1. Introduction
In recent years, as most food products are packaged, the best preservation of food and monitoring of its freshness are mandatory for the consumer’s experience [
1]. In parallel, sustainable food packaging is of the utmost importance for a greener environment with less microplastics [
2]. For these reasons, natural polymer gels, such as chitosan and gelatin gels, are used to produce non-toxic, biodegradable bioactive films for alternative food packaging applications [
3,
4,
5]. Chitosan films, containing anthocyanins from various sources, like vinasse and barberry, can also be used as pH-responsive films due to their ability to change color as a response to pH changes. These films are used to detect the pH changes that occur during the spoilage of food, such as shrimps and meat, meeting the consumer’s need to know about the freshness and safety of the products [
4,
6].
Chitosan is derived from the deacetylation of chitin, a polysaccharide found in abundance in nature and mainly in the shells of crustaceans, such as shrimps, crabs, etc. [
7]. The casting of chitosan gels to prepare films for food packaging applications is well described due to their high biodegradability and their antioxidant and antimicrobial properties [
8]. Additionally, incorporating natural phenolic extracts from winemaking by-products (e.g., grape pomace, wine lees, grape seed) in the film matrix often enhances their antioxidant and antimicrobial properties [
8,
9,
10]. Similarly, chitosan has also been used to produce smart pH-responsive packaging films by adding extracts rich in natural pigments. For example, barberry, aronia, and red cabbage have been successfully utilized as anthocyanin-rich sources in the fabrication of pH-sensitive chitosan-based films [
6,
11,
12]. However, the poor mechanical properties of chitosan films limit their use without the addition of a proper plasticizer such as glycerol.
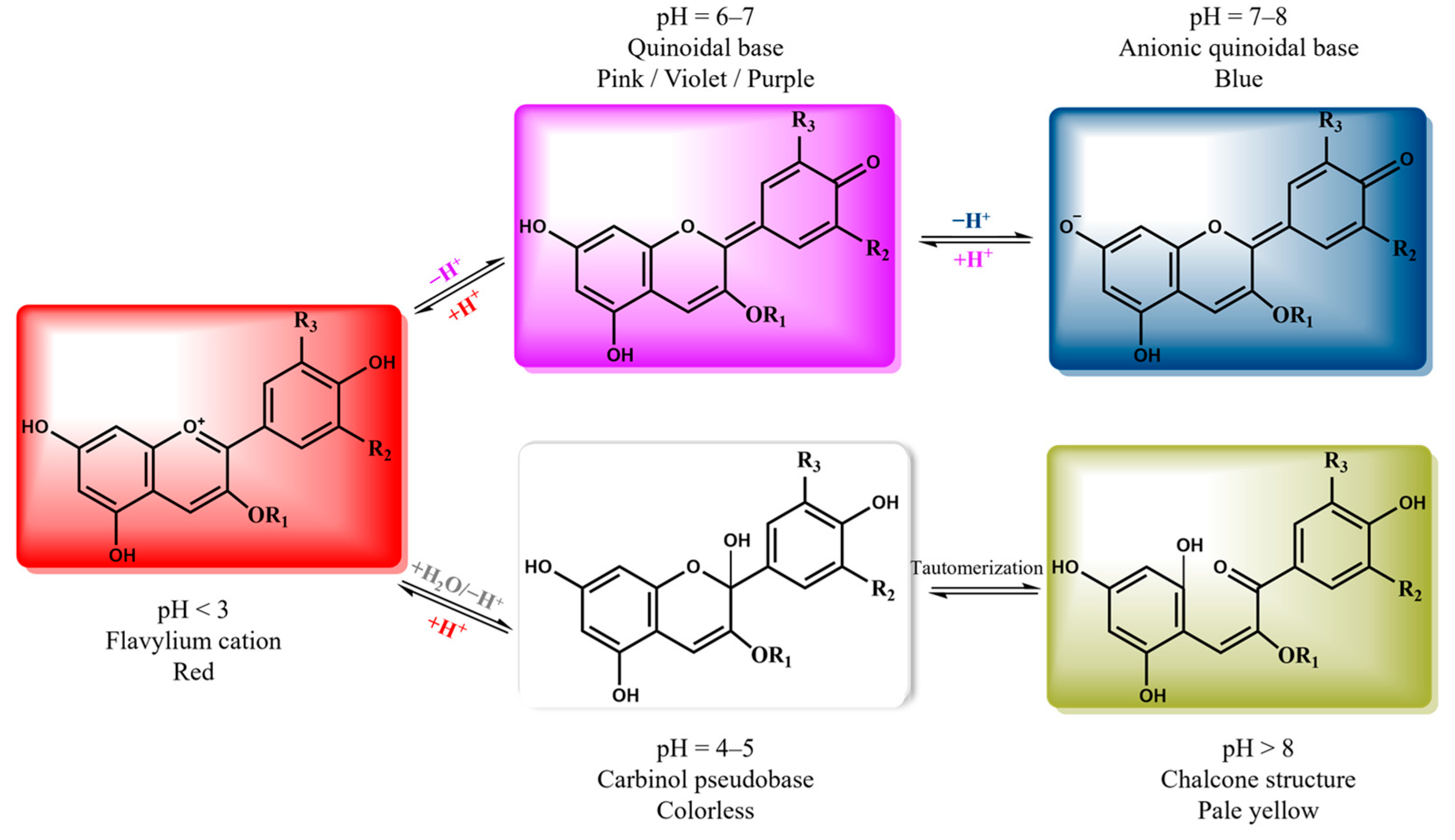
Anthocyanins, a subclass of (poly)phenolic compounds, are natural pigments responsible for the red, blue, and purple colors in plants [
13]. In response to the pH variation, anthocyanins undergo structural changes, resulting in a visible color shift [
14]. Depending on pH, different anthocyanin structures predominate, resulting in pink-red colors under acidic conditions and green to yellow hues under alkaline conditions [
15,
16]. This property makes anthocyanins a promising tool for developing films that can detect pH changes. However, due to the high cost of pure anthocyanins, a sustainable solution is the extraction of anthocyanins from natural sources and agro-industrial wastes, following the principles of zero waste management and the circular economy [
13,
17].
