Amidated Pectin/Nanocellulose Hybrid Cryogel System with a pH-Responsive Release Profile for Small Intestinal Delivery
Abstract
1. Introduction
2. Results and Discussion
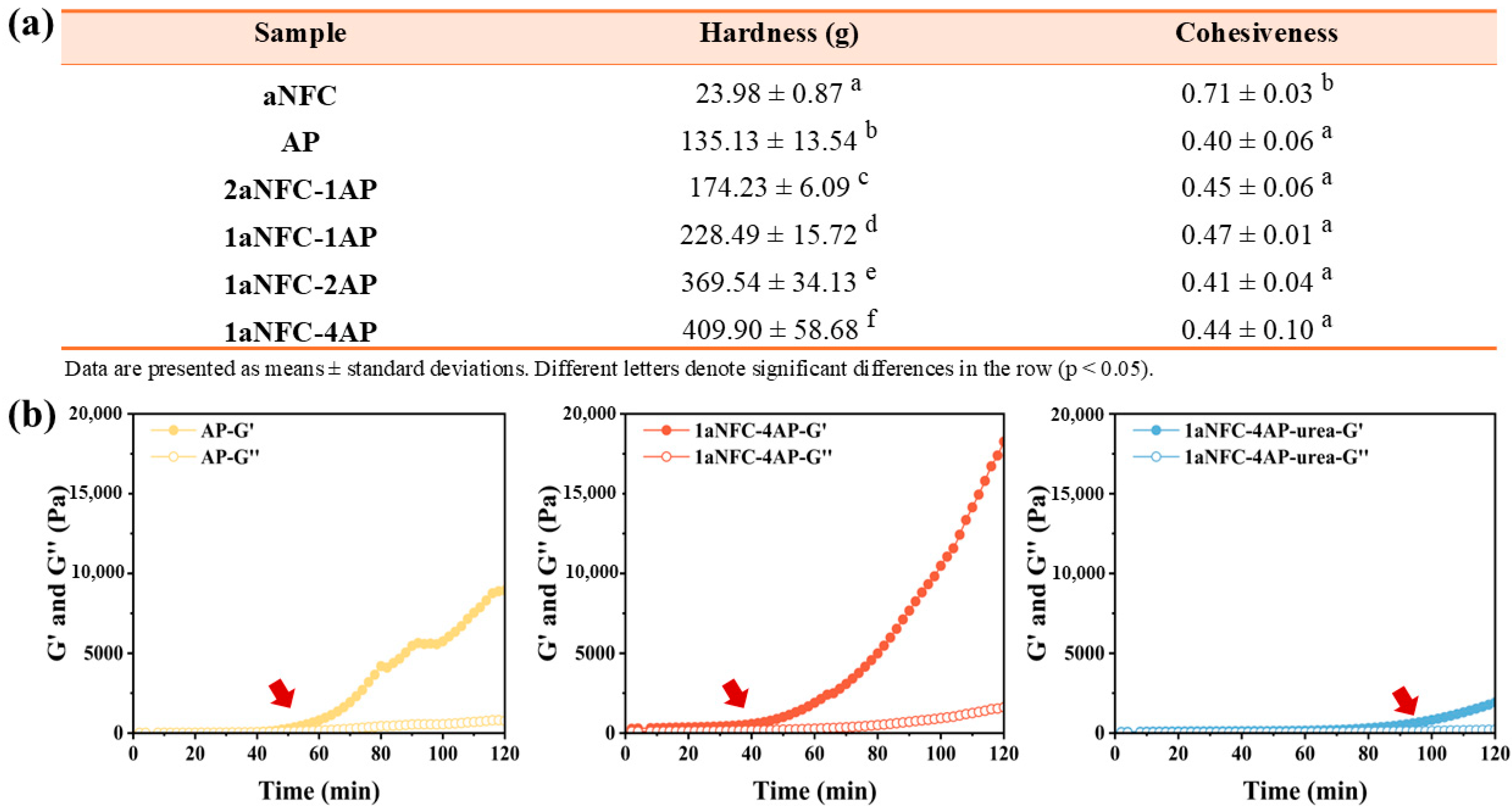
2.1. Textural Properties of aNFC/AP Hybrid Hydrogels
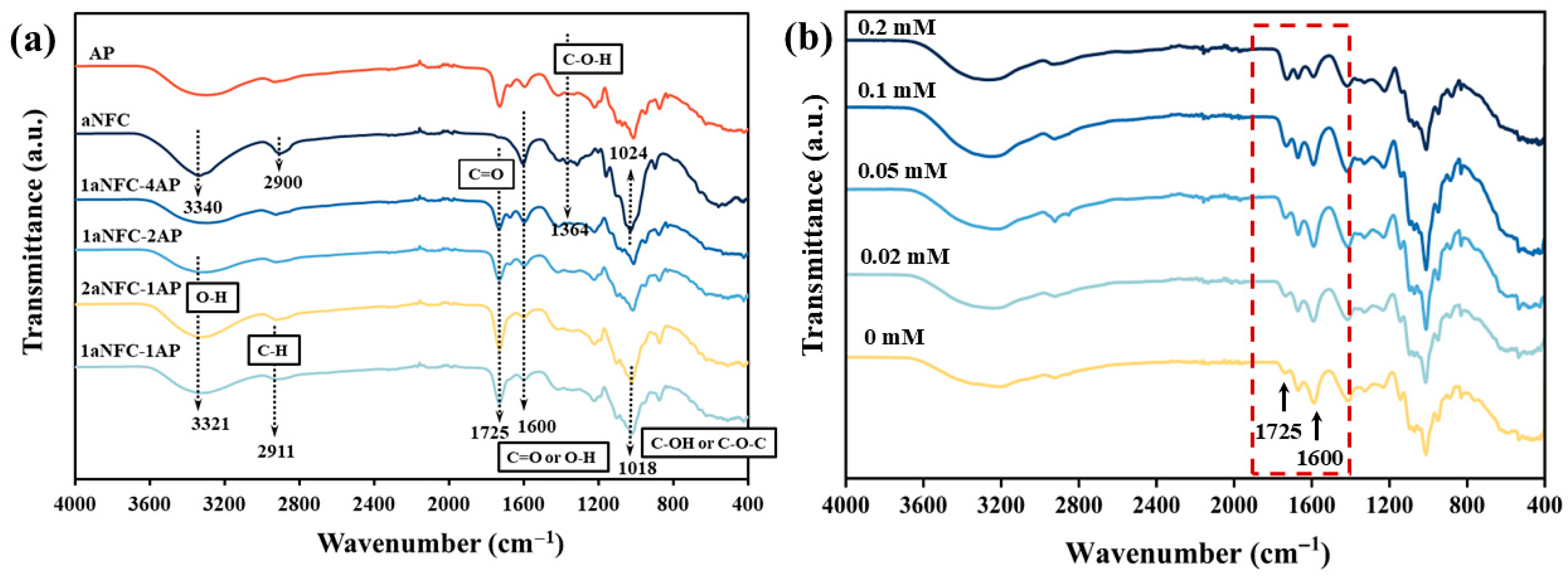
2.2. Structural Analysis of Hybrid aNFC/AP Cryogels
2.3. Morphology of Hybrid aNFC/AP Cryogels
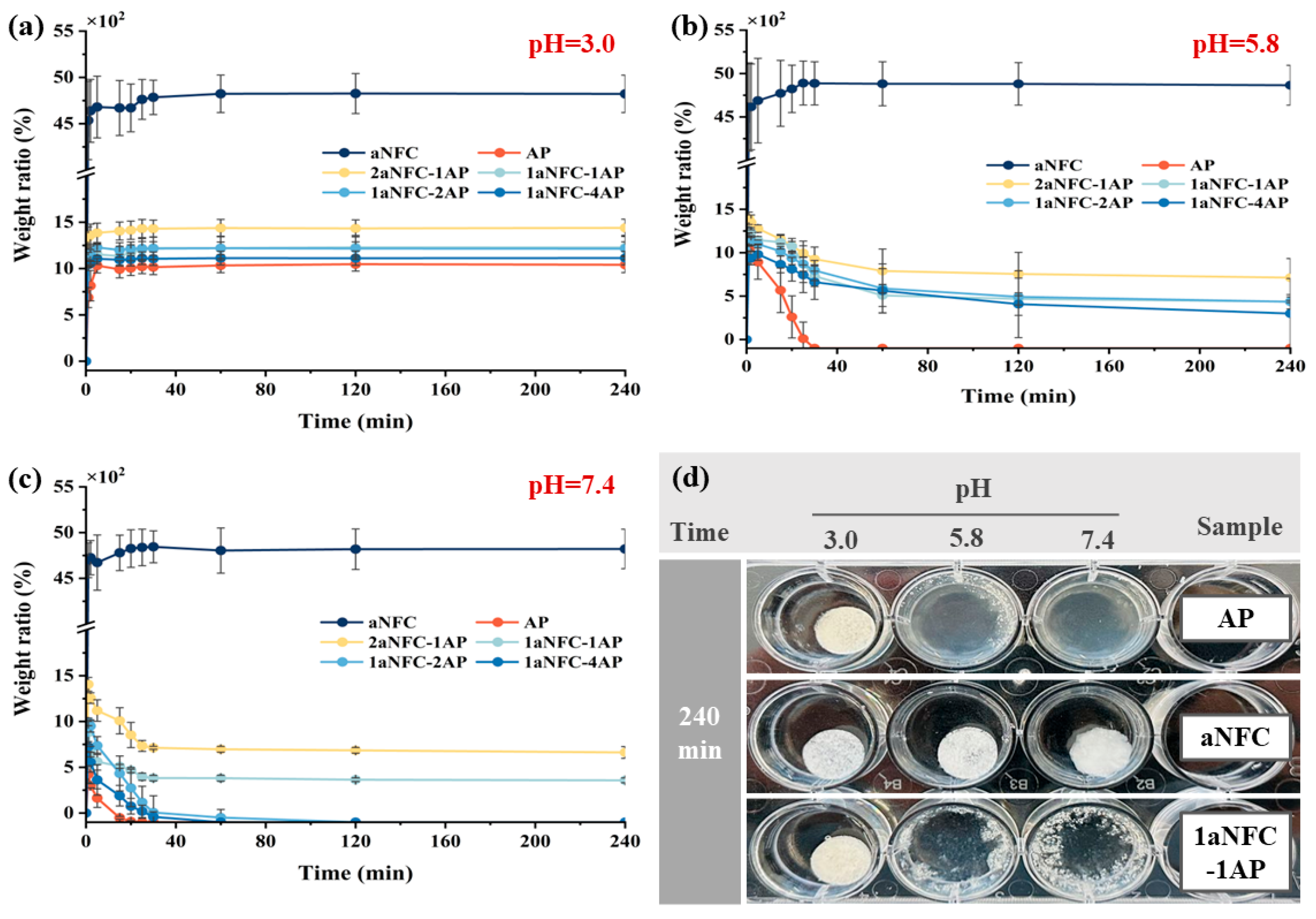
2.4. Swelling of Hybrid aNFC/AP Cryogels Under Difference Acidic Conditions
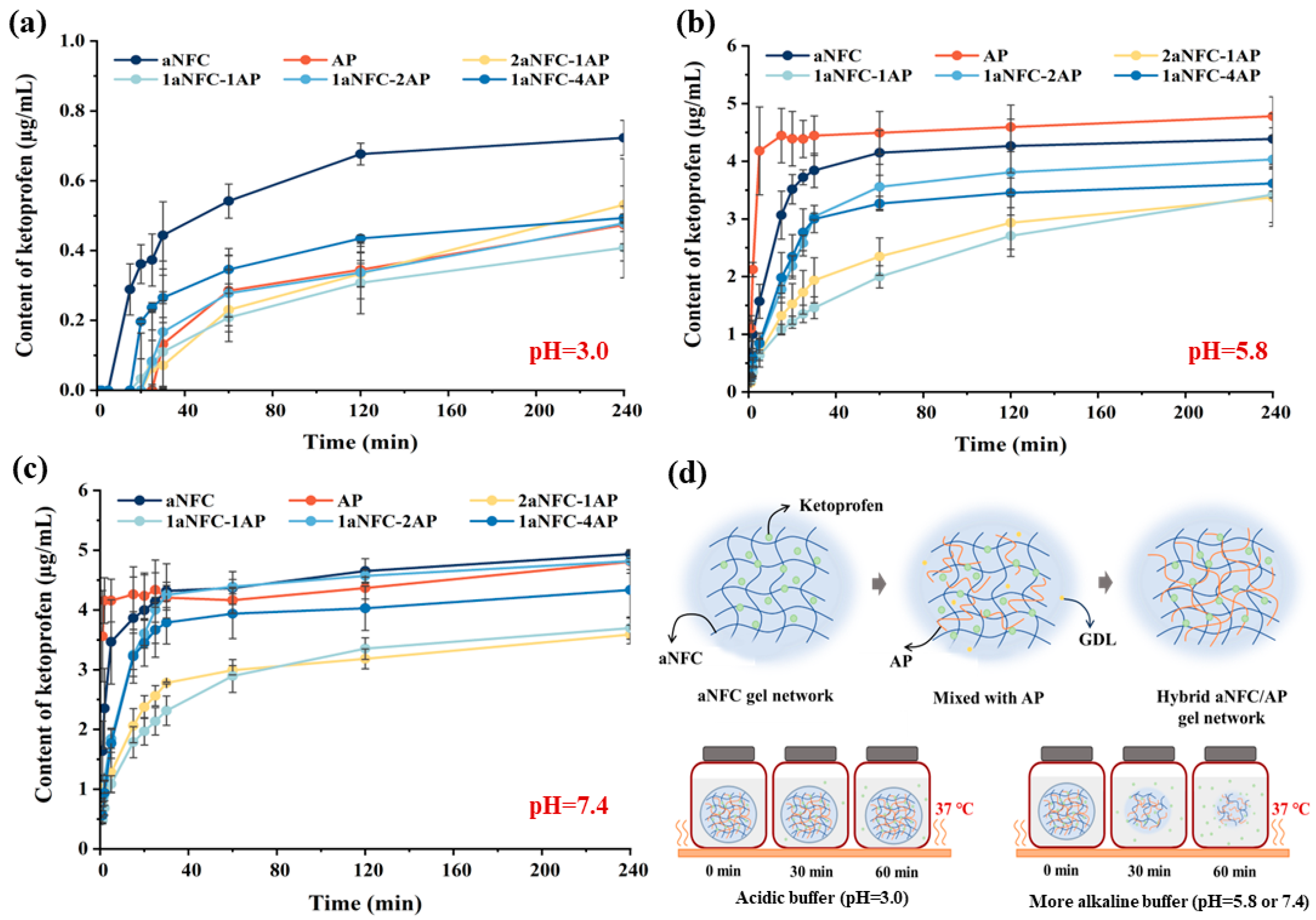
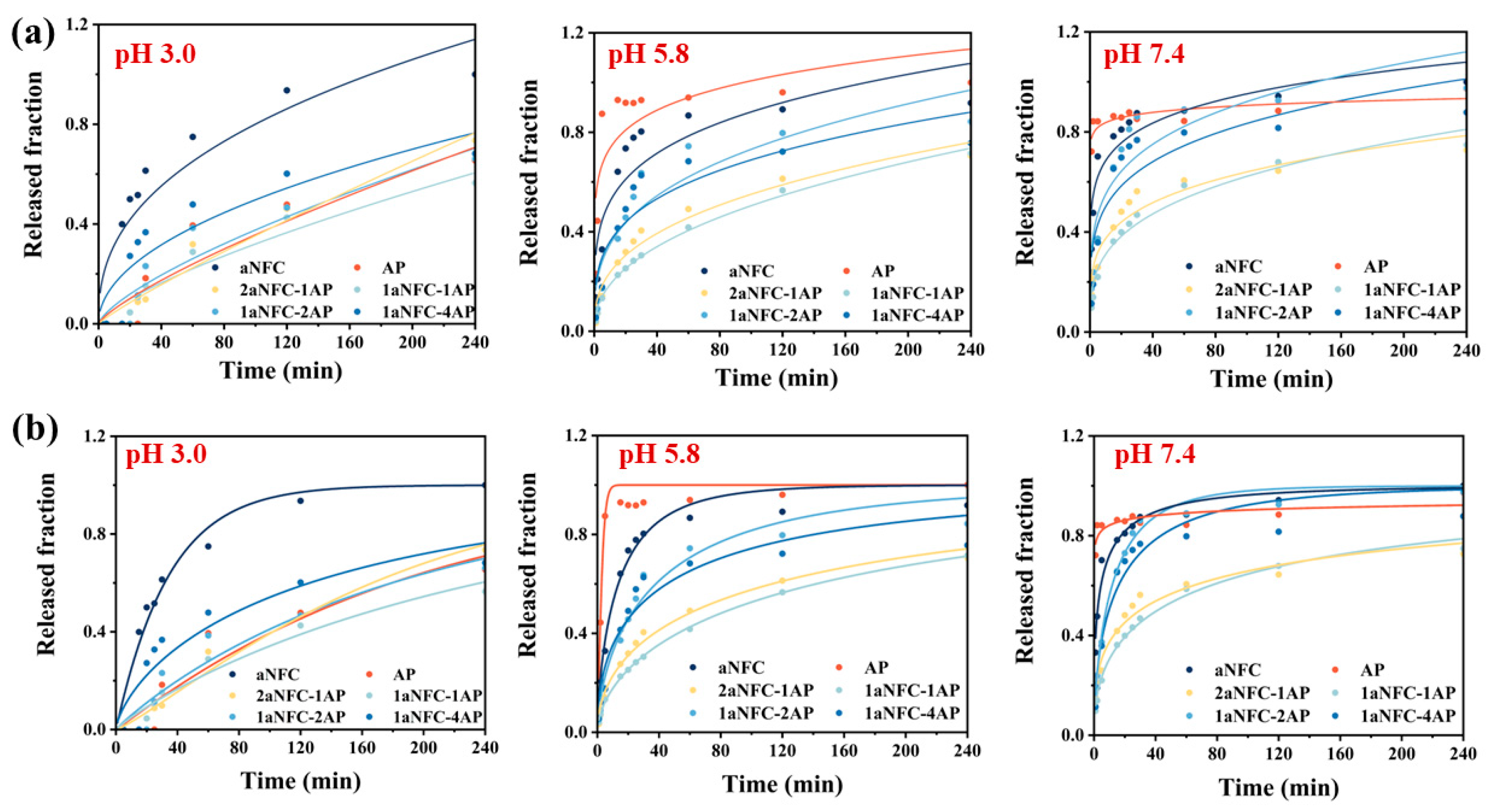
2.5. Release of the Model Compounds in Hybrid aNFC/AP Cryogels
2.6. Non-Linear Release Data Fitting Using Korsmeyer-Peppas and Weibull Model
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the aNFC/AP Hybrid Cryogels
4.3. Textural Analysis of aNFC/AP Hybrid Hydrogels
4.4. Swelling and Degradation Behavior of aNFC/AP Hybrid Cryogels
4.5. In Vitro Drug Release Studies
4.6. Quantification of Released Model Compounds
4.7. Fourier Transform Infrared (FT-IR) Spectroscopy
4.8. Scanning Electron Microscopy (SEM) Measurements
4.9. Scanning Electron Microscopy (SEM) Measurements
4.10. Release Kinetics Modeling
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aNFC | Anionic Nanofibrillar Cellulose |
| AP | Amidated Pectin |
| DDS | Drug Delivery Systems |
| NFC | Nanofibrillar Cellulose |
| GalA | D-Galacturonic Acid |
| HMP | High Methoxyl Pectin |
| LMP | Low Methoxyl Pectin |
| DA | Degree of Amidation |
| GDL | Glucono-δ-Lactone |
References
- Borawake, D.D.; Pande, V.V.; Giri, M.A. Mesoporous Silica Nanoparticles as Theranostic Platform for Smart Drug Delivery: A Review. J. Nanosci. Nanomed. 2017, 2017, JNAN-125. [Google Scholar]
- Heragh, B.K.; Javanshir, S.; Mahdavinia, G.R.; Naimi-Jamal, M.R. Development of pH-sensitive biomaterial-based nanocomposite for highly controlled drug release. Results Mater. 2022, 16, 100324. [Google Scholar] [CrossRef]
