Novel GelMA/GelMA-AEMA Hydrogel Blend with Enhanced Printability as a Carrier for iPSC-Derived Chondrocytes In Vitro
Abstract
1. Introduction
2. Results
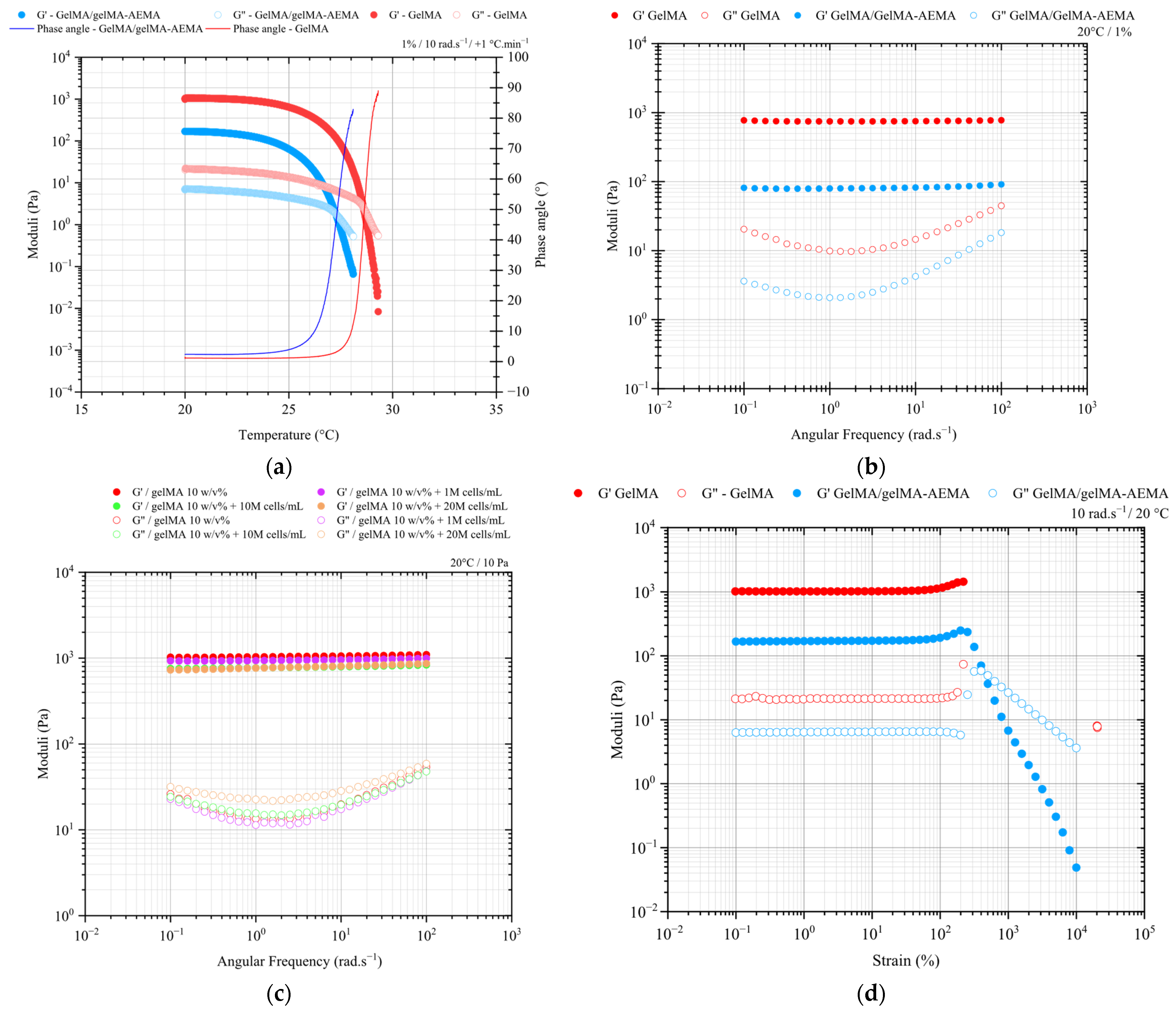
2.1. Bulk Shear Rheology
2.1.1. Oscillatory Shear Measurements of Cell-Free and Cell-Laden Hydrogels
2.1.2. Shear Creep Measurements and Determination of the Burgers Viscoelastic Model Parameters
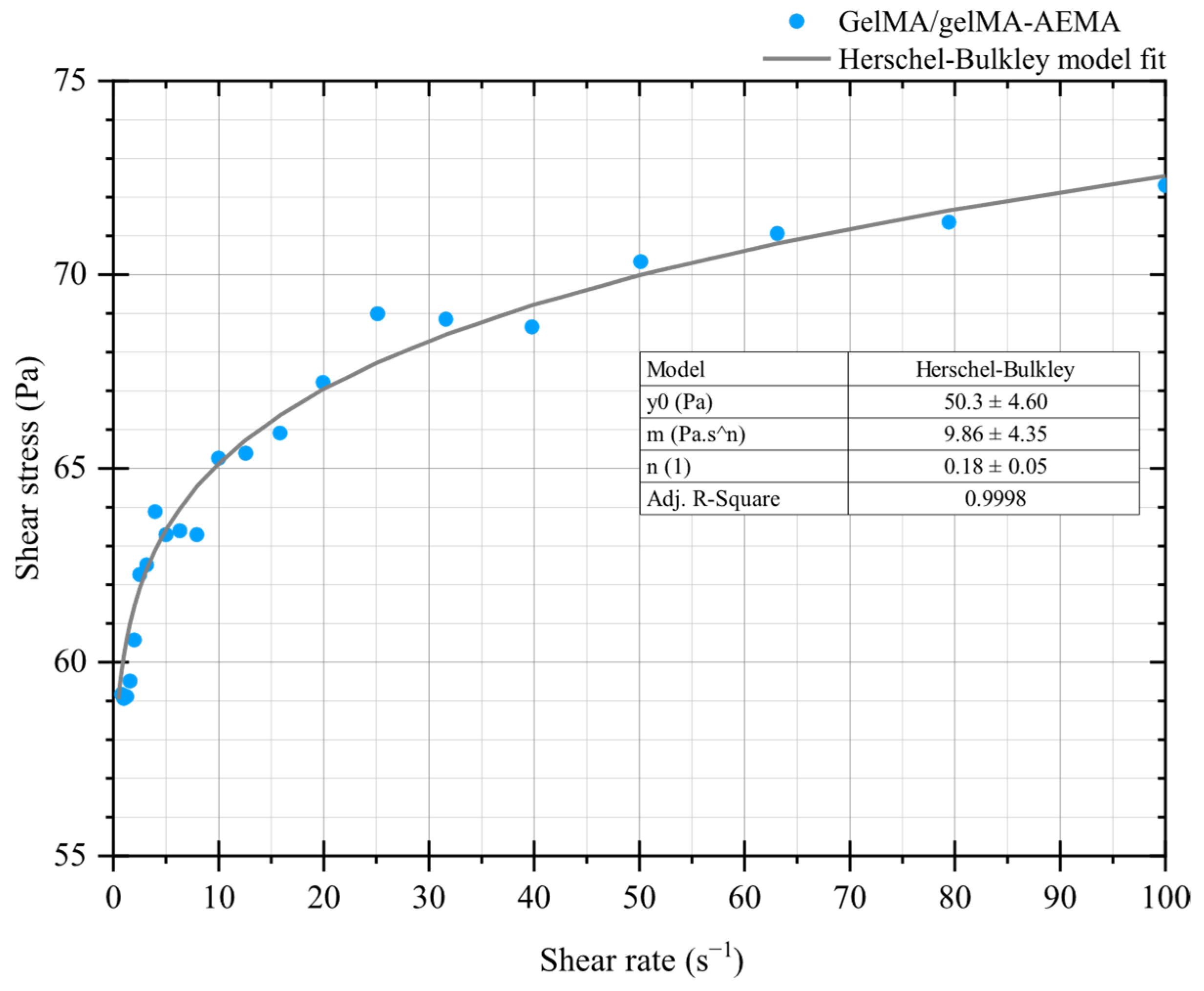
2.1.3. Steady-State Rotational Measurements and the Determination of the Herschel–Bulkley Model Parameters
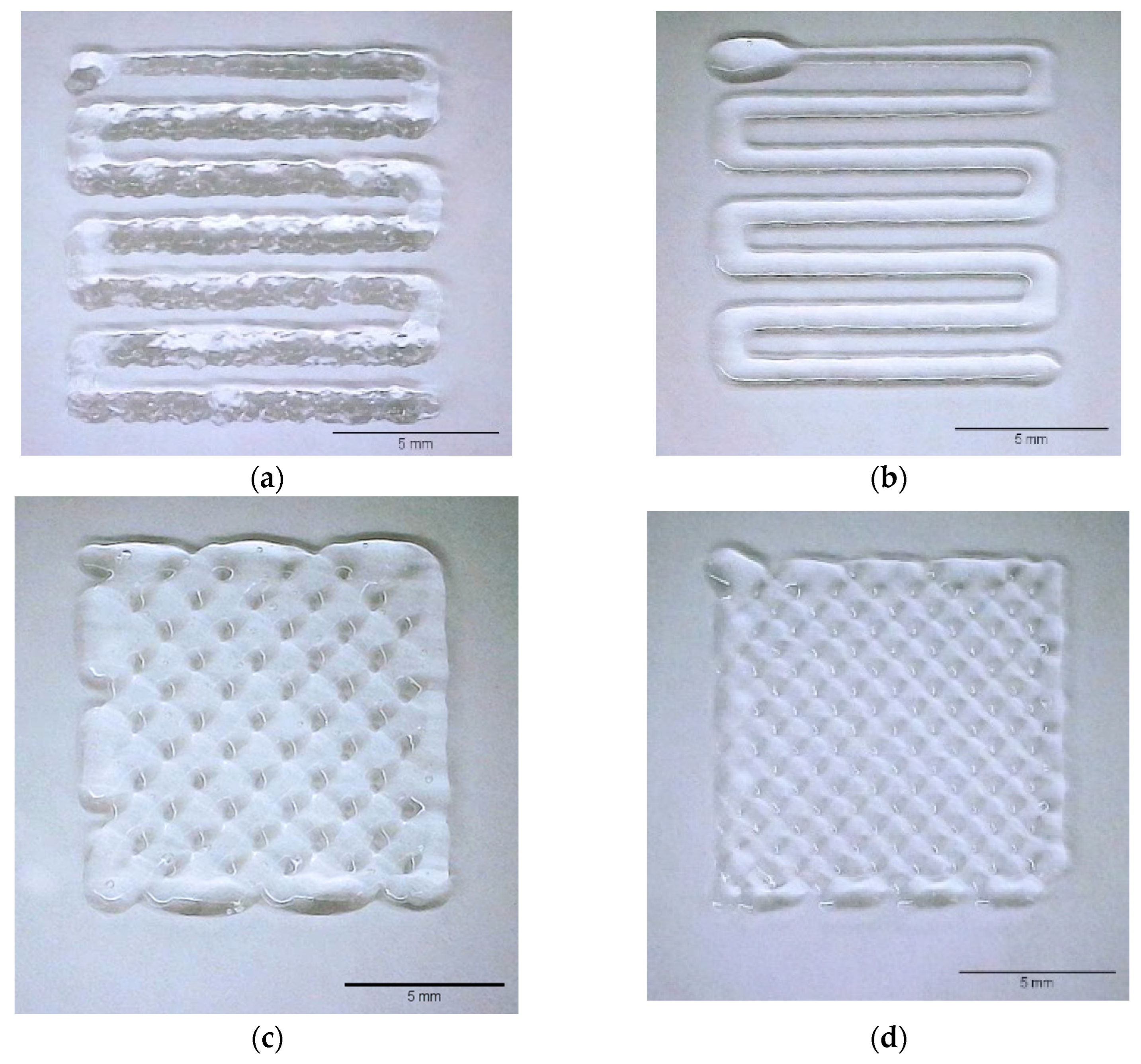
2.2. 3D-Printing Validation
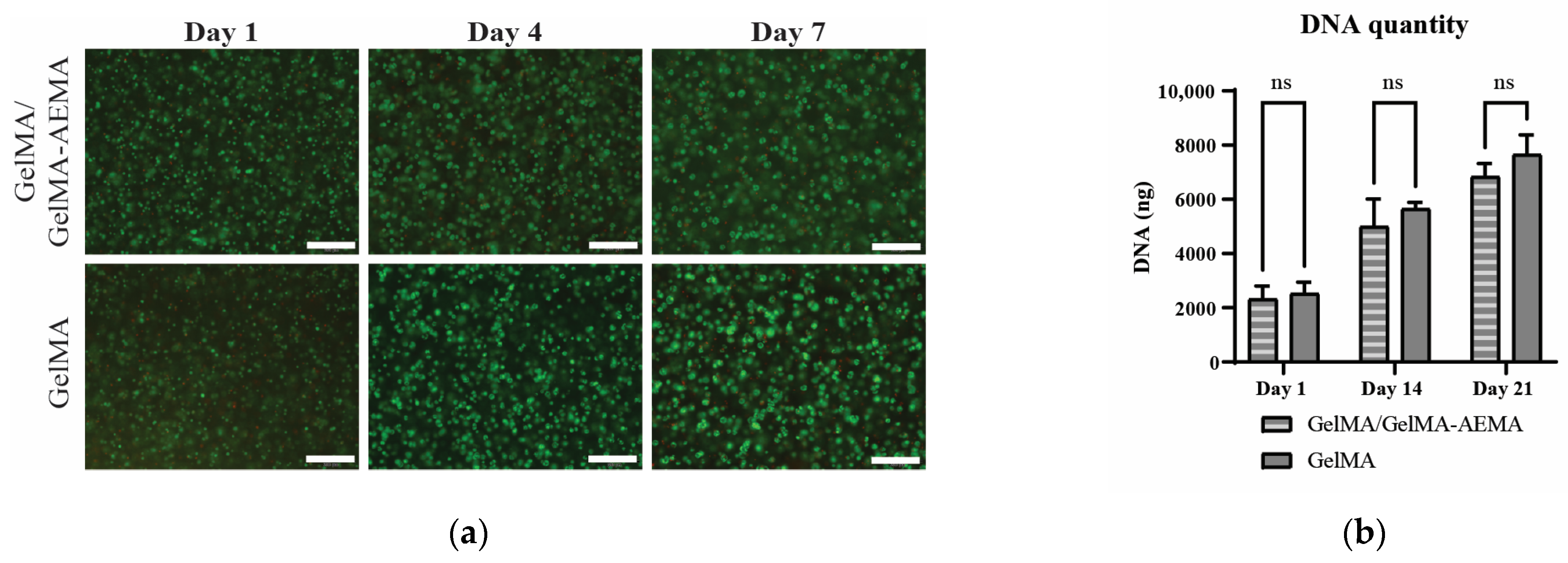
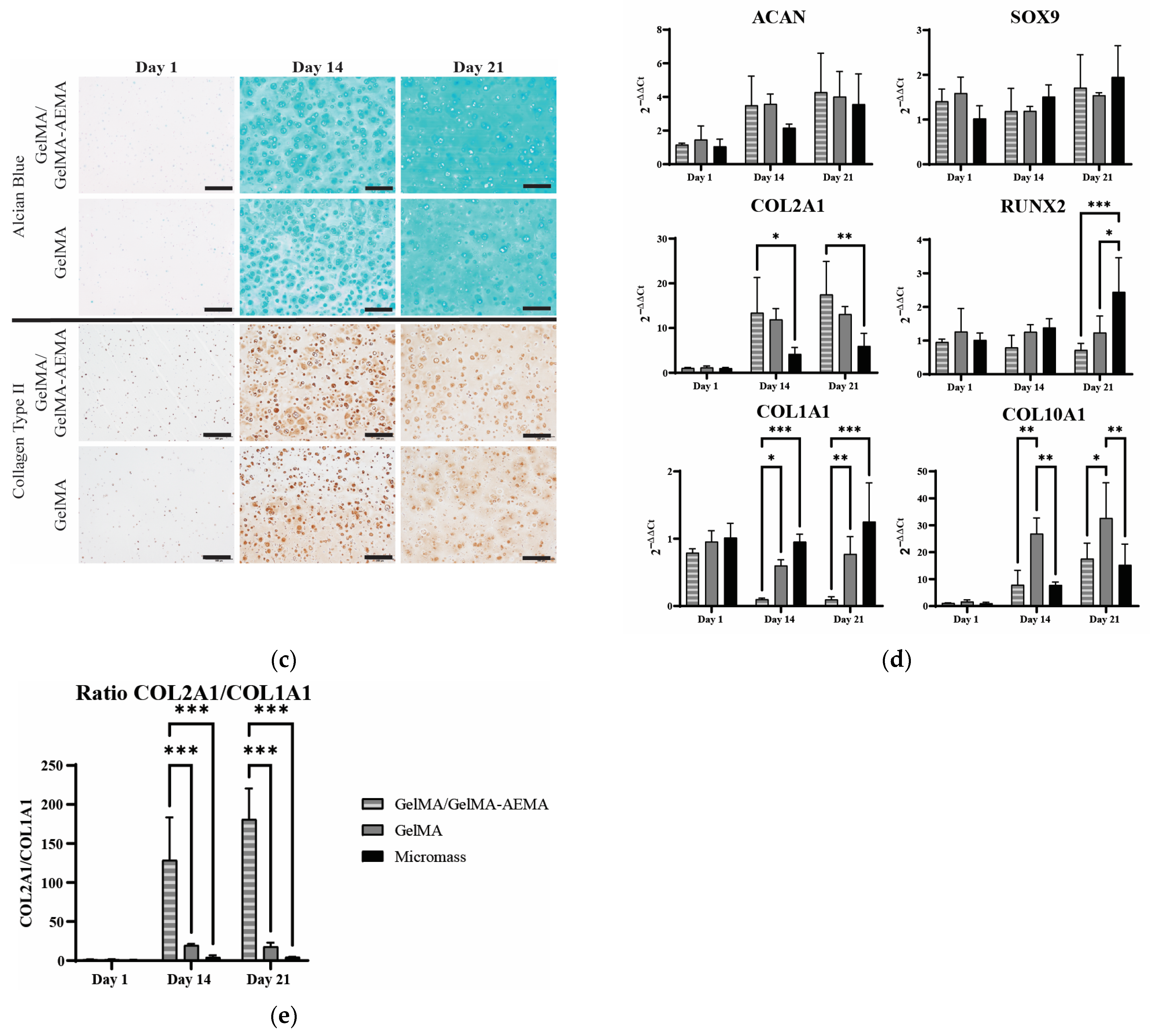
2.3. Biological Evaluation of Encapsulated iPSC-Derived Chondrocytes
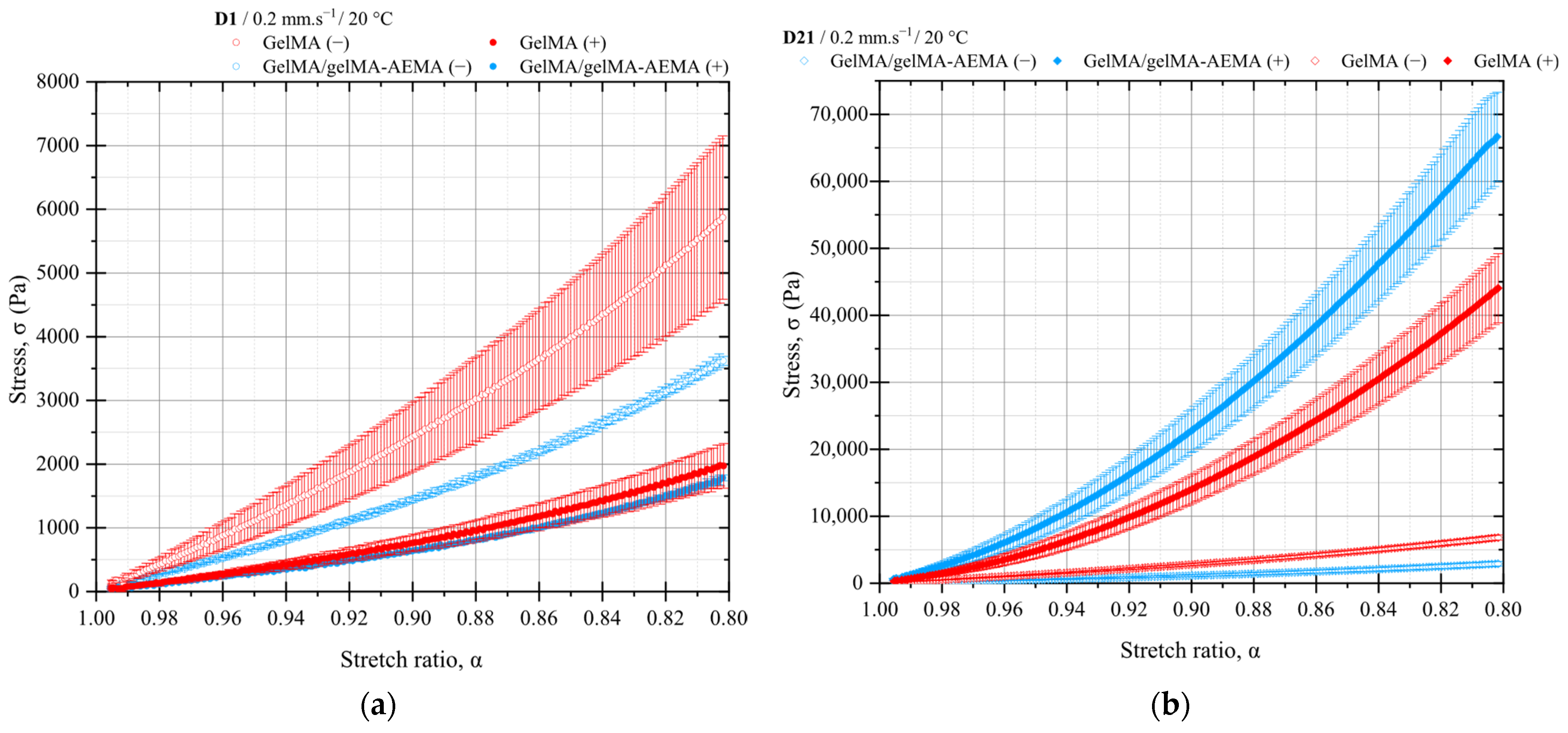
2.4. Mechanical Properties of UV-Cured Constructs
2.4.1. Mechanical Testing of Cell-Free Constructs
2.4.2. Mechanical Testing of Cell-Laden Constructs
2.4.3. Comparative Analysis Between Materials
2.4.4. Structural Evolution During Culture
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Solutions and Physical Hydrogels Preparation
5.2. Bulk Shear Rheology
5.2.1. Oscillatory Shear Measurements with and Without Encapsulated Human Periosteum-Derived Cells and Their Expansion Protocol
5.2.2. Shear Creep and Recovery Measurements and Determination of the Parameters of the Burgers Viscoelastic Model
5.2.3. Steady-State Rotational Shear Measurements and Determination of the Herschel–Bulkley Model Parameters
5.3. 3D-Printing Validation
5.4. Biological Evaluation of Encapsulated iPSC-Derived Chondrocytes
5.4.1. Induced Pluripotent Stem Cells Expansion and Chondrogenic Differentiation
5.4.2. Hydrogel Precursor Preparation and Fabrication of Chondrocyte-Laden Hydrogel Constructs
5.4.3. In Vitro Culture
5.4.4. Cell Viability
5.4.5. Cell-Laden Construct Homogenization
5.4.6. DNA Quantification
5.4.7. RNA Extraction and Gene Expression Analysis
5.4.8. Histology and Immunohistochemistry