The traditional extraction methods for anthocyanins involve the use of hydroalcoholic mixtures, typically ethanol or methanol [
18]. However, according to the EU environmental policy targets, there is a general need to reduce the use of organic volatile solvents because they are flammable and often toxic [
19,
20]. A promising alternative is the use of Natural Deep Eutectic Solvents (NADES). NADES are mainly composed of a hydrogen bond donor (e.g., an alcohol or a carbohydrate) and a hydrogen bond acceptor (e.g., a quaternary ammonium salt), forming a liquid with a lower melting point compared to their parental materials [
21]. They have gained considerable interest owing to their low cost, toxicity, and volatility, as well as their easy preparation and high biodegradability [
22]. Due to their characteristics and the high stability of phenolic compounds within these solvents, NADES already have an extended use in the extraction of bioactive compounds from numerous natural sources [
22,
23].
Wine lees, a by-product of the winemaking process that precipitates on the barrel bottom, are an excellent source of phenolic compounds, including anthocyanins [
24]. Many non-conventional extraction processes, such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and extraction using NADES, have already been proposed for extracting bioactive compounds from wine-related by-products, with recovery yields superior to conventional extraction methods [
23,
24]. For example, various choline chloride-based NADES have been used effectively to extract anthocyanins from wine lees using UAE [
25]. Similarly, choline chloride- and betaine-based NADES were used for grape pomace anthocyanins recovery with promising results [
26,
27].
In parallel, the applications of NADES are not limited to the extraction of bioactive compounds from natural sources. Chitosan films are often characterized by poor mechanical properties, especially low elongation [
28,
29]. Traditionally, common plasticizers (e.g., polyols, such as glycerol) or other additives, like proteins and lipids, are used to enhance the flexibility of these films [
28]. Therefore, NADES have been used as plasticizers in natural polymer films, serving as sustainable alternatives to conventional plasticizers and resulting in improved mechanical properties [
29,
30,
31]. This plasticizing effect is primarily attributed to the hydrogen bond network of the NADES, which increases the free space between the polymeric chains of chitosan, thereby leading to enhanced polymer mobility [
32,
33].
Taking into account the aforementioned considerations, this study focuses on the development of pH-responsive chitosan films, adding a rich-in-anthocyanins wine lees extract obtained using an NADES/water mixture as an extraction solvent. The extraction conditions were optimized by focusing on the anthocyanins and phenolic compounds recovery, as well as the antioxidant activity of the extracts. Under the optimized conditions, sixteen 50% (v/v) NADES/water mixtures were used to investigate which was the most efficient for wine lees anthocyanins extraction, against 50% (v/v) ethanol/water as a control solvent. The final extract was further characterized and used as an additive in chitosan films. The produced film was characterized in terms of its colorimetric response to pH variations, antioxidant capacity, and mechanical properties, and was subsequently applied for monitoring the freshness of meat. To the best of our knowledge, this is the first report on the direct incorporation of wine lees-derived anthocyanin extract, obtained using NADES, into chitosan films, simultaneously providing colorimetric pH sensitivity and plasticizing functionality.
3. Conclusions
Summarizing the results of this work, we demonstrated the preparation of pH-responsive chitosan films that combine plasticizing properties and pH sensitivity using a natural extract obtained with NADES. Among the different tested NADES for anthocyanins extraction, ChCl:BG was the most effective. The obtained NADES-wine lees extract was used as an additive in chitosan films, enhancing their antioxidant activity and UV-barrier effect, and providing excellent mechanical properties. The pH-sensing ability of the functional film was tested, demonstrating distinct color changes across the tested pH values. Moreover, it is notable that the L, a, b, and ΔE values were recorded using a smartphone application, highlighting the potential for practical, user-friendly commercial deployment. Finally, the film was used to monitor pork freshness, showing a clear color shift during the initial stages of spoilage (ΔE = 15.8). These results indicate that the developed films presented in this work could be effectively used as pH-monitor systems for food packaging applications, paving the way for sustainable and intelligent solutions. However, further research may be necessary to evaluate the long-term storage stability and to investigate their colorimetric response to different meat types. Extended spoilage periods, under different temperatures, should also be tested to evaluate the color response of the films to different acidic or basic volatile compounds. Additionally, further investigation is required to evaluate the biocompatibility and safety of these films for direct applications in meat packaging.
4. Materials and Methods
4.1. Chemicals and Reagents
Folin–Ciocalteu’s phenol reagent, chitosan (75–85% deacetylated, low molecular weight, 20–300 cP), D (+)-Glucose (≥99.5%), D (−)-Fructose (≥99.0%), sodium acetate trihydrate (≥99.0%), choline chloride (>98.0%), lactic acid (>98.0%), acetic acid (99.8%), 2,2- diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium peroxydisulfate (≥99.0%) and potassium chloride (≥99.5%) were purchased from Merck KGaA (Darmstadt, Germany). Gallic acid hydrate (≥98.0%), butylene glycol (>99.0%), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Glycerol (0.5% max water) and ethanol (99.8%) were purchased from Fisher Scientific Co. (Loughborough, UK). Sodium carbonate (≥99.8%) was purchased from Riedel de Haen (Charlotte, NC, USA). Betaine (98.0%) and Propylene glycol (99.0%) were purchased from Acros Organics (Waltham, MA, USA). Ethylene glycol (≥99.5%) was obtained from AppliChem (Darmstadt, Germany). Urea (>99.0%) was purchased from Fluka/Riedel-de Haën (Seelze, Germany). Double-distilled water (ddH2O) was used for all experiments. Pork meat was obtained from the local market.
