Catalytic Interface of rGO-VO2/W5O14 Hydrogel for High-Performance Electrochemical Water Oxidation
Abstract
1. Introduction
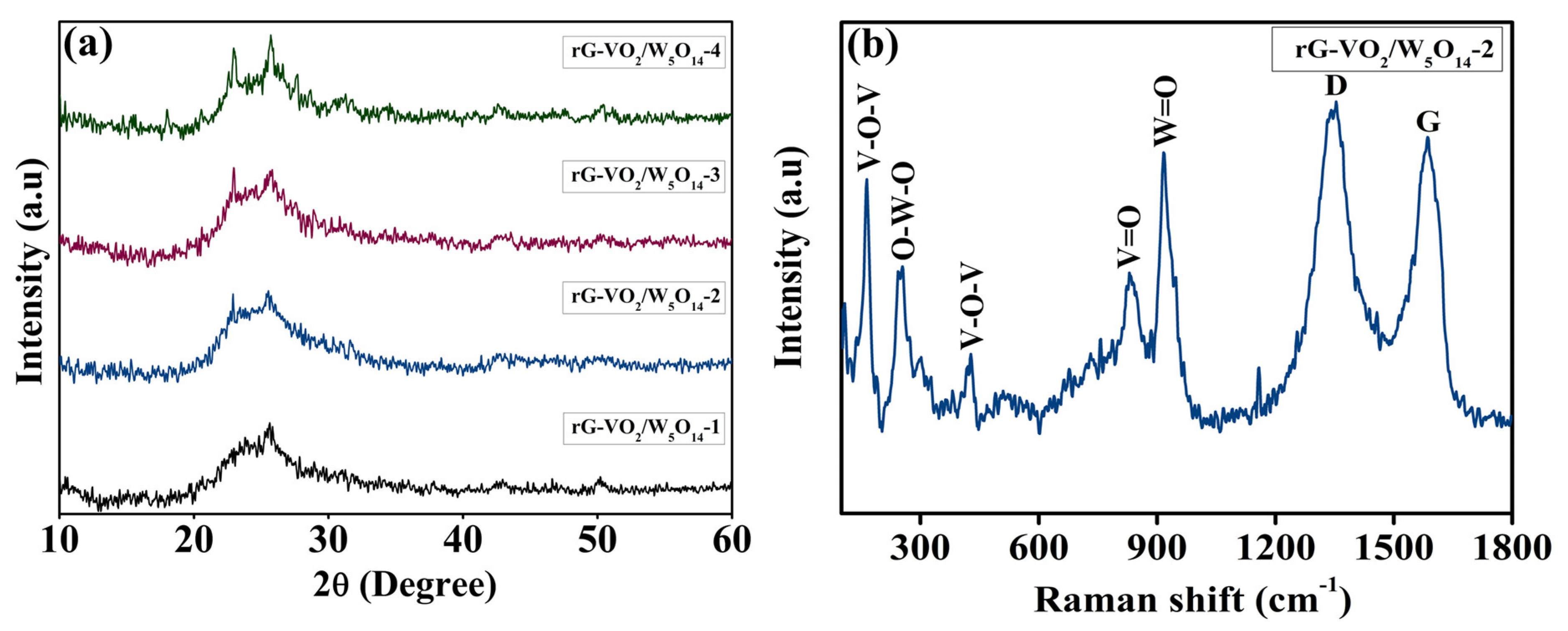
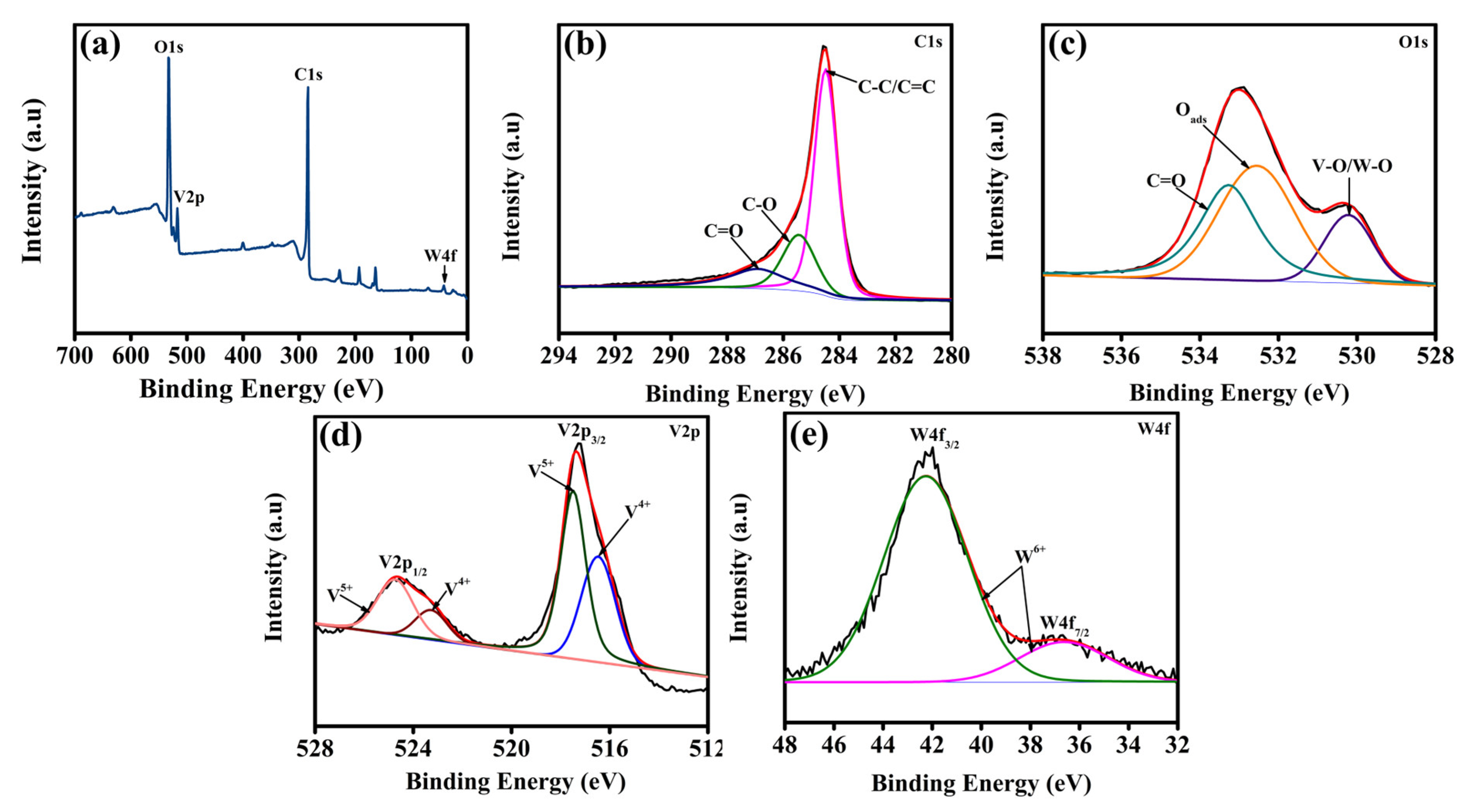
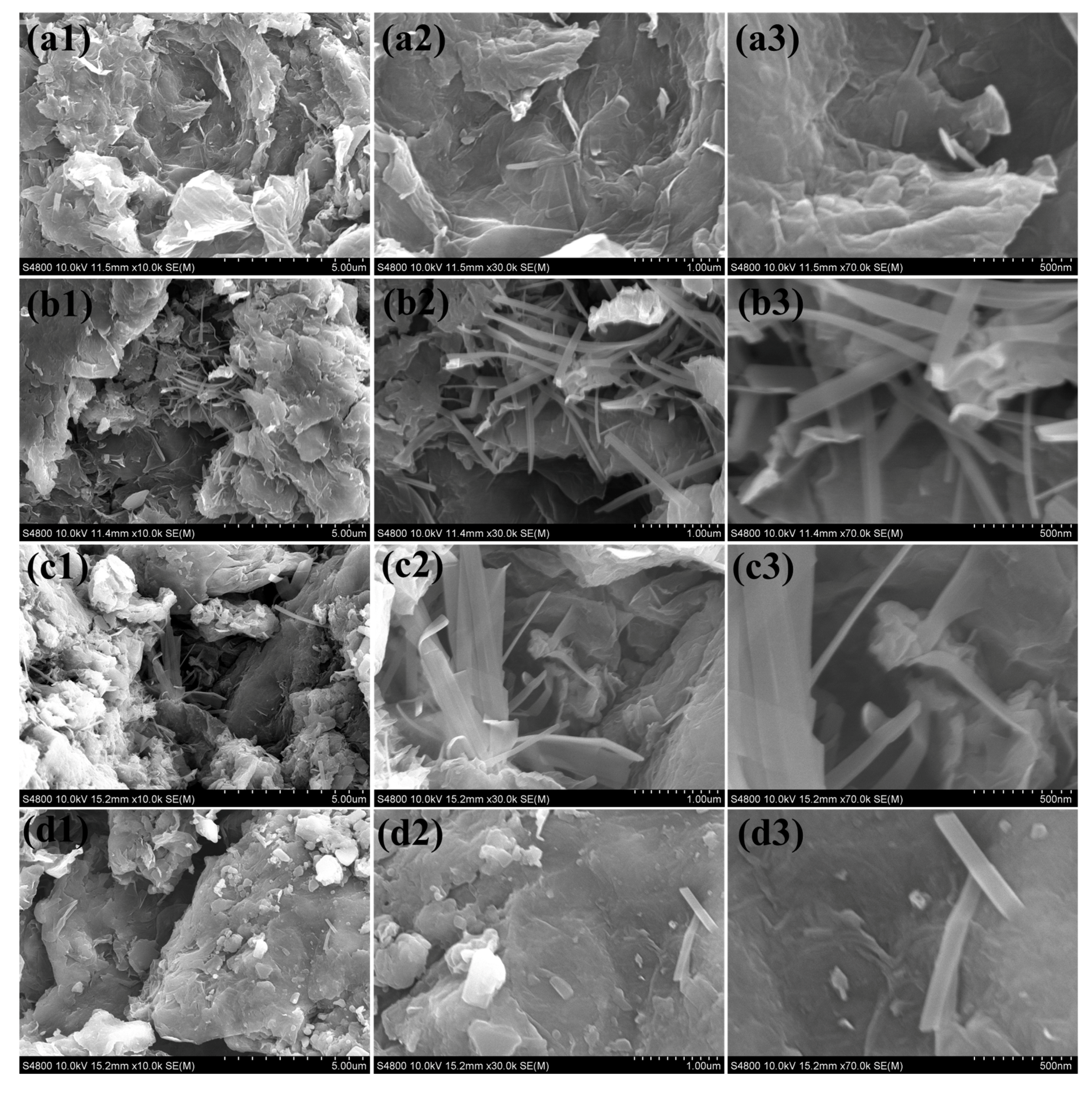
2. Result and Discussion
3. Conclusions
4. Experimental Section
4.1. Chemicals
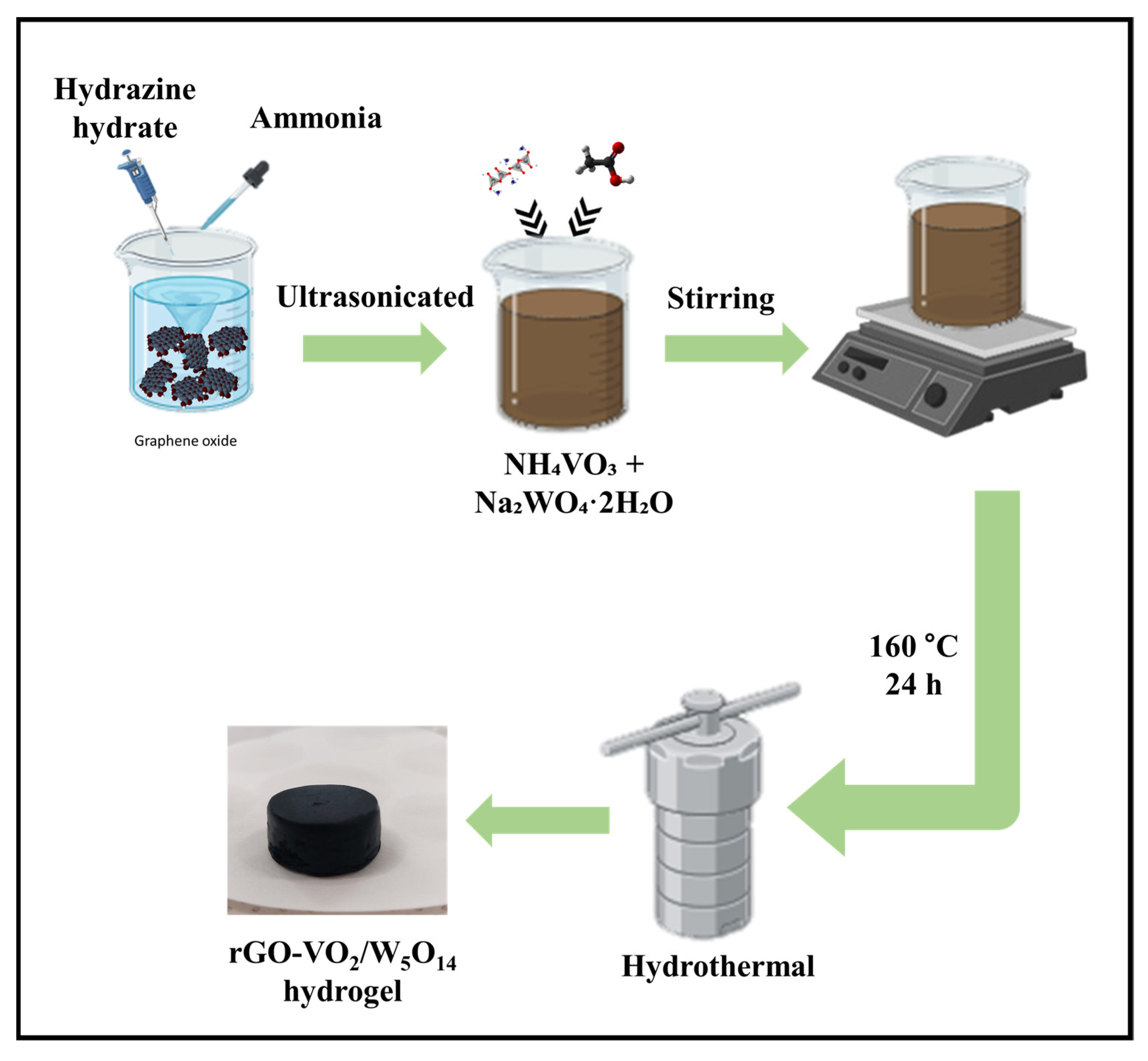
4.2. Preparation of rGO-VO2/W5O14 Composite
4.3. Material Characterization
4.4. Electrochemical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, N.; Wang, Q.; Zhu, J.; Zhou, N.; Chai, X.; Li, M.; Pei, Z.; Hu, K.; Huang, Z.; Chen, B. Interface engineering of Ni3S2 coupled NiFe-LDH heterostructure enables superior overall water splitting. Mater. Today Chem. 2025, 43, 102510. [Google Scholar] [CrossRef]
- Chen, B.; Kim, D.; Zhang, Z.; Lee, M.; Yong, K. MOF-derived NiCoZnP nanoclusters anchored on hierarchical N-doped carbon nanosheets array as bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 422, 130533. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, R.; Wu, X.; Ding, Y.; Zhang, X.; Liang, S.; Deng, C.; Qin, G.; Huang, Z.; Chen, B. One-spot autogenous formation of crystalline-amorphous Ni3S2/NiFeOxHy heterostructure nanosheets array for synergistically boosted oxygen evolution reaction. J. Power Sources 2023, 574, 233163. [Google Scholar] [CrossRef]
- Kang, L.; Li, J.; Wang, Y.; Gao, W.; Hao, P.; Lei, F.; Xie, J.; Tang, B. Dual-oxidation-induced lattice disordering in a Prussian blue analog for ultrastable oxygen evolution reaction performance. J. Colloid Interface Sci. 2023, 630, 257–265. [Google Scholar] [CrossRef]
- Cao, S.; Qi, J.; Lei, F.; Wei, Z.; Lou, S.; Yang, X.; Guo, Y.; Hao, P.; Xie, J.; Tang, B. Reduction-induced surface reconstruction to fabricate cobalt hydroxide/molybdenum oxide hybrid nanosheets for promoted oxygen evolution reaction. Chem. Eng. J. 2021, 413, 127540. [Google Scholar] [CrossRef]