- Lu, H.; Li, X.; Tian, T.; Yang, H.; Quan, G.; Zhang, Y.; Huang, H. The pH-responsiveness carrier of sanxan gel beads crosslinked with CaCl2 to control drug release. Int. J. Biol. Macromol. 2023, 250, 126298. [Google Scholar] [CrossRef]
- He, P.; Dai, L.; Wei, J.; Zhu, X.; Li, J.; Chen, Z.; Ni, Y. Nanocellulose-based hydrogels as versatile drug delivery vehicles: A review. Int. J. Biol. Macromol. 2022, 222, 830–843. [Google Scholar] [CrossRef]
- Chauve, G.; Bras, J. Industrial Point of View of Nanocellulose Materials and Their Possible Applications. In Materials and Energy|Handbook of Green Materials; Oksman, K., Mathew, A.P., Bismarck, A., Rojas, O., Sain, M., Eds.; World Scientific: Singapore, 2014; pp. 233–252. [Google Scholar]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2014, 132, 41719. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, J.; Ren, H.; Jin, J.; He, H.; Jin, P.; Wu, Z.; Zheng, Y. Research progress of nanocellulose-based food packaging. Trends Food Sci. Technol. 2024, 143, 104289. [Google Scholar] [CrossRef]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L.; Laukkanen, A.; Hirvonen, J. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef]
- Wu, T.; Farnood, R.; O’Kelly, K.; Chen, B. Mechanical behavior of transparent nanofibrillar cellulose-chitosan nanocomposite films in dry and wet conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Masruchin, N.; Park, B.D.; Causin, V. Dual-responsive composite hydrogels based on TEMPO-oxidized cellulose nanofibril and poly (N-isopropylacrylamide) for model drug release. Cellulose 2017, 25, 485–502. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, V.V.; Virtanen, J.; Merivaara, A.; Virtanen, V.; Laurén, P.; Tuukkanen, S.; Laaksonen, T. Modulating sustained drug release from nanocellulose hydrogel by adjusting the inner geometry of implantable capsules. J. Drug Deliv. Sci. Technol. 2020, 57, 101625. [Google Scholar] [CrossRef]
