Polymeric Systems as Hydrogels and Membranes Containing Silver Nanoparticles for Biomedical and Food Applications: Recent Approaches and Perspectives
Abstract
1. Introduction
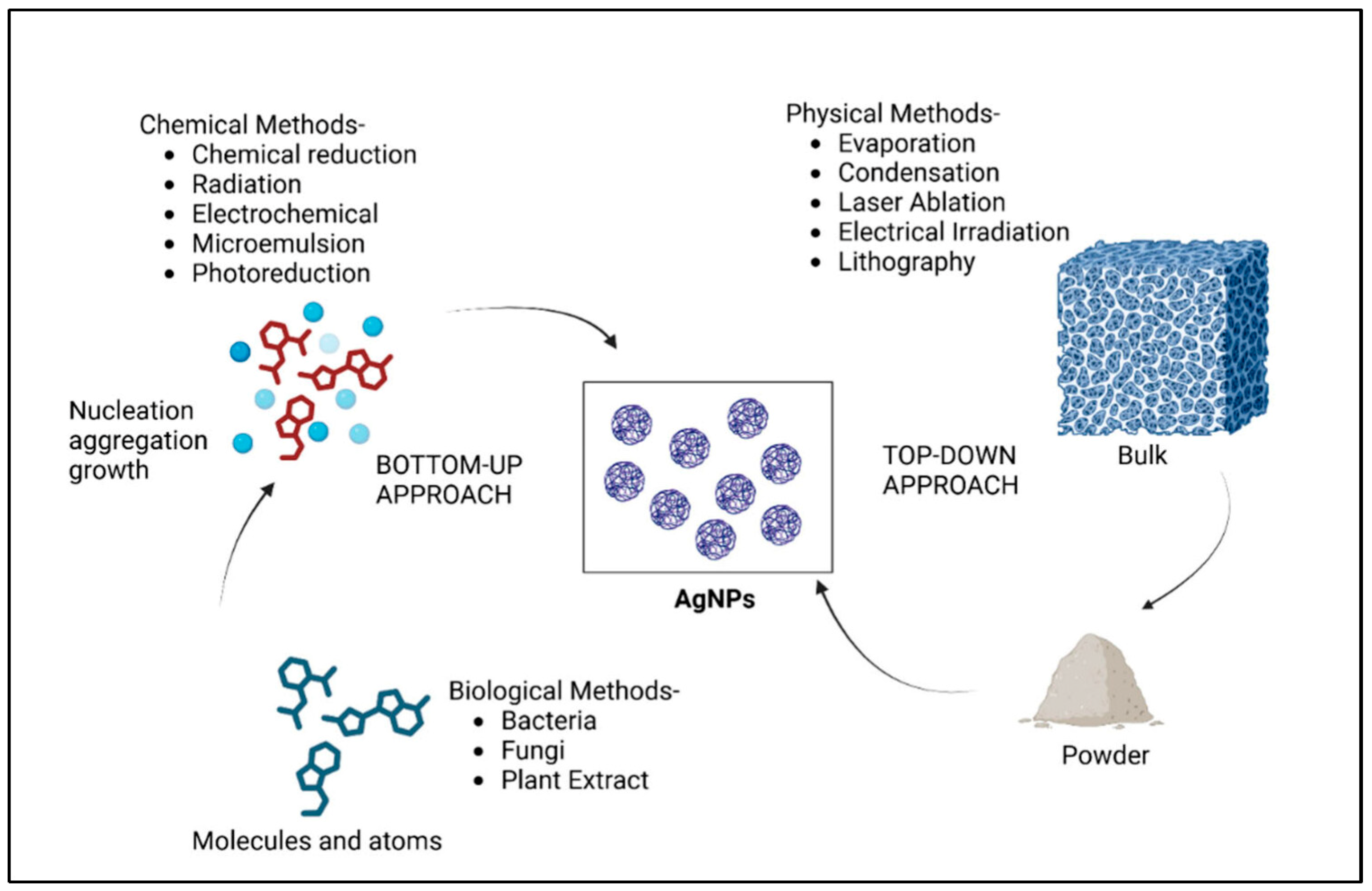
2. Fundamentals of Silver Nanoparticles
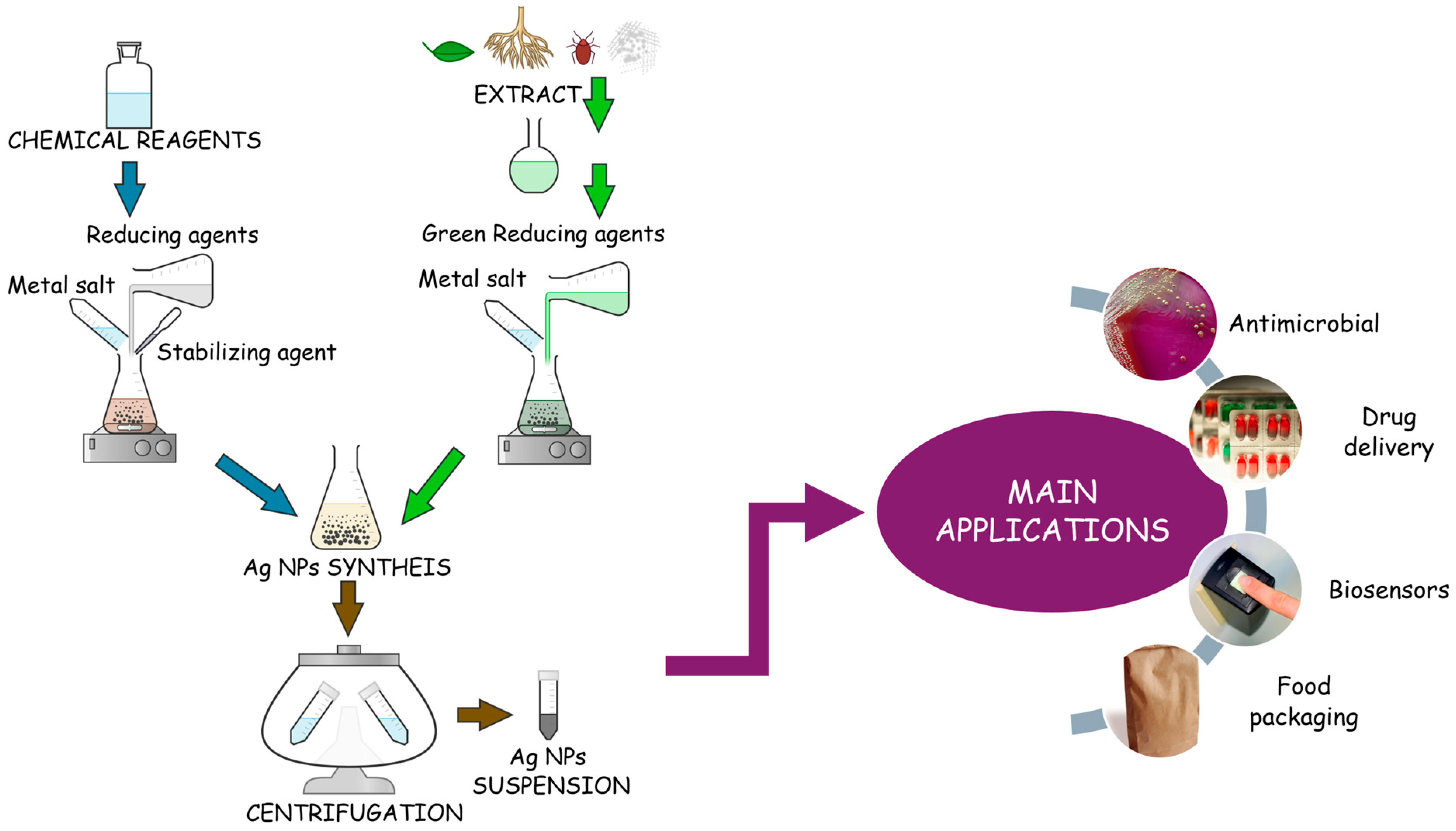
2.1. Synthesis Techniques for AgNPs
2.2. Physicochemical Properties of AgNPs
2.3. Antimicrobial and Biological Activity
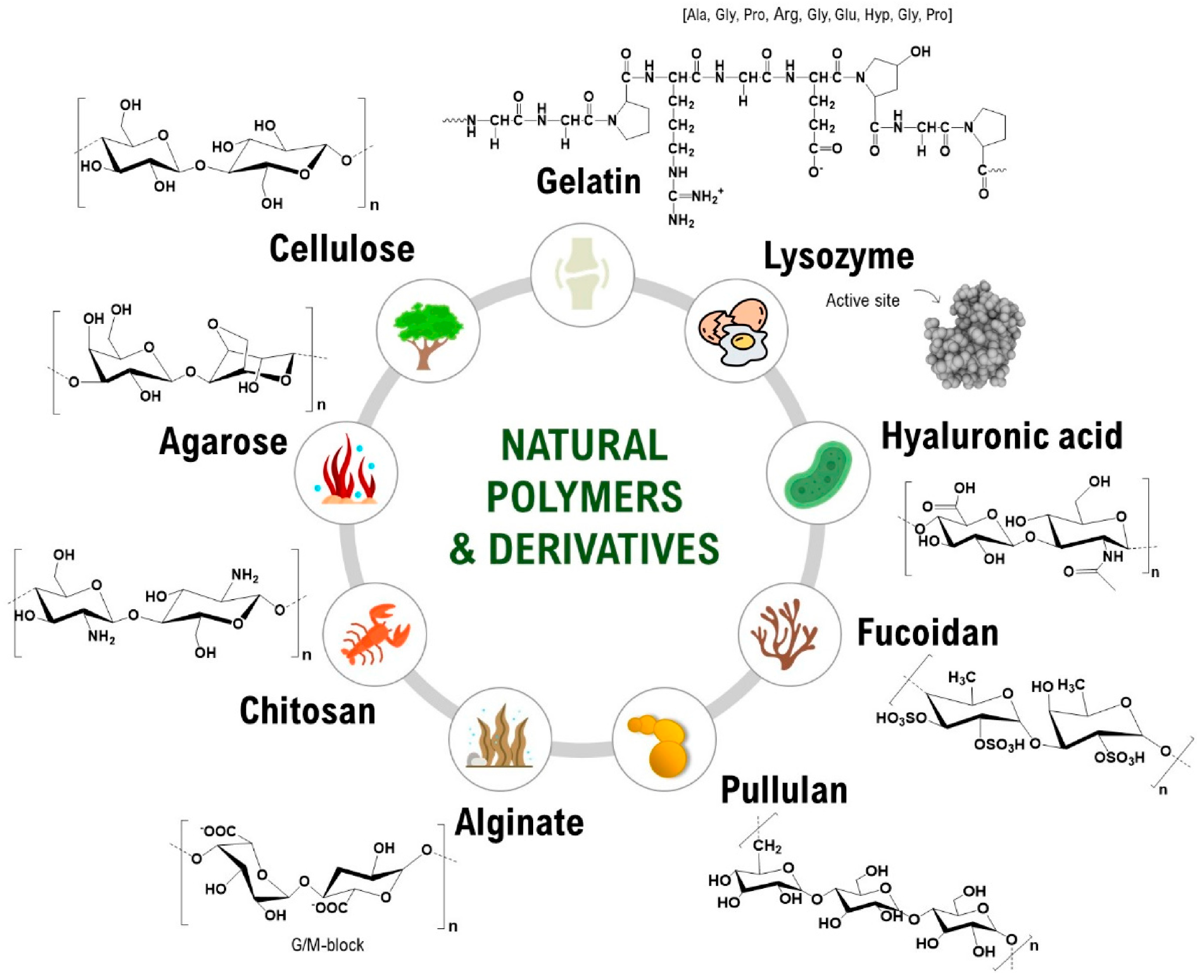
3. Polymeric Matrices for AgNPs Incorporation
3.1. Natural Polymers
3.2. Synthetic Polymers
4. Hybrid AgNPs–Polymer Systems
4.1. Hydrogels
4.2. Membranes and Films
4.3. Scaffolds
4.4. 3D/4D Printed Structures
4.5. Coatings and Sprays
5. Applications for Polymeric Systems Containing AgNPs
5.1. Biomedical Applications
5.1.1. Antibacterial Wound Dressings
5.1.2. Drug Delivery and Controlled Release Systems
5.1.3. Tissue Regeneration and Scaffolding
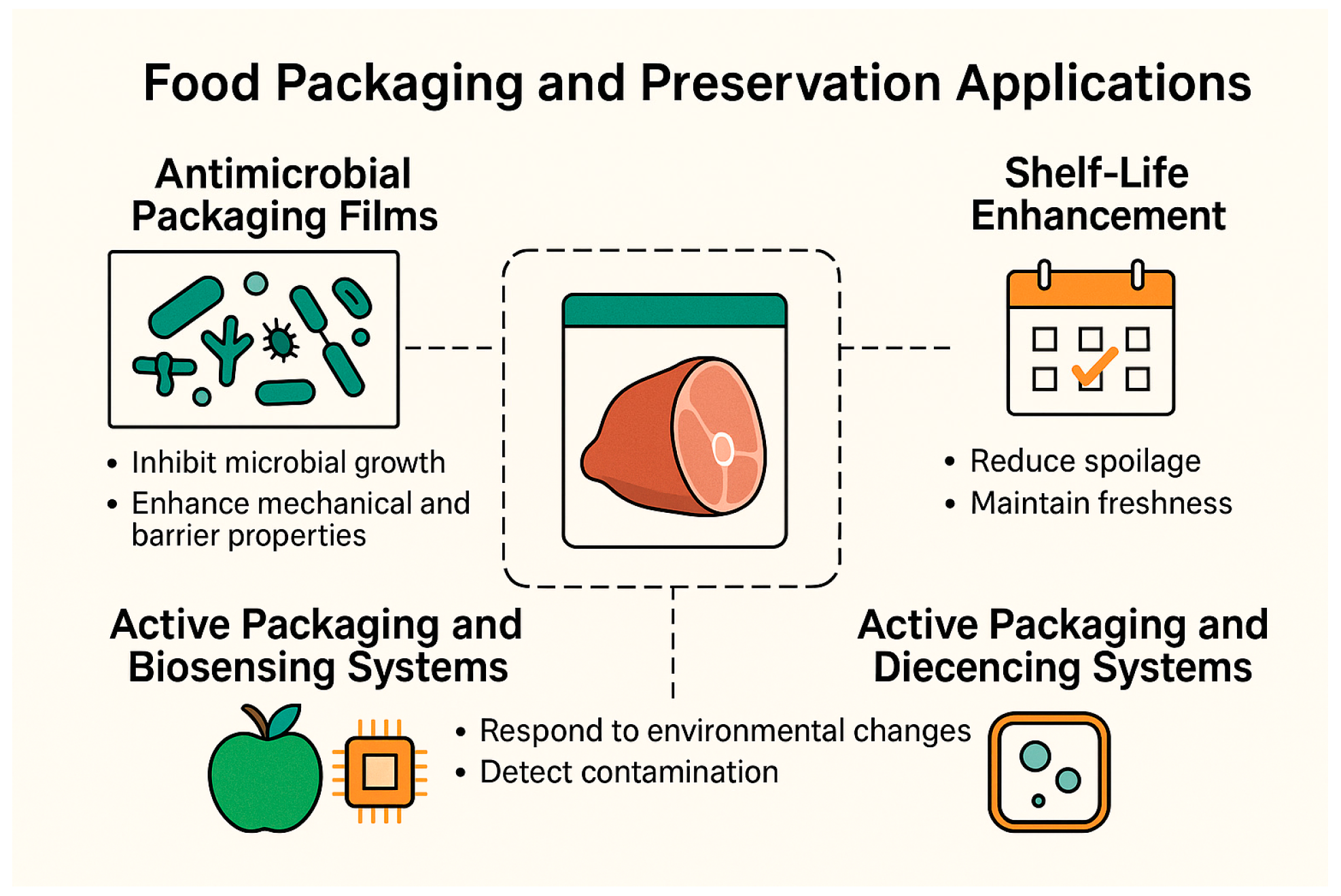
5.2. Food Packaging Applications
5.2.1. Antimicrobial Packaging Films
5.2.2. Shelf-Life Extension Through AgNP–Polymer Packaging
5.2.3. Active Packaging and Biosensing Systems
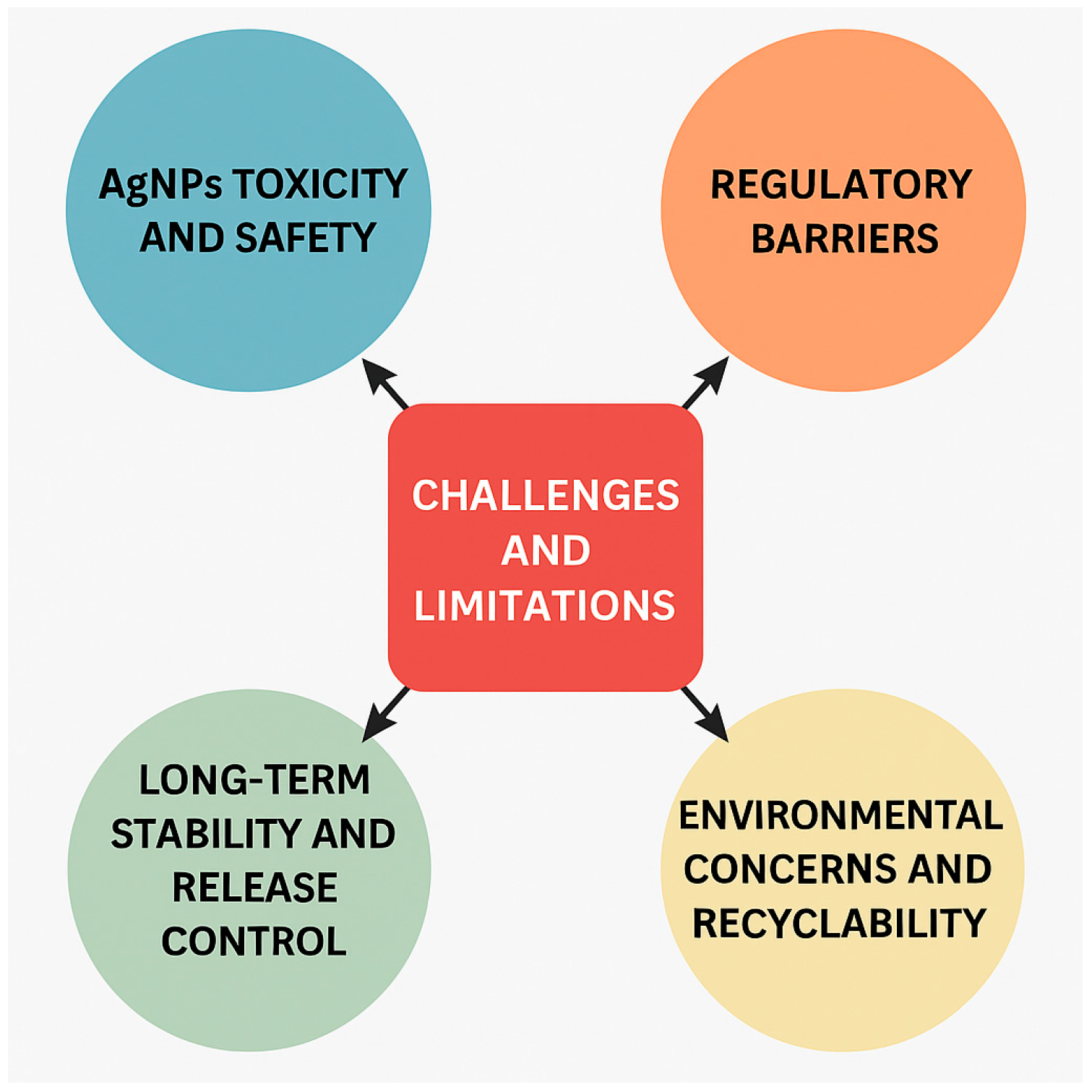
6. Challenges and Limitations
6.1. AgNPs Toxicity and Safety
6.2. Regulatory Barriers
6.3. Recommendations for Standardizing Characterization and Testing
6.4. Long-Term Stability and Release Control
6.5. Environmental Concerns and Recyclability
6.5.1. Ecotoxicity and Recycling Challenges in Wastewater and Biosolids
6.5.2. Mitigation Strategies and Sustainable Design
6.5.3. Environmental Impact Across the Entire Life Cycle
7. Future Perspectives and Opportunities
7.1. Integration with Other Nanomaterials
7.2. Smart Packaging with IoT and Biosensing Integration
7.2.1. Colorimetric Sensor Arrays
7.2.2. IoT-Enabled RFID/NFC Smart Tags
7.3. Emerging Bioinks and Advanced 4D Printing
7.4. Cost Optimization, Scalability, and Commercialization Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of silver nanoparticles in hydrogel matrices for controlling wound infection. J. Burn Care Res. 2021, 42, 785–793. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiol. Res. 2025, 16, 110. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Foyez, T.; Krishna, S.B.N.; Poda, S.; Imran, A.B. Recent advances of silver nanoparticle-based polymer nanocomposites for biomedical applications. RSC Adv. 2025, 15, 8480–8505. [Google Scholar] [CrossRef]
- Alshangiti, D.M.; El-damhougy, T.K.; Zaher, A.; Madani, M.; Mohamady Ghobashy, M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef]
- Yang, A.-L.; Sun, S.-B.; Qu, L.-Y.; Li, X.-Y.; Liu, J.-L.; Zhou, F.; Xu, Y.-J. Polysaccharide hydrogel containing silver nanoparticle@ catechol microspheres with photothermal, antibacterial and anti-inflammatory activities for infected-wounds repair. Int. J. Biol. Macromol. 2024, 265, 130898. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ.-Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Kalakonda, P.; Thudumu, S.; Mynepall, S.L.; Kathi, R.; Yatham, V.R.; Mandal, P.; Banne, S.; Kalakonda, P.B.; Banavoth, M.; Kigozi, M.; et al. Porous micro-/nano-fibrous silver-coated polymeric scaffolds with tunable mechanical properties for wound healing applications. Polym. Bull. 2025, 82, 4443–4457. [Google Scholar] [CrossRef]
- Chandrababu, V.; Parameswaranpillai, J.; Gopi, J.A.; Pathak, C.; Midhun Dominic, C.D.; Feng, N.L.; Krishnasamy, S.; Muthukumar, C.; Hameed, N.; Ganguly, S. Progress in food packaging applications of biopolymer-nanometal composites—A comprehensive review. Biomater. Adv. 2024, 162, 213921. [Google Scholar] [CrossRef]
- Astaneh, M.E.; Fereydouni, N. Silver Nanoparticles in 3D Printing: A New Frontier in Wound Healing. ACS Omega 2024, 9, 41107–41129. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, J. Recent advances in 4D printing hydrogel for biological interfaces. Int. J. Mater. Form. 2023, 16, 55. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver nanoparticles (AgNPs): Comprehensive insights into bio/synthesis, key influencing factors, multifaceted applications, and toxicity—A 2024 update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver nanoparticles: Synthesis, structure, properties and applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A review of silver nanoparticles: Synthesis methods, properties and applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Sudarman, F.; Shiddiq, M.; Armynah, B.; Tahir, D. Silver nanoparticles (AgNPs) synthesis methods as heavy-metal sensors: A review. Int. J. Environ. Sci. Technol. 2023, 20, 9351–9368. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Nemčeková, K.; Dudoňová, P.; Holka, T.; Balážová, S.; Hornychová, M.; Szebellaiová, V.; Naumowicz, M.; Gemeiner, P.; Mackuľak, T.; Gál, M. Silver Nanoparticles for Biosensing and Drug Delivery: A Mechanical Study on DNA Interaction. Biosensors 2025, 15, 331. [Google Scholar] [CrossRef]
- Beck, F.; Loessl, M.; Baeumner, A.J. Signaling strategies of silver nanoparticles in optical and electrochemical biosensors: Considering their potential for the point-of-care. Microchim. Acta 2023, 190, 91. [Google Scholar] [CrossRef] [PubMed]
- Chicea, D.; Nicolae-Maranciuc, A. Metal Nanocomposites as Biosensors for Biological Fluids Analysis. Materials 2025, 18, 1809. [Google Scholar] [CrossRef]
- Geagea, R.; Aubert, P.-H.; Banet, P.; Sanson, N. Signal enhancement of electrochemical biosensors via direct electrochemical oxidation of silver nanoparticle labels coated with zwitterionic polymers. Chem. Commun. 2015, 51, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Wang, L.; Wang, H.-B.; Gan, T.; Wu, Y.-Y.; Li, J.; Liu, Y.-M. Electrochemical biosensor based on silver nanoparticles–polydopamine–graphene nanocomposite for sensitive determination of adenine and guanine. Talanta 2013, 114, 43–48. [Google Scholar] [CrossRef]
