Salt-Induced Gel Formation by Zwitterionic Polymer for Synergistic Methane Hydrate Inhibition
Abstract
1. Introduction
2. Results and Discussion
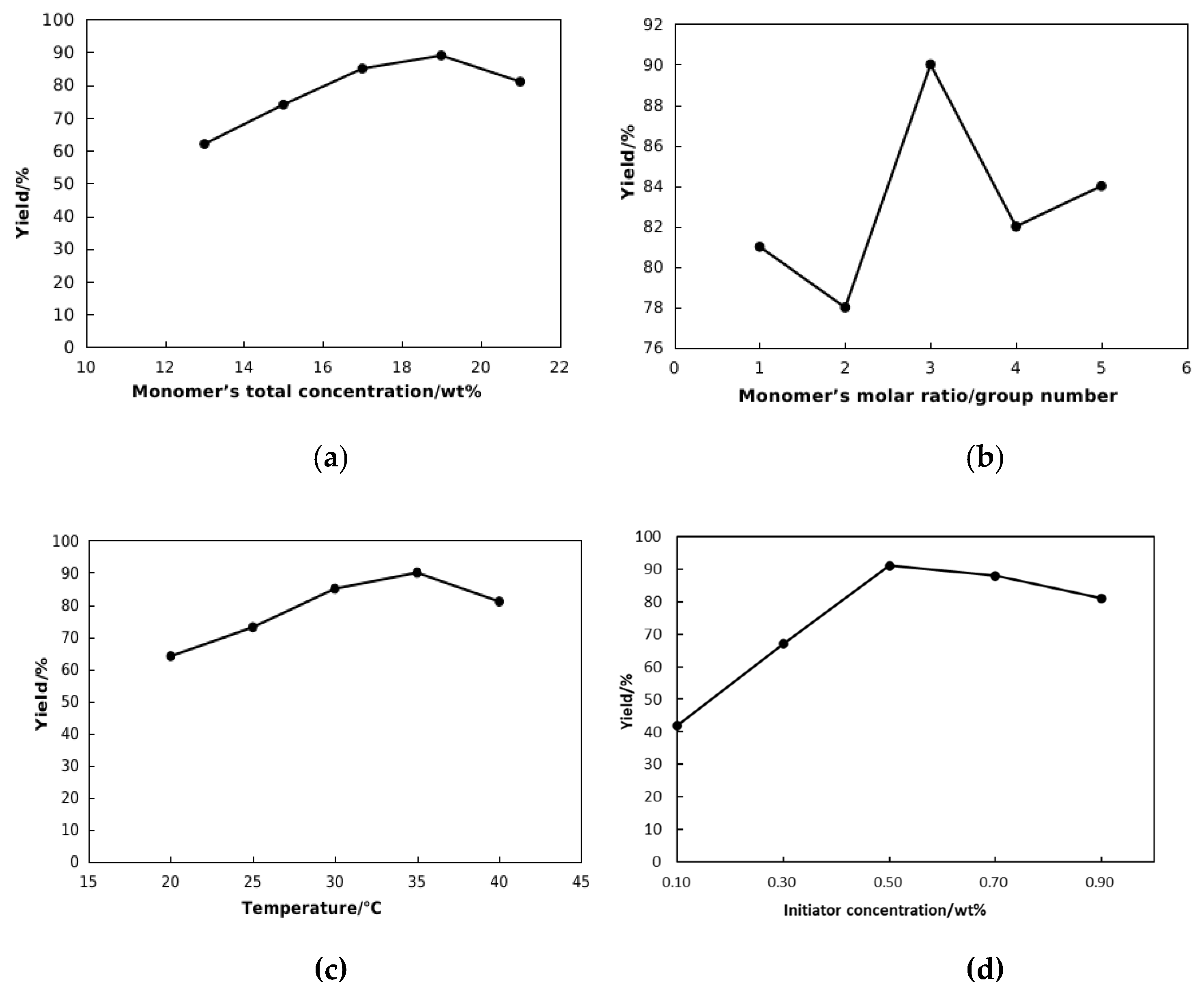
2.1. Optimization of Preparation Conditions
2.1.1. Single Factor Tests
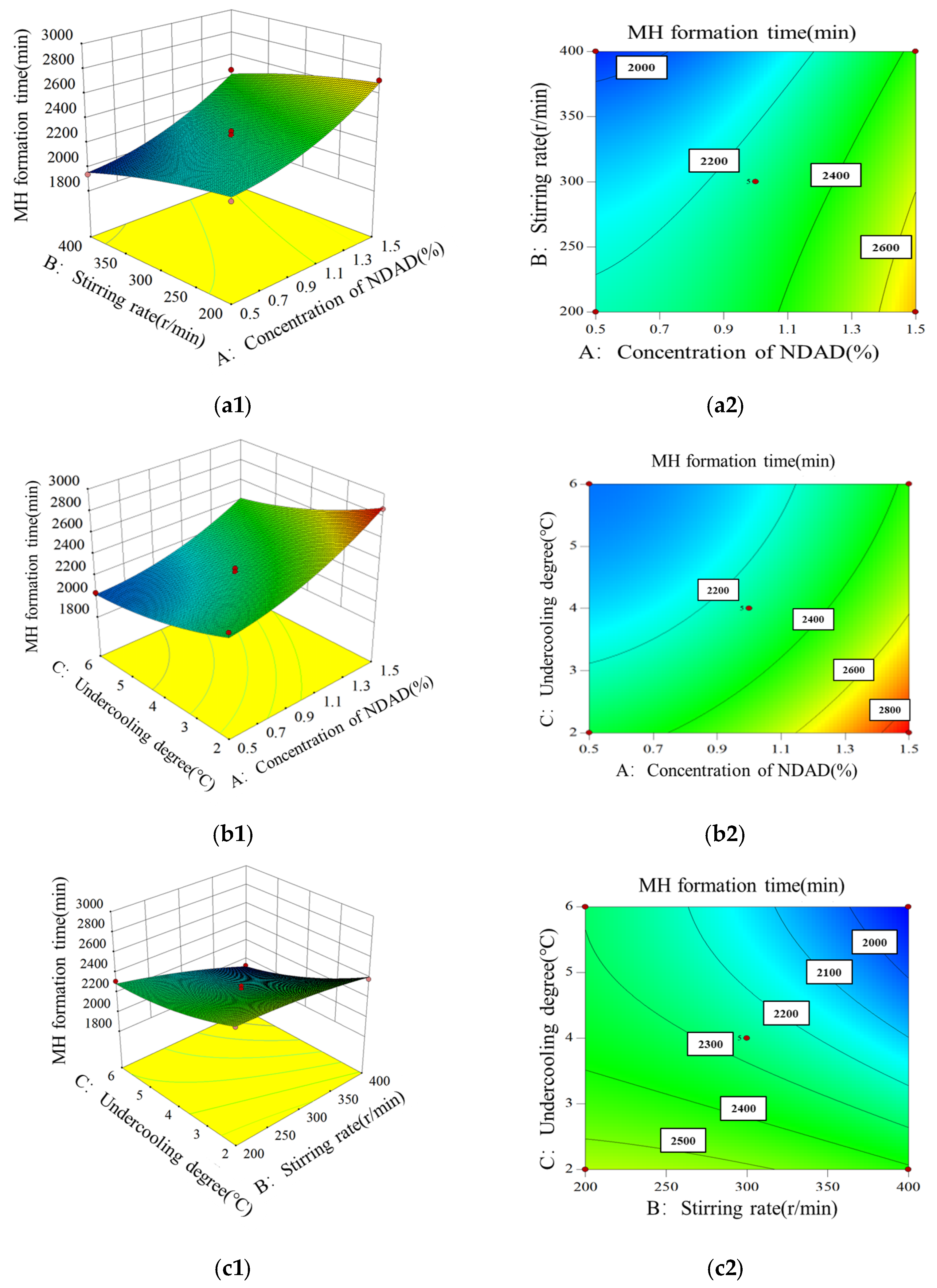
2.1.2. Orthogonal Tests
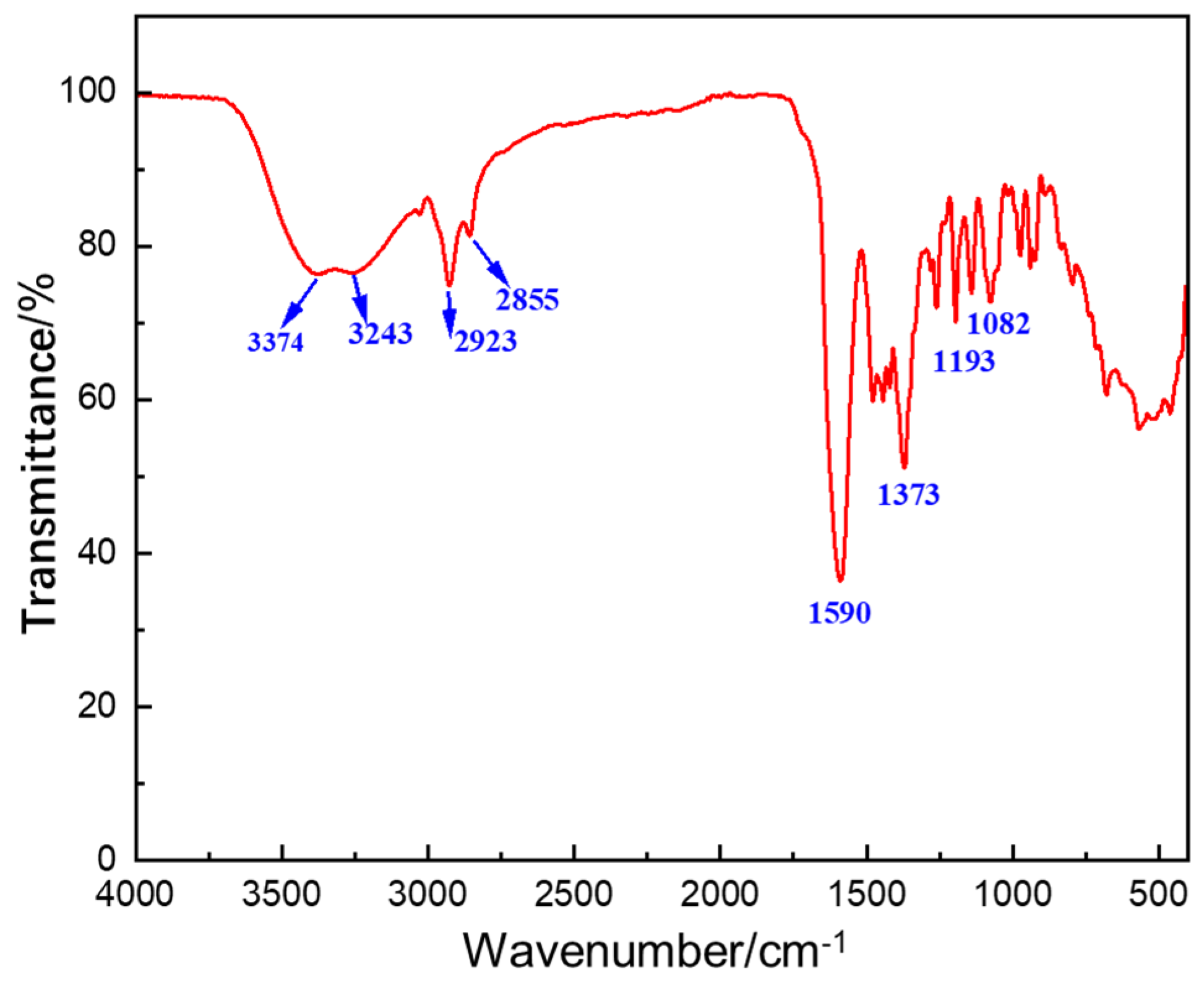
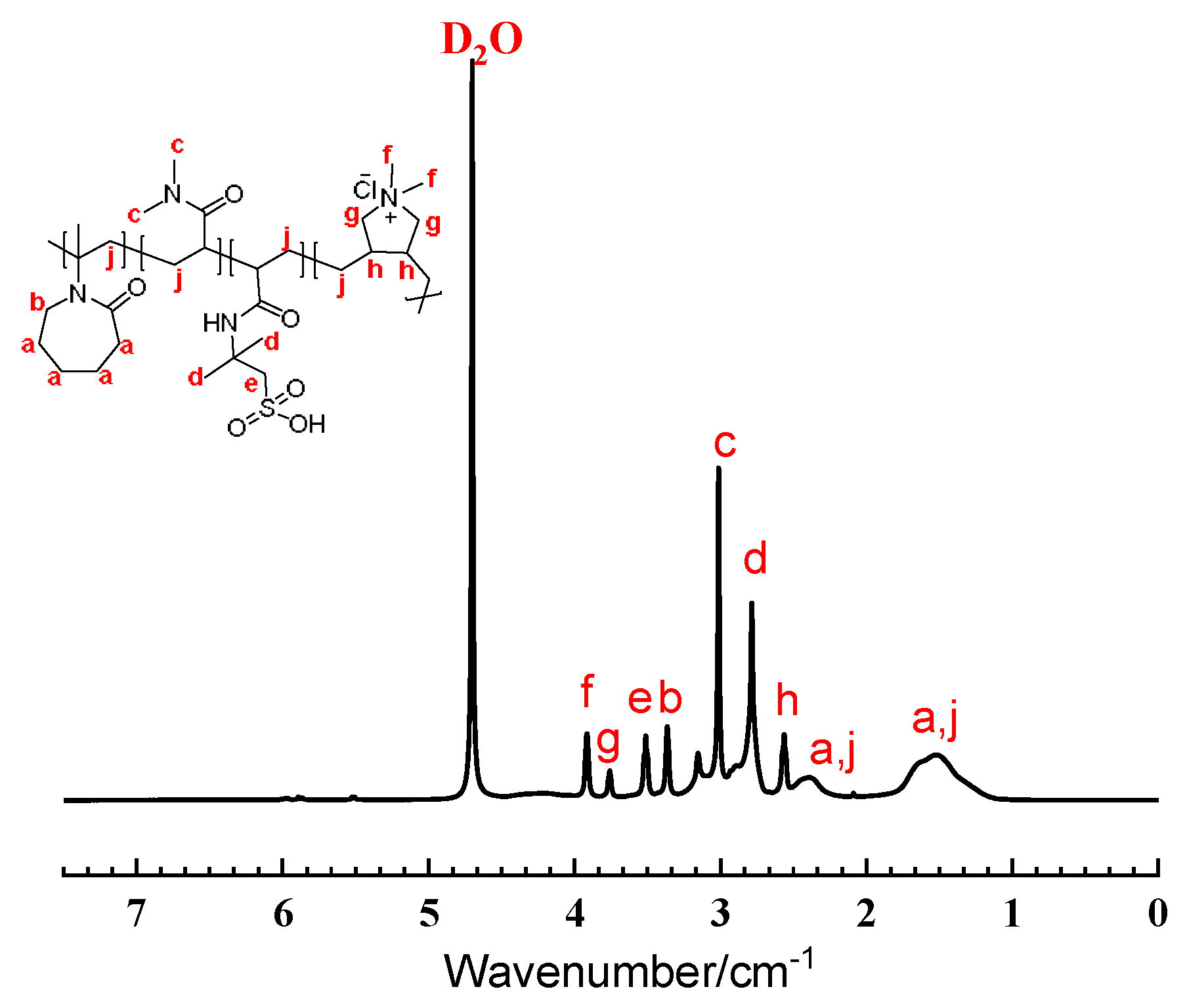
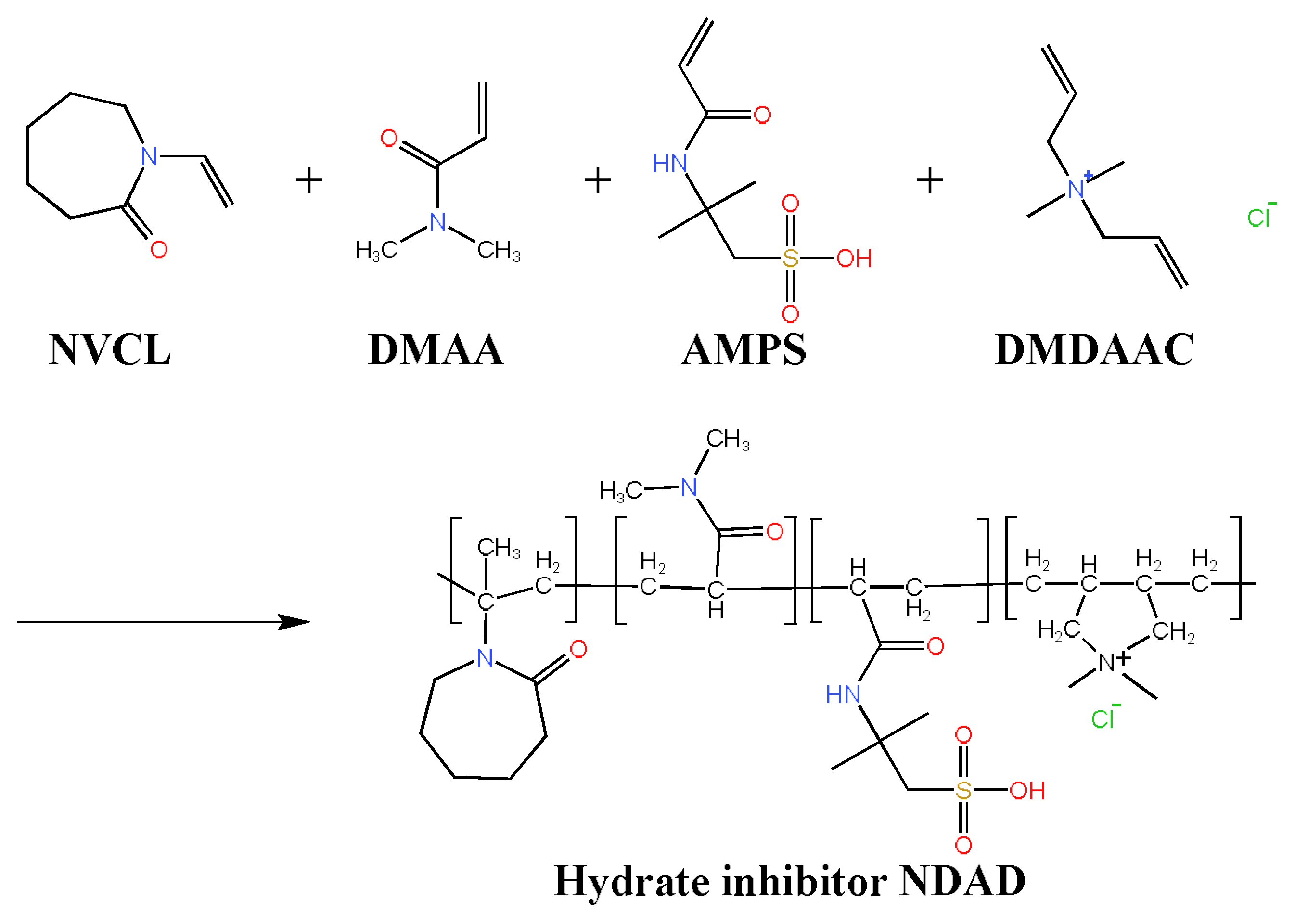
2.2. Molecular Structure Characterization of NDAD
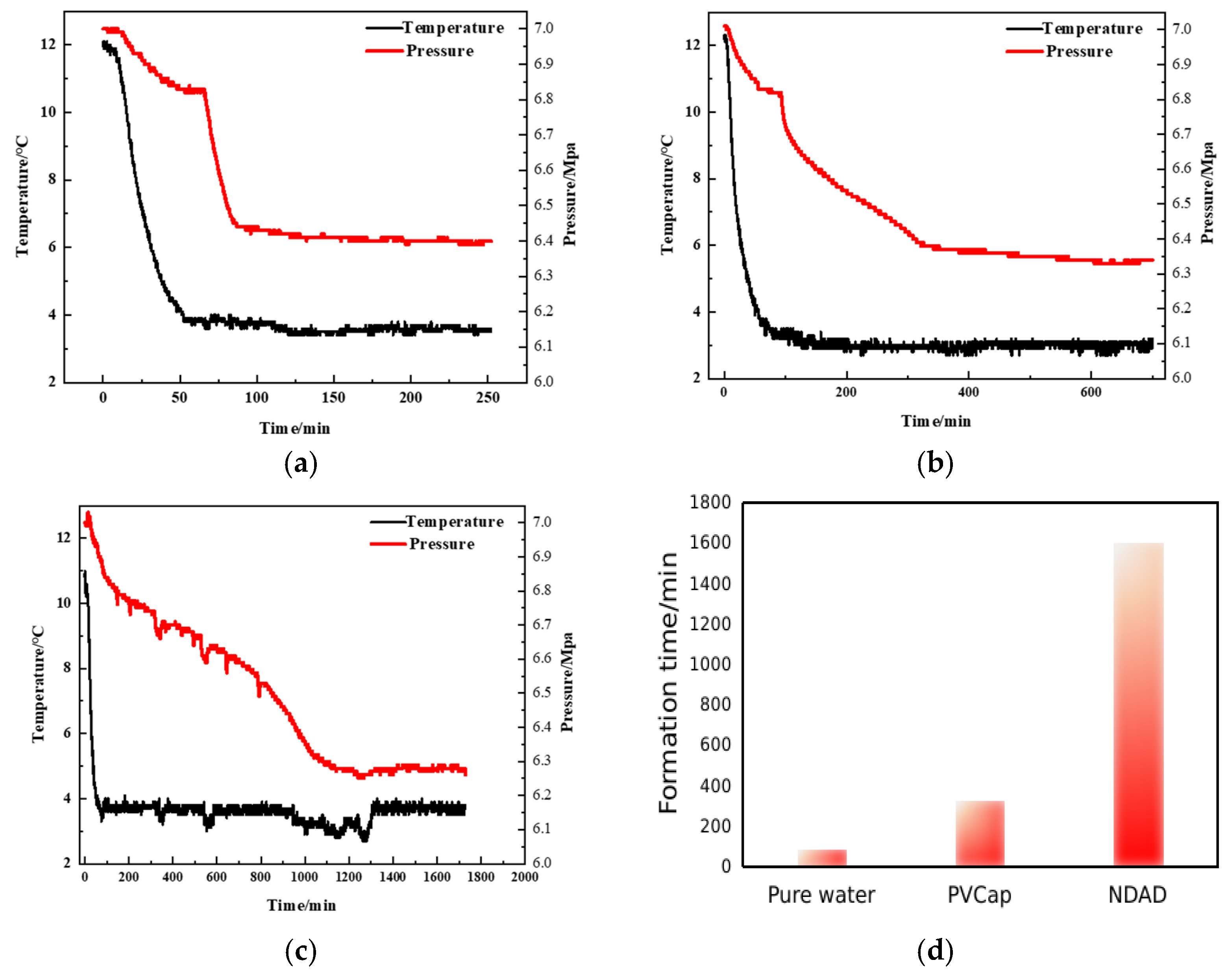
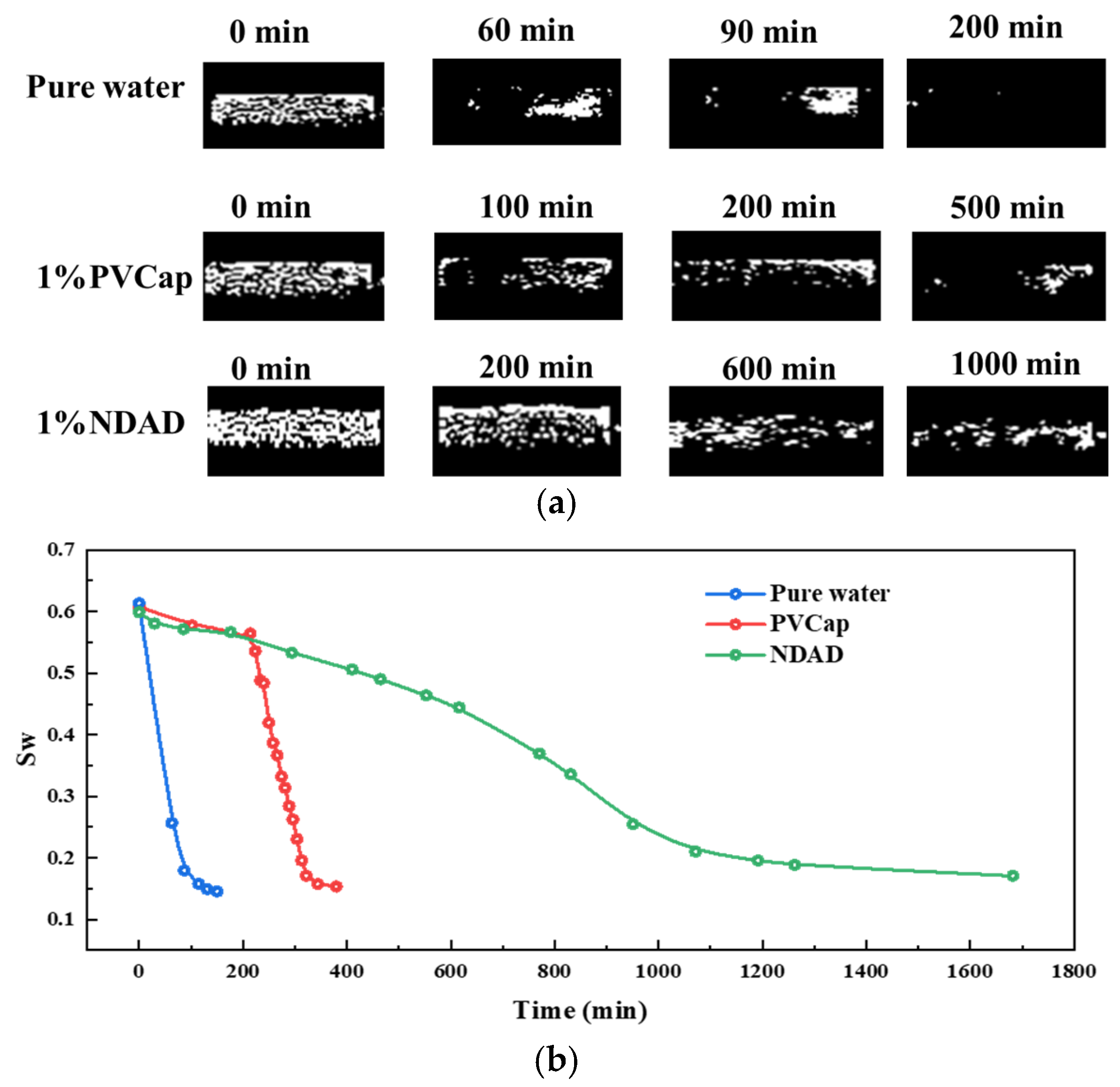
2.3. Hydrate Formation Inhibition Performance of NDAD
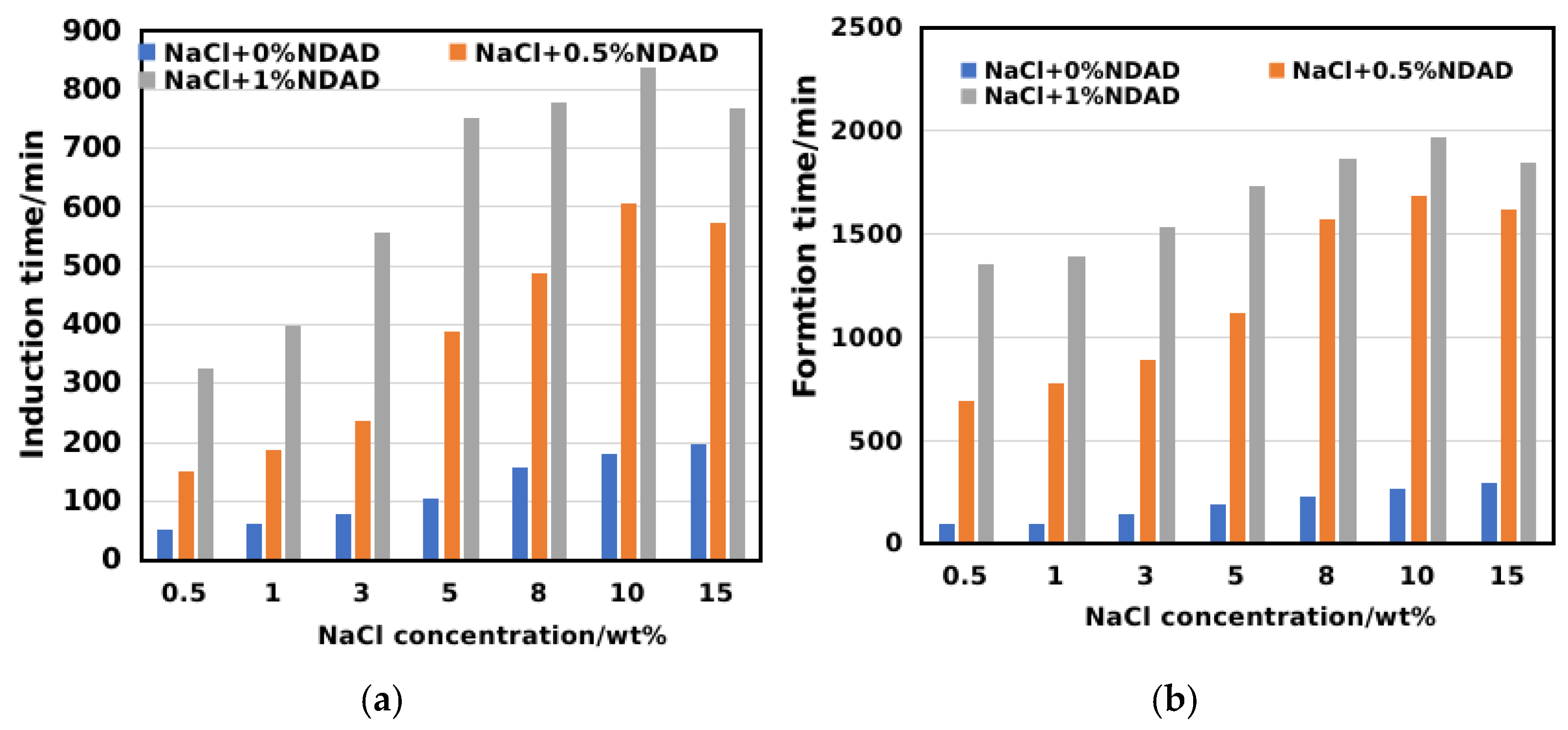
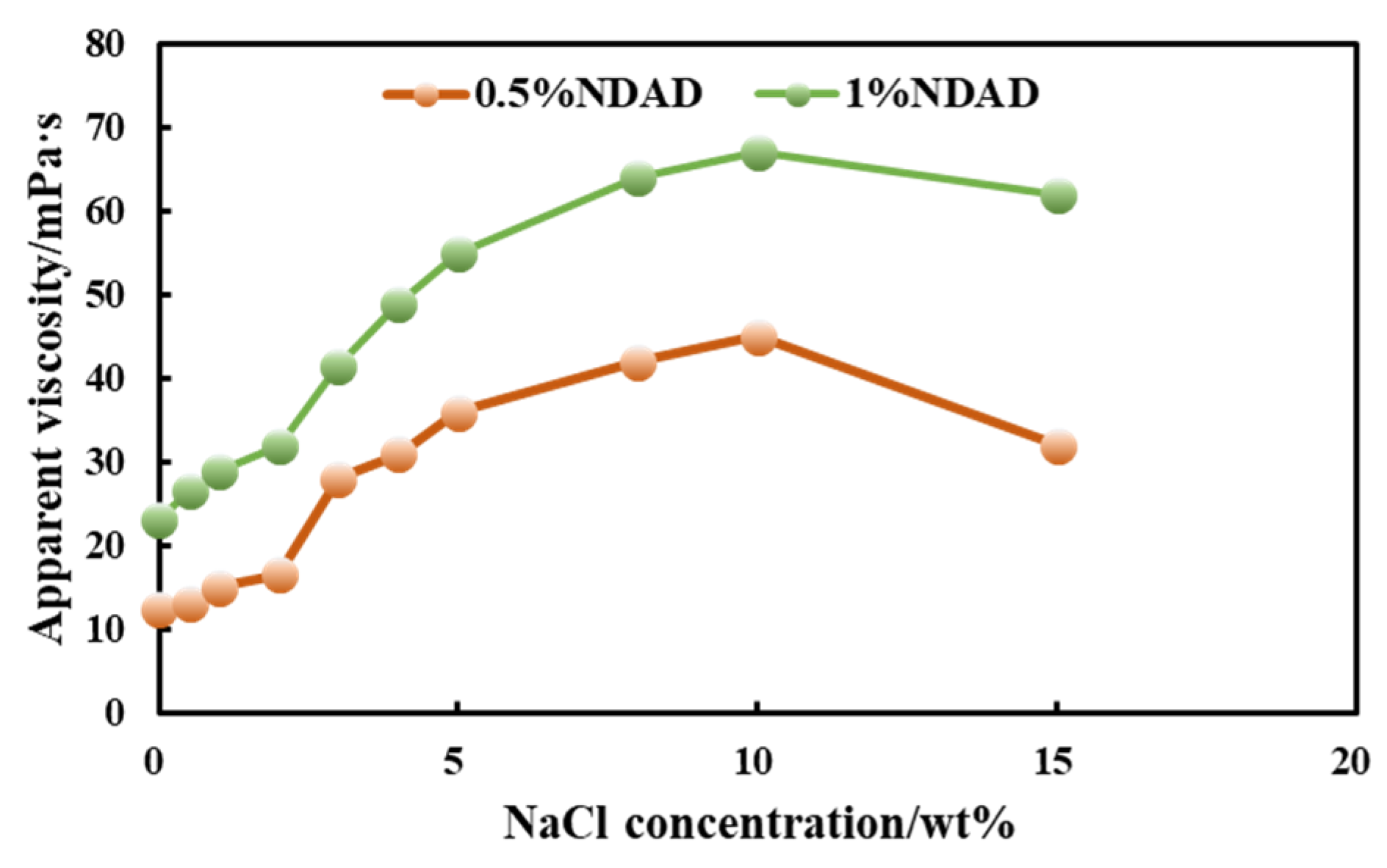
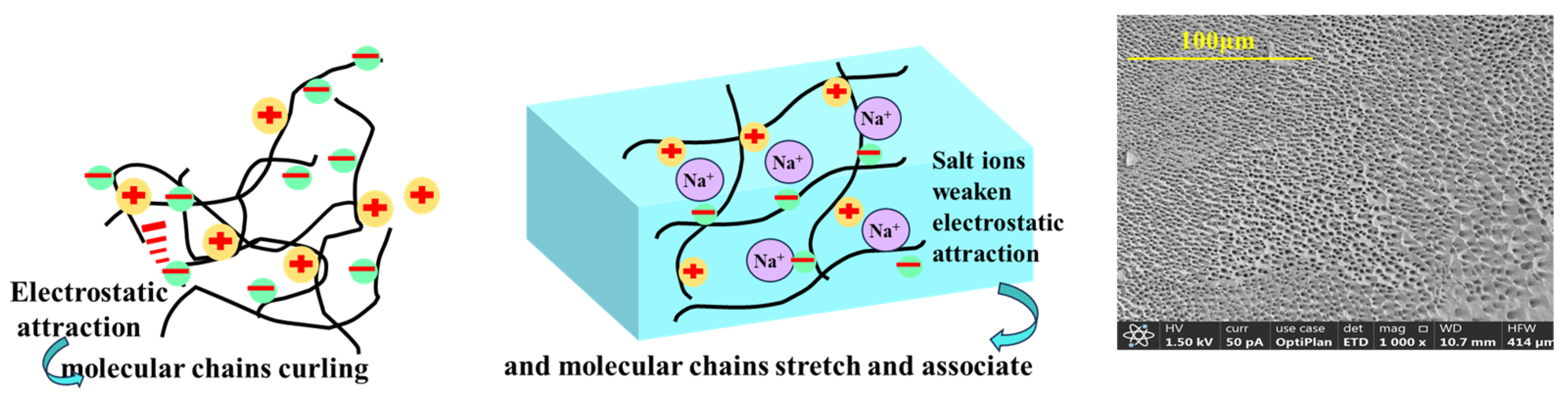
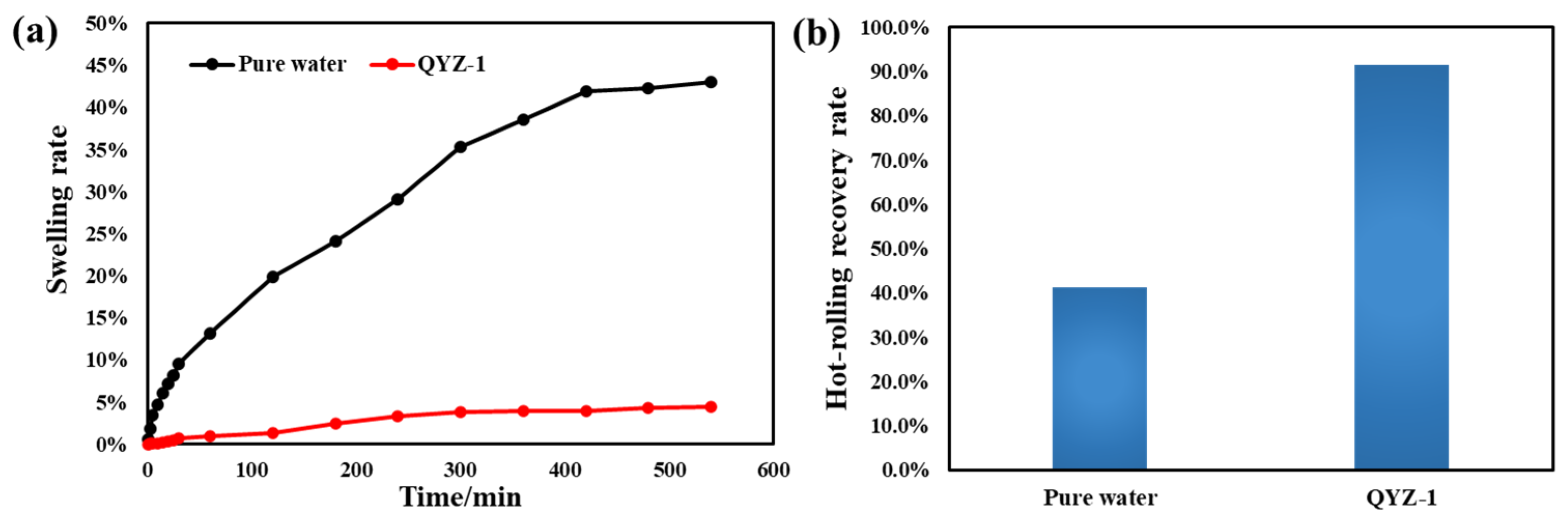
2.4. Salinity Promotes Gel Transformation and Enhances Hydrate Inhibition Performance
2.5. Impact of Hydrate Formation Inhibition Performance of NDAD
2.6. The Feasibility of NDAD in Practical Applications
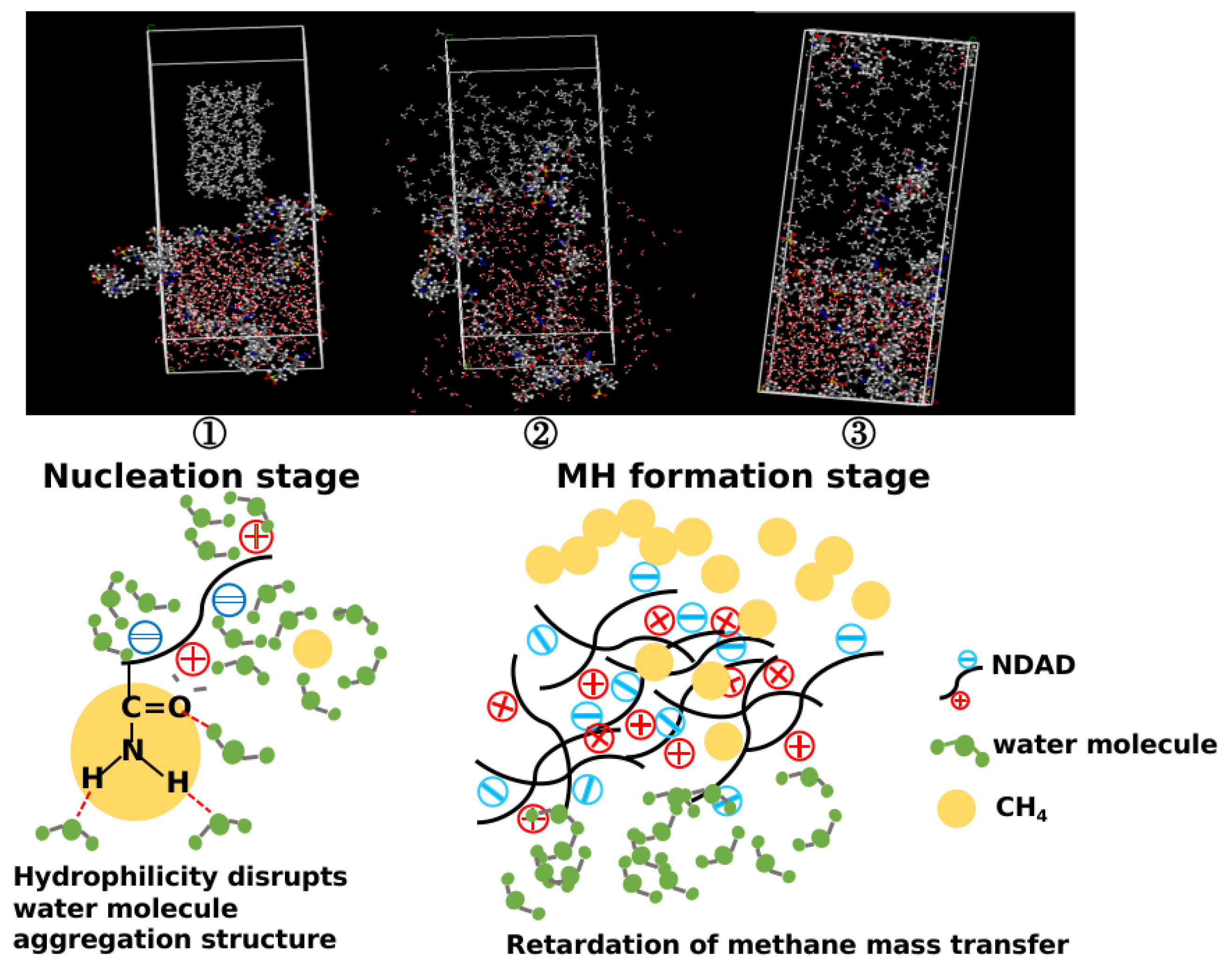
2.7. Hydrate Formation Inhibition Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of NDAD
4.3. Structural Characterizations of NDAD
- (1)
- Fourier infrared spectroscopy (FT-IR)
- (2)
- Nuclear magnetic resonance hydrogen spectroscopy (1H NMR)
- (3)
- Gel Permeation Chromatography (GPC)
4.4. Method of Hydrate Formation
4.5. Evaluation Method for Drilling Fluid
4.6. Molecular Simulation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NDAD | Copolymer of NVCL, DMAA, AMPS, and DMDAAC |
| MRI | Magnetic resonance imaging |
| MH | Methane hydrate |
| RSM | Response surface methodology |
| NVCL | N-Vinylcaprolactam |
| DMAA | N,N-Dimethylacrylamide |
| AMPS | 2-Acrylamido-2-methyl-1-propanesulfonic acid |
| DADMAC | diallyldimethylammo-nium chloride solution |
| API | American Petroleum Institute |
References