- Yang, X.; Kang, L.; Wei, Z.; Lou, S.; Lei, F.; Hao, P.; Xie, J.; Tang, B. A self-sacrificial templated route to fabricate CuFe Prussian blue analogue/Cu(OH)2 nanoarray as an efficient pre-catalyst for ultrastable bifunctional electro-oxidation. Chem. Eng. J. 2021, 422, 130139. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Kang, L.; Li, J.; Wei, Z.; Lei, F.; Hao, P.; Tang, B. Molten-salt-protected pyrolytic approach for fabricating borate-modified cobalt–iron spinel oxide with robust oxygen-evolving performance. ACS Sustain. Chem. Eng. 2021, 9, 14596–14604. [Google Scholar] [CrossRef]
- Xie, J.; Yang, X.; Wang, Y.; Kang, L.; Li, J.; Wei, Z.; Hao, P.; Lei, F.; Wang, Q.; Tang, B. “Pit-dot” ultrathin nanosheets of hydrated copper pyrophosphate as efficient pre-catalysts for robust water oxidation. Chem. Commun. 2021, 57, 11517–11520. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lei, F.; Qi, J.; Cao, S.; Wei, Z.; Lou, S.; Hao, P.; Xie, J.; Tang, B. Molten-salt-protected pyrolysis for fabricating perovskite nanocrystals with promoted water oxidation behavior. ACS Sustain. Chem. Eng. 2020, 8, 16711–16719. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Zhang, H.; Zhang, J.; Li, S.; Wang, R.; Pan, B.; Xie, Y. Intralayered Ostwald ripening to ultrathin nanomesh catalyst with robust oxygen-evolving performance. Adv. Mater. 2017, 29, 1604765. [Google Scholar] [CrossRef]
- Sun, W.; Wei, Z.; Qi, J.; Kang, L.; Li, J.; Xie, J.; Tang, B.; Xie, Y. Rapid and scalable synthesis of Prussian blue analogue nanocubes for electrocatalytic water oxidation. Chin. J. Chem. 2021, 39, 2347–2353. [Google Scholar] [CrossRef]
- Xie, J.; Xin, J.; Wang, R.; Zhang, X.; Lei, F.; Qu, H.; Hao, P.; Cui, G.; Tang, B.; Xie, Y. Sub-3 nm pores in two-dimensional nanomesh promoting the generation of electroactive phase for robust water oxidation. Nano Energy 2018, 53, 74–82. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, S.; Wang, W.; Cheng, Y.; Li, Z.; He, C. Enhanced electrocatalytic performance of LCO-NiFe-C3N4 composite material for highly efficient overall water splitting. J. Colloid Interface Sci. 2025, 680, 787–796. [Google Scholar] [CrossRef]