5.4.9. Statistical Analyses of Biological Testing
5.5. Mechanical Properties of UV-Cured Constructs
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ACAN | Aggrecan |
| ANOVA | Analysis of variance |
| BMP | Bone morphogenetic protein |
| cDNA | Complementary DNA |
| COL10A1 | Collagen type X alpha 1 chain |
| COL1A1 | Collagen type I alpha 1 chain |
| COL2A1 | Collagen type II alpha 1 chain |
| ddH2O | Double-distilled water |
| DMEM | Dulbecco’s modified eagle medium |
| DNA | Deoxyribonucleic acid |
| EBB | Extrusion-based 3D bioprinting |
| FBS | Fetal bovine serum |
| GDF-5 | Growth differentiation factor 5 |
| GelMA | Gelatin methacryloyl |
| GelMA-AEMA | Gelatin methacryloyl-aminoethyl-methacrylate |
| hbFGF | Human basic fibroblast growth factor |
| hESC | Human embryonic stem cells |
| hPDC | Human periosteum-derived cells |
| HPRT1 | Hypoxanthine Phosphoribosyltransferase 1 |
| HRP | Horseradish peroxidase |
| iPSC | Induced-pluripotent stem cell |
| LAP | Lithium phenyl-2,4,6-trimethylbenzoylphosphinate |
| LVR | Linear viscoelastic regime |
| mRNA | Messenger RNA |
| PBS | Phosphate buffered saline |
| PTFE | Polytetrafluoroethylene |
| RH | Relative humidity |
| RNA | Ribonucleic acid |
| RUNX2 | Run-related transcription factor 2 |
| SNL | STO-neo-leukemia |
| SOX9 | Sex determining region Y—box 9 |
| TE | Tissue engineering |
| TGF-β1 | Transforming growth factor beta 1 |
| UV | Ultraviolet light |
References
- Sohn, S.; Van Buskirk, M.; Buckenmeyer, M.J.; Londono, R.; Faulk, D. Whole Organ Engineering: Approaches, Challenges, and Future Directions. Appl. Sci. 2020, 10, 4277. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-Based Hydrogels for Biomedical Applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Yu, C.; Zhang, Y.S. Three-Dimensional Bioprinting of Gelatin Methacryloyl (GelMA). Biodes Manuf. 2018, 1, 215–224. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Tytgat, L.; Dobos, A.; Ottevaere, H.; Van Erps, J.; Thienpont, H.