Constructive Neuroengineering of Axon Polarization Control Using Modifiable Agarose Gel Platforms for Neuronal Circuit Construction
Abstract
1. Introduction
2. Results and Discussion
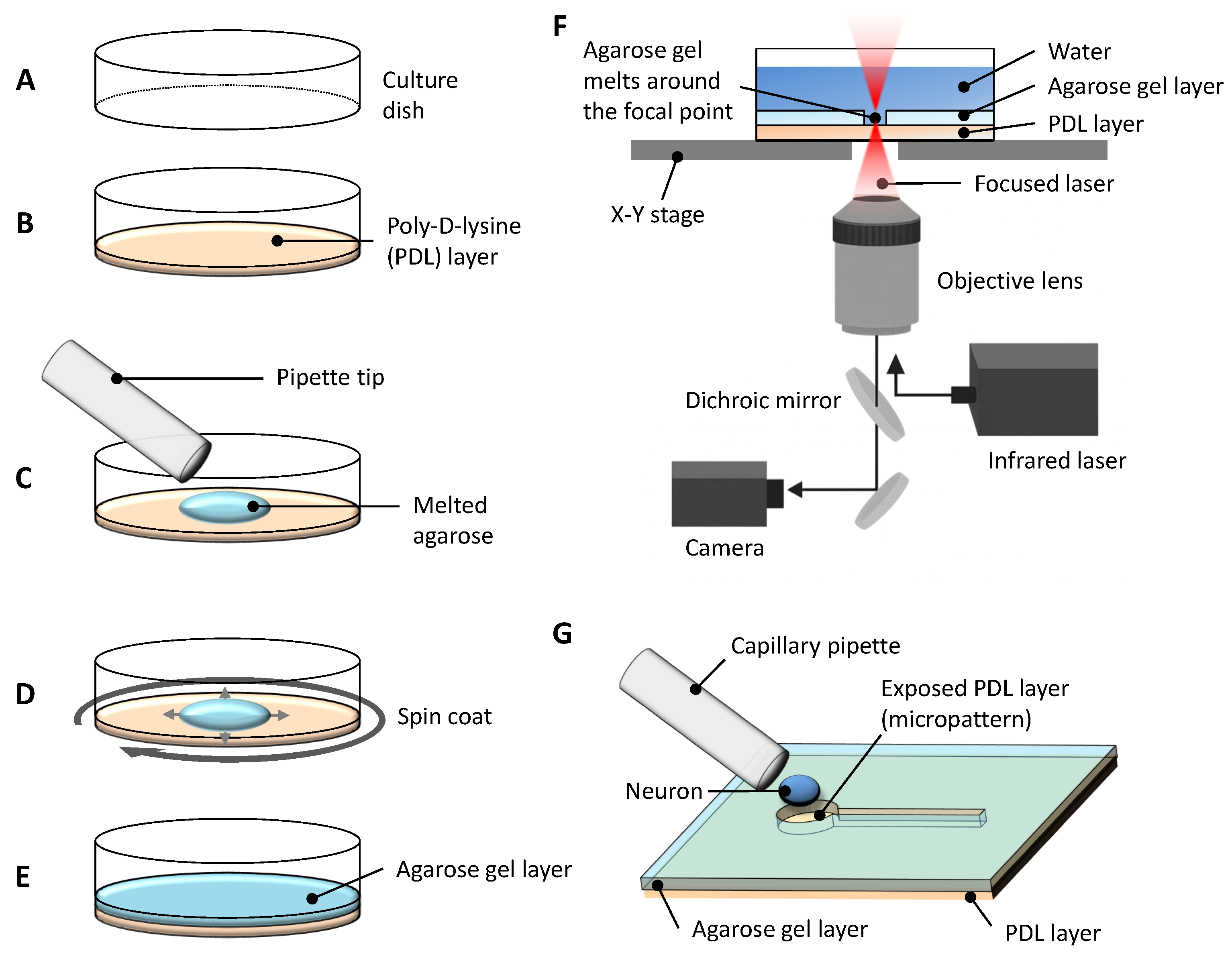
2.1. Constructive Approach for Stepwise Elongation of Neurites in Agarose Gel Microstructures
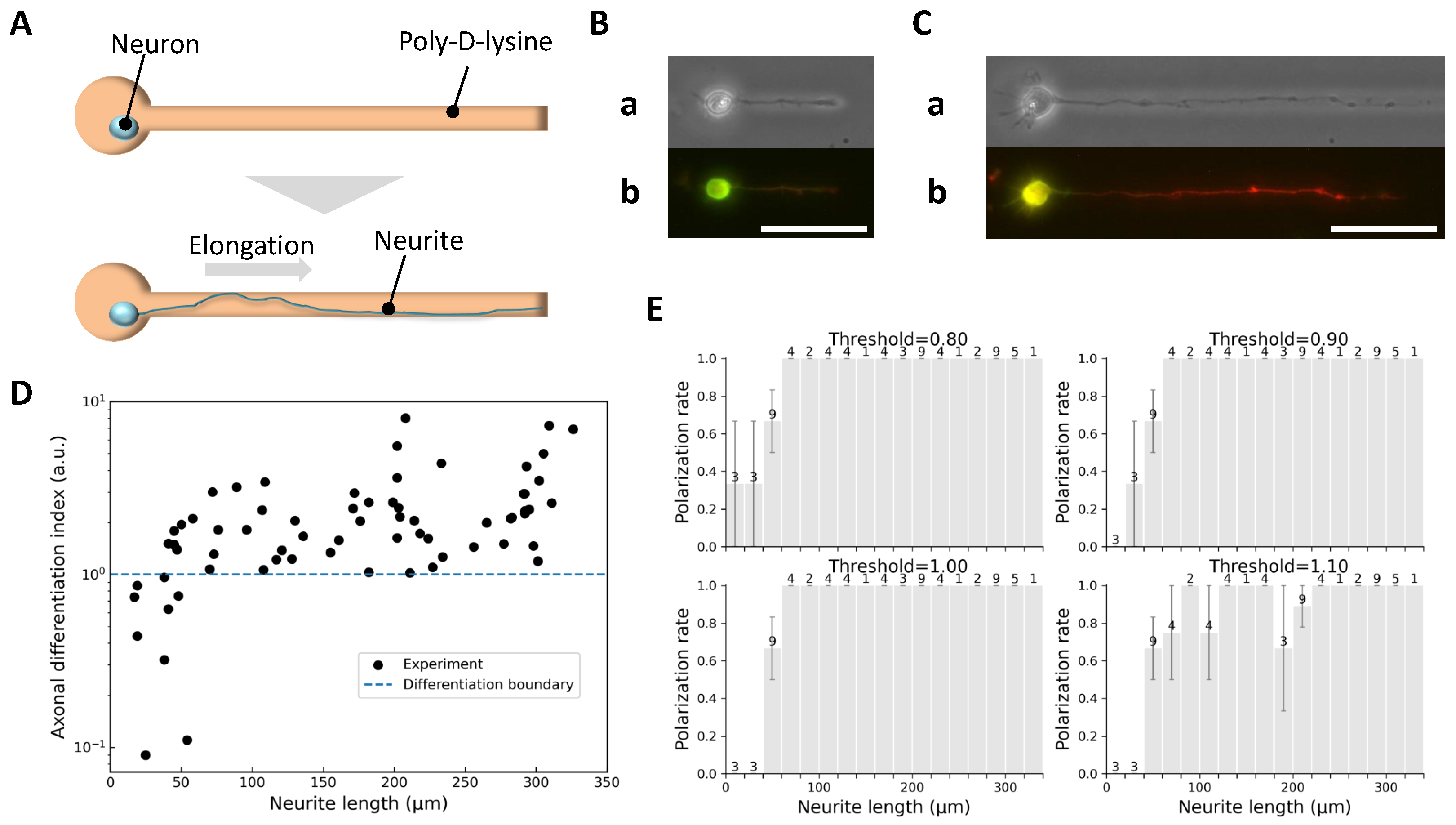
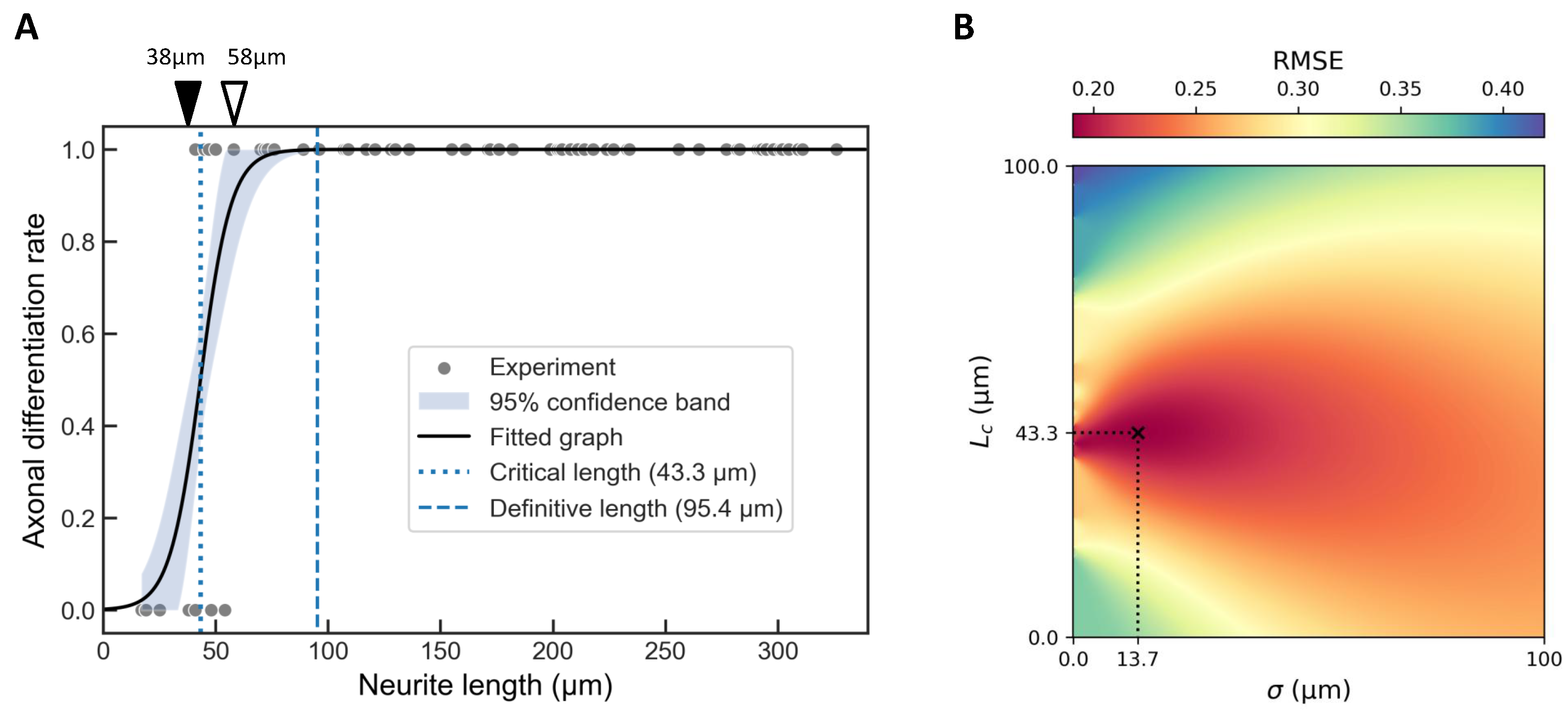
2.2. Critical Length for Axonal Differentiation in Single Neurite Elongation
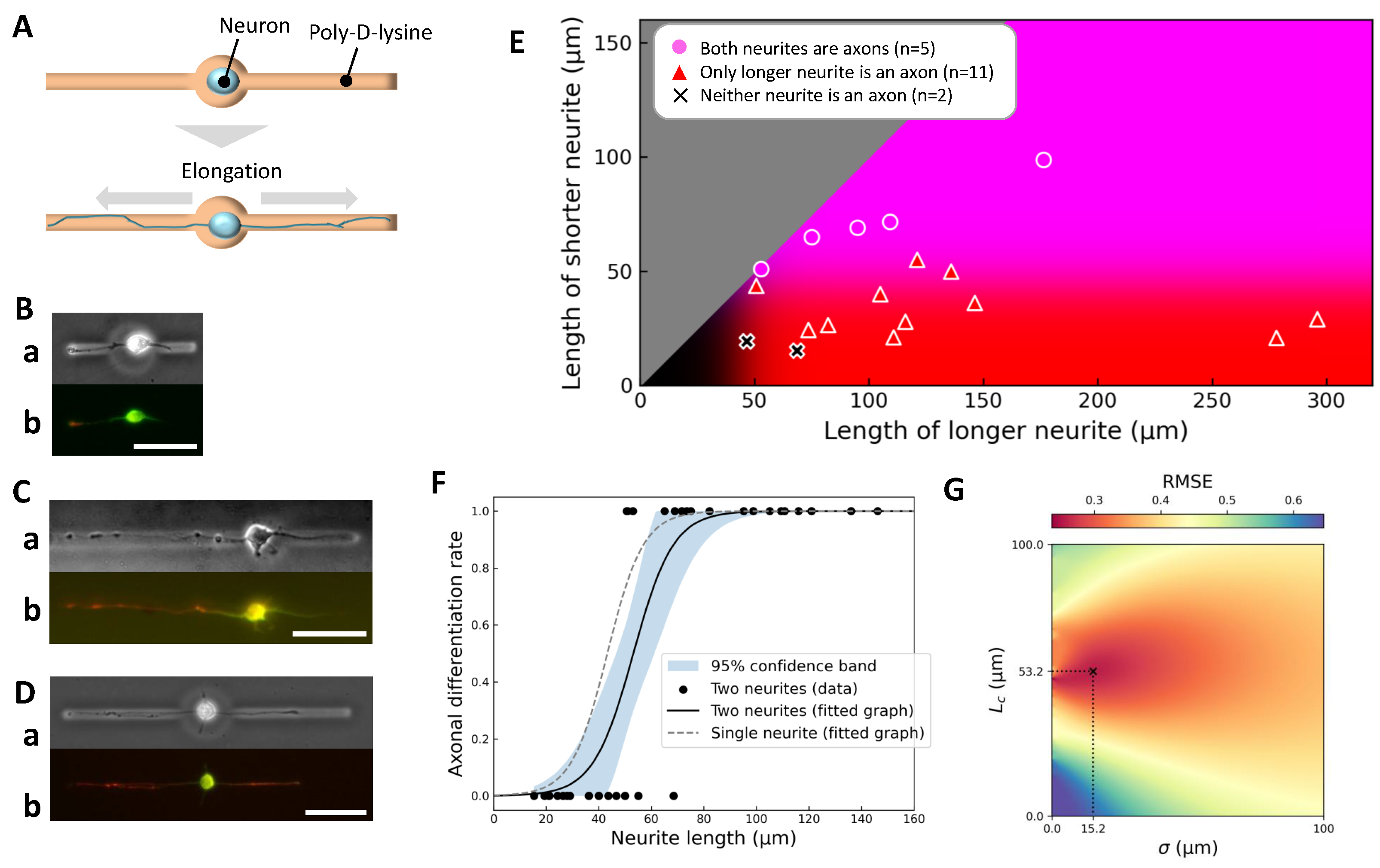
2.3. Effect of Competing Neurites on Axonal Differentiation Thresholds
2.4. Stepwise Control of Elongation Order and Its Effect on Axonal Fate
2.5. Advantages, Limitations, and Mechanistic Implications
3. Conclusions
4. Materials and Methods
4.1. Culture Dish Preparation
4.2. Photo-Thermal Microfabrication of Agarose Gel
4.3. Cell Cultivation
4.4. Cell Observation
4.5. Immunofluorescence Staining
4.6. Quantification and Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef]
- Khan, T.A.; Guo, A.; Martin, J.; Te Chien, C.; Liu, T.; Szczurkowska, J.; Shelly, M. Directed mechanisms for apical dendrite development during neuronal polarization. Dev. Biol. 2022, 490, 110–116. [Google Scholar] [CrossRef]
- Bradke, F.; Dotti, C.C. The role of local actin instability in axon formation. Science 1999, 283, 1931–1934. [Google Scholar] [CrossRef]
- Barnes, A.P.; Polleux, F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 2009, 32, 347–381. [Google Scholar] [CrossRef]