4.2. Preparation of Natural Deep Eutectic Solvents
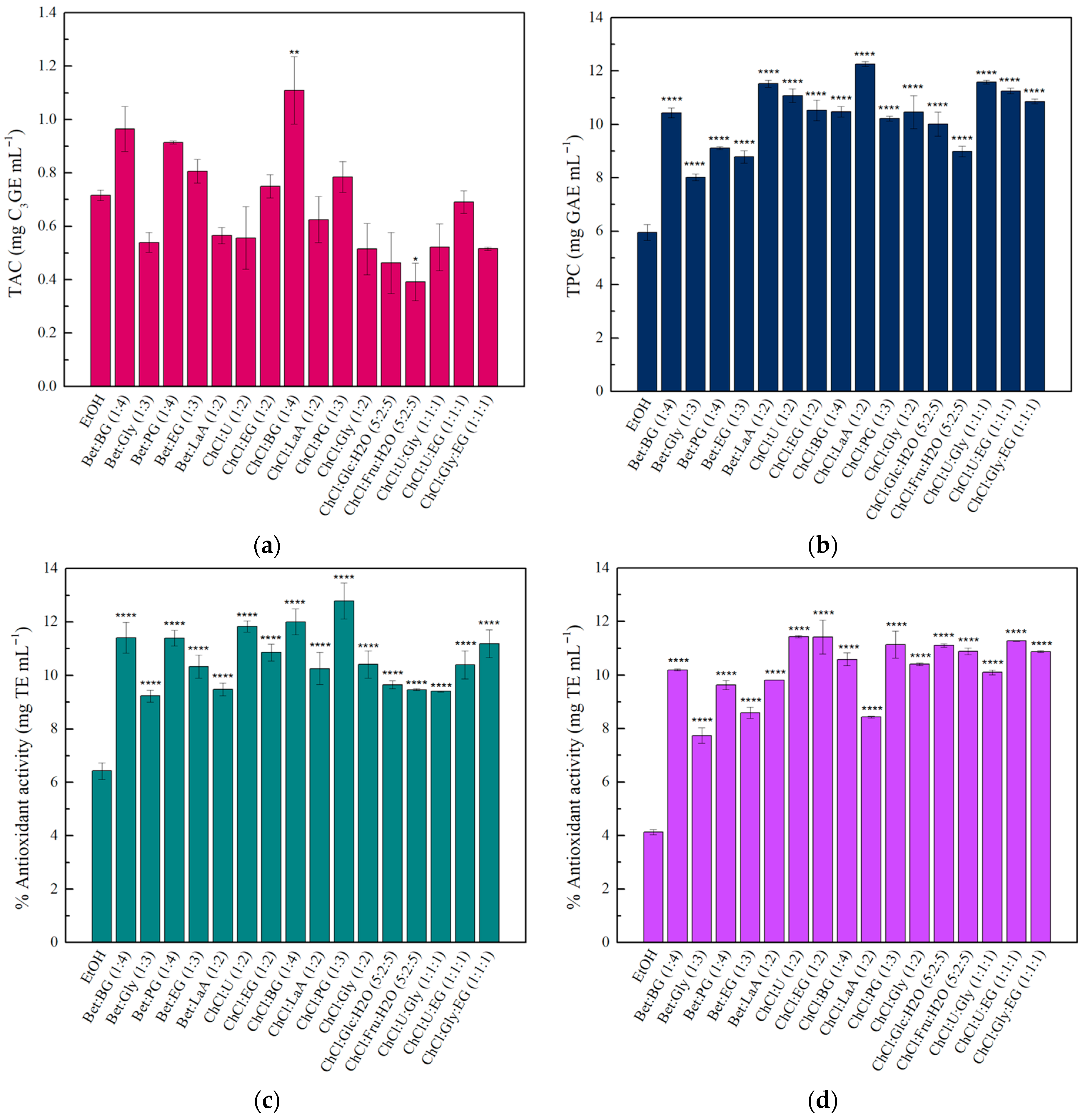
The preparation of NADES was performed according to the literature [
81]. In brief, the hydrogen bond acceptors and the hydrogen bond donors were mixed at proper molar ratios in screw-capped glass vials and incubated at 80 °C under stirring (200 rpm) for 2 h. The formed NADES were used without further treatment, and their composition, abbreviations, and molar ratios are summarized in
Table 6.
4.3. Wine Lees Extractions—Optimization
Red wine lees were provided from a local winery (Ioannina, Epirus, Greece), and their main characteristics are described in our previous work [
2]. Before extraction, wine lees were centrifuged at 9500 rpm at 4 °C for 10 min, and the pellet was freeze-dried, ground, and stored at −20 °C until use.