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. (Photo-)Crosslinkable Gelatin Derivatives for Biofabrication Applications. Acta Biomater. 2019, 97, 46–73. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in Organ-on-a-Chip Engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Schuurman, W.; Klein, T.J.; Dhert, W.J.A.; van Weeren, P.R.; Hutmacher, D.W.; Malda, J. Cartilage Regeneration Using Zonal Chondrocyte Subpopulations: A Promising Approach or an Overcomplicated Strategy? J. Tissue Eng. Regen. Med. 2015, 9, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D Printing of Gelatin Methacrylamide Cell-Laden Tissue-Engineered Constructs with High Cell Viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-Linking Strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef]
- Townsend, J.M.; Beck, E.C.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow Behavior Prior to Crosslinking: The Need for Precursor Rheology for Placement of Hydrogels in Medical Applications and for 3D Bioprinting. Prog. Polym. Sci. 2019, 91, 126–140. [Google Scholar] [CrossRef]
- Mendoza-Cerezo, L.; Rodríguez-Rego, J.M.; Macías-García, A.; Callejas-Marín, A.; Sánchez-Guardado, L.; Marcos-Romero, A.C. Three-Dimensional Bioprinting of GelMA Hydrogels with Culture Medium: Balancing Printability, Rheology and Cell Viability for Tissue Regeneration. Polymers 2024, 16, 1437. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A Review of Trends and Limitations in Hydrogel-Rapid Prototyping for Tissue Engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, C.G.; Baruffaldi, D.; Pirri, C.F.; Napione, L.; Frascella, F. GelMA Synthesis and Sources Comparison for 3D Multimaterial Bioprinting. Front. Bioeng. Biotechnol. 2024, 12, 1383010. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Feng, Z.; He, W.; Li, C.; Han, S.; Li, Z.; Guo, R. Novel 3D-Printing Bilayer GelMA-Based Hydrogel Containing BP, β-TCP and Exosomes for Cartilage–Bone Integrated Repair. Biofabrication 2023, 16, 015008. [Google Scholar] [CrossRef]