- Paukkonen, H.; Kunnari, M.; Lauren, P.; Hakkarainen, T.; Auvinen, V.V.; Oksanen, T.; Koivuniemi, R.; Yliperttula, M.; Laaksonen, T. Nanofibrillar cellulose hydrogels and reconstructed hydrogels as matrices for controlled drug release. Int. J. Pharm. 2017, 532, 269–280. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Tuning bio-aerogel properties for controlling theophylline delivery. Part 1: Pectin aerogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112148. [Google Scholar] [CrossRef]
- Itoh, K.; Hatakeyama, T.; Shimoyama, T.; Miyazaki, S.; D’Emanuele, A.; Attwood, D. In situ gelling formulation based on methylcellulose/pectin system for oral-sustained drug delivery to dysphagic patients. Drug Dev. Ind. Pharm. 2011, 37, 790–797. [Google Scholar] [CrossRef]
- Munjeri, O.; Collett, J.H.; Fell, J.T. Amidated Pectin Hydrogel Beads for Colonic Drug Delivery—An In Vitro Study. Drug Deliv. 1997, 4, 207–211. [Google Scholar] [CrossRef]
- Itoh, K.; Yahaba, M.; Takahashi, A.; Tsuruya, R.; Miyazaki, S.; Dairaku, M.; Togashi, M.; Mikami, R.; Attwood, D. In situ gelling xyloglucan/pectin formulations for oral sustained drug delivery. Int. J. Pharm. 2008, 356, 95–101. [Google Scholar] [CrossRef]
- Capel, F.; Nicolai, T.; Durand, D.; Boulenguer, P.; Langendorff, V. Calcium and acid induced gelation of (amidated) low methoxyl pectin. Food Hydrocoll. 2006, 20, 901–907. [Google Scholar] [CrossRef]