- Nicolae-Maranciuc, A.; Chicea, D.; Chicea, L.M. Ag nanoparticles for biomedical applications—Synthesis and characterization—A review. Int. J. Mol. Sci. 2022, 23, 5778. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of silver nanoparticles: From conventional to ‘modern’ methods—A review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A. The recent progress on silver nanoparticles: Synthesis and electronic applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- De Muijlder, T.; Voué, M.; Leclère, P. Laser Ablation Synthesis of Silver Nanoparticles for Polymer Nanocomposites. Energies 2023, 16, 4625. [Google Scholar] [CrossRef]
- Ali, W.A.; Richards, S.E.; Alzard, R.H. Unlocking the potential of ball milling for nanomaterial Synthesis: An overview. J. Ind. Eng. Chem. 2025, 149, 63–93. [Google Scholar] [CrossRef]
- Haynes, C.L.; Van Duyne, R.P. Nanosphere Lithography: A Versatile Nanofabrication Tool for Studies of Size-Dependent Nanoparticle Optics; ACS Publications: Washington, DC, USA, 2001; pp. 5599–5611. [Google Scholar]
- Mwenze, N.M.; Juma, M.; Maaza, M.; Birech, Z.; Dhlamini, M. Laser liquid ablation for silver nanoparticles synthesis and conjugation with hydroxychloroquine for COVID-19 treatment. Mater. Today Proc. 2023, in press. [CrossRef]
- Abdulraheem, I.M.; Khodair, Z.T.; Habeeb, A.A. Preparation and characterization of silver nanoparticles (Agnps) by pulsed laser ablation in liquid. Macromol. Symp. 2023, 407, 2100376. [Google Scholar] [CrossRef]
- Kováčová, M.; Daneu, N.; Tkáčiková, Ľ.; Búreš, R.; Dutková, E.; Stahorský, M.; Bujňáková, Z.L.; Baláž, M. Sustainable One-Step Solid-State Synthesis of Antibacterially Active Silver Nanoparticles Using Mechanochemistry. Nanomaterials 2020, 10, 2119. [Google Scholar] [CrossRef]
- Ahani, M.; Khatibzadeh, M. Optimisation of significant parameters through response surface methodology in the synthesis of silver nanoparticles by chemical reduction method. Micro Nano Lett. 2017, 12, 705–710. [Google Scholar] [CrossRef]
- Althobaiti, F.; Abu Ali, O.A.; Kamal, I.; Alfaifi, M.Y.; Shati, A.A.; Fayad, E.; Elbehairi, S.E.I.; Elshaarawy, R.F.; El-Fattah, W.A. New ionic liquid microemulsion-mediated synthesis of silver nanoparticles for skin bacterial infection treatments. Antibiotics 2023, 12, 247. [Google Scholar] [CrossRef]
- Durdu, S.; Yalçin, E.; Altinkök, A.; Çavuşoğlu, K. Characterization and investigation of electrochemical and biological properties of antibacterial silver nanoparticle-deposited TiO2 nanotube array surfaces. Sci. Rep. 2023, 13, 4699. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, E.; Roto, R.; Novarita, D.; Suwondo, K.; Kuswandi, B. Preparation of TiO2/AgNPs by photodeposition method using Ag(I) present in radiophotography wastewater and their antibacterial activity in visible light illumination. J. Environ. Chem. Eng. 2019, 7, 103178. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Ahmed, A.N.; Gafur, M.A.; Seong, G.; Hossain, M.Z. Size-Controlled Facile Synthesis of Silver Nanoparticles by Chemical Reduction Method and Analysis of Their Antibacterial Performance. ChemistrySelect 2021, 6, 9714–9720. [Google Scholar] [CrossRef]
- Horne, J.; De Bleye, C.; Lebrun, P.; Kemik, K.; Van Laethem, T.; Sacré, P.-Y.; Hubert, P.; Hubert, C.; Ziemons, E. Optimization of silver nanoparticles synthesis by chemical reduction to enhance SERS quantitative performances: Early characterization using the quality by design approach. J. Pharm. Biomed. Anal. 2023, 233, 115475. [Google Scholar] [CrossRef]
- Fu, L.-M.; Hsu, J.-H.; Shih, M.-K.; Hsieh, C.-W.; Ju, W.-J.; Chen, Y.-W.; Lee, B.-H.; Hou, C.-Y. Process optimization of silver nanoparticle synthesis and its application in mercury detection. Micromachines 2021, 12, 1123. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Zahran, M.; Beltagi, A.M.; Rabie, M.; Maher, R.; Hathoot, A.A.; Azzem, M.A. Biosynthesized silver nanoparticles for electrochemical detection of bromocresol green in river water. R. Soc. Open Sci. 2023, 10, 221621. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, I.; Khan, H.; Rahman, F.U.; Rahman, M.U.; Saleem, M.A.; Nazir, S.; Ali, A.; Ullah, A. Green synthesis of AgNPs from leaves extract of Saliva Sclarea, their characterization, antibacterial activity, and catalytic reduction ability. Z. für Phys. Chem. 2024, 238, 931–947. [Google Scholar] [CrossRef]
- Alzoubi, F.; Ahmad, A.A.; Aljarrah, I.A.; Migdadi, A.; Al-Bataineh, Q.M. Localize surface plasmon resonance of silver nanoparticles using Mie theory. J. Mater. Sci. Mater. Electron. 2023, 34, 2128. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J.; Walker, H.W. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef] [PubMed]
- El-Zahry, M.R.; Mahmoud, A.; Refaat, I.H.; Mohamed, H.A.; Bohlmann, H.; Lendl, B. Antibacterial effect of various shapes of silver nanoparticles monitored by SERS. Talanta 2015, 138, 183–189. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Adv. Powder Technol. 2018, 29, 407–415. [Google Scholar] [CrossRef]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo, on their antibacterial efficacy. Nanomaterials 2017, 7, 178. [Google Scholar] [CrossRef]
- Secario, M.K.; Truong, T.T.V.; Chen, C.-C.; Lai, J.-Y.; Lue, S.J. Size-dependent antibacterial efficacy of silver nanoparticles from a green synthesis method: Effects of extract quantity and origin. J. Taiwan Inst. Chem. Eng. 2024, 161, 105511. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, S.; Fu, Y.; Wang, Y.; Mi, J.; Lu, T.; Wang, X.; Lü, C. Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng. C 2020, 110, 110735. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green synthesis of silver nanoparticles (AgNPs), structural characterization, and their antibacterial potential. Dose-Response 2022, 20, 15593258221088709. [Google Scholar] [CrossRef]