- Pourazariyan, A.; Shahgholi, M.; Karimipour, A. The Effect of Initial Temperature and ZnO Nanoparticle Volume Fractions on the Stability of Sodium Alginate Hydrogel Nanocomposite Using Molecular Dynamics Simulation. Int. Commun. Heat. Mass. Transf. 2025, 162, 108641. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Liska, R.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. Cross-Linkable Gelatins with Superior Mechanical Properties Through Carboxylic Acid Modification: Increasing the Two-Photon Polymerization Potential. Biomacromolecules 2017, 18, 3260–3272. [Google Scholar] [CrossRef]
- Jain, T.; Baker, H.B.; Gipsov, A.; Fisher, J.P.; Joy, A.; Kaplan, D.S.; Isayeva, I. Impact of Cell Density on the Bioprinting of Gelatin Methacrylate (GelMA) Bioinks. Bioprinting 2021, 22, e00131. [Google Scholar] [CrossRef]
- Majumder, N.; Mishra, A.; Ghosh, S. Effect of Varying Cell Densities on the Rheological Properties of the Bioink. Bioprinting 2022, 28, e00241. [Google Scholar] [CrossRef]
- Groot, R.D.; Bot, A.; Agterof, W.G.M. Molecular Theory of Strain Hardening of a Polymer Gel: Application to Gelatin. J. Chem. Phys. 1996, 104, 9202–9219. [Google Scholar] [CrossRef]
- Michon, C.; Chapuis, C.; Langendorff, V.; Boulenguer, P.; Cuvelier, G. Strain-Hardening Properties of Physical Weak Gels of Biopolymers. Food Hydrocoll. 2004, 18, 999–1005. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1980; ISBN 9780471048947. [Google Scholar]
- Calafel, M.I.; Criado-Gonzalez, M.; Aguirresarobe, R.; Fernández, M.; Mijangos, C. From Rheological Concepts to Additive Manufacturing Assessment of Hydrogel-Based Materials for Advanced Bioprinting Applications. Mater. Adv. 2025, 6, 4566–4597. [Google Scholar] [CrossRef]
- Radi, B.; Wellard, R.M.; George, G.A. Effect of Dangling Chains on the Structure and Physical Properties of a Tightly Crosslinked Poly(Ethylene Glycol) Network. Soft Matter 2013, 9, 3262–3271. [Google Scholar] [CrossRef]