- Tamura, C.; Nakauma, M.; Furusawa, H.; Kadota, T.; Kamata, Y.; Nishijima, M.; Itoh, S.; Sugita-Konishi, Y. Formulation of a pectin gel that efficiently traps mycotoxin deoxynivalenol and reduces its bioavailability. Carbohydr. Polym. 2013, 93, 747–752. [Google Scholar] [CrossRef]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- Vithanage, C.R.; Grimson, M.J.; Wills, P.R.; Harrison, P.; Smith, B.G. Rheological and Structural Properties of High-Methoxyl Esterified, Low-Methoxyl Esterified and Low-Methoxyl Amidated Pectin Gels. J. Texture Stud. 2010, 41, 899–927. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Ma, Y.; Bi, J. The role of amide groups in the mechanism of acid-induced pectin gelation: A potential pH-sensitive hydrogel based on hydrogen bond interactions. Food Hydrocoll. 2023, 141, 108741. [Google Scholar] [CrossRef]
- Mishra, R.K.; Datt, M.; Pal, K.; Banthia, A.K. Preparation and characterization of amidated pectin based hydrogels for drug delivery system. J. Mater. Sci. Mater. Med. 2008, 19, 2275–2280. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/Gelatin Composite Cryogels for Controlled Drug Release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Jakubowska, E.; Lulek, J. The application of freeze-drying as a production method of drug nanocrystals and solid dispersions—A review. J. Drug Deliv. Sci. Technol. 2021, 62, 102357. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Bao, Y.; Cui, H.; Li, J.; Song, B.; Wang, M.; Li, H.; Cui, X.; Chen, Y.; et al. Colon-targeted delivery of polyphenols: Construction principles, targeting mechanisms and evaluation methods. Crit. Rev. Food. Sci. Nutr. 2025, 65, 64–86. [Google Scholar] [CrossRef]
- Cacic, A.; Amidzic Klaric, D.; Keser, S.; Radikovic, M.; Rukavina, Z.; Joraholmen, M.W.; Uzelac, L.; Kralj, M.; Skalko-Basnet, N.; Segvic Klaric, M.; et al. A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel. Pharmaceutics 2023, 15, 1356. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2011, 125, 180–188. [Google Scholar] [CrossRef]