- Skomorokhova, E.; Sankova, T.; Orlov, I.; Savelev, A.; Magazenkova, D.; Pliss, M.; Skvortsov, A.; Sosnin, I.; Kirilenko, D.; Grishchuk, I. Size-Dependent bioactivity of silver nanoparticles: Antibacterial properties, influence on copper status in mice, and whole-body turnover. Nanotechnol. Sci. Appl. 2020, 13, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Wang, Z.X.; Chen, C.Y.; Wang, Y.; Li, F.X.Z.; Huang, J.; Luo, Z.W.; Rao, S.S.; Tan, Y.J.; Liu, Y.W.; Yin, H. Ångstrom-scale silver particles as a promising agent for low-toxicity broad-spectrum potent anticancer therapy. Adv. Funct. Mater. 2019, 29, 1808556. [Google Scholar] [CrossRef]
- Liu, H.-L.; Dai, S.A.; Fu, K.-Y.; Hsu, S.-H. Antibacterial properties of silver nanoparticles in three different sizes and their nanocomposites with a new waterborne polyurethane. Int. J. Nanomed. 2010, 5, 1017–1028. [Google Scholar] [CrossRef]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver Nanoparticle-Based Nanocomposites for Combating Infectious Pathogens: Recent Advances and Future Prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, W.; Liu, Q.; Wang, Q.; Chen, X.; Chang, C.; Rao, S.; Zheng, G.; Shi, Z.; Meng, Y. One-step construction of silver nanoparticles immersed hydrogels by triple-helix β-glucans and the application in infectious wound healing. Int. J. Biol. Macromol. 2024, 282, 137146. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Alawam, K.A.; Binshaya, A.S.; Alduraywish, S.A. Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications. Molecules 2023, 28, 3241. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Han, J.; Wang, Z.; Zhang, S. On the developmental toxicity of silver nanoparticles. Mater. Des. 2021, 203, 109611. [Google Scholar] [CrossRef]
- Anozie, U.C.; Dalhaimer, P. Molecular links among non-biodegradable nanoparticles, reactive oxygen species, and autophagy. Adv. Drug Deliv. Rev. 2017, 122, 65–73. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.-R.; Chae, H.-J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Yin, S.; Zhou, X.; Jiang, Y.; Shao, L. The lysosome-mitochondrion crosstalk engaged in silver nanoparticles-disturbed mitochondrial homeostasis. Sci. Total Environ. 2023, 889, 164078. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.; Wang, Y.; Ma, C.; Bi, L.; Song, M.; Jiang, G. Silver Nanoparticles Induce Apoptosis in HepG2 Cells through Particle-Specific Effects on Mitochondria. Environ. Sci. Technol. 2022, 56, 5706–5713. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.-X. Uptake, intracellular dissolution, and cytotoxicity of silver nanowires in cell models. Chemosphere 2021, 281, 130762. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Bujňáková, Z.L.; Balážová, Ľ.; Tkáčiková, Ľ. Green Synthesis of Silver Nanoparticles with Antibacterial Activity Using Various Medicinal Plant Extracts: Morphology and Antibacterial Efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef]
- Shanmugam, J.; Dhayalan, M.; Umar, M.R.S.; Gopal, M.; Khan, M.A.; Simal-Gandara, J.; Cid-Samamed, A. Green Synthesis of Silver Nanoparticles Using Allium cepa var. Aggregatum Natural Extract: Antibacterial and Cytotoxic Properties. Nanomaterials 2022, 12, 1725. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 67–89. [Google Scholar]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef]
- Song, E.H.; Shang, J.; Ratner, D.M. 9.08-Polysaccharides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 137–155. [Google Scholar]
- Pinto, R.J.B.; Martins, M.A.; Lucas, J.M.F.; Vilela, C.; Sales, A.J.M.; Costa, L.C.; Marques, P.A.A.P.; Freire, C.S.R. Highly Electroconductive Nanopapers Based on Nanocellulose and Copper Nanowires: A New Generation of Flexible and Sustainable Electrical Materials. ACS Appl. Mater. Interfaces 2020, 12, 34208–34216. [Google Scholar] [CrossRef]
- Faria, M.; Vilela, C.; Mohammadkazemi, F.; Silvestre, A.J.D.; Freire, C.S.R.; Cordeiro, N. Poly(glycidyl methacrylate)/bacterial cellulose nanocomposites: Preparation, characterization and post-modification. Int. J. Biol. Macromol. 2019, 127, 618–627. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A. A Review of Chitosan-Based Materials for Biomedical, Food, and Water Treatment Applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, S.; Liu, Q.; Zeng, D.; Wang, K.; Chang, C.; Zhang, B.; Zhang, L.; Shi, Z.; Meng, Y. Construction of AgNPs-loaded oriented hydrogel based on Periostracum Cicadae chitosan by electro-assembly for rapid hemostasis and wound healing. Carbohydr. Polym. 2025, 358, 123500. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.P.; de Andrade, L.R.M.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque, R.L.C., Jr.; Pereira da Costa, L.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Li, Q.; Ai, R.; Fan, J.; Fu, X.; Zhu, L.; Zhou, Q.; Chen, L.; Ma, W.; Li, Y.; Liu, L. AgNPs-loaded chitosan/sodium alginate hydrogel film by in-situ green reduction with tannins for enhancing antibacterial activity. Mater. Today Commun. 2024, 38, 107927. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Cheng, Z. Research on the Application of Synthetic Polymer Materials in Contemporary Public Art. Polymers 2022, 14, 1208. [Google Scholar] [CrossRef]
- Shrivastava, A. 1-Introduction to Plastics Engineering. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 1–16. [Google Scholar]
- Zhu, Y.; Yang, K.; Cheng, R.; Xiang, Y.; Yuan, T.; Cheng, Y.; Sarmento, B.; Cui, W. The current status of biodegradable stent to treat benign luminal disease. Mater. Today 2017, 20, 516–529. [Google Scholar] [CrossRef]
- Mačák, L.; Velgosova, O.; Múdra, E.; Vojtko, M.; Dolinská, S.; Kromka, F. Preparation of Green Silver Nanoparticles and Eco-Friendly Polymer–AgNPs Nanocomposites: A Study of Toxic Properties across Multiple Organisms. Polymers 2024, 16, 1865. [Google Scholar] [CrossRef]