- Amin, D.; Wang, Z. Nonlinear Rheology and Dynamics of Supramolecular Polymer Networks Formed by Associative Telechelic Chains under Shear and Extensional Flows. J. Rheol. 2020, 64, 581–600. [Google Scholar] [CrossRef]
- Kokol, V.; Pottathara, Y.B.; Mihelčič, M.; Perše, L.S. Rheological Properties of Gelatine Hydrogels Affected by Flow- and Horizontally-Induced Cooling Rates during 3D Cryo-Printing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126356. [Google Scholar] [CrossRef]
- Elharfaoui, N.; Djabourov, M.; Babel, W. Molecular Weight Influence on Gelatin Gels: Structure, Enthalpy and Rheology. Macromol. Symp. 2007, 256, 149–157. [Google Scholar] [CrossRef]
- Djabourov, M. Gelation of Physical Gels: The Gelatin Gels. In Physics of Finely Divided Matter; Boccara, N., Daoud, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 21–23. ISBN 978-3-642-93303-5. [Google Scholar]
- Ross-Murphy, S.B. Structure and Rheology of Gelatin Gels: Recent Progress. Polymer 1992, 33, 2622–2627. [Google Scholar] [CrossRef]
- Picout, D.R.; Ross-Murphy, S.B. Rheology of Biopolymer Solutions and Gels. Sci. World J. 2003, 3, 105–121. [Google Scholar] [CrossRef]
- Yang, Z.; Hemar, Y.; Hilliou, L.; Gilbert, E.P.; McGillivray, D.J.; Williams, M.A.K.; Chaieb, S. Nonlinear Behavior of Gelatin Networks Reveals a Hierarchical Structure. Biomacromolecules 2016, 17, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.M.; Melchels, F.P.W.; Visser, J.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Yield Stress Determines Bioprintability of Hydrogels Based on Gelatin-Methacryloyl and Gellan Gum for Cartilage Bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, R.; Rodríguez-Rego, J.M.; Macías-García, A.; Mendoza-Cerezo, L.; Díaz-Parralejo, A. Relationship between Shear-Thinning Rheological Properties of Bioinks and Bioprinting Parameters. Int. J. Bioprint 2023, 9, 687. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar] [CrossRef]