- Witte, H.; Neukirchen, D.; Bradke, F. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 2008, 180, 619–632. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Zhang, J.; Li, J.; Cheng, L.; Chen, L.; Cha, C.; Guo, G. Spastin Interacts with CRMP2 to Regulate Neurite Outgrowth by Controlling Microtubule Dynamics through Phosphorylation Modifications. CNS Neurol. Disord.-Drug Targets 2020, 20, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Deurloo, M.H.; Eide, S.; Turlova, E.; Li, Q.; Spijker, S.; Sun, H.S.; Groffen, A.J.; Feng, Z.P. Rasal1 regulates calcium dependent neuronal maturation by modifying microtubule dynamics. Cell Biosci. 2024, 14, 13. [Google Scholar] [CrossRef]
- Nishimura, T.; Kato, K.; Yamaguchi, T.; Fukata, Y.; Ohno, S.; Kaibuchi, K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 2004, 6, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wang, Q.J.; Hu, H.S.; Yu, P.C.; Zhu, J.; Drewes, G.; Piwnica-Worms, H.; Luo, Z.G. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc. Natl. Acad. Sci. USA 2006, 103, 8534–8539. [Google Scholar] [CrossRef]
- Xiao, Q.; Hu, X.; Wei, Z.; Tam, K.Y. Cytoskeleton molecular motors: Structures and their functions in neuron. Int. J. Biol. Sci. 2016, 12, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Tas, R.P.; Chazeau, A.; Cloin, B.M.; Lambers, M.L.; Hoogenraad, C.C.; Kapitein, L.C. Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport. Neuron 2017, 96, 1264–1271.e5. [Google Scholar] [CrossRef]
- Friesland, A.; Zhao, Y.; Chen, Y.H.; Wang, L.; Zhou, H.; Lu, Q. Small molecule targeting Cdc42-intersectin interaction disrupts Golgi organization and suppresses cell motility. Proc. Natl. Acad. Sci. USA 2013, 110, 1261–1266. [Google Scholar] [CrossRef]