- Li, M.; Ma, D.; Feng, X.; Zhi, C.; Jia, Y.; Zhang, J.; Zhang, Y.; Chen, Y.; Shi, L.; Shi, J.W. Design and Modification of Layered Double Hydroxides-Based Compounds in Electrocatalytic Water Splitting: A Review. Small 2025, 21, 2412576. [Google Scholar] [CrossRef]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpää, M.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci. Total Environ. 2024, 912, 169160. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Chen, F.-Y.; Li, B.; Yu, S.-W.; Finfrock, Y.Z.; Meira, D.M.; Yan, Q.-Q.; Zhu, P.; Chen, M.-X.; Song, T.-W. Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Nat. Mater. 2023, 22, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ye, B.; Ouyang, B.; Zhang, T.; Tang, T.; Qiu, Z.; Li, S.; Li, Y.; Chen, R.; Wen, W. Dual Doping of N and F on Co3O4 to Activate the Lattice Oxygen for Efficient and Robust Oxygen Evolution Reaction. Adv. Mater. 2025, 37, 2501381. [Google Scholar] [CrossRef]
- Qasim, M.; Atta, M.S.; Makasana, J.; Rekha, M.; Kumar, G.S.; Al-Anber, M.A.; Das, S.N.; Chaudhary, R.; Kumar, A.; Oza, A.D. Enhancement in performance of CuMnO2 anchored over rGO for water splitting. J. Phys. Chem. Solids 2025, 206, 112838. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Graphene-based electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 21401–21418. [Google Scholar] [CrossRef]
- Baby, N.; Thangarasu, S.; Murugan, N.; Kim, Y.A.; Oh, T.-H. MOF derived Fe3O4/NiO decorated rGO-BN for efficient electrochemical water splitting. Int. J. Hydrogen Energy 2025, 130, 127–138. [Google Scholar] [CrossRef]
- Han, G.-Q.; Liu, Y.-R.; Hu, W.-H.; Dong, B.; Li, X.; Shang, X.; Chai, Y.-M.; Liu, Y.-Q.; Liu, C.-G. Three dimensional nickel oxides/nickel structure by in situ electro-oxidation of nickel foam as robust electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2015, 359, 172–176. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Y.; Wang, H.; Li, J.; Wang, Y.; Du, X.; Liu, G. Transition metal induced metal oxide lattice strain for efficient and stable alkaline water splitting. Chem. Eng. J. 2025, 511, 161686. [Google Scholar] [CrossRef]