- Kumar, S.; Kwon, H.T.; Choi, K.H.; Lim, W.; Cho, J.H.; Tak, K.; Moon, I. LNG: An eco-friendly cryogenic fuel for sustainable development. Appl. Energy 2011, 88, 4264–4273. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, F.; Du, M.; Du, Q.; Zhou, S. Fluctuation in the global oil market, stock market volatility, and economic policy uncertainty: A study of the US and China. Q. Rev. Econ. Financ. 2023, 87, 377–387. [Google Scholar] [CrossRef]
- Xiong, P.; Shaokun, Y.; Ming, Z.; Jinsong, L. Deep-Water Fan Systems and Petroleum Resources on the Northern Slope of the South China Sea. Acta Geologica Sinica 2004, 78, 626–631. [Google Scholar] [CrossRef]
- Li, J.; Tan, Z.; Jing, Q.; Mei, W.; Shen, W.; Feng, L.; Dong, T.; Hao, Z. Research and Performance Evaluation of Low-Damage Plugging and Anti-Collapse Water-Based Drilling Fluid Gel System Suitable for Coalbed Methane Drilling. Gels 2025, 11, 473. [Google Scholar] [CrossRef]
- Randolph, M.F.; Gaudin, C.; Gourvenec, S.M.; White, D.J.; Boylan, N.; Cassidy, M.J. Recent advances in offshore geotechnics for deep water oil and gas developments. Ocean Eng. 2011, 38, 818–834. [Google Scholar] [CrossRef]
- Zhu, W.; Zhong, K.; Li, Y.; Xu, Q.; Fang, D. Characteristics of hydrocarbon accumulation and exploration potential of the northern South China Sea deepwater basins. Chin. Sci. Bull. 2012, 57, 3121–3129. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.; Liu, L.; Wang, J.; Feng, Z.; Zhang, D.; Gao, S.; Wang, J.; Ren, H.; Hui, B. Investigation on Filtration Control of Zwitterionic Polymer AADN in High Temperature High Pressure Water-Based Drilling Fluids. Gels 2022, 8, 826. [Google Scholar] [CrossRef]