- Takano, T.; Xu, C.; Funahashi, Y.; Namba, T.; Kaibuchi, K. Neuronal polarization. Development 2015, 142, 2088–2093. [Google Scholar] [CrossRef]
- De Anda, F.C.; Pollarolo, G.; Da Silva, J.S.; Camoletto, P.G.; Feiguin, F.; Dotti, C.G. Centrosome localization determines neuronal polarity. Nature 2005, 436, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Lindhout, F.W.; Portegies, S.; Kooistra, R.; Herstel, L.J.; Stucchi, R.; Hummel, J.J.A.; Scheefhals, N.; Katrukha, E.A.; Altelaar, M.; MacGillavry, H.D.; et al. Centrosome-mediated microtubule remodeling during axon formation in human iPSC-derived neurons. EMBO J. 2021, 40, e106798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Meka, D.P.; Scharrenberg, R.; König, T.; Schwanke, B.; Kobler, O.; Windhorst, S.; Kreutz, M.R.; Mikhaylova, M.; Calderon De Anda, F. Microtubules Modulate F-actin Dynamics during Neuronal Polarization. Sci. Rep. 2017, 7, 9583. [Google Scholar] [CrossRef] [PubMed]
- Higgs, V.E.; Das, R.M. Establishing neuronal polarity: Microtubule regulation during neurite initiation. Oxf. Open Neurosci. 2022, 1, kvac007. [Google Scholar] [CrossRef]
- Leterrier, C. The Axon Initial Segment: An Updated Viewpoint. J. Neurosci. 2018, 38, 2135. [Google Scholar] [CrossRef]
- Wilson, C.; Moyano, A.L.; Cáceres, A. Perspectives on Mechanisms Supporting Neuronal Polarity From Small Animals to Humans. Front. Cell Dev. Biol. 2022, 10, 878142. [Google Scholar] [CrossRef]
