Preparation of Uniform-Pore Ceramics from Highly Stable Emulsions via the Sol–Gel Method
Abstract
1. Introduction
- (a)
- the ease with which the template can be incorporated into the continuous phase by simple agitation or mixing,
- (b)
- the ability to achieve very small droplet/pore sizes when using immiscible liquids with low interfacial tension, and
- (c)
- the mild conditions required for template removal [8].
2. Results and Discussion
2.1. Preliminary Study of Emulsion Formation and Kinetic Stability
2.2. Thermodynamic Size Distribution of Droplets Plotted as a Function of Time
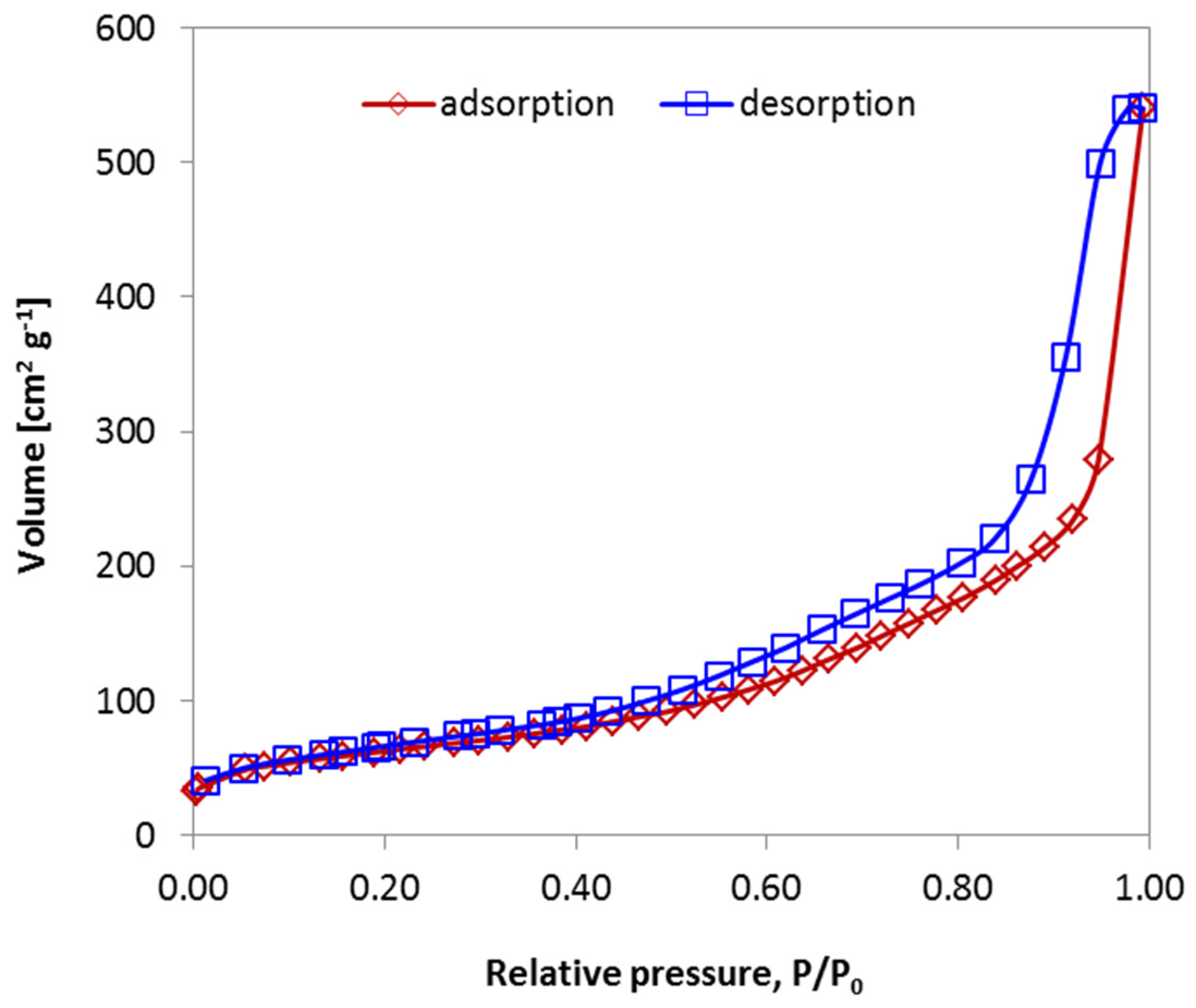
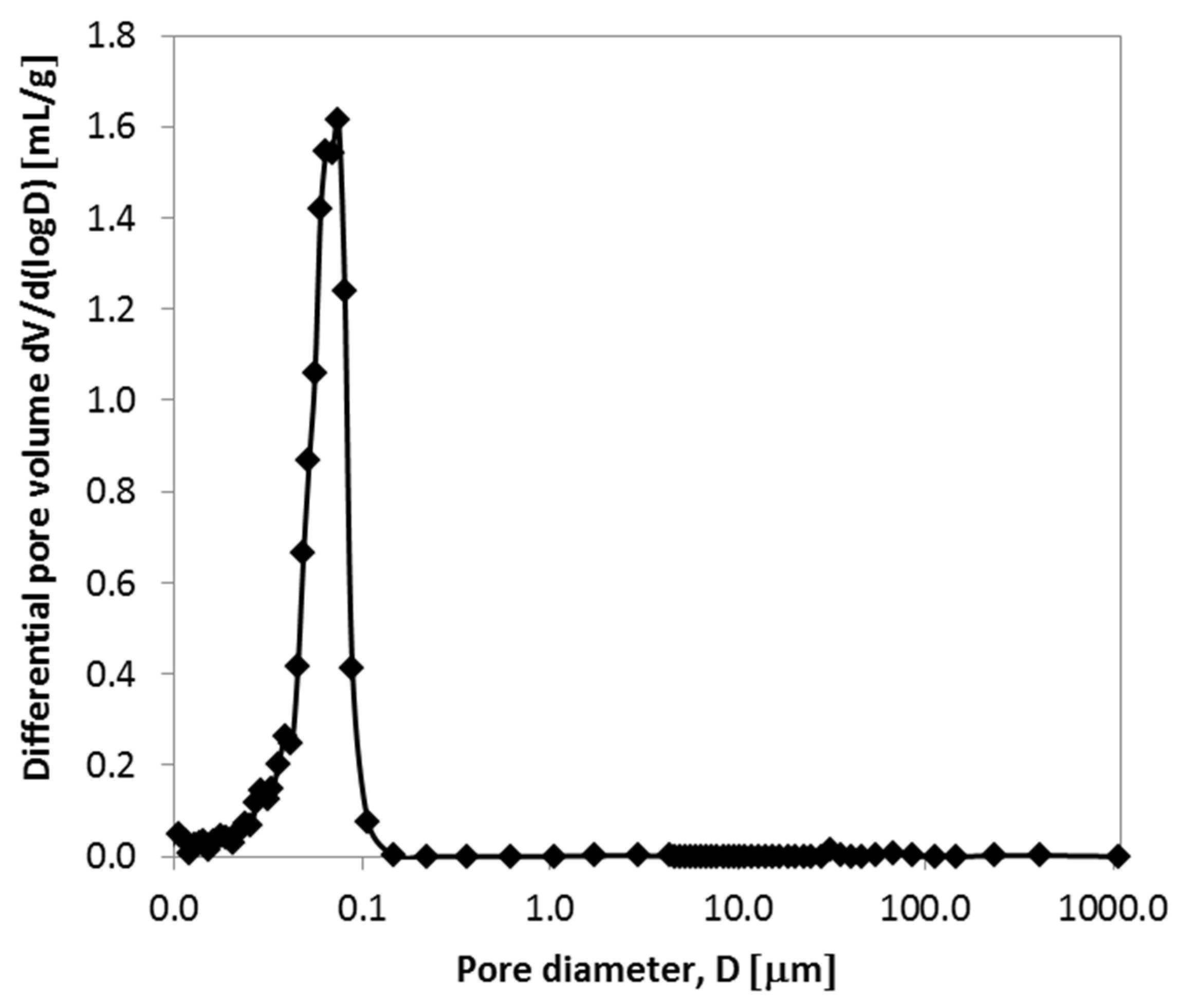
2.3. Porous Characteristics of Alumina Prepared from Stable Emulsions via the Sol–Gel Technique
- A triplet of small, overlapping endothermic peaks at 112 °C, 134 °C, and 160 °C corresponds to the desorption of water, gel dehydration, phase transitions, and the melting of the secondarily formed product (NH4NO3). These peaks appear superimposed on a broad endothermic background that begins at approximately 61 °C, attributed to the evaporation of residual hexanol. This process is accompanied by a mass loss of approximately 19%;
- The gaseous decomposition products of CTAB and NH4NO3 react violently (explosively) in the presence of atmospheric oxygen, producing a sharp exothermic peak at 324 °C. The TG curve indicates a weight loss of 6%, which is attributed to the combustion of carbon soot. The gaseous reaction products include water vapor, CO2, N2, and Br2;
- The rigid residue consists of partially hydrated Al2O3 in the form of AlOOH, as evidenced by a broad exothermic peak beginning at approximately 374 °C. This peak corresponds to the phase transition to η-Al2O3 [31] and is accompanied by a weight loss of about 5%;
- The transformation of η-Al2O3 to θ-Al2O3 is marked by a small exothermic peak with a maximum near 850 °C.