- Teper, P.; Oleszko-Torbus, N.; Bochenek, M.; Hajduk, B.; Kubacki, J.; Jałowiecki, Ł.; Płaza, G.; Kowalczuk, A.; Mendrek, B. Hybrid nanolayers of star polymers and silver nanoparticles with antibacterial activity. Colloids Surf. B Biointerfaces 2022, 213, 112404. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, X.; Lin, X.; Yi, W.; Jiang, J. An injectable metal nanoparticle containing cellulose derivative-based hydrogels: Evaluation of antibacterial and in vitro-vivo wound healing activity in children with burn injuries. Int. Wound J. 2022, 19, 666–678. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Fagir, M.H.; El Dein, D.K. Green synthesized silver nanoparticles loaded in polysaccharide hydrogel applied to chronic wound healing in mice models. Gels 2023, 9, 646. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug delivery systems and other biomedical applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Munni, A.; Bashammakh, M.A.; Bellier, M.; Ansari, A.; Ali, M.E.; Karahan, H.E.; Islam, R.; Boyer, T.H.; Perreault, F. Surface characteristics of thin film composite polyamide membranes dictate silver nanoparticle loading efficacy. RSC Appl. Interfaces 2024, 1, 1186–1197. [Google Scholar] [CrossRef]
- Velgosova, O.; Mačák, L.; Múdra, E.; Vojtko, M.; Lisnichuk, M. Preparation, structure, and properties of PVA–AgNPs nanocomposites. Polymers 2023, 15, 379. [Google Scholar] [CrossRef]
- Amin, N.A.A.M.; Mokhter, M.A.; Salamun, N.; Mohamad, M.F.B.; Mahmood, W.M.A.W. Anti-fouling electrospun organic and inorganic nanofiber membranes for wastewater treatment. S. Afr. J. Chem. Eng. 2023, 44, 302–317. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications—A review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Xu, S.; Zhang, W. Enhanced Anti-Biofouling Properties of BWRO Membranes via the Deposition of Poly (Catechol/Polyamine) and Ag Nanoparticles. Membranes 2023, 13, 530. [Google Scholar] [CrossRef]
- Molleman, B.; Hiemstra, T. Surface structure of silver nanoparticles as a model for understanding the oxidative dissolution of silver ions. Langmuir 2015, 31, 13361–13372. [Google Scholar] [CrossRef]
- Băjan, M.; Cursaru, D.L.; Mihai, S. The Impact of Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles (GrO/AgNP) on the Cellulose Acetate (CA) Membrane Matrix Used for Hydrocarbon Removal from Water. Membranes 2025, 15, 158. [Google Scholar] [CrossRef]
- Tang, L.; Livi, K.J.T.; Chen, K.L. Polysulfone Membranes Modified with Bioinspired Polydopamine and Silver Nanoparticles Formed in Situ To Mitigate Biofouling. Environ. Sci. Technol. Lett. 2015, 2, 59–65. [Google Scholar] [CrossRef]
- Municoy, S.; Antezana, P.E.; Bellino, M.G.; Desimone, M.F. Development of 3D-printed collagen scaffolds with in-situ synthesis of silver nanoparticles. Antibiotics 2022, 12, 16. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 607099. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111525. [Google Scholar] [CrossRef]
- Iurilli, M.; Porrelli, D.; Turco, G.; Lagatolla, C.; Camurri Piloni, A.; Medagli, B.; Nicolin, V.; Papa, G. Electrospun Collagen-Coated Nanofiber Membranes Functionalized with Silver Nanoparticles for Advanced Wound Healing Applications. Membranes 2025, 15, 39. [Google Scholar] [CrossRef]
- Menotti, F.; Scutera, S.; Maniscalco, E.; Coppola, B.; Bondi, A.; Costa, C.; Longo, F.; Mandras, N.; Pagano, C.; Cavallo, L. Is Silver Addition to Scaffolds Based on Polycaprolactone Blended with Calcium Phosphates Able to Inhibit Candida albicans and Candida auris Adhesion and Biofilm Formation? Int. J. Mol. Sci. 2024, 25, 2784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mi, M.; Hu, Z.; Li, L.; Chen, Z.; Gao, X.; Liu, D.; Xu, B.; Liu, Y. Polydopamine-based biomaterials in orthopedic therapeutics: Properties, applications, and future perspectives. Drug Des. Dev. Ther. 2024, 18, 3765–3790. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Han, J.; Guo, Z.; Mu, C.; Yu, C.; Ji, Z.; Sun, L.; Wang, Y.; Wang, J. Antibacterial 3D-printed silver nanoparticle/poly lactic-co-glycolic acid (PLGA) scaffolds for bone tissue engineering. Materials 2023, 16, 3895. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Lee, W.-K.; Hsu, J.-T.; Shih, J.-Y.; Ma, T.-L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in bone tissue engineering: A comprehensive review of innovations in scaffold fabrication and surface modifications. J. Funct. Biomater. 2024, 15, 243. [Google Scholar] [CrossRef]
- Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-dimensional (3D) printed silver nanoparticles/alginate/nanocrystalline cellulose hydrogels: Study of the antimicrobial and cytotoxicity efficacy. Nanomaterials 2020, 10, 844. [Google Scholar] [CrossRef]
- Ahmed, S.; Hussain, R.; Khan, A.; Batool, S.A.; Mughal, A.; Nawaz, M.H.; Irfan, M.; Wadood, A.; Avcu, E.; Rehman, M.A.U. 3D printing assisted fabrication of copper–silver mesoporous bioactive glass nanoparticles reinforced sodium alginate/poly (vinyl alcohol) based composite scaffolds: Designed for skin tissue engineering. ACS Appl. Bio Mater. 2023, 6, 5052–5066. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, S.; Peng, L.; Wang, Y.; Shang, S.; Miao, D.; Guo, R. AgNps-PVA–coated woven cotton fabric: Preparation, water repellency, shielding properties and antibacterial activity. J. Ind. Text. 2019, 48, 1545–1565. [Google Scholar] [CrossRef]
- Niyonshuti, I.I.; Krishnamurthi, V.R.; Okyere, D.; Song, L.; Benamara, M.; Tong, X.; Wang, Y.; Chen, J. Polydopamine Surface Coating Synergizes the Antimicrobial Activity of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 40067–40077. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Chitosan-based composites: Development and perspective in food preservation and biomedical applications. Polymers 2023, 15, 3150. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Li, X.; Liu, Z.; Zhang, Z. Application of photo-crosslinkable gelatin methacryloyl in wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1303709. [Google Scholar] [CrossRef]