- Johnson, D.M.; Abi-Mansour, J.P.; Maurer, J.A. Spatial confinement instigates environmental determination of neuronal polarity. Integr. Biol. 2012, 4, 1034–1037. [Google Scholar] [CrossRef]
- Yamamoto, H.; Demura, T.; Morita, M.; Banker, G.A.; Tanii, T.; Nakamura, S. Differential neurite outgrowth is required for axon specification by cultured hippocampal neurons. J. Neurochem. 2012, 123, 904–910. [Google Scholar] [CrossRef]
- Dotti, C.G.; Banker, G.A. Experimentally induced alteration in the polarity of developing neurons. Nature 1987, 330, 254–256. [Google Scholar] [CrossRef]
- Lamoureux, P.; Ruthel, G.; Buxbaum, R.E.; Heidemann, S.R. Mechanical tension can specify axonal fate in hippocampal neurons. J. Cell Biol. 2002, 159, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Moriguhi, H.; Ishiwata, S.; Yasuda, K. A 1480/1064 nm dual wavelength photo-thermal etching system for non-contact three-dimensional microstructure generation into agar microculture chip. Sens. Actuators B Chem. 2004, 100, 455–462. [Google Scholar] [CrossRef]
- Suzuki, I.; Sugio, Y.; Jimbo, Y.; Yasuda, K. Stepwise pattern modification of neuronal network in photo-thermally-etched agarose architecture on multi-electrode array chip for individual-cell-based electrophysiological measurement. Lab Chip 2005, 5, 241–247. [Google Scholar] [CrossRef]
- Tanaka, Y.; Watanabe, H.; Shimoda, K.; Sakamoto, K.; Hondo, Y.; Sentoku, M.; Sekine, R.; Kikuchi, T.; Yasuda, K. Stepwise neuronal network pattern formation in agarose gel during cultivation using non-destructive microneedle photothermal microfabrication. Sci. Rep. 2021, 11, 14656. [Google Scholar] [CrossRef]