- Kao, Y.-T.; Young, C. Trimetallic oxide catalysts from metal-organic frameworks on Ti3C2Tx MXene for enhanced water splitting. Int. J. Hydrogen Energy 2025, 100, 704–712. [Google Scholar] [CrossRef]
- Fester, J.; Makoveev, A.; Grumelli, D.; Gutzler, R.; Sun, Z.; Rodríguez-Fernández, J.; Kern, K.; Lauritsen, J.V. The structure of the cobalt oxide/Au catalyst interface in electrochemical water splitting. Angew. Chem. 2018, 130, 12069–12073. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Xie, Y. Preferential microstructure design of two-dimensional electrocatalysts for boosted oxygen evolution reaction. ChemCatChem 2019, 11, 4662–4670. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Qi, J.; Zhang, J.; Zheng, X.; Zhang, X.; Lei, F.; Sun, X.; Tang, B.; Xie, J. Two-dimensional confined topotactic transformation to produce Co-Pi/Co3O4 hybrid porous nanosheets for promoted water oxidation. J. Colloid Interface Sci. 2025, 677, 406–416. [Google Scholar] [CrossRef]
- Cummins, D.R.; Martinez, U.; Sherehiy, A.; Kappera, R.; Martinez-Garcia, A.; Schulze, R.K.; Jasinski, J.; Zhang, J.; Gupta, R.K.; Lou, J. Efficient hydrogen evolution in transition metal dichalcogenides via a simple one-step hydrazine reaction. Nat. Commun. 2016, 7, 11857. [Google Scholar] [CrossRef]
- Shang, X.; Chi, J.-Q.; Liu, Z.-Z.; Dong, B.; Yan, K.-L.; Gao, W.-K.; Zeng, J.-B.; Chai, Y.-M.; Liu, C.-G. Ternary Ni-Fe-V sulfides bundles on nickel foam as free-standing hydrogen evolution electrodes in alkaline medium. Electrochim. Acta 2017, 256, 241–251. [Google Scholar] [CrossRef]
- Dong, B.; Zhao, X.; Han, G.-Q.; Li, X.; Shang, X.; Liu, Y.-R.; Hu, W.-H.; Chai, Y.-M.; Zhao, H.; Liu, C.-G. Two-step synthesis of binary Ni–Fe sulfides supported on nickel foam as highly efficient electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 13499–13508. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Wang, J.; Shao, M.; Chai, J.; Wang, S.; Yang, M.; Yang, Y.; Wang, N.; Wang, S. 3D heterostructured pure and N-Doped Ni3S2/VS2 nanosheets for high efficient overall water splitting. Electrochim. Acta 2018, 269, 55–61. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Q.; Wang, D.; Yang, X.; Sun, X. Self-supported nickel nitride as an efficient high-performance three-dimensional cathode for the alkaline hydrogen evolution reaction. Electrochim. Acta 2016, 191, 841–845. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Huang, Y.; Qiu, W.; Yang, H.; Ji, H.; Tong, Y. Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting. Mater. Today 2017, 20, 425–451. [Google Scholar] [CrossRef]
- Li, W.; Gao, X.; Wang, X.; Xiong, D.; Huang, P.-P.; Song, W.-G.; Bao, X.; Liu, L. From water reduction to oxidation: Janus Co-Ni-P nanowires as high-efficiency and ultrastable electrocatalysts for over 3000 h water splitting. J. Power Sources 2016, 330, 156–166. [Google Scholar] [CrossRef]
- Wan, H.; Li, L.; Chen, Y.; Gong, J.; Duan, M.; Liu, C.; Zhang, J.; Wang, H. One pot synthesis of Ni12P5 hollow nanocapsules as efficient electrode materials for oxygen evolution reactions and supercapacitor applications. Electrochim. Acta 2017, 229, 380–386. [Google Scholar] [CrossRef]
- Usmani, A.B.S.; Rana, S.; Arora, A.; Yadav, K.K.; Sammi, H.; Sardana, N.; Jha, M. Electrochemical oxygen generation from VO2 nanoflakes decorated onto graphite sheet. J. Alloys Compd. 2024, 976, 173058. [Google Scholar] [CrossRef]
- Karahan, O.; Tufani, A.; Unal, S.; Misirlioglu, I.B.; Menceloglu, Y.Z.; Sendur, K. Synthesis and morphological control of VO2 nanostructures via a one-step hydrothermal method. Nanomaterials 2021, 11, 752. [Google Scholar] [CrossRef]
- Goodenough, J.B. The two components of the crystallographic transition in VO2. J. Solid State Chem. 1971, 3, 490–500. [Google Scholar] [CrossRef]
- Sreekanth, T.; Tamilselvan, M.; Yoo, K.; Kim, J. Microwave-assisted in situ growth of VO2 nanoribbons on Ni foam as inexpensive bifunctional electrocatalysts for the methanol oxidation and oxygen evolution reactions. Appl. Surf. Sci. 2021, 570, 151119. [Google Scholar] [CrossRef]