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Uniformly Porous Al2O3 via the Sol–Gel Processing of Stable Emulsions
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| CTAB | hexadecyltrimethylammonium bromide |
References
- Ren, Y.; Ma, Z.; Morris, R.E.; Liu, Z.; Jiao, F.; Dai, S.; Bruce, P.G. A solid with a hierarchical tetramodal micro-meso-macro pore size distribution. Nat. Commun. 2013, 4, 2015. [Google Scholar] [CrossRef]
- Burg, A.; Yadav, K.K.; Meyerstein, D.; Kornweitz, H.; Shamir, D.; Albo, Y. Effect of Sol–Gel Silica Matrices on the Chemical Properties of Adsorbed/Entrapped Compounds. Gels 2024, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Romanczuk-Ruszuk, E.; Sztorch, B.; Oksiuta, Z.; Przekop, R.E. Metallic Strontium as a Precursor of the Al2O3/SrCO3 Xerogels Obtained by the One-Pot Sol–Gel Method. Gels 2022, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, H.; Liu, Y.; Liu, X.; Zhao, Y.; Zhou, X.; Kang, W.; Zhuang, X.; Cheng, B. Facile construction of hierarchical porous ultrafine alumina fibers (HPAFs) and its application for dye adsorption. Microporous Mesoporous Mater. 2020, 308, 110544. [Google Scholar] [CrossRef]
- Liu, J.; Huo, W.; Ren, B.; Gan, K.; Lu, Y.; Zhang, X.; Tang, X.; Yang, J. A novel approach to fabricate porous alumina ceramics with excellent properties via pore-forming agent combined with sol impregnation technique. Ceram. Int. 2018, 44, 16751–16757. [Google Scholar] [CrossRef]
- Mei, J.; Shao, Y.; Lu, S.; Ma, Y.; Ren, L. Synthesis of Al2O3 with tunable pore size for efficient formaldehyde oxidation degradation performance. J. Mater. Sci. 2018, 53, 3375–3387. [Google Scholar] [CrossRef]
- Pana, D.; Chena, W.; Huang, X.; Zhang, J.; Yang, Y. Solvothermal-assisted evaporation-induced self-assembly of ordered mesoporous alumina with improved performance. J. Colloid Interface Sci. 2018, 529, 432–443. [Google Scholar] [CrossRef]
- Hammel, E.C.; Ighodaro, O.L.-R.; Okoli, O.I. Processing and properties of advanced porous ceramics: An application-based review. Ceram. Int. 2014, 40, 15351–15370. [Google Scholar] [CrossRef]
- Vijayan, S.; Narasimman, R.; Prabhakaran, K. Freeze gelcasting of hydrogenated vegetable oil-in-aqueous alumina slurry emulsions for the preparation of macroporous ceramics. J. Eur. Ceram. Soc. 2014, 34, 4347–4354. [Google Scholar] [CrossRef]
- Petrolini, D.D.; Urquieta-González, E.A.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. Emulsion-mediated synthesis of hierarchical mesoporous-macroporous Al-Mg hydrotalcites. Microporous Mesoporous Mater. 2017, 240, 149–158. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, A.; Wang, X.; Gao, P.; Wang, X.; Zhang, T. Synthesis, characterization and catalytic applications of mesoporous γ-alumina from boehmite sol. Microporous Mesoporous Mater. 2008, 111, 323–333. [Google Scholar] [CrossRef]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 864943. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Alves Rosa, M.A.; Pulcinelli, S.H.; Santilli, C.V. Preparation of hierarchically structured porous aluminas by a dual soft template method. Microporous Mesoporous Mater. 2010, 132, 268–275. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.-H.; Guan, W.-M.; Fu, M.-J.; Liu, L.-J. Emulsion-templated high porosity mullite ceramics with sericite induced textured structures. Mater. Des. 2016, 89, 1041–1047. [Google Scholar] [CrossRef]
- Vinogradov, V.V.; Vinogradov, A.V.; Kraev, A.S.; Agafonov, A.V.; Kessler, V.G. Sol–gel synthesis, characterization and catalytic activity of γ-alumina with bimodal mesopore distribution. J. Sol-Gel Sci. Technol. 2013, 68, 155–161. [Google Scholar] [CrossRef]