- Takada, N.; Hagiwara, S.; Abe, N.; Yamazaki, R.; Tsuneishi, K.; Yasuda, K. Open-End Control of Neurite Outgrowth Lengths with Steep Bending Confinement Microchannel Patterns for Miswiring-Free Neuronal Network Formation. Micromachines 2024, 15, 1374. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Tsuneishi, K.; Takada, N.; Yasuda, K. Constructive Neuroengineering of Crossing Multi-Neurite Wiring Using Modifiable Agarose Gel Platforms. Gels 2025, 11, 419. [Google Scholar] [CrossRef]
- Tomba, C.; Braïni, C.; Wu, B.; Gov, N.S.; Villard, C. Tuning the adhesive geometry of neurons: Length and polarity control. Soft Matter 2014, 10, 2381–2387. [Google Scholar] [CrossRef]
- Thome, C.; Kelly, T.; Yanez, A.; Schultz, C.; Engelhardt, M.; Cambridge, S.B.; Both, M.; Draguhn, A.; Beck, H.; Egorov, A.V. Axon-carrying dendrites convey privileged synaptic input in hippocampal neurons. Neuron 2014, 83, 1418–1430. [Google Scholar] [CrossRef]
- Toriyama, M.; Sakumura, Y.; Shimada, T.; Ishii, S.; Inagaki, N. A diffusion-based neurite length-sensing mechanism involved in neuronal symmetry breaking. Mol. Syst. Biol. 2010, 6, 394. [Google Scholar] [CrossRef]
- Namba, T.; Kibe, Y.; Funahashi, Y.; Nakamuta, S.; Takano, T.; Ueno, T.; Shimada, A.; Kozawa, S.; Okamoto, M.; Shimoda, Y.; et al. Pioneering axons regulate neuronal polarization in the developing cerebral cortex. Neuron 2014, 81, 814–829. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, Y.; Namba, T.; Nakamuta, S.; Kaibuchi, K. Neuronal polarization in vivo: Growing in a complex environment. Curr. Opin. Neurobiol. 2014, 27, 215–223. [Google Scholar] [CrossRef] [PubMed]