- Cui, R.; Yu, S.; Han, P.; Wu, Y.; Li, Y.; Dang, Y.; Zhou, Y.; Zhu, J.-J. Novel vacancy-rich Co3O4/VO2 nanohybrids for enhanced electrocatalytic performance and application as oxygen evolution electrocatalysts. J. Alloys Compd. 2021, 876, 160129. [Google Scholar] [CrossRef]
- Gowrisankar, A.; Thangavelu, S. Effect of β-MnO2 on controlled polymorphism of VO2(x) (x= A, B, M polymorphs) microstructures anchored on two-dimensional reduced graphene oxide nanosheets for overall water splitting. J. Phys. Chem. C 2022, 126, 3419–3431. [Google Scholar] [CrossRef]
- Madhura, T.R.; Kumar, G.G.; Ramaraj, R. Reduced graphene oxide supported 2D-NiO nanosheets modified electrode for urea detection. J. Solid State Electrochem. 2020, 24, 3073–3081. [Google Scholar] [CrossRef]
- Kumar, N.; Upadhyay, S.; Chetana, S.; Joshi, N.C.; Priyadarshi, N.; Hossain, I.; Sen, A. Hydrothermal growth of 1D VO2 for oxygen evolution reaction. Mater. Lett. 2023, 335, 133810. [Google Scholar] [CrossRef]
- Saqib, M.; Jelenc, J.; Pirker, L.; Škapin, S.D.; De Pietro, L.; Ramsperger, U.; Knápek, A.; Müllerová, I.; Remškar, M. Field emission properties of single crystalline W5O14 and W18O49 nanowires. J. Electron. Spectrosc. Relat. Phenom. 2020, 241, 146837. [Google Scholar] [CrossRef]
- Le, T.K.; Kang, M.; Kim, S.W. Relation of photoluminescence and sunlight photocatalytic activities of pure V2O5 nanohollows and V2O5/RGO nanocomposites. Mater. Sci. Semicond. Process. 2019, 100, 159–166. [Google Scholar] [CrossRef]
- Bhosale, M.; Thangarasu, S.; Magdum, S.S.; Jeong, C.; Oh, T.-H. Enhancing the electrocatalytic performance of vanadium oxide by interface interaction with rGO and NiO nanostructures for electrochemical water oxidation. Int. J. Hydrogen Energy 2024, 54, 1449–1460. [Google Scholar] [CrossRef]
- Bhosale, M.; Thangarasu, S.; Murugan, N.; Kim, Y.A.; Oh, T.-H. Engineering 2D heterostructured VS2-rGO-Ni nanointerface to stimulate electrocatalytic water splitting and supercapacitor applications. J. Energy Storage 2023, 73, 109133. [Google Scholar] [CrossRef]