- Sulastri, E.; Lesmana, R.; Zubair, M.S.; Mohammed, A.F.A.; Elamin, K.M.; Wathoni, N. Ulvan/Silver nanoparticle hydrogel films for burn wound dressing. Heliyon 2023, 9, e18044. [Google Scholar] [CrossRef]
- Xiang, J.; Ma, L.; Su, H.; Xiong, J.; Li, K.; Xia, Q.; Liu, G. Layer-by-layer assembly of antibacterial composite coating for leather with cross-link enhanced durability against laundry and abrasion. Appl. Surf. Sci. 2018, 458, 978–987. [Google Scholar] [CrossRef]
- Takeda, E.; Xu, W.; Terakawa, M.; Niidome, T. Tailored Structure and Antibacterial Properties of Silica-Coated Silver Nanoplates by Pulsed Laser Irradiation. ACS Omega 2022, 7, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Kopecki, Z.; Sutton, A.T.; Garg, S.; Cowin, A.J.; Vasilev, K. pH-responsive “smart” hydrogel for controlled delivery of silver nanoparticles to infected wounds. Antibiotics 2021, 10, 49. [Google Scholar] [CrossRef]
- Shen, T.; Liu, Y.; Zhu, Y.; Yang, D.-Q.; Sacher, E. Improved adhesion of Ag NPs to the polyethylene terephthalate surface via atmospheric plasma treatment and surface functionalization. Appl. Surf. Sci. 2017, 411, 411–418. [Google Scholar] [CrossRef]
- Unuma, H.; Miyauchi, N.; Yano, S.; Hirayama, N.; Harashima, S.; Nakamura, K. Preparation of a coating solution for antibacterial Ag nanoparticle layers. J. Ceram. Soc. Jpn. 2024, 132, 193–195. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The emerging progress on wound dressings and their application in clinic wound management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef] [PubMed]
- Sethuram, L.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Eugenol micro-emulsion reinforced with silver nanocomposite electrospun mats for wound dressing strategies. Mater. Adv. 2021, 2, 2971–2988. [Google Scholar] [CrossRef]
- Long, L.; Hu, C.; Liu, W.; Wu, C.; Lu, L.; Yang, L.; Wang, Y. Injectable multifunctional hyaluronic/methylcellulose hydrogels for chronic wounds repairing. Carbohydr. Polym. 2022, 289, 119456. [Google Scholar] [CrossRef] [PubMed]
- Alipour, A.; Nejati, O.; Yaşayan, G.; Girgin, A.; Zaman, B.T.; Giray, B.; Karal-Yılmaz, O.; Bakırdere, S.; Bal-Öztürk, A. Multilayer Antibacterial Hydrogel Wound Dressings Incorporated with Green Synthesized Silver Nanoparticles. Drug Dev. Res. 2025, 86, e70102. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Sayyar, Z.; Mahdavinia, G.R.; Khataee, A. Dual-drug (Curcumin/Ciprofloxacin) loading and release from chitosan-based hydrogels embedded with magnetic Montmorillonite/Hyaluronic acid for enhancing wound healing. J. Biol. Eng. 2023, 17, 66. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Ditta, S.A.; Zaffar Bukhari, S.Z.; Yousaf, M.J.; Hassan, Z.; Nasir, M.; Rashid, M.; Tanvir, F.; Naz, M.; Haider, H.; Yaqub, A. Nanogels-empowered amino acid-capped silver nanoparticles for enhanced skin tissue regeneration. J. Drug Deliv. Sci. Technol. 2025, 107, 106751. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Li, T.; Huang, Z.; Tsui, G.C.-P.; Tang, C.-Y.; Deng, Y. Recent advances in 4D printing of hydrogels. Rev. Adv. Mater. Sci. 2024, 63, 20240028. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals 2022, 15, 1282. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, X.; Fang, C.; Chen, J.; Zhang, X.; Cao, C.; Wang, J. Antimicrobial Properties of Carboxymethyl Cellulose/Starch/N’N Methylenebisacrylamide Membranes Endowed by Ultrasound and Their Potential Application in Antimicrobial Packaging. Polymers 2024, 16, 1282. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Q.; Gao, Y.; Wan, S.; Meng, F.; Weng, W.; Zhang, Y. Characterization of silver nanoparticles loaded chitosan/polyvinyl alcohol antibacterial films for food packaging. Food Hydrocoll. 2023, 136, 108305. [Google Scholar] [CrossRef]
- Li, S.; Wei, N.; Wei, J.; Fang, C.; Feng, T.; Liu, F.; Liu, X.; Wu, B. Curcumin and silver nanoparticles loaded antibacterial multifunctional pectin/gelatin films for food packaging applications. Int. J. Biol. Macromol. 2024, 266, 131248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Lin, S.; Wang, Q. Preparation and properties of glutaraldehyde crosslinked poly(vinyl alcohol) membrane with gradient structure. J. Polym. Res. 2020, 27, 228. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, T.; Singhal, R. Silver doped Polypyrrole nanocomposite-based gas sensor for enhanced ammonia gas sensing performance at room temperature. Chem. Phys. Impact 2024, 9, 100722. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Fan, B.; He, Y.-C.; Ma, C. Antibacterial, antioxidant and fruit packaging ability of biochar-based silver nanoparticles-polyvinyl alcohol-chitosan composite film. Int. J. Biol. Macromol. 2024, 256, 128297. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, H.; Rastegar, H.; Taherian, M.; Samadi, M.; Rostami, H. The effect of nano-silver packaging in increasing the shelf life of nuts: An in vitro model. Ital. J. Food Saf. 2017, 6, 6874. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Yousuf, O.; Islam, R.U.; Younis, K. Silver nanoparticles as an active packaging ingredient and its toxicity. Packag. Technol. Sci. 2021, 34, 653–663. [Google Scholar] [CrossRef]
- Herrera-Rivera, M.d.R.; Torres-Arellanes, S.P.; Cortés-Martínez, C.I.; Navarro-Ibarra, D.C.; Hernández-Sánchez, L.; Solis-Pomar, F.; Pérez-Tijerina, E.; Román-Doval, R. Nanotechnology in food packaging materials: Role and application of nanoparticles. RSC Adv. 2024, 14, 21832–21858. [Google Scholar] [CrossRef]