- Kong, L.; Chen, H.; Ping, H.; Zhai, P.; Liu, Y.; Zhu, J. Formation pressure modeling in the Baiyun Sag, northern South China Sea: Implications for petroleum exploration in deep-water areas. Mar. Pet. Geol. 2018, 97, 154–168. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, J.; Liu, Z.; Jiang, S. Challenges and Solutions for Deepwater Drilling in the South China Sea. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2013. [Google Scholar] [CrossRef]
- Maiti, M.; Bhaumik, A.K.; Mandal, A. Performance of water-based drilling fluids for deepwater and hydrate reservoirs: Designing and modelling studies. Pet. Sci. 2021, 18, 1709–1728. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Wang, M.; Xu, J.; Huang, W. Experimental investigation of the effect of drilling fluid on wellbore stability in shallow unconsolidated formations in deep water. J. Pet. Sci. Eng. 2019, 175, 595–603. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Q.; Wang, Z.; Wang, J.; Sun, X.; Liu, Z.; Sun, B.; Sun, J. Prediction of hydrate formation and plugging in the trial production pipes of offshore natural gas hydrates. J. Clean. Prod. 2021, 316, 128262. [Google Scholar] [CrossRef]
- Wei, J.; Liang, J.; Lu, J.; Zhang, W.; He, Y. Characteristics and dynamics of gas hydrate systems in the northwestern South China Sea—Results of the fifth gas hydrate drilling expedition. Mar. Pet. Geol. 2019, 110, 287–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-S.; Wang, Y.; Chen, Z.-Y.; Yan, K.-F. Decomposition conditions of methane hydrate in marine sediments from South China Sea. Fluid Phase Equilibria 2016, 413, 110–115. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Ouyang, Y.; Han, Q. Synthesis of hydrophobically modified polyampholyte based on epoxidized soybean oil as shale inhibitor in water-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126664. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Wu, T.; Liu, J.; Fan, Y. Synthesis of carboxymethyl chitosan as an eco-friendly amphoteric shale inhibitor in water-based drilling fluid and an assessment of its inhibition mechanism. Appl. Clay Sci. 2020, 193, 105637. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Zhang, D.; Tang, Z.; Huang, W.; Wang, W. Inhibiting shale hydration and dispersion with amine-terminated polyamidoamine dendrimers. J. Nat. Gas Sci. Eng. 2016, 28, 52–60. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Huang, W.; Sun, D.; Zhang, D.; Cao, J. Synergistic stabilization of shale by a mixture of polyamidoamine dendrimers modified bentonite with various formations in water-based drilling fluid. Appl. Clay Sci. 2015, 114, 359–369. [Google Scholar] [CrossRef]
- Du, W.; Slaný, M.; Wang, X.; Chen, G.; Zhang, J. The Inhibition Property and Mechanism of a Novel Low Molecular Weight Zwitterionic Copolymer for Improving Wellbore Stability. Polymers 2020, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, K.; Li, M.; Lyu, X.; Zhu, S.; Huang, Y. Hydration inhibition and physical plugging to enhance shale stability using zwitterionic polymer/nano-silica composite. J. Mol. Liq. 2023, 387, 122642. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhou, L.; Wan, X.C.; Tang, Y.F.; Liu, Q.; Li, W.; Liao, J.B. Synthesis and characterization of a temperature sensitive microcapsule gelling agent for high-temperature acid release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qiu, Z.; Zhang, Y.; Zhong, H.; Huang, W.; Tang, Z. Zwitterionic Polymer P(AM-DMC-AMPS) as a Low-Molecular-Weight Encapsulator in Deepwater Drilling Fluid. Appl. Sci. 2017, 7, 594. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Zhang, F.; Bai, Y.; Lv, K.; Wang, J.; Zhou, X.; Wang, B. Salt-Responsive Zwitterionic Polymer Brush Based on Modified Silica Nanoparticles as a Fluid-Loss Additive in Water-Based Drilling Fluids. Energy Fuels 2020, 34, 1669–1679. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Øvsthus, J.; Tomita, T.; Chosa, J. Studies on some zwitterionic surfactant gas hydrate anti-agglomerants. Chem. Eng. Sci. 2006, 61, 4048–4059. [Google Scholar] [CrossRef]