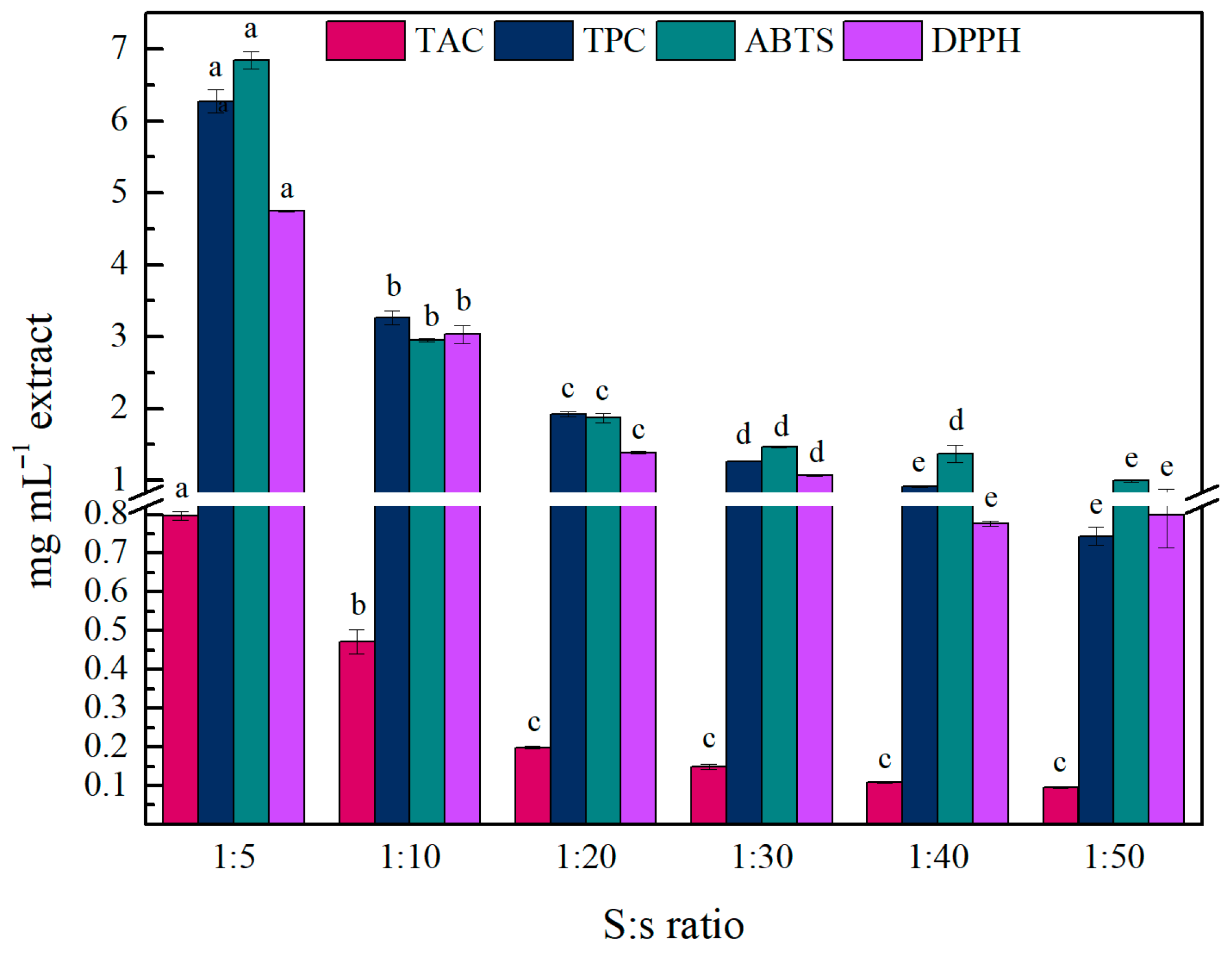
The optimization of the extraction process was conducted using ethanol as a common solvent for anthocyanin extraction. The extractions were performed using an ATPIO XO-SM50 ultrasound and microwave collaborative system (Nanjing Xianou Instruments Manufacture Co., Ltd., Nanjing, China), where only the ultrasound function was used. The ultrasonic power was set at 200 watts (ultrasonic on delay: 3 s., ultrasonic turn-off time: 1 s.), and the solvent volume was 20 mL. After the extraction, the extract was centrifuged at 9500 rpm at 4 °C, for 10 min, and the supernatant was filtered through Whatman filter paper and stored at 4 °C until further use. The effect of S:s ratio, extraction time (min), and ethanol content (%) on the TPC, TAC, and AA was investigated, and the different trials are described in
Table 7. After determining the optimized extraction conditions, the different NADES were tested under the same conditions as alternative solvents for ethanol.
4.4. Characterization of the Extracts
4.4.1. Total Anthocyanin Content
The TAC of the liquid extracts was estimated using the pH-differential method, but the final volume was adjusted to 1 mL [
82,
83]. In brief, the dilution factor (DF) of each sample was estimated by diluting the extract with potassium chloride buffer (0.025 M, pH 1.0) to reach an absorbance in the linear range of the spectrophotometer at 520 nm. In the next step, each sample was diluted (according to the estimated DF) in potassium chloride buffer (0.025 M, pH 1.0) and sodium acetate buffer (0.4 M, pH 4.5) and left to stabilize for at least 15 min. The absorbance of both solutions was measured at 520 nm and 700 nm using a spectrophotometer (Agilent Cary 60, Santa Clara, CA, USA). The results are expressed as mg of cyanidin-3-glucoside equivalents per mL of the extract (mg C
3GE mL
−1) according to the following Equation (1).
where A = (A
520–A
700)
pH1.0 − (A
520–A
700)
pH4.5, and A
520 and A
700 are the absorbance of the solution at 520 nm and 700 nm, respectively, MW is the molecular weight of cyanidin-3-glucoside (449.2 g mol
−1), DF is the dilution factor, and ε is the molar absorptivity (26,900 L cm
−1 mol
−1). The equation presented above assumes a path length of 1 cm.
4.4.2. Total Phenolic Content
The TPC of the extracts was determined by the Folin–Ciocalteu assay as described in our previous work [
9]. Briefly, water, a proper volume of the extract, and 10 μL of Folin–Ciocalteu reagent were mixed in a final volume of 180 μL in a 96-well plate, and the samples were incubated at room temperature for 3 min. Then, 20 μL of a 20%
w/
v Na
2CO
3 aqueous solution were added, and the samples were incubated for 1 h in the dark. The absorbance of the samples was measured at 725 nm using a UV–Vis Multiskan SkyHigh Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA). The results are expressed in terms of gallic acid equivalents per mL of the extract (mg GAE mL
−1) using a gallic acid standard curve.
4.4.3. Antioxidant Activity DPPH—ABTS
The AA of the extracts was estimated using DPPH
• and ABTS
•+ free radicals as described in our previous work [
84]. In brief, a proper volume of the extracts was mixed with the DPPH
• and ABTS
•+ free radicals’ solutions, and the samples were incubated in the dark for 30 min. The absorbance of the samples was measured at 517 nm and 734 nm, respectively. The AA is expressed as mg Trolox equivalents per mL of extract (mg TE mL
−1) for both assays using Trolox standard curves.
