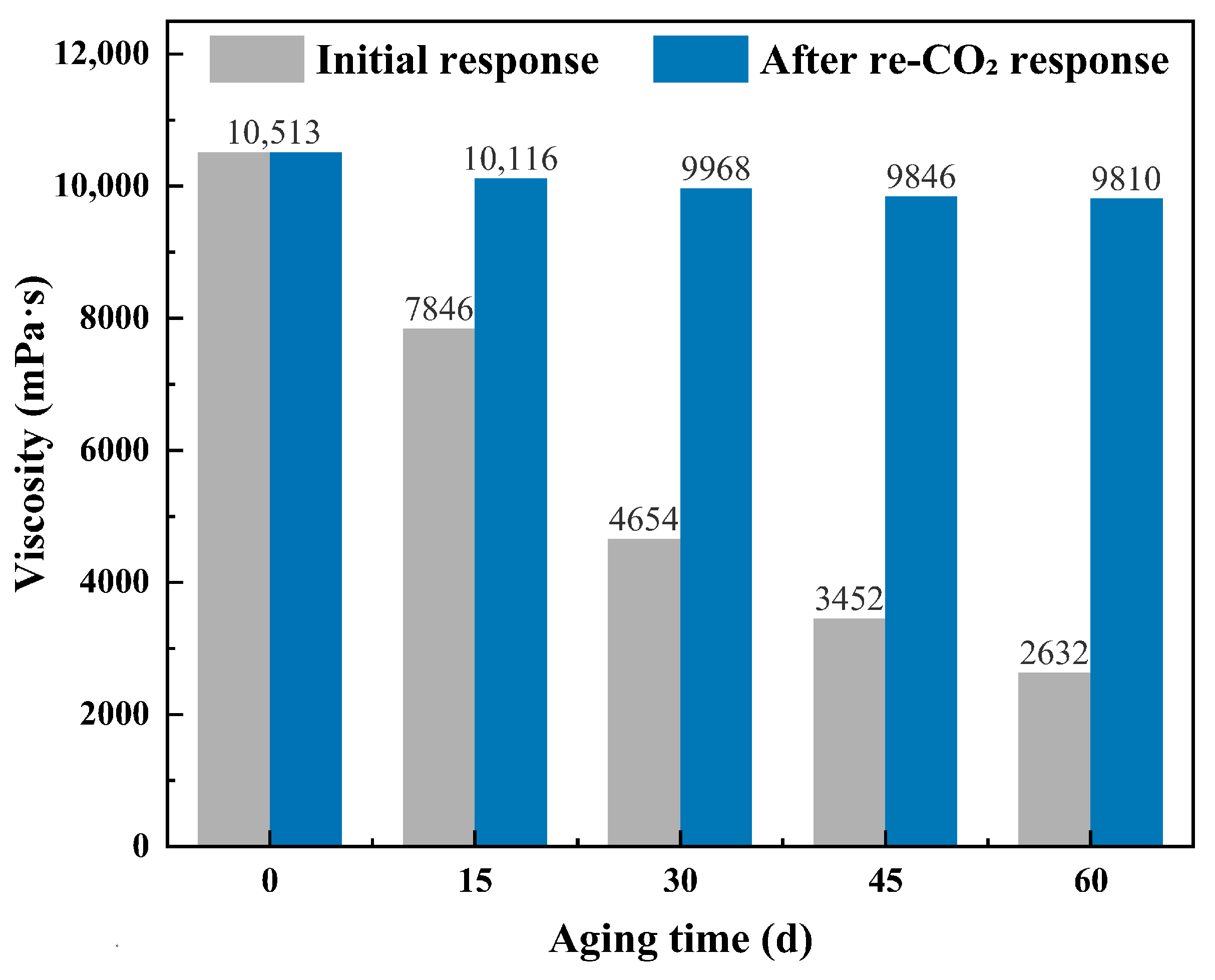
Thermal Aging Test (90 °C): Experiments were performed on the CO2-responsive TMPDA–SDS–SiO2 gel system with the optimized formulation (7.39 wt% SDS, 1.69 wt% TMPDA, and 0.1 wt% SiO2). Gel samples were sealed and aged in an oven at 90 °C for 0, 15, 30, 45, and 60 days. At each aging time point, the samples were cooled to 25 °C, and their apparent viscosities were measured under identical conditions using a digital viscometer. Immediately afterward, CO2 was introduced into the same samples at 25 °C at a rate of 0.1 L·min−1 until a stable viscosity was obtained, and the post-response viscosity was recorded.