- Cesconeto, F.R.; Frade, J.R. Cellular ceramics by slip casting of emulsified suspensions. J. Eur. Ceram. Soc. 2020, 40, 4949–4954. [Google Scholar] [CrossRef]
- Tang, Y.; Mao, M.; Qiu, S.; Zhao, K. Fabrication of porous ceramics with double-pore structure by stepwise freeze casting using water/diphenyl methane emulsion. Ceram. Int. 2018, 44, 1187–1192. [Google Scholar] [CrossRef]
- Vijayan, S.; Narasimman, R.; Prabhakaran, K. Freeze gelcasting of naphthalene-in-aqueous alumina slurry emulsions for the preparation of macroporous alumina ceramics. Ceram. Int. 2015, 41, 1487–1494. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Yao, J. Recent advances in the direct fabrication of millimeter-sized hierarchical porous materials. RSC Adv. 2016, 84, 80840–80846. [Google Scholar] [CrossRef]
- Kuibao Zhang, K.; Fu, Z.; Nakayama, T.; Suzuki, T.; Suematsu, H.; Niihara, K. One-pot synthesis of hierarchically macro/mesoporous Al2O3 monoliths from a facile sol–gel process. Mater. Res. Bull. 2011, 46, 2155–2162. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Zdarta, J.; Paukszta, D.; Jesionowski, T. The influence of addition of a catalyst and chelating agent on the properties of titanium dioxide synthesized via the sol–gel method. J. Sol-Gel Sci. Technol. 2015, 75, 264–278. [Google Scholar] [CrossRef]
- Passos, A.R.; Pulcinelli, S.H.; Briois, V.; Santilli, C.V. High surface area hierarchical porous Al2O3 prepared by the integration of sol-gel transition and phase separation. RSC Adv. 2016, 6, 7217–7226. [Google Scholar] [CrossRef]
- Imhof, A.; Pine, D.J. Ordered macroporous materials by emulsion templating. Nature 1977, 389, 948–951. [Google Scholar] [CrossRef]
- Ghosh, S.; Bose, P.; Basak, S.; Naskar, M.K. Solvothermal-assisted evaporation-induced self-assembly process for significant improvement in the textural properties of γ-Al2O3, and study dye adsorption efficiency. J. Asian Ceram. Soc. 2015, 3, 198–205. [Google Scholar] [CrossRef]
- Jia, C.; Batterman, S. A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. Int. J. Environ. Res. Public Health 2010, 7, 2903–2939. [Google Scholar] [CrossRef]
- Filley, C.M.; Halliday, W.; Kleinschmidt-DeMasters, B.K. The effects of toluene on the central nervous system. J. Neuropathol. Exp. Neurol. 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, K.; Ádám, P.; Sinkó, K. Chemical tailoring of porous aluminum oxide xerogels. J. Non-Cryst. Solids 2018, 499, 394–400. [Google Scholar] [CrossRef]
- Giacobello, F.; Mollica-Nardo, V.; Foti, C.; Ponterio, R.C.; Saija, F.; Trusso, S.; Sponer, J.; Cassone, G.; Giuffrè, O. Hydrolysis of Al3+ in Aqueous Solutions: Experiments and Ab Initio Simulations. Liquids 2022, 2, 26–38. [Google Scholar] [CrossRef]
- Gunawan, R.; Zhang, D. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. J. Hazard. Mater. 2009, 165, 751–758. [Google Scholar] [CrossRef]
- Vahdat Vasei, H.; Masoudpanah, S.M.; Adeli, M.; Aboutalebi, M.R. Solution combustion synthesis of ZnO powders using CTAB as fuel. Ceram. Int. 2018, 44, 7741–7745. [Google Scholar] [CrossRef]
- Fedoročková, A.; Sučik, G.; Plešingerová, B.; Popovič, Ľ.; Kovaľaková, M.; Vavra, M. Simplified waste-free process for synthesis of nanoporous compact alumina under technologically advantageous conditions. RSC Adv. 2020, 10, 32423–32435. Available online: https://pubs.rsc.org/en/content/articlehtml/2020/ra/d0ra06544g (accessed on 4 August 2025).