4.4.4. Color Response of the Extract in pH Changes—UV–Vis Characterization
The pH response of the final extract was evaluated by diluting the extract in buffer solutions in a pH range 2–12. The buffer solutions were prepared according to the literature [
85]. In brief, the extract was diluted with each buffer (DF = 50) and left to stabilize for 15 min at room temperature. Then, 300 μL of each solution were transferred to a 96-well UV-ELISA plate, and the spectra were recorded in the 200–800 nm range using a UV–Vis Multiskan SkyHigh Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA).
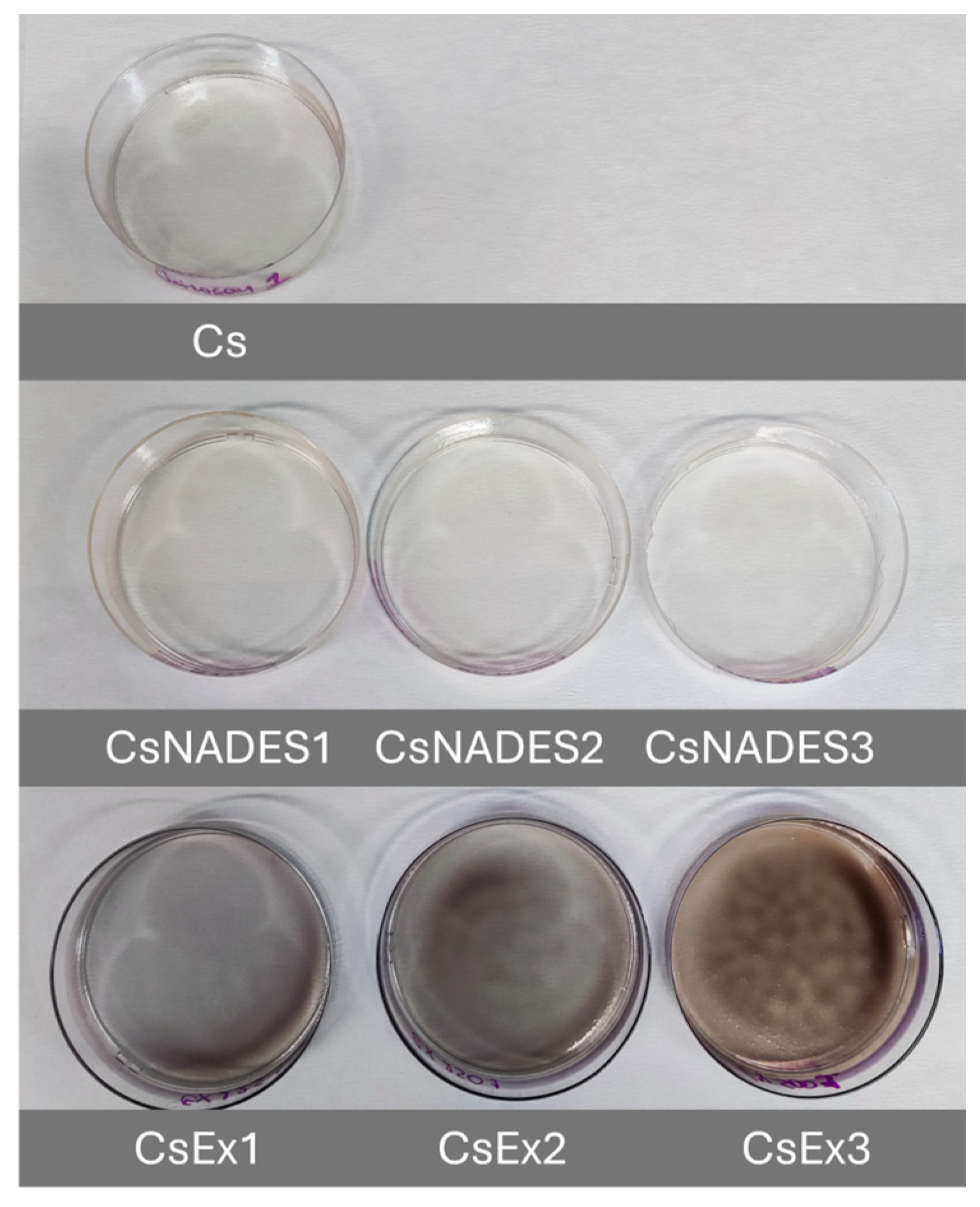
4.5. Preparation of pH-Responsive Chitosan Films
The preparation of chitosan films was conducted as described in our previous work with some modifications [
84]. Briefly, a chitosan solution (1.5%
w/
v) was prepared in acetic acid (1%
v/
v) under stirring, at 75 °C for 30 min. After cooling, the solution was centrifuged (8000 rpm, 10 min) to remove any insoluble material. Then, 10 mL of Cs solution were mixed with 125, 250, or 500 μL (corresponding to 1.25, 2.5, or 5%
v/
v, respectively) of the final extract, cast in 50 mm plastic Petri dishes, and then dried at 30 °C for 24 h. Cs films without any addition and Cs films with the addition of 1.25, 2.5, or 5%
v/
v of extraction solvent were used as control samples.
4.6. Characterization of pH-Responsive Chitosan Films
4.6.1. Antioxidant Activity of the Chitosan Films
The AA of the formed films was evaluated as described in our previous work with minor modifications [
9]. In brief, 0.5 cm × 0.5 cm pieces of the films were mixed with the DPPH
• or ABTS
•+ free radical solutions, and the absorbance of the mixtures was measured after 30 or 20 min of incubation, at 517 nm and 734 nm, respectively. The results are presented as % antioxidant activity through the following Equation (2).
where A
control and A
sample represent the absorbance of the free radical without and after the interaction with the extract, respectively.