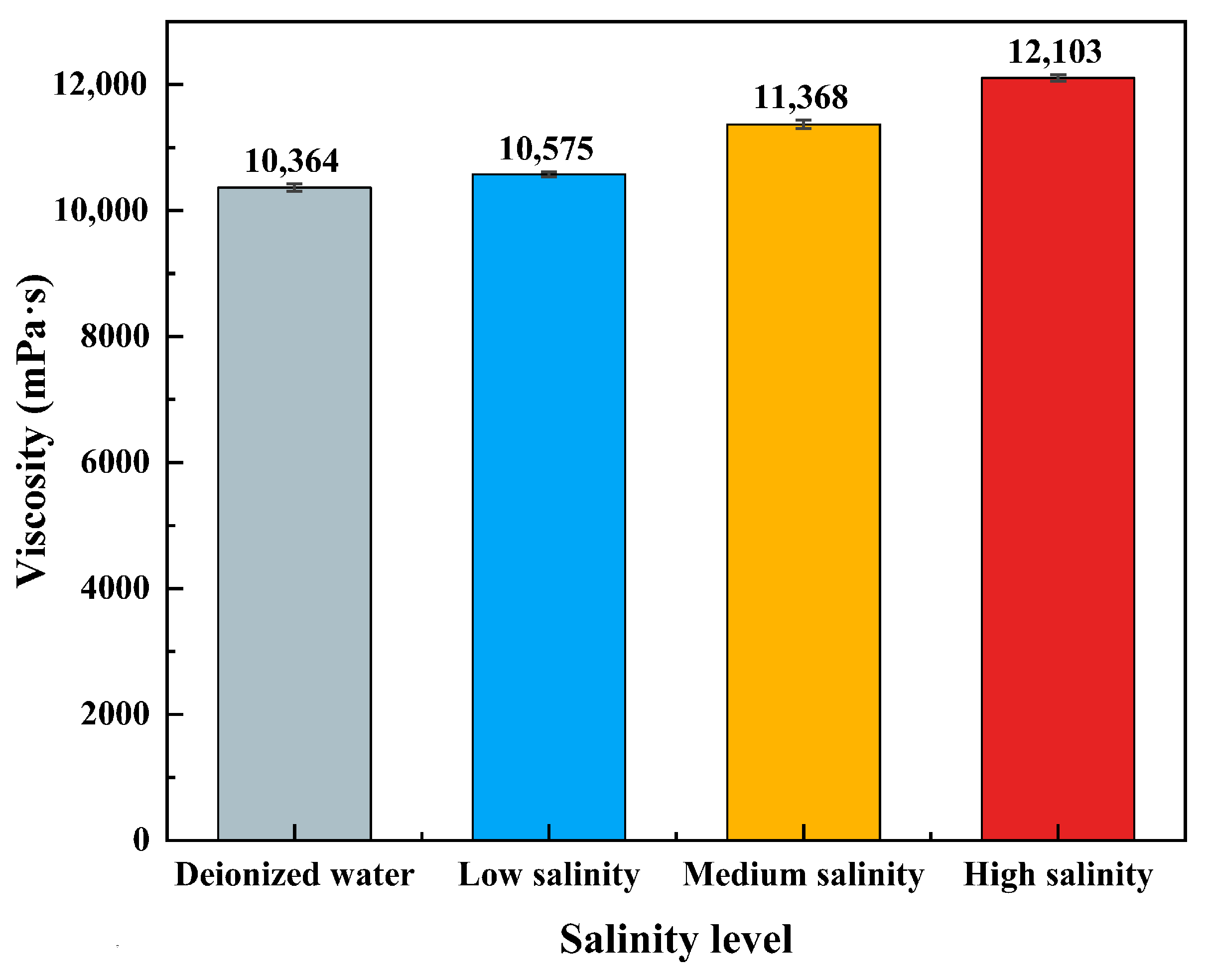
Salt Tolerance: Synthetic brines with low (5275.1 mg·L−1), medium (21,000 mg·L−1), and high (52,780.1 mg·L−1) salinities were prepared using NaCl, CaCl2, and MgCl2, with deionized water as the zero-salinity control. Gel solutions were formulated using these brines, and CO2 was injected at 25 °C at a rate of 0.1 L·min−1 until the viscosity stabilized. The apparent viscosity was then measured at 25 °C using a digital viscometer.
4.6. Plugging Performance and Heterogeneous Core Flooding
Relationship between Injected PV and Plugging Efficiency: Experiments were conducted in sand-packed tubes at 40 °C under a backpressure of 5 MPa and a confining pressure of 20 MPa, using the CO2-responsive TMPDA–SDS–SiO2 gel system (7.39 wt% SDS, 1.69 wt% TMPDA, and 0.1 wt% SiO2). The procedure was as follows: (1) Quartz sand was packed into the tubes, and the pore volume (PV) was determined by water saturation; (2) Initial CO2 permeability (k0) was measured at an injection rate of 2 mL·min−1; (3) Gel solutions were injected at different pore volumes (0.10, 0.12, 0.14, 0.15, 0.25, and 0.50 PV); (4) CO2 was reinjected at 2 mL·min−1 while continuously monitoring inlet/outlet pressures and outlet flow rate. Once steady state was reached, permeability after gel injection (k1) was calculated. Plugging efficiency was then determined using the plugging ratio (1–k1/k0).