- Zhang, Q.; Kelland, M.A.; Lewoczko, E.M.; Bohannon, C.A.; Zhao, B. Non-amide based zwitterionic poly(sulfobetaine methacrylate)s as kinetic hydrate inhibitors. Chem. Eng. Sci. 2021, 229, 116031. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, J.; Lv, K.; Liao, B.; Wang, Z.; Bai, Y.; Wang, R.; Sun, J. Experimental and molecular dynamics studies of zwitterionic inhibitors of methane hydrate dissociation. Fuel 2022, 318, 123059. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Zhen, Z.; Sun, J.; Wang, Z.; Maeda, N. Preparation of thermo-responsive polymer and its application for plugging in hydrate-bearing sediments. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132210. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Qiu, Z.; Jia, J.; Wang, Y.; Li, X. Inhibiting the surface hydration of shale formation using preferred surfactant compound of polyamine and twelve alkyl two hydroxyethyl amine oxide for drilling. J. Pet. Sci. Eng. 2017, 159, 791–798. [Google Scholar] [CrossRef]
- Polat, S.; Sayan, P. Application of Response Surface Methodology with A Box–Behnken Design for Struvite Precipitation. Adv. Powder Technol. 2019, 30, 2396–2407. [Google Scholar] [CrossRef]
- Salehnezhad, L.; Heydari, A.; Fattahi, M. Experimental Investigation and Rheological Behaviors of Water—Based Drilling Mud Contained Starch ZnO Nanofluids through Response Surface Methodology. J. Mol. Liq. 2019, 276, 417–430. [Google Scholar] [CrossRef]
- Gulbrandsen, A.C.; Svartås, T.M. Effects of PVCap on Gas Hydrate Dissociation Kinetics and the Thermodynamic Stability of the Hydrates. Energy Fuels 2017, 31, 9863–9873. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Z.; Sun, B.; Xu, J.; Chen, L.; Wang, X. Rheological Properties of Methane Hydrate Slurry in the Presence of Xanthan Gum. SPE J. 2020, 25, 2341–2352. [Google Scholar] [CrossRef]