- Baronskiy, M.G.; Tsybulya, S.V.; Snytnikov, V.N. Structural properties investigation of different alumina polymorphs (η-, γ-, χ-, θ-, α-Al2O3) using Cr3+ as a luminescent probe. J. Lumin. 2022, 242, 118554. [Google Scholar] [CrossRef]
- Gholizadeh, Z.; Aliannezhadi, M.; Ghominejad, M.; Tehrani, F.S. Novel boehmite and η-alumina nanostructures synthesized using a green ultrasonic-assisted hydrothermal method by clove extract for water treatment. J. Water Process Eng. 2024, 65, 105786. [Google Scholar] [CrossRef]
- Ludescher, L.; Morak, R.; Braxmeier, S.; Putz, F.; Hüsing, N.; Reichenauer, G.; Paris, O. Hierarchically organized materials with ordered mesopores: Adsorption isotherm and adsorption-induced deformation from small-angle scattering. Phys. Chem. Chem. Phys. 2020, 22, 12713. [Google Scholar] [CrossRef]
- Gu, D.; Schüth, F. Synthesis of non-siliceous mesoporous oxides. Chem. Soc. Rev. 2014, 43, 313–344. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-, BET- and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
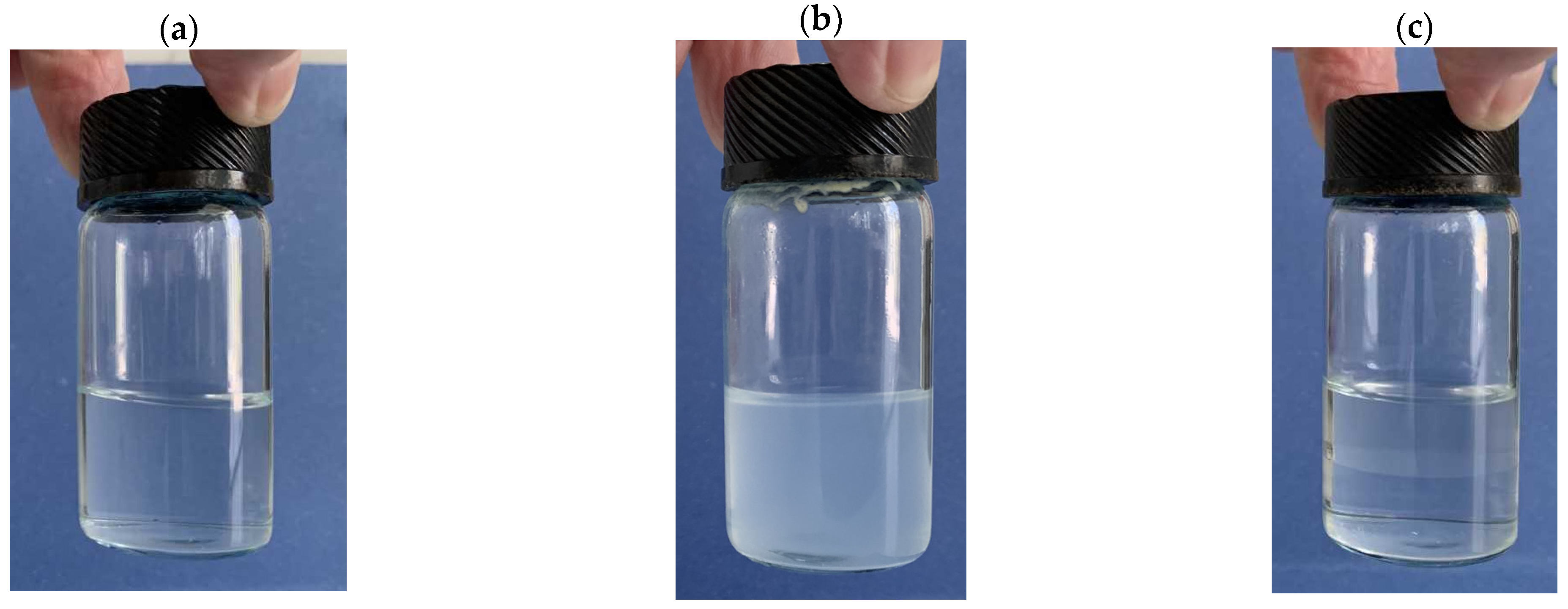
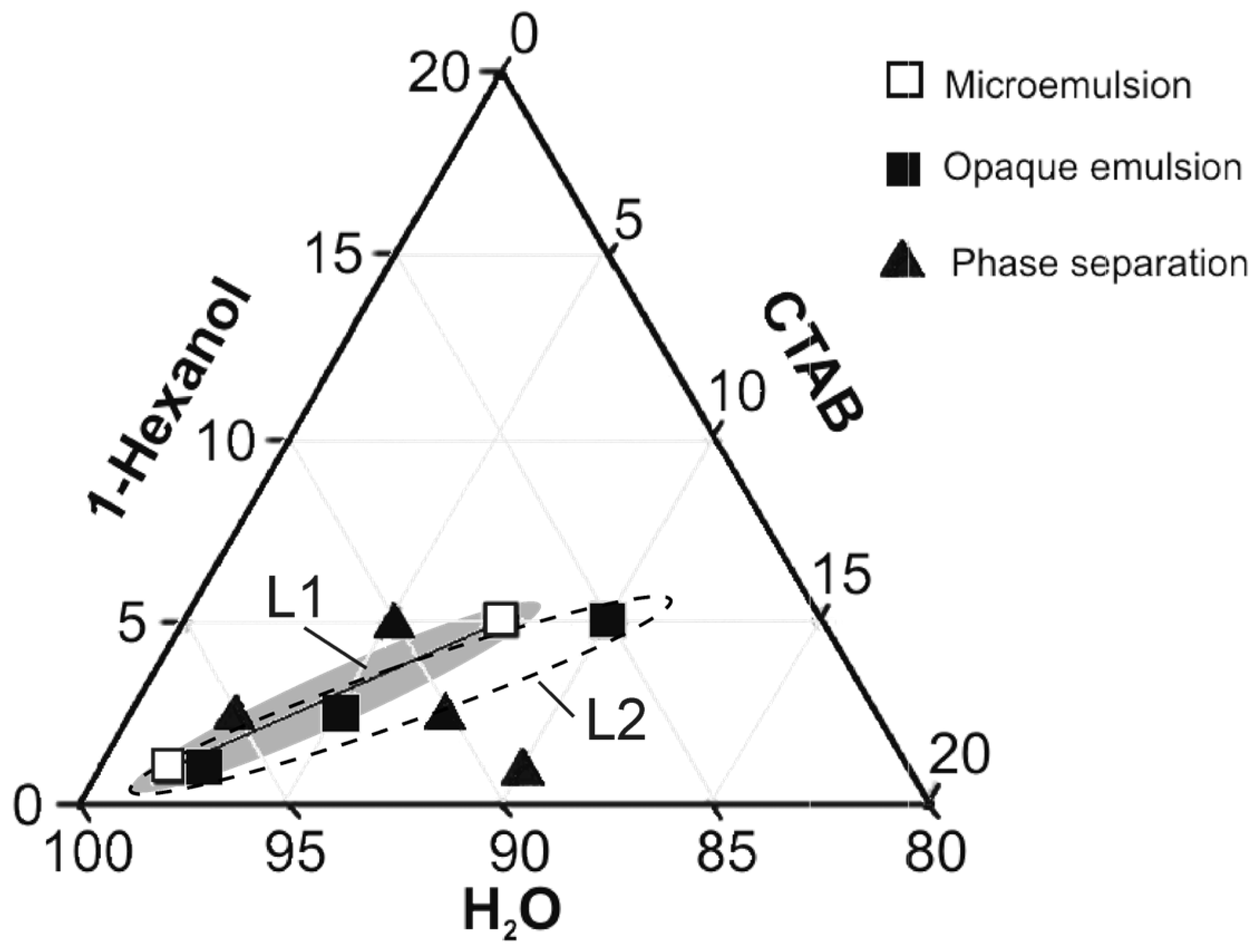



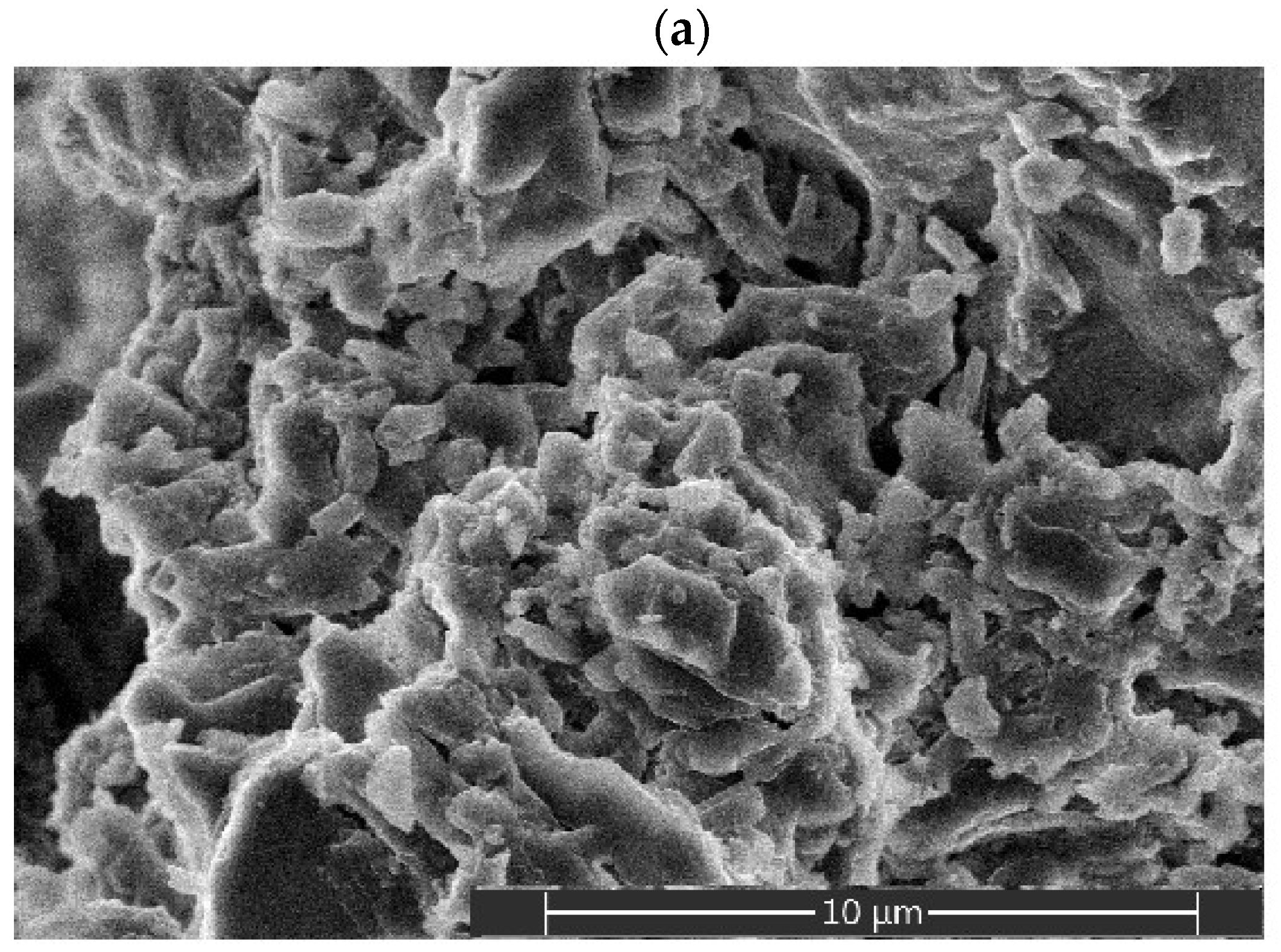
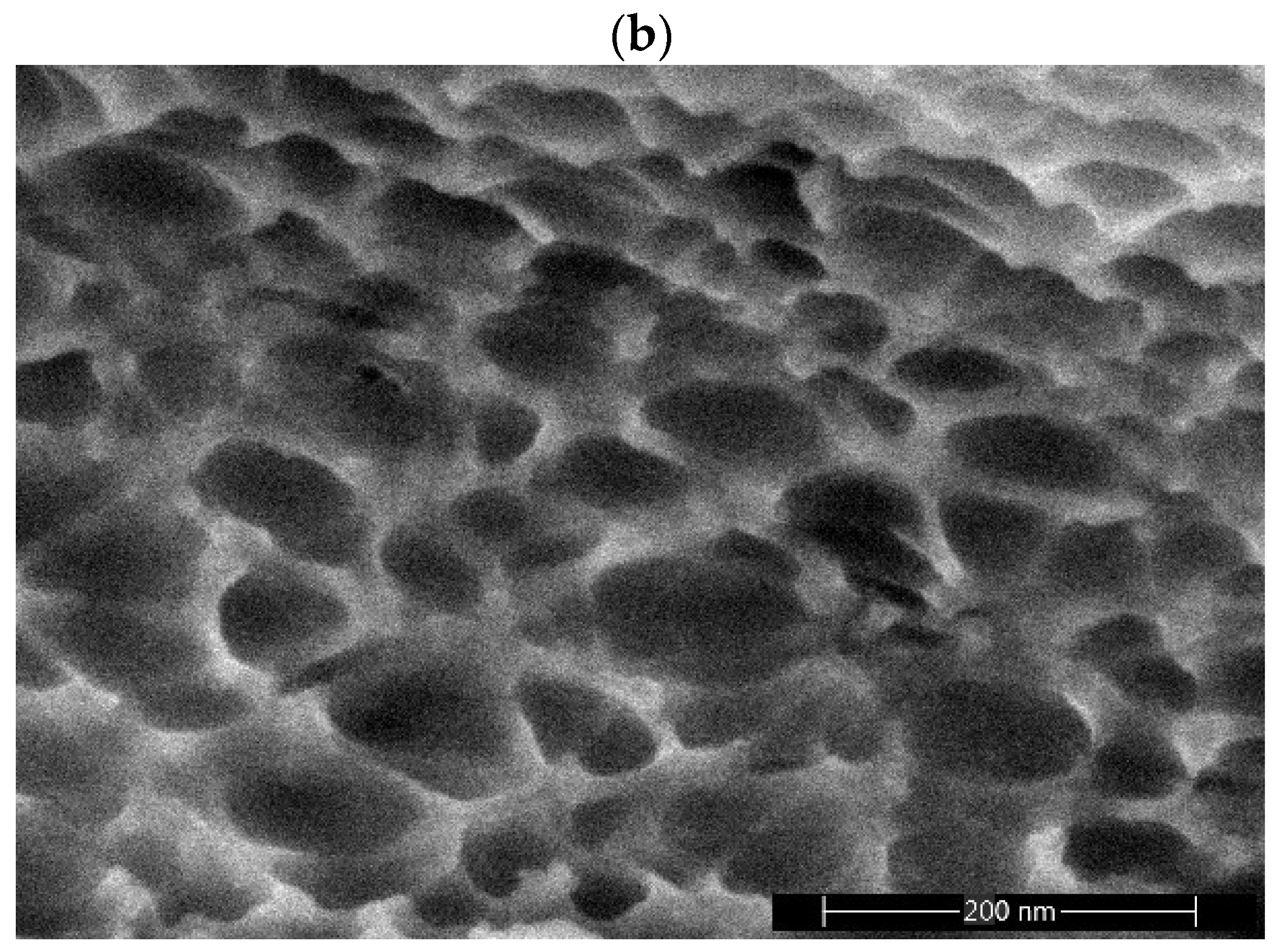
| Emulsion Composition (wt.%) | ||||
|---|---|---|---|---|
| CTAB | Hexanol | Water | Region | |
| 2.5 | 2.5 | 95 | 1 | Phase separation |
| 5 | 5 | 90 | 1 | Phase separation |
| 4 | 3.5 | 92.5 | 1.14 | Phase separation |
| 7.5 | 5 | 87.5 | 1.5 | L1 |
| 1.75 | 1 | 97.25 | 1.75 | L1 |
| 5 | 2.5 | 92.5 | 2 | L2 |
| 10 | 5 | 85 | 2 | L2 |
| 2.5 | 1 | 96.5 | 2.5 | L2 |
| 7.5 | 2.5 | 90 | 3 | Phase separation |
| 10 | 1 | 89 | 10 | Phase separation |
| Day | Peak Mean (nm) | Z-Avg. (nm) | PDI | Relative kcoal (Days 1–7) (Arb. Units) |
|---|---|---|---|---|
| 1 | 173.1 | 139.3 | 0.222 | - |
| 3 | 164.1 | 140.3 | 0.177 | - |
| 7 | 148.7 | 119.0 | 0.236 | ~−1.15 × 10−6 |
| Method | Al3+ Source | Additive(s) | Treatment | Properties of Al2O3 | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aging T (°C)/ Time (h) | Drying T (°C)/ Time (d) | Calcination T (°C)/ Time (h) | SA (m2·g−1) | Pore Diameter | |||||