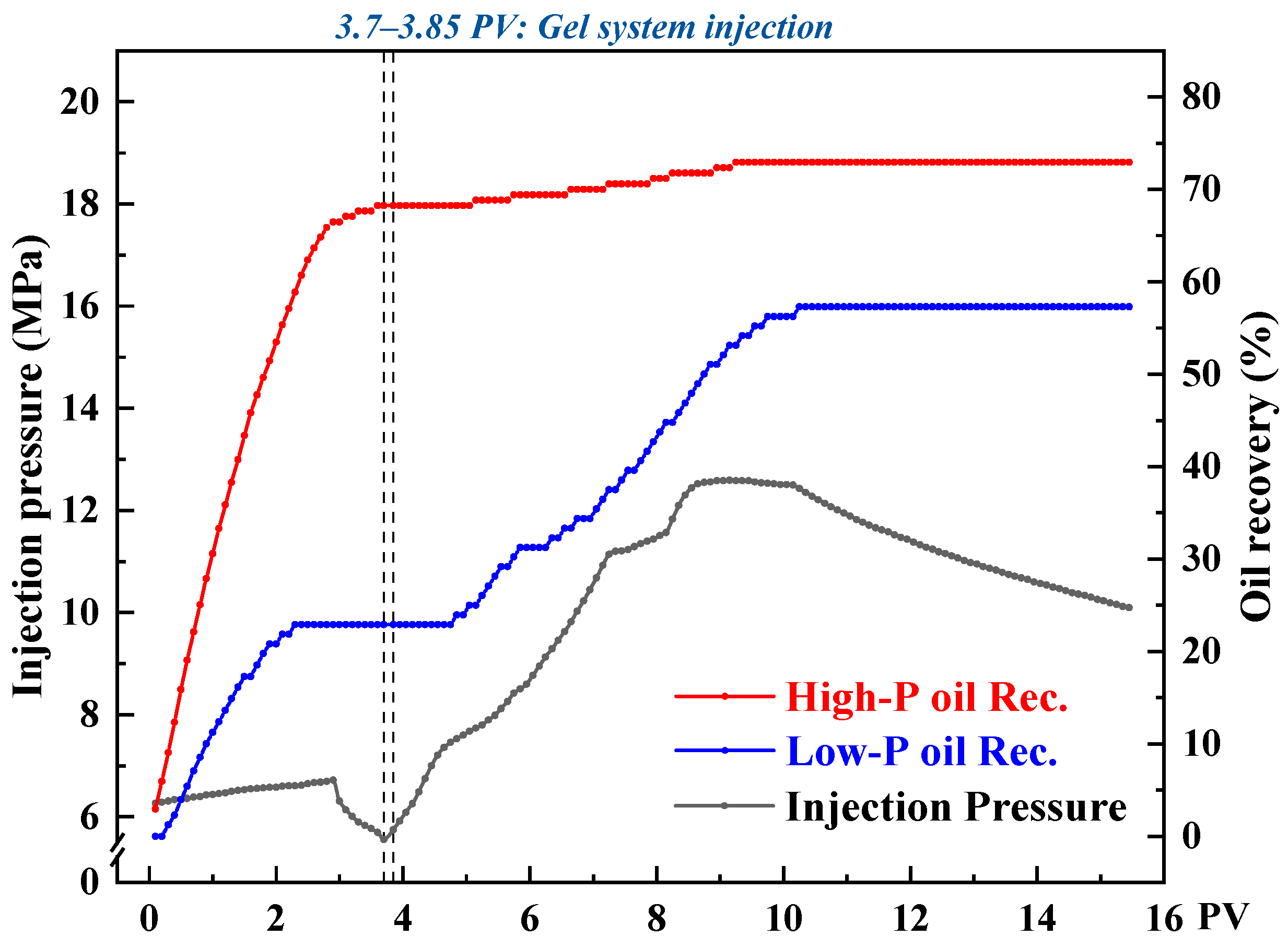
Heterogeneous Dual-Core Parallel Flooding: These experiments were performed in dual-core systems with permeability contrast ratios of 10 (high: 300 mD; low: 30 mD), 20 (high: 600 mD; low: 30 mD), and 30 (high: 600 mD; low: 20 mD). The procedure was as follows: (1) High- and low-permeability cores were oven-dried and aged for 6 h, then sequentially saturated with deionized water and simulated oil; (2) The cores were mounted in the flooding apparatus, with the experimental conditions set to 40 °C, 5 MPa backpressure, and 20 MPa confining pressure; (3) CO2 was injected at 2 mL·min−1 until no further fluid was produced; (4) 0.15 PV of the TMPDA–SDS–SiO2 solution was injected; (5) A second CO2 flooding was carried out until no additional fluid was produced, concluding the experiment. Throughout the entire process, inlet/outlet pressures, cumulative oil production, and CO2 volume were recorded at every 0.1 PV of injection.
A schematic flowchart of the experimental procedures, including gel preparation, structural and rheological characterization, and core-flooding tests, is shown in
Figure 20.