- Wang, D.; Yang, H.; Lu, X.; Wu, Y.; Blasi, F. The Inhibitory Effect of Chitosan Based Films, Incorporated with Essential Oil of Perilla frutescens Leaves, against Botrytis cinerea during the Storage of Strawberries. Processes 2022, 10, 706. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; He, J.; Luo, J.; Cai, J.; Wei, J.; Li, P.; Zhong, H. Preparation of curcumin-loaded chitosan/polyvinyl alcohol intelligent active films for food packaging and freshness monitoring. Int. J. Biol. Macromol. 2024, 276, 133807. [Google Scholar] [CrossRef]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yalamarty, S.S.K.; Filipczak, N.; Jin, Y.; Li, X. Nano Silver-Induced Toxicity and Associated Mechanisms. Int. J. Nanomed. 2022, 17, 1851–1864. [Google Scholar] [CrossRef]
- Tran, Q.H.; Le, A.-T. Corrigendum: Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives (Adv. Nat. Sci: Nanosci. Nanotechnol. 4 033001). Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 049501. [Google Scholar] [CrossRef]
- Kim, W.I.; Pak, S.W.; Lee, S.J.; Park, S.H.; Shin, I.S.; Moon, C.; Yu, W.J.; Kim, S.H.; Kim, J.C. In vitro study of silver nanoparticles-induced embryotoxicity using a rat whole embryo culture model. Toxicol. Res. 2025, 41, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, A.C.; Lynch, I.; Valsami-Jones, E. Silver nanoparticle induced toxicity and cell death mechanisms in embryonic zebrafish cells. Nanoscale 2021, 13, 6142–6161. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Phan, T.L.; Le, T.T.; Doan, V.D.; Vu Truong, H.A.; Le, V.T. Size-dependent catalytic and antibacterial effects of phytogenically synthesized silver nanoparticles. Kuwait J. Sci. 2025, 52, 100366. [Google Scholar] [CrossRef]
- Kim, K.-T.; Truong, L.; Wehmas, L.; Tanguay, R.L. Silver nanoparticle toxicity in the embryonic zebrafish is governed by particle dispersion and ionic environment. Nanotechnology 2013, 24, 115101. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, E.; Cho, H.-J.; Kang, T.; Kim, B.; Kim, M.Y.; Kim, Y.S.; Song, N.W.; Lee, J.-S.; Jeong, J. The Relationship between Dissolution Behavior and the Toxicity of Silver Nanoparticles on Zebrafish Embryos in Different Ionic Environments. Nanomaterials 2018, 8, 652. [Google Scholar] [CrossRef] [PubMed]
- Kuempel, E.D.; Roberts, J.R.; Roth, G.; Dunn Kevin, L.; Zumwalde, R.D.; Hubbs, A.F.; Trout, D.; Holdsworth, G. Current Intelligence Bulletin 70: Health effects of Occupational Exposure to Silver Nanomaterials; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupa-tional Safety and Health, DHHS (NIOSH) Publishing: Cincinnati, OH, USA, 2021; pp. 19–27. [CrossRef]
- McCourt, K.M.; Cochran, J.; Abdelbasir, S.M.; Carraway, E.R.; Tzeng, T.-R.J.; Tsyusko, O.V.; Vanegas, D.C. Potential Environmental and Health Implications from the Scaled-Up Production and Disposal of Nanomaterials Used in Biosensors. Biosensors 2022, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Park, S.; Sung, J.H. Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model. Int. J. Environ. Res. Public Health 2020, 17, 9218. [Google Scholar] [CrossRef]
- Karakurt, S.; Bilgiseven, I.M.; Cinazr, S.; Bilecen, D.S.; Kandir, S. Size-Dependent Effects of Silver Nanoparticles in Colorectal Cancer Treatment: Apoptosis Activation, Anti-Metastatic Properties, and Tissue Accumulation. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Rezghi Rami, M.; Forouzandehdel, S.; Aalizadeh, F. Enhancing biodegradable smart food packaging: Fungal-synthesized nanoparticles for stabilizing biopolymers. Heliyon 2024, 10, e37692. [Google Scholar] [CrossRef]
- Afewerki, S.; Harb, S.V.; Stocco, T.D.; Ruiz-Esparza, G.U.; Lobo, A.O. Chapter 5-Polymers for surgical sutures. In Advanced Technologies and Polymer Materials for Surgical Sutures; Thomas, S., Coates, P., Whiteside, B., Joseph, B., Nair, K., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 95–128. [Google Scholar]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials; Enzymes and Processing Aids (CEP); Aids, P.; Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; et al. Safety assessment of the substance silver nanoparticles for use in food contact materials. EFSA J. 2021, 19, e06790. [Google Scholar] [CrossRef]
- Committee, E.S.; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA J. 2021, 19, e06769. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Andreassen, M.; Aquilina, G.; Bastos, M.L.; Boon, P.; Castle, L.; Fallico, B.; FitzGerald, R.; Frutos Fernandez, M.J.; Grasl-Kraupp, B.; et al. Follow-up of the re-evaluation of silver (E 174) as a food additive (EFSA-Q-2023-00169). EFSA J. 2025, 23, e9316. [Google Scholar] [CrossRef] [PubMed]
- FDA’s Approach to Regulation of Nanotechnology Products. Available online: https://www.fda.gov/science-research/nanotechnology-programs-fda/fdas-approach-regulation-nanotechnology-products (accessed on 22 June 2025).
- Food Packaging Forum. Available online: https://foodpackagingforum.org/?s=Silver (accessed on 22 June 2025).
- Morais, L.d.O.; Macedo, E.V.; Granjeiro, J.M.; Delgado, I.F. Critical evaluation of migration studies of silver nanoparticles present in food packaging: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3083–3102. [Google Scholar] [CrossRef]
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In Vitro Release Study of the Polymeric Drug Nanoparticles: Development and Validation of a Novel Method. Pharmaceutics 2020, 12, 732. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Chen, J.; Tan, Z.; Wang, C. ICP-MS based detection method combined with Au NP and Ag NP labeling for bacteremia diagnosis. Anal. Biochem. 2024, 692, 115559. [Google Scholar] [CrossRef]
- Addo Ntim, S.; Goodwin, D.G.; Sung, L.; Thomas, T.A.; Noonan, G.O. Long-term wear effects on nanosilver release from commercially available food contact materials. Food Addit. Contam. Part A 2019, 36, 1757–1768. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Yan, Y. Molecular dynamics study of the migration of Bisphenol A from polycarbonate into food simulants. Chem. Phys. Lett. 2020, 741, 137125. [Google Scholar] [CrossRef]