- Liang, H.; Guan, D.; Shi, K.; Yang, L.; Zhang, L.; Zhao, J.; Song, Y. Characterizing Mass-Transfer mechanism during gas hydrate formation from water droplets. Chem. Eng. J. 2022, 428, 132626. [Google Scholar] [CrossRef]
- Gbadamosi, A.; Murtaza, M.; Patil, S.; Kamal, M.S.; Hussain, S.M.S. Zwitterionic Surfactant as Shale Swelling Inhibition Additive in Water-Based Drilling Mud. In Proceedings of the International Petroleum Technology Conference, Dhahran, Saudi Arabia, 12 February 2024. [Google Scholar] [CrossRef]
- Gao, F.; Xu, P.; Zhang, Y. Preparation of zwitterionic polymer and its application for dual-functional inhibition in deepwater drilling. J. Mol. Liq. 2024, 417, 126335. [Google Scholar] [CrossRef]
- Saleh, T.A.; Rana, A.; Arfaj, M.K.; Ibrahim, M.A. Hydrophobic polymer-modified nanosilica as effective shale inhibitor for water-based drilling mud. J. Pet. Sci. Eng. 2022, 209, 109868. [Google Scholar] [CrossRef]
| Level | Factor A | Factor B | Factor C | Factor D |
|---|---|---|---|---|
| Total Monomer Concentration/% | Monomer’s Molar Ratio | Temperature /°C | Initiator Concentration /% | |
| 1 | 15% | 3:3:2:2 | 25 | 0.3 |
| 2 | 17% | 2:2:3:3 | 30 | 0.5 |
| 3 | 19% | 2:4:2:2 | 35 | 0.7 |
| 4 | 21% | 4:2:2:2 | 40 | 0.9 |
| Run | A | B | C | D | Yield /% | Hydrate Inhibition Rate/% | Clay Hydration Inhibition Rate/% | X | Y | Z | H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 63.8 | 230% | 52% | 16.2% | 13.7% | 32.7% | 0.6263 |
| 2 | 1 | 2 | 2 | 2 | 74.2 | 524% | 68% | 50.5% | 68.5% | 65.1% | 1.8406 |
| 3 | 1 | 3 | 3 | 3 | 78.9 | 601% | 75% | 66.0% | 82.8% | 80.0% | 2.2877 |
| 4 | 1 | 4 | 4 | 4 | 70.1 | 479% | 60% | 37.0% | 60.1% | 49.1% | 1.4617 |