- Suenaga, S.; Osada, M. Self-Sustaining Cellulose Nanofiber Hydrogel Produced by Hydrothermal Gelation without Additives. ACS Biomater. Sci. Eng. 2018, 4, 1536–1545. [Google Scholar] [CrossRef]
- Benhamou, K.; Dufresne, A.; Magnin, A.; Mortha, G.; Kaddami, H. Control of size and viscoelastic properties of nanofibrillated cellulose from palm tree by varying the TEMPO-mediated oxidation time. Carbohydr. Polym. 2014, 99, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bhattacharya, S. Compressive textural attributes, opacity and syneresis of gels prepared from gellan, agar and their mixtures. J. Food Eng. 2011, 102, 287–292. [Google Scholar] [CrossRef]
- Ashori, A.; Sepahvand, S.; Jonoobi, M. Development of biodegradable nanofiber filters based on surface-modified cellulose nanofibers with graphene oxide for high removal of airborne particulate matter. Int. J. Biol. Macromol. 2024, 261, 129687. [Google Scholar] [CrossRef]
- Ardjoum, N.; Shankar, S.; Chibani, N.; Salmieri, S.; Lacroix, M. In situ synthesis of silver nanoparticles in pectin matrix using gamma irradiation for the preparation of antibacterial pectin/silver nanoparticles composite films. Food Hydrocoll. 2021, 121, 107000. [Google Scholar] [CrossRef]
- Wang, S.; Attah, R.; Li, J.; Chen, Y.; Chen, R. A pH-Responsive Amphiphilic Hydrogel Based on Pseudopeptides and Poly(ethylene glycol) for Oral Delivery of Hydrophobic Drugs. ACS Biomater. Sci. Eng. 2018, 4, 4236–4243. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Wang, Y.; Higgins, W.; Chen, J.; Chen, Y.; Kharlampieva, E. pH-triggered shape response of cubical ultrathin hydrogel capsules. Soft Matter 2012, 8, 25641. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Lv, P.; Barou, F.; Le Guilcher, C.; Aid, R.; David, B.; Letourneur, D. Interplay between crosslinking and ice nucleation controls the porous structure of freeze-dried hydrogel scaffolds. Biomater. Adv. 2022, 139, 212973. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, F.; Lin, K.; Fang, J.; Chen, F.; Ru, Y.; Weng, H.; Xiao, Q.; Yang, Q.; Xiao, A. Anhydride esterification to regulate water migration and reduce ice crystal formation in κ-carrageenan gel during freezing. Food Hydrocoll. 2024, 150, 109726. [Google Scholar] [CrossRef]
- Muhr, A.H.; Blanshard, J.M.V. Effect of polysaccharide stabilizers on the rate of growth of ice. Int. J. Food Sci. Technol. 2015, 21, 683–710. [Google Scholar] [CrossRef]
- Němec, T. Estimation of ice–water interfacial energy based on pressure-dependent formulation of classical nucleation theory. Chem. Phys. Lett. 2013, 583, 64–68. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Ma, Y.; Bi, J. Study on the ice crystals growth under pectin gels with different crosslinking strengths by modulating the degree of amidation in HG domain. Food. Chem. 2023, 428, 136758. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Yang, W.; Jiang, W.; Chen, F.; Fu, Q. Enhancement of mechanical property and absorption capability of hydrophobically associated polyacrylamide hydrogels by adding cellulose nanofiber. Mater. Res. Express 2020, 7, 6373. [Google Scholar] [CrossRef]
- Shaheen, A.; Maswal, M.; Dar, A.A. Synergistic effect of various metal ions on the mechanical, thixotropic, self-healing, swelling and water retention properties of bimetallic hydrogels of alginate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127223. [Google Scholar] [CrossRef]
- Ghorbani, M.; Roshangar, L. Construction of collagen/nanocrystalline cellulose based-hydrogel scaffolds: Synthesis, characterization, and mechanical properties evaluation. Int. J. Polym. Mater. Polym. Biomater. 2019, 70, 142–148. [Google Scholar] [CrossRef]