| EISA | Al(C3H7O)3 | 0.5 (EtOH) | Pluronic P123 | 60/2 | 400/4 900/1 1000/1 | 419.6 220.9 146.6 | 3.92 nm 3.93 nm 3.94 nm | [24] | |
| EISA solvoth. | Al(C3H7O)3 | 0.5 (EtOH) | Pluronic P123 | l80/24 100/24 130/24 | 60/2 | 400/4 900/1 1000/1 | 742.9 422.8 182.6 | 10.08 nm 5.81 nm 6.82 nm | |
| Sol–gel | Al(C3H7O)3 | 1.5 (H2O) | Pluronic P123 n-PentOH Decalin NH4OH | -/168 | 50/2 | 600/2 | 225–473 | Bimodal 8–10 nm 0.1–6 μm | [13] |
| Sol–gel | Al(C3H7O)3 | 0.8 (H2O) | HNO3 MMA SDS APS | -/3 | 110 | 600/3 | 228–236 | Bimodal 3.8 nm 25.7 nm | [15] |
| Sol–gel | Al(C3H7O)3 | 0.5 (EtOH) | Pluronic P123 PEG | -/24 | 60/2 | 450/5 | 199–287 | 13–29 nm | [20] |
| Sol–gel | Al(C3H7O)3 | 2 (EtOH +H2O) | PEO | 40/24 | 50/2 | 600/2 | 427–675 | 6.5–15.1 nm | [22] |
| Sol–gel | AlCl3 | - (EtOH) | PEG | 40/24 | 50/1 | 600/6 | 273–565 | 4.31–14.36 nm | [6] |
| Sol–gel | Al(NO3)3 | - (n-PrOH) | CA HMTA Carbamide EtOAc PO | 80/- | 500/2 | 110–290 | 0.5–120 μm | [27] | |
| Lyoph. | 150–310 | 0.5–15 μm | |||||||