- Wu, X.; Tao, Y.; Dong, L.; Wang, Z.; Hu, Z. Preparation of VO2 nanowires and their electric characterization. Mater. Res. Bull. 2005, 40, 315–321. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, Q.; Zhang, W.; Qi, P.; Xiao, Q.; Yu, Y. Facile preparation of W5O14 nanosheet arrays with large crystal channels as high-performance negative electrode for supercapacitor. Electrochim. Acta 2020, 330, 135209. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, X.; Wang, H.-E. Enhanced pseudocapacitive lithium-ion storage in a coherent rGO/VO2-R heterojunction. Energy Fuels 2024, 38, 4689–4698. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.; Dai, P.; Tang, X.; Li, G.; Yang, L. RuNi single-atom alloy anchored on rGO as an outstanding bifunctional catalyst for efficient electrochemical water splitting. New J. Chem. 2024, 48, 3942–3951. [Google Scholar] [CrossRef]
- Zhou, W.; Li, X.; Li, X.; Shao, J.; Yang, H.; Chai, X.; Hu, Q.; He, C. Crafting amorphous VO2–crystalline NiS2 heterostructures as bifunctional electrocatalysts for efficient water splitting: The different cocatalytic function of VO2. Chem. Eng. J. 2023, 470, 144146. [Google Scholar] [CrossRef]
- Yu, W.; Shen, Z.; Peng, F.; Lu, Y.; Ge, M.; Fu, X.; Sun, Y.; Chen, X.; Dai, N. Improving gas sensing performance by oxygen vacancies in sub-stoichiometric WO3−x. RSC Adv. 2019, 9, 7723–7728. [Google Scholar] [CrossRef]
- Remškar, M.; Kovac, J.; Viršek, M.; Mrak, M.; Jesih, A.; Seabaugh, A. W5O14 Nanowires. Adv. Funct. Mater. 2007, 17, 1974–1978. [Google Scholar] [CrossRef]
- Cogal, G.C.; Karaca, G.Y.; Uygun, E.; Kuralay, F.; Oksuz, L.; Remskar, M.; Oksuz, A.U. RF plasma-enhanced conducting Polymer/W5O14 based self-propelled micromotors for miRNA detection. Anal. Chim. Acta 2020, 1138, 69–78. [Google Scholar] [CrossRef]