- Livney, Y.D.; Portnaya, I.; Faupin, B.; Fahoum, L.; Ramon, O.; Cohen, Y.; Mizrahi, S.; Cogan, U. Interactions of glucose and polyacrylamide in solutions and gels. J. Polym. Sci. B Polym. Phys. 2003, 41, 3053–3063. [Google Scholar] [CrossRef]
- van der Sman, R.G.M.; Vergeldt, F.J.; Van As, H.; van Dalen, G.; Voda, A.; van Duynhoven, J.P.M. Multiphysics pore-scale model for the rehydration of porous foods. Innov. Food Sci. Emerg. Technol. 2014, 24, 69–79. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, D.; Liu, Y.; Liu, J. Study on the influence of sample size and test conditions on the capillary water absorption coefficient of porous building materials. J. Build. Eng. 2021, 43, 103120. [Google Scholar] [CrossRef]
- O’Grady, J.; Murphy, C.L.; Barry, L.; Shanahan, F.; Buckley, M. Defining gastrointestinal transit time using video capsule endoscopy: A study of healthy subjects. Endosc. Int. Open 2020, 8, e396–e400. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Skalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Fosca, M.; Rau, J.V.; Uskokovic, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Ramesh, S.; Zvonicek, V.; Pecek, D.; Pislova, M.; Beranek, J.; Hofmann, J.; Dumicic, A. Acid-Neutralizing Omeprazole Formulation for Rapid Release and Absorption. Pharmaceutics 2025, 17, 161. [Google Scholar] [CrossRef]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Razzaq Chaudhry, W.; Akbar, A. Effects of Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]

| pH | Sample | Korsmeyer–Peppas | Weibull | ||||
|---|---|---|---|---|---|---|---|
| k | n | r2 | λ | β | r2 | ||
| 3.0 | aNFC | 0.12 | 0.404 | 0.857 | 34.48 | 0.413 | 0.97 |
| AP | 0.009 | 0.782 | 0.865 | 195.057 | 1.036 | 0.9 | |
| 2aNFC–1AP | 0.006 | 0.884 | 0.951 | 179.341 | 1.192 | 0.97 | |
| 1aNFC–1AP | 0.011 | 0.728 | 0.934 | 260.828 | 0.91 | 0.95 | |
| 1aNFC–2AP | 0.013 | 0.716 | 0.891 | 196.779 | 0.942 | 0.92 | |
| 1aNFC–4AP | 0.051 | 0.493 | 0.831 | 142.517 | 0.704 | 0.87 | |