4.6.2. Water Swelling, and Water Solubility Assays
The water swelling (WSw), and the water solubility (WS) of the films were estimated according to the methods described in our previous work [
84].
4.6.3. Film Thickness
The thickness of the films was determined using a digital caliper with a resolution of 0.01 mm. At least three different measurements were performed in three different areas of the films.
4.6.4. Mechanical Properties
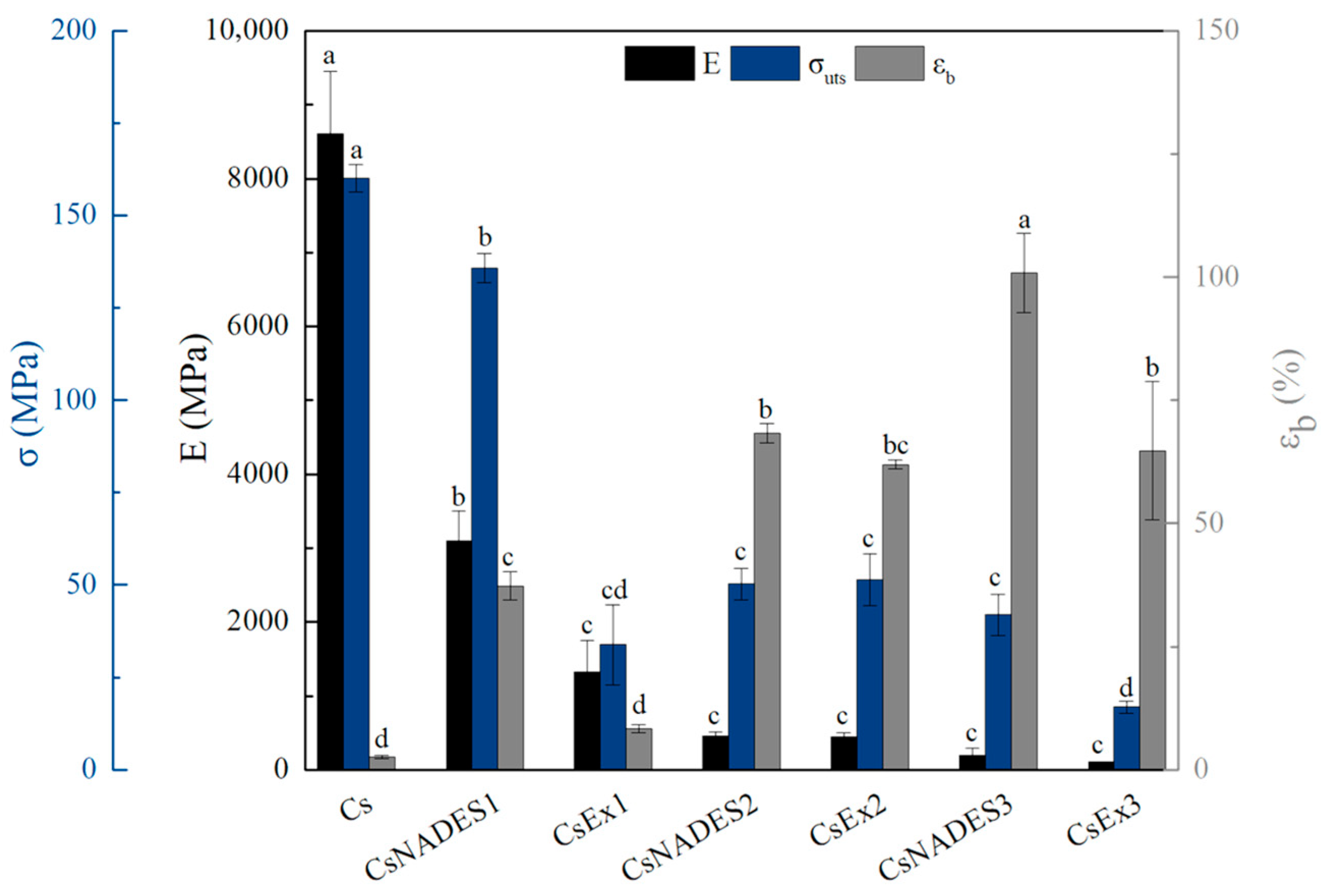
Tensile tests were performed on strip films with a width of 10 mm and a gauge length of 30 mm using a mini tester equipped with a 1000 lb load cell. The mini tester used in this study was made by Fulam USA, while the tests were performed according to ASTM D882. Specifically, after measuring the thickness of 2 to 3 samples of each formulation with a digital micrometer, the films were clamped between the grips and tensed at a deformation speed of 10 mm min−1 at 25 °C. Force and deformation were recorded during the tensile experiment, and based on these data and the dimensions of the specimens (cross-sectional area and gauge length), Young’s modulus (E), tensile strength (σuts), and % strain at break (εb) were calculated.