- Gavriil, G.; Kanavouras, A.; Coutelieris, F.A. Food-packaging migration models: A critical discussion. Crit. Rev. Food Sci. Nutr. 2018, 58, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, Y.V.; Balyakin, I.A.; Popov, I.D.; Schummer, B.; Sochor, B.; Rempel, S.V.; Rempel, A.A. Ag2S interparticle interaction in an aqueous solution: Mechanism of steric and electrostatic stabilization. J. Mol. Liq. 2021, 335, 116130. [Google Scholar] [CrossRef]
- Aouay, M.; Aguado, R.J.; Bayés, G.; Fiol, N.; Putaux, J.-L.; Boufi, S.; Delgado-Aguilar, M. In-situ synthesis and binding of silver nanoparticles to dialdehyde and carboxylated cellulose nanofibrils, and active packaging therewith. Cellulose 2024, 31, 5687–5706. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Manikandan, M.; Shajahan, A.; Mariadoss, A.V.A.; Wang, M.-H. Chapter 4—Core-shell silver nanoparticles: Synthesis, characterization, and applications. In Green Synthesis of Silver Nanomaterials; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–97. [Google Scholar]
- Pourzahedi, L.; Eckelman, M.J. Comparative life cycle assessment of silver nanoparticle synthesis routes. Environ. Sci. Nano 2015, 2, 361–369. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of Metallic Silver Nanoparticles in a Pilot Wastewater Treatment Plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef]
- Sánchez-Martínez, A.; Mínguez-García, D.; Díaz-García, P.; Gisbert-Payá, J. Environmental Impact of Nanosilver on the Biodegradability of Polylactic Acid Nonwovens. J. Polym. Environ. 2025, 33, 3897–3918. [Google Scholar] [CrossRef]
- Molleman, B.; Hiemstra, T. Time, pH, and size dependency of silver nanoparticle dissolution: The road to equilibrium. Environ. Sci. Nano 2017, 4, 1314–1327. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S.; Oladijo, O.P.; Dhakal, H.N. Development of sustainable biopolymer-based composites for lightweight applications from agricultural waste biomass: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 436–450. [Google Scholar] [CrossRef]
- Bafana, A.; Kumar, S.V.; Temizel-Sekeryan, S.; Dahoumane, S.A.; Haselbach, L.; Jeffryes, C.S. Evaluating microwave-synthesized silver nanoparticles from silver nitrate with life cycle assessment techniques. Sci. Total Environ. 2018, 636, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Pourzahedi, L.; Eckelman, M.J. Environmental Life Cycle Assessment of Nanosilver-Enabled Bandages. Environ. Sci. Technol. 2015, 49, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.; Fayad, A.; Mohamed, A.-M.O.; Al Nahyan, M.T. The Role of E-Waste in Sustainable Mineral Resource Management. Waste 2025, 3, 27. [Google Scholar] [CrossRef]
- Walser, T.; Demou, E.; Lang, D.J.; Hellweg, S. Prospective Environmental Life Cycle Assessment of Nanosilver T-Shirts. Environ. Sci. Technol. 2011, 45, 4570–4578. [Google Scholar] [CrossRef]
- Potera, C. Transformation of silver nanoparticles in sewage sludge. Environ. Health Perspect 2010, 118, A526–A527. [Google Scholar] [CrossRef] [PubMed]
- Alkhallawi, M.F.H.; Mohammed, M.H.; Hemmatzadeh, F.; Petrovski, K. Exploring Metal Ions as Potential Antimicrobial Agents to Combat Future Drug Resistance in Mycoplasma bovis. Microorganisms 2025, 13, 169. [Google Scholar] [CrossRef]
- Hailan, S.Y.; Al-Khatieeb, M.M. Antimicrobial efficacy of silver, zinc oxide, and titanium dioxide nanoparticles incorporated in orthodontic bonding agent. J. Baghdad Coll. Dent. 2019, 31, 10–16. [Google Scholar] [CrossRef]
- Amini, E.; Azadfallah, M.; Layeghi, M.; Talaei-Hassanloui, R. Silver-nanoparticle-impregnated cellulose nanofiber coating for packaging paper. Cellulose 2016, 23, 557–570. [Google Scholar] [CrossRef]
- Somsesta, N.; Jinnapat, A.; Fakpiam, S.; Suksanguan, C.; Wongsan, V.; Ouneam, W.; Wattanaeabpun, S.; Hongrattanavichit, I. Antimicrobial and biodegradable hydrogel based on nanocellulose/alginate incorporated with silver nanoparticles as active packaging for poultry products. Sci. Rep. 2024, 14, 27135. [Google Scholar] [CrossRef]
- Yu, J.; Huang, M.; Tian, H.; Xu, X. Silver Nanoparticle Sensor Array-Based Meat Freshness Inspection System. Foods 2023, 12, 3814. [Google Scholar] [CrossRef]
- Zuo, J.; Feng, J.; Gameiro, M.G.; Tian, Y.; Liang, J.; Wang, Y.; Ding, J.; He, Q. RFID-based sensing in smart packaging for food applications: A review. Future Foods 2022, 6, 100198. [Google Scholar] [CrossRef]
- Douaki, A.; Ahmed, M.; Longo, E.; Windisch, G.; Riaz, R.; Inam, S.; Tran, T.N.; Papadopoulou, E.L.; Athanassiou, A.; Boselli, E.; et al. Battery-Free, Stretchable, and Autonomous Smart Packaging. Adv. Sci. 2025, 12, 2417539. [Google Scholar] [CrossRef]
- Chakraborty, A.; Roy, A.; Ravi, S.P.; Paul, A. Exploiting the role of nanoparticles for use in hydrogel-based bioprinting applications: Concept, design, and recent advances. Biomater. Sci. 2021, 9, 6337–6354. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, T.; Kelson, T.J.; Ray, A.; Malyarenko, A.E.; Kopelman, R. Hydrogel Nanoparticles with Thermally Controlled Drug Release. ACS Macro Lett. 2014, 3, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, A.; Suhagia, T.A.; Sharma, C.; Jaganathan, S.K.; Purohit, S.D. Carbon Based Polymeric Nanocomposite Hydrogel Bioink: A Review. Polymers 2024, 16, 3318. [Google Scholar] [CrossRef]
- Lin, X.Z.; Terepka, A.D.; Yang, H. Synthesis of Silver Nanoparticles in a Continuous Flow Tubular Microreactor. Nano Lett. 2004, 4, 2227–2232. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- Alli, Y.A.; Anuar, H.; Manshor, M.R.; Bankole, O.M.; Rahman, N.A.A.; Olatunde, S.K.; Omotola, E.O.; Oladoye, P.O.; Ejeromedoghene, O.; Suhr, J.; et al. Influence of nanocomposites in extrusion-based 3D printing: A review. Hybrid Adv. 2023, 3, 100069. [Google Scholar] [CrossRef]
- Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-Based Synergistic Antimicrobial Composites. A Critical Review. Nanomaterials 2021, 11, 1687. [Google Scholar] [CrossRef]