- Pan, U.N.; Singh, T.I.; Paudel, D.R.; Gudal, C.C.; Kim, N.H.; Lee, J.H. Covalent doping of Ni and P on 1T-enriched MoS2 bifunctional 2D-nanostructures with active basal planes and expanded interlayers boosts electrocatalytic water splitting. J. Mater. Chem. A 2020, 8, 19654–19664. [Google Scholar] [CrossRef]
- Tang, C.; Zhong, L.; Xiong, R.; Xiao, Y.; Cheng, B.; Lei, S. Regulable in-situ autoredox for anchoring synergistic Ni/NiO nanoparticles on reduced graphene oxide with boosted alkaline electrocatalytic oxygen evolution. J. Colloid Interface Sci. 2023, 648, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sui, S.; Zhai, Y. Preparation and characterization of Ir/TiC catalyst for oxygen evolution. J. Power Sources 2008, 177, 470–477. [Google Scholar] [CrossRef]
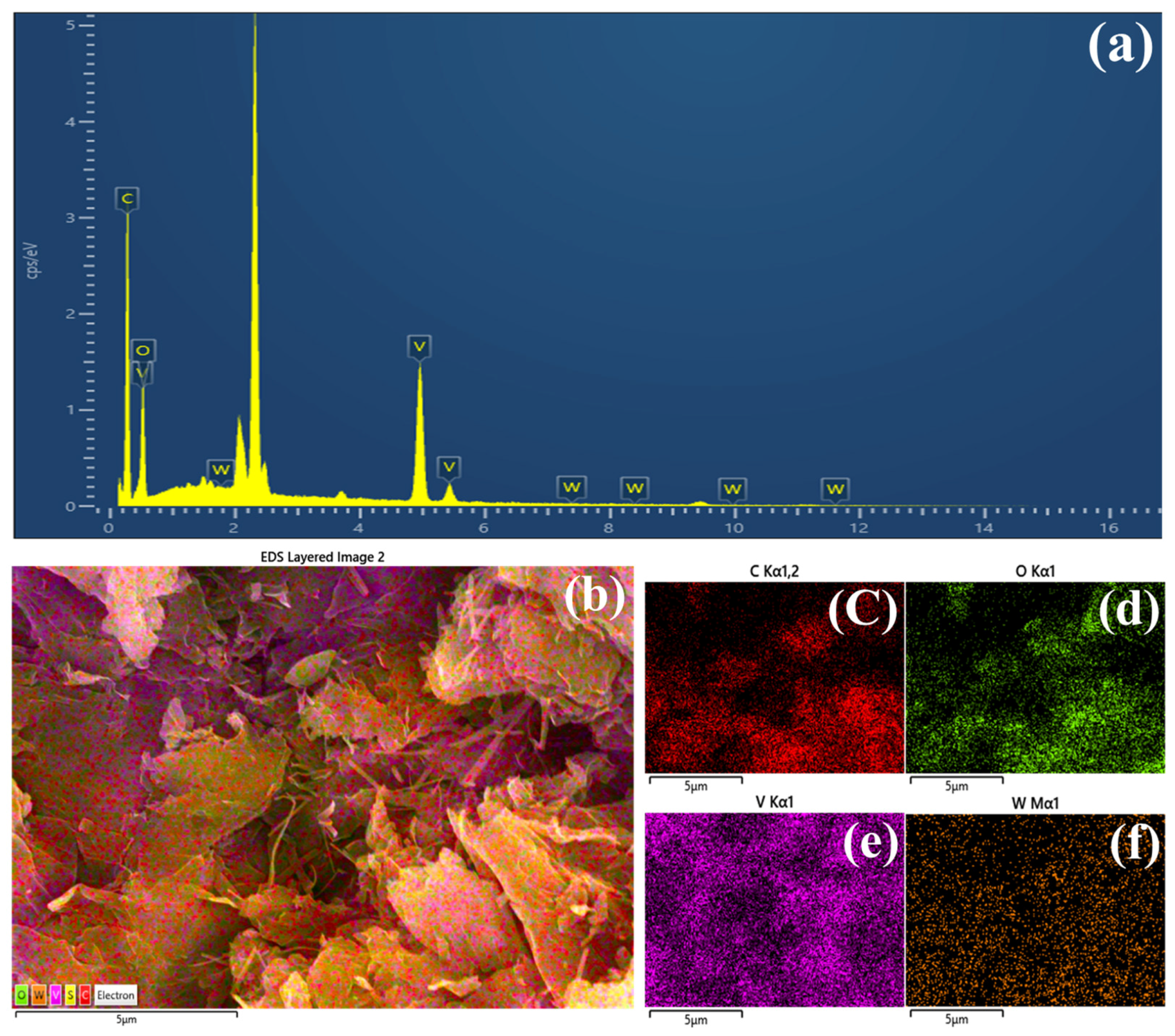

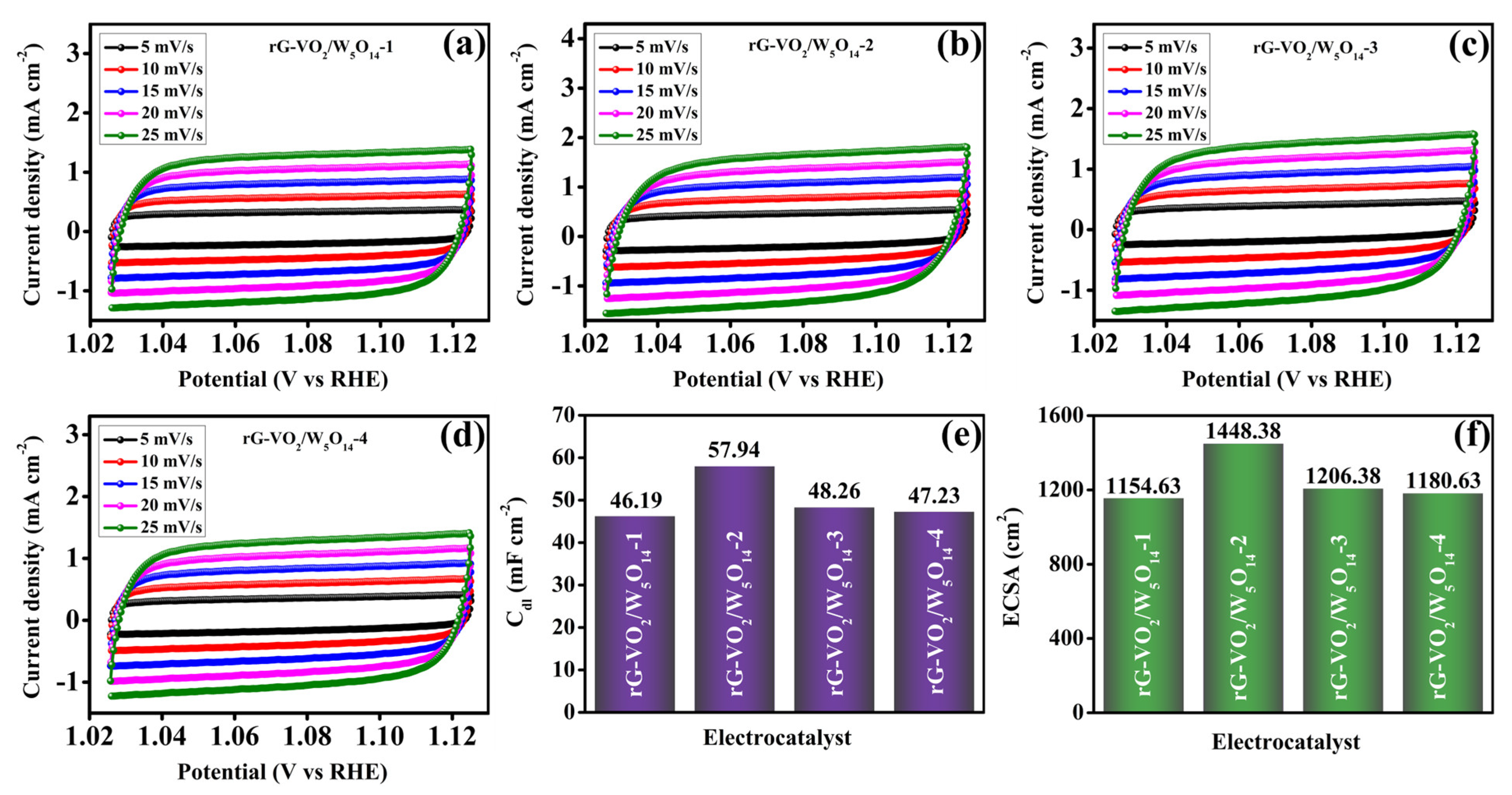
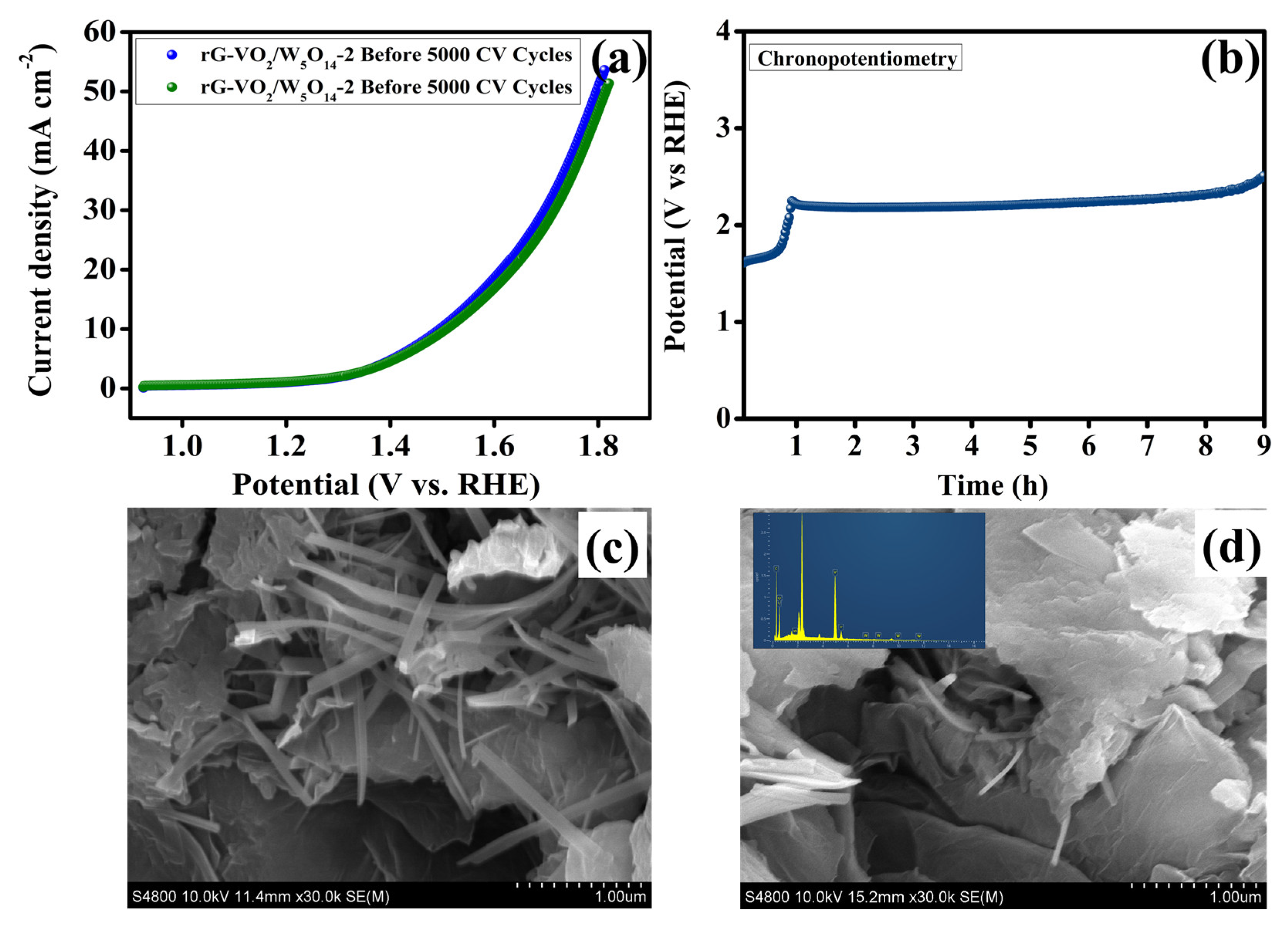
| Electrocatalysts | C (Wt%) | O (Wt%) | V (Wt%) | W (Wt%) |
|---|---|---|---|---|
| rG-VO2/W5O14-1 | 39.77 | 29.7 | 30.13 | 0.40 |
| rG-VO2/W5O14-2 | 38.35 | 28.97 | 31.80 | 0.88 |
| rG-VO2/W5O14-3 | 37.78 | 29.91 | 30.76 | 1.55 |
| rG-VO2/W5O14-4 | 38.15 | 30.79 | 28.26 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, M.; Amate, R.U.; Morankar, P.J.; Jeon, C.-W. Catalytic Interface of rGO-VO2/W5O14 Hydrogel for High-Performance Electrochemical Water Oxidation. Gels 2025, 11, 670. https://doi.org/10.3390/gels11080670
Bhosale M, Amate RU, Morankar PJ, Jeon C-W. Catalytic Interface of rGO-VO2/W5O14 Hydrogel for High-Performance Electrochemical Water Oxidation. Gels. 2025; 11(8):670. https://doi.org/10.3390/gels11080670
Chicago/Turabian StyleBhosale, Mrunal, Rutuja U. Amate, Pritam J. Morankar, and Chan-Wook Jeon. 2025. "Catalytic Interface of rGO-VO2/W5O14 Hydrogel for High-Performance Electrochemical Water Oxidation" Gels 11, no. 8: 670. https://doi.org/10.3390/gels11080670
APA StyleBhosale, M., Amate, R. U., Morankar, P. J., & Jeon, C.-W. (2025). Catalytic Interface of rGO-VO2/W5O14 Hydrogel for High-Performance Electrochemical Water Oxidation. Gels, 11(8), 670. https://doi.org/10.3390/gels11080670