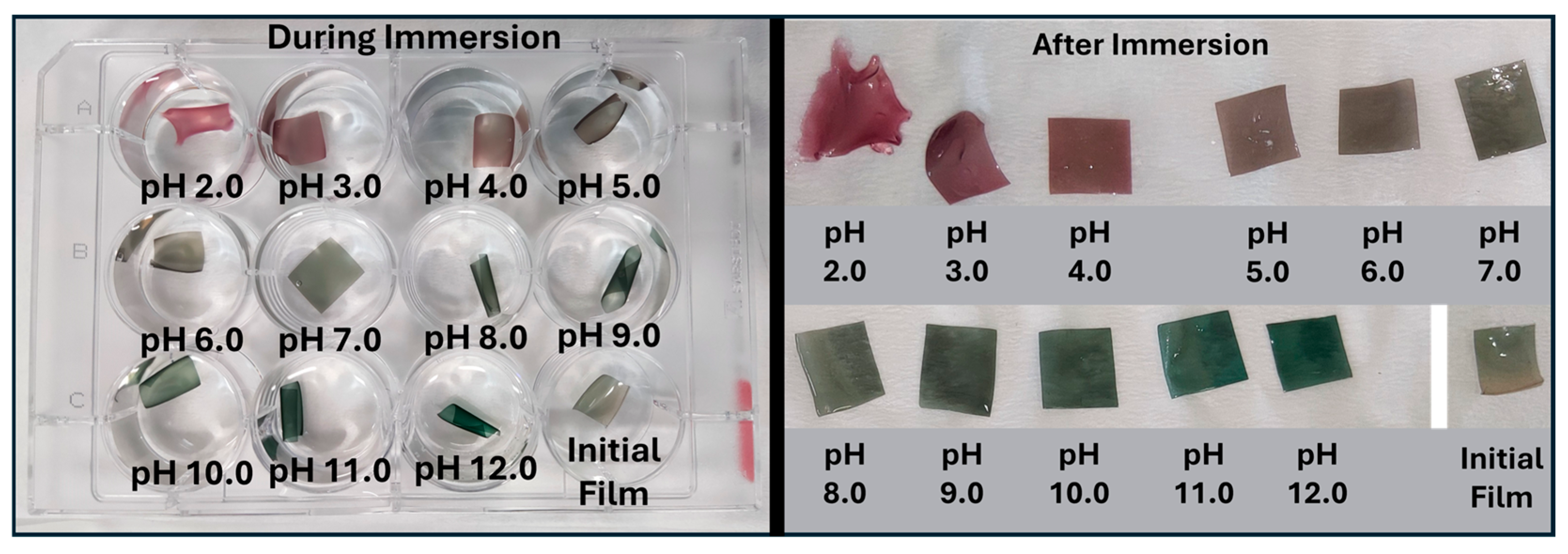
4.6.5. Color Response to pH Changes
To determine the pH response of the selected film, 1 cm × 1 cm pieces were immersed for 2 min in the same buffer solutions as mentioned in paragraph 4.4.4. Then, the film pieces were placed on top of a filter paper to remove the excess buffer solutions. The L (darkness/lightness), a (greenness/redness), and b (blueness/yellowness) values were determined using the Color Grab™ app (Version 3.9.2, © 2021 Loomatix Ltd., Haifa, Israel) [
86], and the ΔE was calculated using the following Equation (3).
where L
0, a
0, and b
0 are the values of the initial film, and L, a, and b are the values of the film after the interaction with the different buffer solutions. All the photos were taken in a custom-made lightbox, using a smartphone with a 48-megapixel camera. The white font of the lightbox was used as a calibration color.
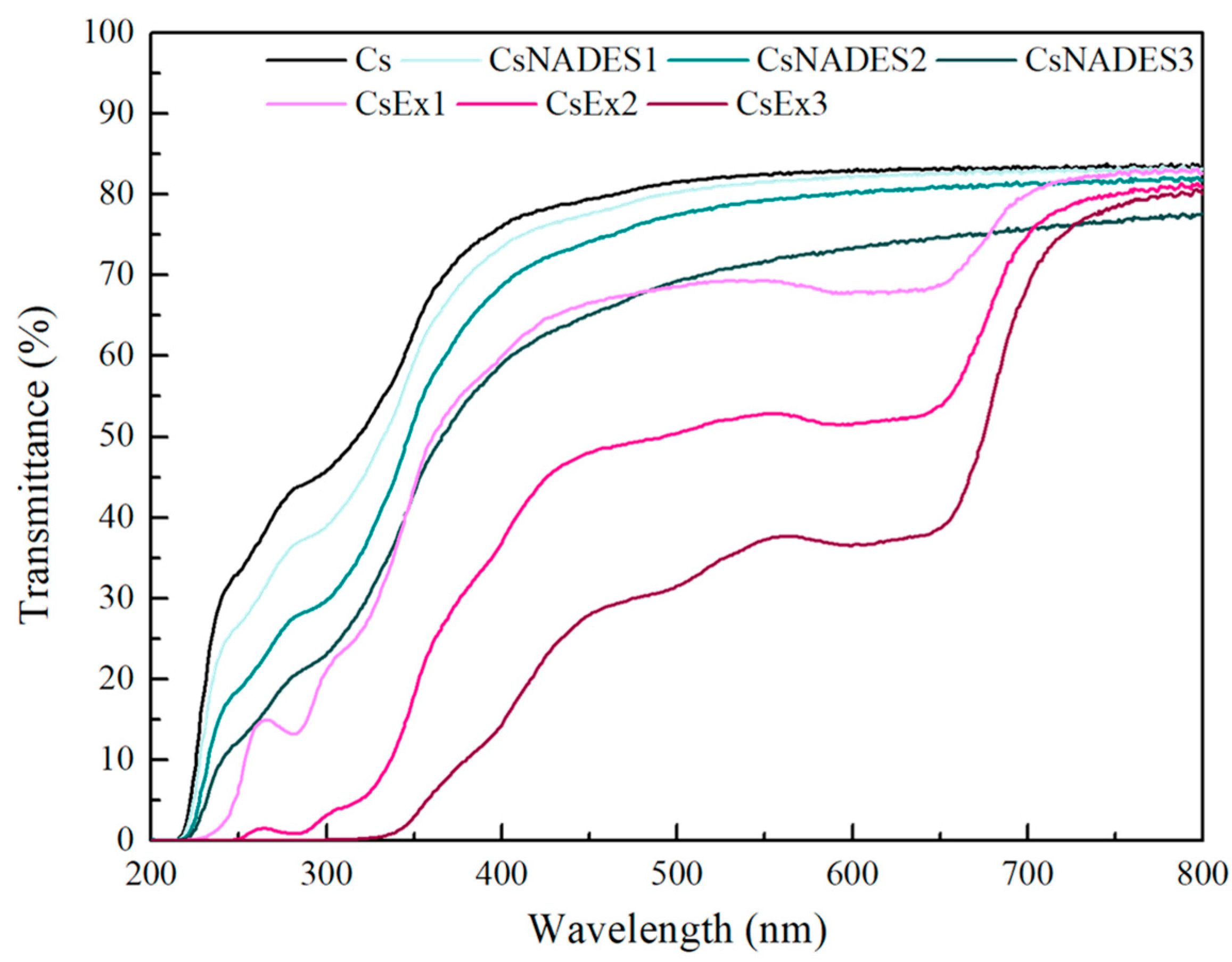
4.6.6. Optical Properties of the Films
The UV–Vis spectra of the films (wavelength range 200–800 nm) were obtained using a UV–Vis Multiskan SkyHigh Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA). The film samples were cut into circular pieces and placed on a UV–ELISA plate. An empty well was used as a reference. The opacity of the films was calculated through the following equation [
5,
87].