| Sol–gel | Al(NO3)3 | 1.4 (H2O) | HexOH CTAB | Lyoph. | 500/2 | 225 | 60–110 nm | This work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedoročková, A.; Ivánová, D.; Sučik, G.; Kubovčíková, M. Preparation of Uniform-Pore Ceramics from Highly Stable Emulsions via the Sol–Gel Method. Gels 2025, 11, 638. https://doi.org/10.3390/gels11080638
Fedoročková A, Ivánová D, Sučik G, Kubovčíková M. Preparation of Uniform-Pore Ceramics from Highly Stable Emulsions via the Sol–Gel Method. Gels. 2025; 11(8):638. https://doi.org/10.3390/gels11080638
Chicago/Turabian StyleFedoročková, Alena, Dana Ivánová, Gabriel Sučik, and Martina Kubovčíková. 2025. "Preparation of Uniform-Pore Ceramics from Highly Stable Emulsions via the Sol–Gel Method" Gels 11, no. 8: 638. https://doi.org/10.3390/gels11080638
APA StyleFedoročková, A., Ivánová, D., Sučik, G., & Kubovčíková, M. (2025). Preparation of Uniform-Pore Ceramics from Highly Stable Emulsions via the Sol–Gel Method. Gels, 11(8), 638. https://doi.org/10.3390/gels11080638