- May-Simera, H.; Liu, C. Neuronal Polarity and Neurological Disorders. J. Neurol. Transl. Neurosci. 2013, 2, 1026. [Google Scholar]
- Sasakit, T.; Matsuki, N.; Ikegaya, Y. Targeted axon-attached recording with fluorescent patch-clamp pipettes in brain slices. Nat. Protoc. 2012, 7, 1228–1234. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; Volume 1, pp. 56–61. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagiwara, S.; Tsuneishi, K.; Takada, N.; Yasuda, K. Constructive Neuroengineering of Axon Polarization Control Using Modifiable Agarose Gel Platforms for Neuronal Circuit Construction. Gels 2025, 11, 668. https://doi.org/10.3390/gels11080668
Hagiwara S, Tsuneishi K, Takada N, Yasuda K. Constructive Neuroengineering of Axon Polarization Control Using Modifiable Agarose Gel Platforms for Neuronal Circuit Construction. Gels. 2025; 11(8):668. https://doi.org/10.3390/gels11080668
Chicago/Turabian StyleHagiwara, Soya, Kazuhiro Tsuneishi, Naoya Takada, and Kenji Yasuda. 2025. "Constructive Neuroengineering of Axon Polarization Control Using Modifiable Agarose Gel Platforms for Neuronal Circuit Construction" Gels 11, no. 8: 668. https://doi.org/10.3390/gels11080668
APA StyleHagiwara, S., Tsuneishi, K., Takada, N., & Yasuda, K. (2025). Constructive Neuroengineering of Axon Polarization Control Using Modifiable Agarose Gel Platforms for Neuronal Circuit Construction. Gels, 11(8), 668. https://doi.org/10.3390/gels11080668