where A
600 and Th represent the absorbance at 600 nm and the thickness (mm) of the film, respectively.
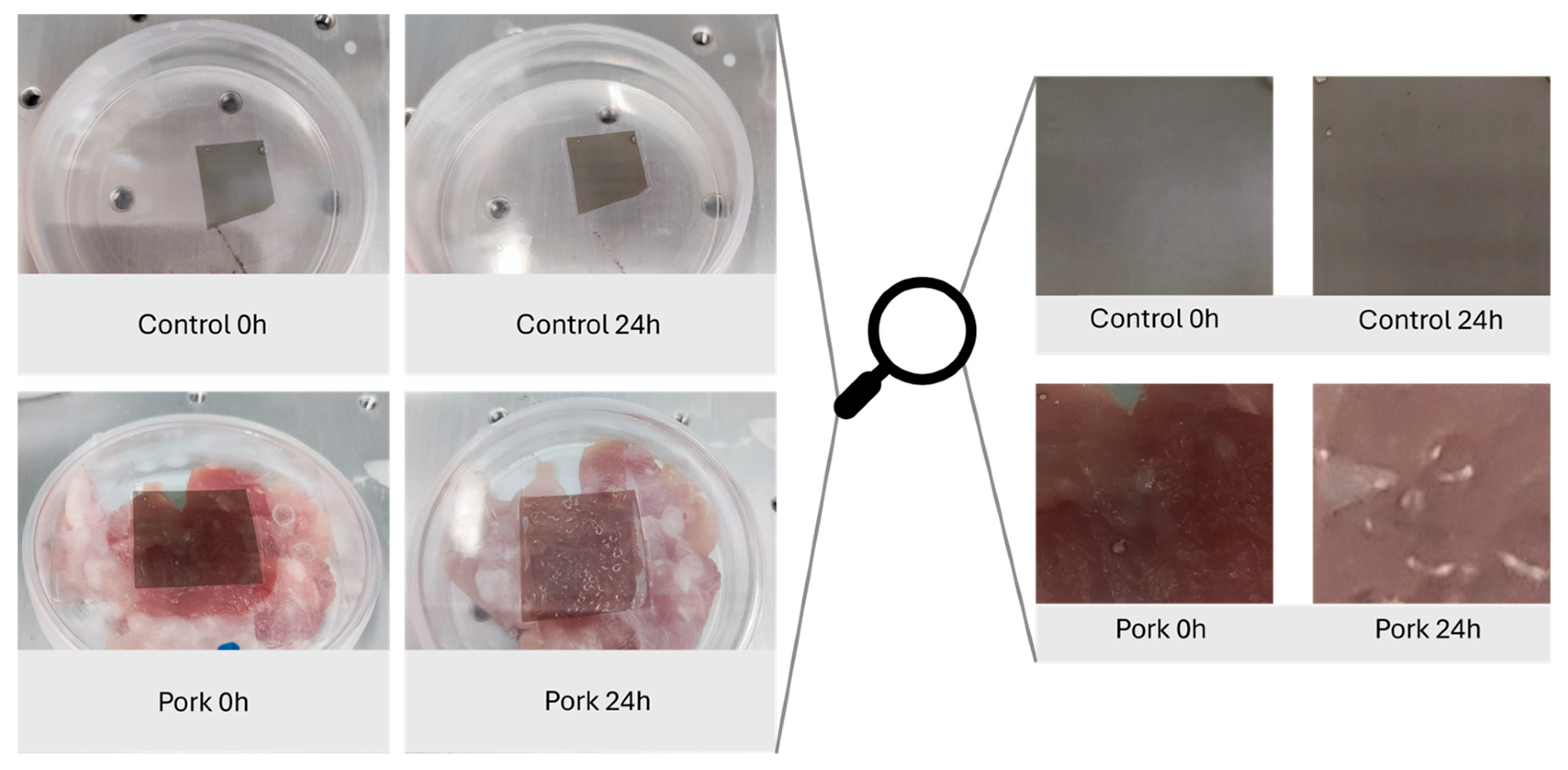
4.7. Monitor of Meat Freshness
The spoilage of the meat was monitored according to Chayavanich et al. [
88]. In brief, 10 g of pork meat was placed in a 50 mm petri dish. One 2 cm × 2 cm piece of the film was placed on the inside of the plastic cap, and the plate was incubated at 30 °C for 24 h. Pictures of the plate were taken at 0, 1, 2, 4, and 24 h to monitor the optical color difference of the films during the spoilage.
4.8. Statistical Analysis
The experimental data were analyzed using one-way ANOVA analysis and Tukey’s or Dunnett’s multiple comparison tests. They were carried out using IBM SPSS Statistics version 21 (SPSS Inc., Chicago, IL, USA) to compare the mean values of each treatment and to determine the statistical significance (p < 0.05).











_Stamatis.png)