| 5.8 | aNFC | 0.308 | 0.228 | 0.776 | 15.443 | 0.685 | 0.97 |
| AP | 0.538 | 0.136 | 0.572 | 2.922 | 1.3 | 0.95 | |
| 2aNFC–1AP | 0.101 | 0.368 | 0.953 | 133.777 | 0.521 | 0.98 | |
| 1aNFC–1AP | 0.069 | 0.431 | 0.993 | 164.65 | 0.584 | 0.99 | |
| 1aNFC–2AP | 0.166 | 0.322 | 0.861 | 43.509 | 0.634 | 0.95 | |
| 1aNFC–4AP | 0.189 | 0.281 | 0.814 | 53.712 | 0.495 | 0.9 | |
| 7.4 | aNFC | 0.493 | 0.143 | 0.83 | 5.524 | 0.413 | 0.96 |
| AP | 0.774 | 0.034 | 0.63 | 0.025 | 0.102 | 0.63 | |
| 2aNFC–1AP | 0.218 | 0.234 | 0.91 | 82.061 | 0.36 | 0.95 | |
| 1aNFC–1AP | 0.155 | 0.302 | 0.96 | 91.66 | 0.455 | 0.99 | |
| 1aNFC–2AP | 0.329 | 0.224 | 0.81 | 93.33 | 0.675 | 0.99 | |
| 1aNFC–4AP | 0.301 | 0.221 | 0.76 | 142.517 | 0.56 | 0.92 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Laurén, P.; Zini, J.; Gounani, Z.; Bi, J.; Yi, J.; Laaksonen, T. Amidated Pectin/Nanocellulose Hybrid Cryogel System with a pH-Responsive Release Profile for Small Intestinal Delivery. Gels 2025, 11, 700. https://doi.org/10.3390/gels11090700
Feng S, Laurén P, Zini J, Gounani Z, Bi J, Yi J, Laaksonen T. Amidated Pectin/Nanocellulose Hybrid Cryogel System with a pH-Responsive Release Profile for Small Intestinal Delivery. Gels. 2025; 11(9):700. https://doi.org/10.3390/gels11090700
Chicago/Turabian StyleFeng, Shuhan, Patrick Laurén, Jacopo Zini, Zahra Gounani, Jinfeng Bi, Jianyong Yi, and Timo Laaksonen. 2025. "Amidated Pectin/Nanocellulose Hybrid Cryogel System with a pH-Responsive Release Profile for Small Intestinal Delivery" Gels 11, no. 9: 700. https://doi.org/10.3390/gels11090700
APA StyleFeng, S., Laurén, P., Zini, J., Gounani, Z., Bi, J., Yi, J., & Laaksonen, T. (2025). Amidated Pectin/Nanocellulose Hybrid Cryogel System with a pH-Responsive Release Profile for Small Intestinal Delivery. Gels, 11(9), 700. https://doi.org/10.3390/gels11090700