- Smith, P.T.; Basu, A.; Saha, A.; Nelson, A. Chemical Modification and Printability of Shear-Thinning Hydrogel Inks for Direct-Write 3D Printing. Polymer 2018, 152, 42–50. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion Bioprinting of Soft Materials: An Emerging Technique for Biological Model Fabrication. Appl. Phys. Rev. 2019, 6, 011310–011330. [Google Scholar] [CrossRef]
- Du, D.; Sugita, N.; Liu, Z.; Moriguchi, Y.; Nakata, K.; Myoui, A.; Yoshikawa, H. Repairing Osteochondral Defects of Critical Size Using Multiple Costal Grafts: An Experimental Study. Cartilage 2015, 6, 241–251. [Google Scholar] [CrossRef]
- Morrison, F.A. Understanding Rheology; Oxford University Press: New York, NY, USA, 2001; ISBN 0195141660. [Google Scholar]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The Bio in the Ink: Cartilage Regeneration with Bioprintable Hydrogels and Articular Cartilage-Derived Progenitor Cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, O.; Henrionnet, C.; Bourge, K.; Loeuille, D.; Gillet, P.; Pinzano, A. Stem Cells and Extrusion 3D Printing for Hyaline Cartilage Engineering. Cells 2020, 10, 2. [Google Scholar] [CrossRef]
- Visser, J.; Gawlitta, D.; Benders, K.E.; Toma, S.M.; Pouran, B.; Ren van Weeren, P.; Dhert, W.J.; Malda, J. Endochondral Bone Formation in Gelatin Methacrylamide Hydrogel with Embedded Cartilage-Derived Matrix Particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Pirosa, A.; Gottardi, R.; Alexander, P.G.; Puppi, D.; Chiellini, F.; Tuan, R.S. An in Vitro Chondro-Osteo-Vascular Triphasic Model of the Osteochondral Complex. Biomaterials 2021, 272, 120773. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R. Aging and Osteoarthritis: The Role of Chondrocyte Senescence and Aging Changes in the Cartilage Matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Castro-Viñuelas, R.; Sanjurjo-Rodríguez, C.; Piñeiro-Ramil, M.; Hermida-Gómez, T.; Fuentes-Boquete, I.M.; de Toro-Santos, F.J.; Blanco-García, F.J.; Díaz-Prado, S.M. Induced Pluripotent Stem Cells for Cartilage Repair: Current Status and Future Perspectives. Eur. Cell Mater. 2018, 36, 96–109. [Google Scholar] [CrossRef]