| 5 | 2 | 1 | 2 | 3 | 77.8 | 574% | 72% | 62.4% | 77.7% | 74.2% | 2.1430 |
| 6 | 2 | 2 | 1 | 4 | 59.6 | 157% | 43% | 2.3% | 0.0% | 12.4% | 0.1467 |
| 7 | 2 | 3 | 4 | 1 | 65.3 | 248% | 58% | 21.1% | 17.1% | 44.0% | 0.8224 |
| 8 | 2 | 4 | 3 | 2 | 86.9 | 609% | 83% | 92.4% | 84.4% | 96.0% | 2.7276 |
| 9 | 3 | 1 | 3 | 4 | 76.2 | 562% | 68% | 57.1% | 75.5% | 65.5% | 1.9803 |
| 10 | 3 | 2 | 4 | 3 | 70.8 | 515% | 63% | 39.3% | 66.8% | 54.5% | 1.6060 |
| 11 | 3 | 3 | 1 | 2 | 68.8 | 479% | 65% | 32.7% | 60.2% | 59.6% | 1.5248 |
| 12 | 3 | 4 | 2 | 1 | 89.2 | 693% | 85% | 100% | 100% | 100% | 3.0000 |
| 13 | 4 | 1 | 4 | 2 | 66.4 | 297% | 50% | 24.8% | 26.2% | 27.6% | 0.7860 |
| 14 | 4 | 2 | 3 | 1 | 72.9 | 507% | 62% | 46.2% | 65.3% | 53.5% | 1.6491 |
| 15 | 4 | 3 | 2 | 4 | 62.8 | 218% | 62% | 12.9% | 11.5% | 51.6% | 0.7598 |
| 16 | 4 | 4 | 1 | 3 | 58.9 | 169% | 37% | 0.0% | 2.3% | 0.0% | 0.0226 |
| Kavg | 1 | 1.55 | 1.38 | 0.58 | 1.52 | ||||||
| 2 | 1.46 | 1.31 | 1.94 | 1.72 | |||||||
| 3 | 2.03 | 1.35 | 2.16 | 1.51 | |||||||
| 4 | 0.8 | 1.8 | 1.17 | 1.09 | |||||||
| optimum factor | 3 | 4 | 3 | 2 | |||||||
| R | 1.22 | 0.49 | 1.58 | 0.63 | |||||||
| Level | Factor A NDAD Concentration/% | Factor B Stirring Rate/(r/min) | Factor C Supercooling Degree/°C |
|---|---|---|---|
| −1 | 0.5 | 200 | 2 |
| 0 | 1.0 | 300 | 4 |
| 1 | 1.5 | 400 | 6 |
| Run | Actual Level of Variables | Response Value | ||
|---|---|---|---|---|
| Factor A NDAD Concentration/% | Factor B Stirring Rate/(r/min) | Factor C Supercooling Degree/°C | MH Formation Time /min | |
| 1 | 1 | 400 | 2 | 2390 |
| 2 | 0.5 | 300 | 6 | 2038 |
| 3 | 1.5 | 400 | 4 | 2466 |
| 4 | 1 | 300 | 4 | 2248 |
| 5 | 1.5 | 300 | 2 | 2864 |
| 6 | 0.5 | 300 | 2 | 2378 |
| 7 | 1 | 400 | 6 | 1920 |
| 8 | 1 | 200 | 2 | 2548 |
| 9 | 1 | 300 | 4 | 2262 |
| 10 | 1 | 200 | 6 | 2320 |
| 11 | 0.5 | 200 | 4 | 2189 |
| 12 | 1.5 | 300 | 6 | 2384 |