- Olenin, A.Y.; Krutyakov, Y.A.; Kudrinskii, A.A.; Lisichkin, G.V. Formation of surface layers on silver nanoparticles in aqueous and water-organic media. Colloid J. 2008, 70, 71–76. [Google Scholar] [CrossRef]
- Mahmud, J.; Sarmast, E.; Shankar, S.; Lacroix, M. Advantages of nanotechnology developments in active food packaging. Food Res. Int. 2022, 154, 111023. [Google Scholar] [CrossRef] [PubMed]
- APMEX. Silver Spot Price. Available online: https://www.apmex.com/silver-price (accessed on 21 August 2025).
- JM Bullion. Live Silver Prices. Available online: https://www.jmbullion.com/charts (accessed on 21 August 2025).
- PSPI-1000 Silver Nanoparticle Ink—Price Tiers. Available online: https://novacentrix.com/product/pspi-1000-silver-nanoparticles (accessed on 21 August 2025).
- Inframat Advances Materials. Available online: http://www.inframat.com/products/30N-0801.htm (accessed on 21 August 2025).
- MSE Supplies. Available online: https://www.msesupplies.com (accessed on 21 August 2025).
- Titanium Oxide (Rutile) Nanopowder. Available online: https://www.ottokemi.com/oxide-nanoparticles/titanium-oxide-rutile-nanopowder-aps-50-nm-99.aspx (accessed on 21 August 2025).
- Dhiman, N.K.; Agnihotri, S.; Shukla, R. Silver-Based Polymeric Nanocomposites as Antimicrobial Coatings for Biomedical Applications. In Nanotechnology in Modern Animal Biotechnology: Recent Trends and Future Perspectives; Singh, S., Maurya, P.K., Eds.; Springer: Singapore, 2019; pp. 115–171. [Google Scholar]
- Alhaj, M.; Narayan, R. Scalable continuous manufacturing process of stereocomplex PLA by twin-screw extrusion. Polymers 2023, 15, 922. [Google Scholar] [CrossRef] [PubMed]




| Synthesis Method | Sizes | Key Aspects | References |
|---|---|---|---|
| Green synthesis | 4.06 nm | Strong antibacterial effect on foodborne pathogens | [52] |
| Green synthesis | 40/58 nm | Stronger antibacterial effect was proved for the smallest AgNPs using microscopy | [49] |
| Green synthesis | 18 nm | Antibacterial activity confirmed using disk diffusion method: once the AgNPs concentration is increasing, the bactericidal effect is also higher | [53] |
| Chemical synthesis | 10/20/75 nm | Antibacterial activity and cytotoxicity showed a higher effect for the smallest AgNPs | [54] |
| Chemical synthesis | 5–100 nm | Antibacterial test performed on four different strains showed the bactericidal effect to be size and dose-dependent | [55] |
| Physical synthesis | 6.6. nm | AgNPs proved a good antibacterial activity for various bacteria Cancer therapy | [56] |
| Physical synthesis | 4/7/40 nm | Incapsulated in a polyurethane membrane, the smallest AgNPs proved the best bactericidal and cytotoxic effect on bacteria and cells | [57] |
| Method | Advantages | Inconveniences | References |
|---|---|---|---|
| In situ | Uniform NPs distribution; good integration | Requires precise control of reduction | [92] |
| Ex situ | Tunable NPs characteristics | Poor dispersion; leaching risk | [93,94] |
| Application | Material/Approach | References |
|---|---|---|
| Bone regeneration | PLGA/AgNPs scaffolds via direct ink writing | [107] |
| Wound healing | Collagen/AgNPs patches via UV-assisted bioprinting | [101] |
| Skin scaffolds | Alginate/PVA/MBGN composite hydrogels | [111] |
| Biofilm-resistant devices | PDA-functionalized PLA/PCL with AgNPs | [106] |
| Dynamic (4D) systems | Thermo-responsive hydrogel matrices with AgNPs | [136] |
| Region/ Country | Regulatory Agency | Approval Status | Key Conditions/Restrictions | References |
|---|---|---|---|---|
| European Union (EU) | EFSA/EC | Not approved for general use | Case-by-case approval required. AgNPs not listed in Regulation (EU) No. 10/2011 Annex I. Migration limit: 0.05 mg/kg food; nanospecific data required | [166,167] |
| United States (US) | FDA/EPA | Not GRAS or FCN-cleared | Requires FDA or GRAS approvement. EPA regulates if marketed as antimicrobial | [169] |
| Canada | Health Canada | No specific provisions for nanomaterials | Regulated under general food contact standards. Nanosilver is not yet individually assessed or listed | [170] |
| China | National Health Commission | Not approved | Only bulk silver (Ag0) allowed in limited uses. Nanoscale forms are not permitted in food contact polymers | [170] |
| Brazil | ANVISA | Excluded from positive list | AgNPs not listed in Ordinance 105/2021 for food contact plastic additives | [170] |
| India | FSSAI | No nano-specific regulation | General material safety standards apply. No official list or migration thresholds for nanomaterials | [170] |
| Australia and New Zealand | FSANZ | No formal approval | Evaluated case-by-case FSANZ encourages nanospecific data but lacks dedicated regulatory framework | [170] |
| Japan | MHLW | Cautious stance | Approves substances individually. AgNPs not listed in positive lists under Japanese FCM laws | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolae-Maranciuc, A.; Chicea, D. Polymeric Systems as Hydrogels and Membranes Containing Silver Nanoparticles for Biomedical and Food Applications: Recent Approaches and Perspectives. Gels 2025, 11, 699. https://doi.org/10.3390/gels11090699
Nicolae-Maranciuc A, Chicea D. Polymeric Systems as Hydrogels and Membranes Containing Silver Nanoparticles for Biomedical and Food Applications: Recent Approaches and Perspectives. Gels. 2025; 11(9):699. https://doi.org/10.3390/gels11090699
Chicago/Turabian StyleNicolae-Maranciuc, Alexandra, and Dan Chicea. 2025. "Polymeric Systems as Hydrogels and Membranes Containing Silver Nanoparticles for Biomedical and Food Applications: Recent Approaches and Perspectives" Gels 11, no. 9: 699. https://doi.org/10.3390/gels11090699
APA StyleNicolae-Maranciuc, A., & Chicea, D. (2025). Polymeric Systems as Hydrogels and Membranes Containing Silver Nanoparticles for Biomedical and Food Applications: Recent Approaches and Perspectives. Gels, 11(9), 699. https://doi.org/10.3390/gels11090699