- Lee, J.; Taylor, S.E.B.; Smeriglio, P.; Lai, J.; Maloney, W.J.; Yang, F.; Bhutani, N. Early Induction of a Prechondrogenic Population Allows Efficient Generation of Stable Chondrocytes from Human Induced Pluripotent Stem Cells. FASEB J. 2015, 29, 3399–3410. [Google Scholar] [CrossRef]
- Agten, H.; Van Hoven, I.; Viseu, S.R.; Van Hoorick, J.; Van Vlierberghe, S.; Luyten, F.P.; Bloemen, V. In Vitro and in Vivo Evaluation of 3D Constructs Engineered with Human IPSC-Derived Chondrocytes in Gelatin Methacryloyl Hydrogel. Biotechnol. Bioeng. 2022, 119, 2950–2963. [Google Scholar] [CrossRef]
- Bachmann, B.; Spitz, S.; Schädl, B.; Teuschl, A.H.; Redl, H.; Nürnberger, S.; Ertl, P. Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation. Front. Bioeng. Biotechnol. 2020, 8, 373. [Google Scholar] [CrossRef]
- Schuh, E.; Hofmann, S.; Stok, K.; Notbohm, H.; Müller, R.; Rotter, N. Chondrocyte Redifferentiation in 3D: The Effect of Adhesion Site Density and Substrate Elasticity. J. Biomed. Mater. Res. A 2012, 100, 38–47. [Google Scholar] [CrossRef]
- Amorim, P.A.; d’Ávila, M.A.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on Shear Rheology of Inks for Extrusion-Based 3D Bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Eyckmans, J.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. A Clinically Relevant Model of Osteoinduction: A Process Requiring Calcium Phosphate and BMP/Wnt Signalling. J. Cell Mol. Med. 2010, 14, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Barros, J. Tensile Creep of a Structural Epoxy Adhesive: Experimental and Analytical Characterization. Int. J. Adhes. Adhes. 2015, 59, 115–124. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of Scaffoldless Hyaline Cartilaginous Tissue from Human IPSCs. Stem Cell Rep. 2015, 4, 404–418. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, Preparation and Use of Cell-Laden Gelatin Methacryloyl-Based Hydrogels as Modular Tissue Culture Platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, UK, 2003; ISBN 019852059X. [Google Scholar]