| 13 | 1 | 300 | 4 | 2289 |
| 14 | 0.5 | 400 | 4 | 1938 |
| 15 | 1 | 300 | 4 | 2240 |
| 16 | 1.5 | 200 | 4 | 2710 |
| 17 | 1 | 300 | 4 | 2265 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 944,400.21 | 9 | 104,933.36 | 71.33 | <0.0001 | ** |
| A, NDAD dosage | 442,270.13 | 1 | 442,270.13 | 300.64 | <0.0001 | ** |
| B, stirring rate | 138,601.13 | 1 | 138,601.13 | 94.22 | <0.0001 | ** |
| C, supercooling degree | 288,040.50 | 1 | 288,040.50 | 195.80 | <0.0001 | ** |
| AB | 12.25 | 1 | 12.25 | 0.01 | 0.9298 | |
| AC | 4900.00 | 1 | 4900.00 | 3.33 | 0.1107 | |
| BC | 14,641.00 | 1 | 14,641.00 | 9.95 | 0.016 | * |
| A2 | 35,812.42 | 1 | 35,812.42 | 24.34 | 0.0017 | ** |
| B2 | 3608.53 | 1 | 3608.53 | 2.45 | 0.1613 | |
| C2 | 15,654.53 | 1 | 15,654.53 | 10.64 | 0.0138 | * |
| Residual | 10,297.55 | 7 | 1471.08 | |||
| Lack of fit | 8242.75 | 3 | 2747.58 | 5.35 | 0.0695 | Not significant |
| Pure error | 2054.80 | 4 | 513.70 | |||
| Cor total | 954,697.76 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Tang, S.; Xu, P.; Wu, J.; Li, X. Salt-Induced Gel Formation by Zwitterionic Polymer for Synergistic Methane Hydrate Inhibition. Gels 2025, 11, 637. https://doi.org/10.3390/gels11080637
Gao F, Tang S, Xu P, Wu J, Li X. Salt-Induced Gel Formation by Zwitterionic Polymer for Synergistic Methane Hydrate Inhibition. Gels. 2025; 11(8):637. https://doi.org/10.3390/gels11080637
Chicago/Turabian StyleGao, Fei, Shijun Tang, Peng Xu, Jiancheng Wu, and Xinru Li. 2025. "Salt-Induced Gel Formation by Zwitterionic Polymer for Synergistic Methane Hydrate Inhibition" Gels 11, no. 8: 637. https://doi.org/10.3390/gels11080637
APA StyleGao, F., Tang, S., Xu, P., Wu, J., & Li, X. (2025). Salt-Induced Gel Formation by Zwitterionic Polymer for Synergistic Methane Hydrate Inhibition. Gels, 11(8), 637. https://doi.org/10.3390/gels11080637