- Toda, M.; Morita, H. Rubber Elasticity of Realizable Ideal Networks. AIP Adv. 2018, 8, 125005. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 1–845. [Google Scholar] [CrossRef]
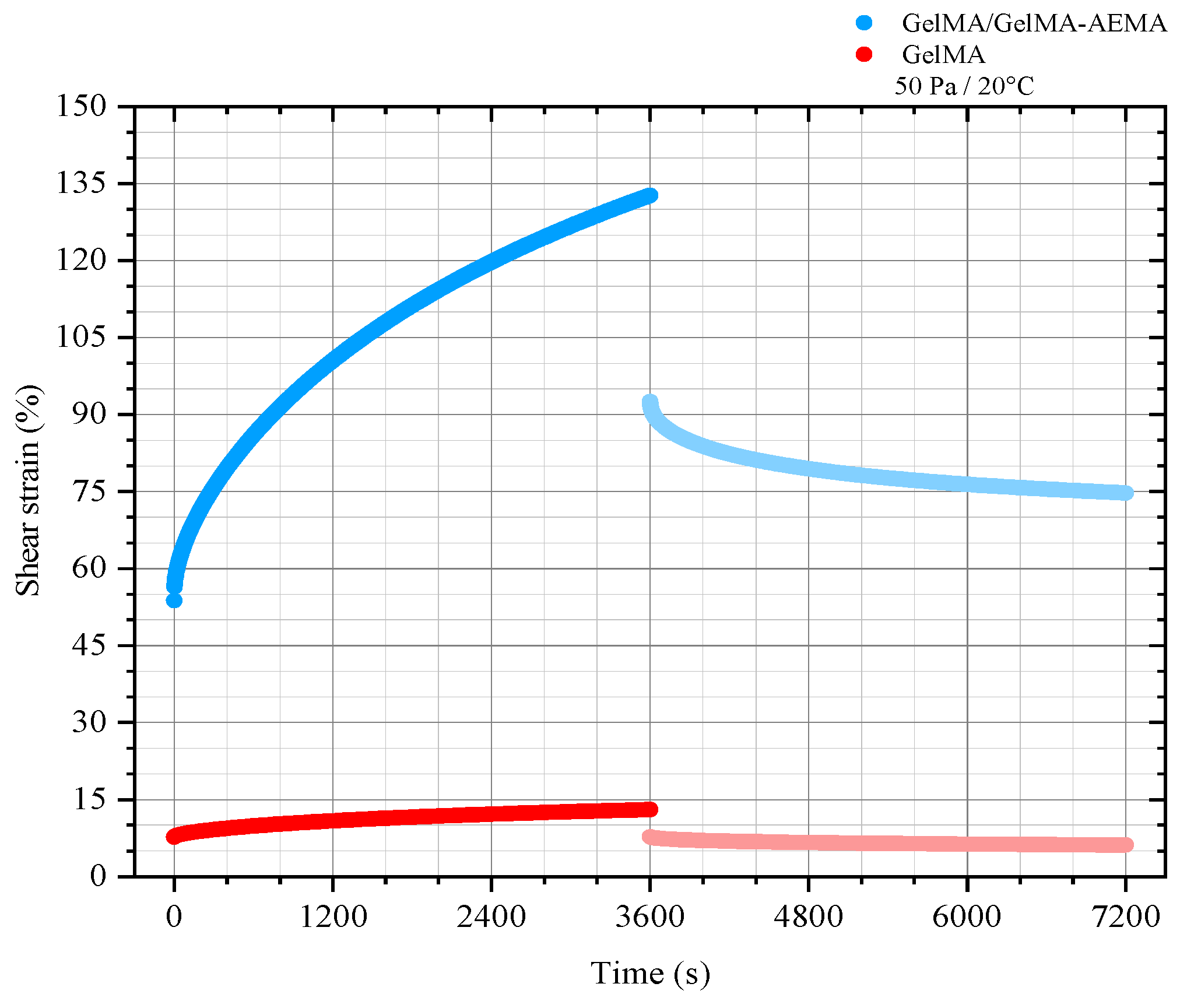
| Material | GM (Pa) | GK (Pa) | ηM (Pa.s) | ηK (Pa.s) | λ (s) |
|---|---|---|---|---|---|
| GelMA | 650 | 1700 | 7 × 106 | 1.2 × 106 | 712 |
| GelMA/GelMA-AEMA | 90 | 100 | 5 × 105 | 6.4 × 104 | 644 |
| Material | Culturing Period (Day) | Initial Cell Density (mL−1) | Sample Size, n |
|---|---|---|---|
| GelMA 10 w/v% | 1 | 2 × 107 | 5 |
| 0 | 5 | ||
| 21 | 2 × 107 | 4 | |
| 0 | 5 | ||
| GelMA/GelMA-AEMA 1:1, 10 w/v% | 1 | 2 × 107 | 5 |
| 0 | 5 | ||
| 21 | 2 × 107 | 6 | |
| 0 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, P.A.; Agten, H.; Vermeulen, M.; Van Vlierberghe, S.; Geris, L.; Bloemen, V. Novel GelMA/GelMA-AEMA Hydrogel Blend with Enhanced Printability as a Carrier for iPSC-Derived Chondrocytes In Vitro. Gels 2025, 11, 698. https://doi.org/10.3390/gels11090698
Amorim PA, Agten H, Vermeulen M, Van Vlierberghe S, Geris L, Bloemen V. Novel GelMA/GelMA-AEMA Hydrogel Blend with Enhanced Printability as a Carrier for iPSC-Derived Chondrocytes In Vitro. Gels. 2025; 11(9):698. https://doi.org/10.3390/gels11090698
Chicago/Turabian StyleAmorim, Paulo A., Hannah Agten, Margaux Vermeulen, Sandra Van Vlierberghe, Liesbet Geris, and Veerle Bloemen. 2025. "Novel GelMA/GelMA-AEMA Hydrogel Blend with Enhanced Printability as a Carrier for iPSC-Derived Chondrocytes In Vitro" Gels 11, no. 9: 698. https://doi.org/10.3390/gels11090698
APA StyleAmorim, P. A., Agten, H., Vermeulen, M., Van Vlierberghe, S., Geris, L., & Bloemen, V. (2025). Novel GelMA/GelMA-AEMA Hydrogel Blend with Enhanced Printability as a Carrier for iPSC-Derived Chondrocytes In Vitro. Gels, 11(9), 698. https://doi.org/10.3390/gels11090698









