Quantum-Dot-Based Molecularly Imprinted Hydrogel for Rapid Detection of Homocysteine
Abstract
1. Introduction
2. Results and Discussion
2.1. Fabrication of Hcy Molecularly Imprinted Hydrogel
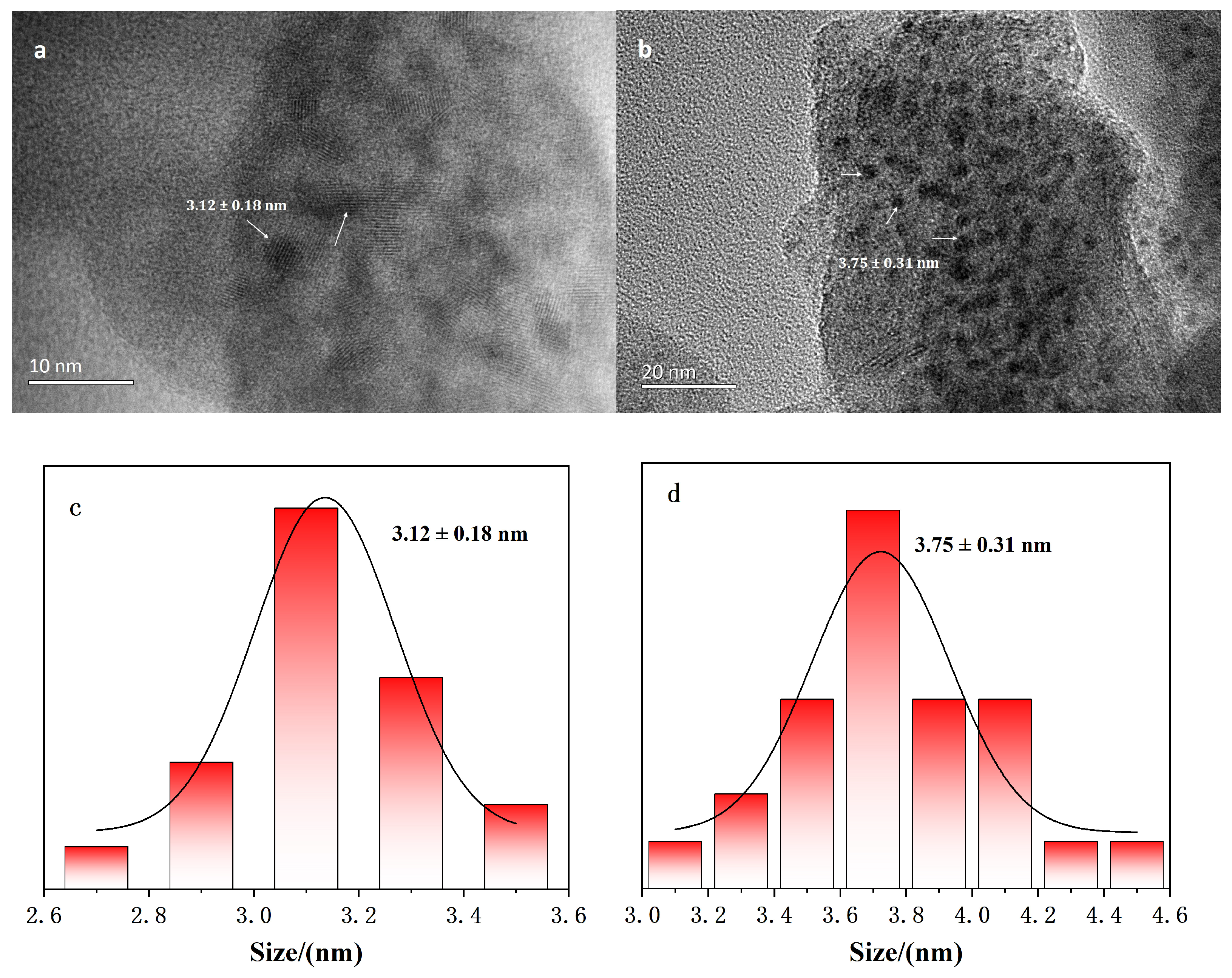
2.2. Characterization
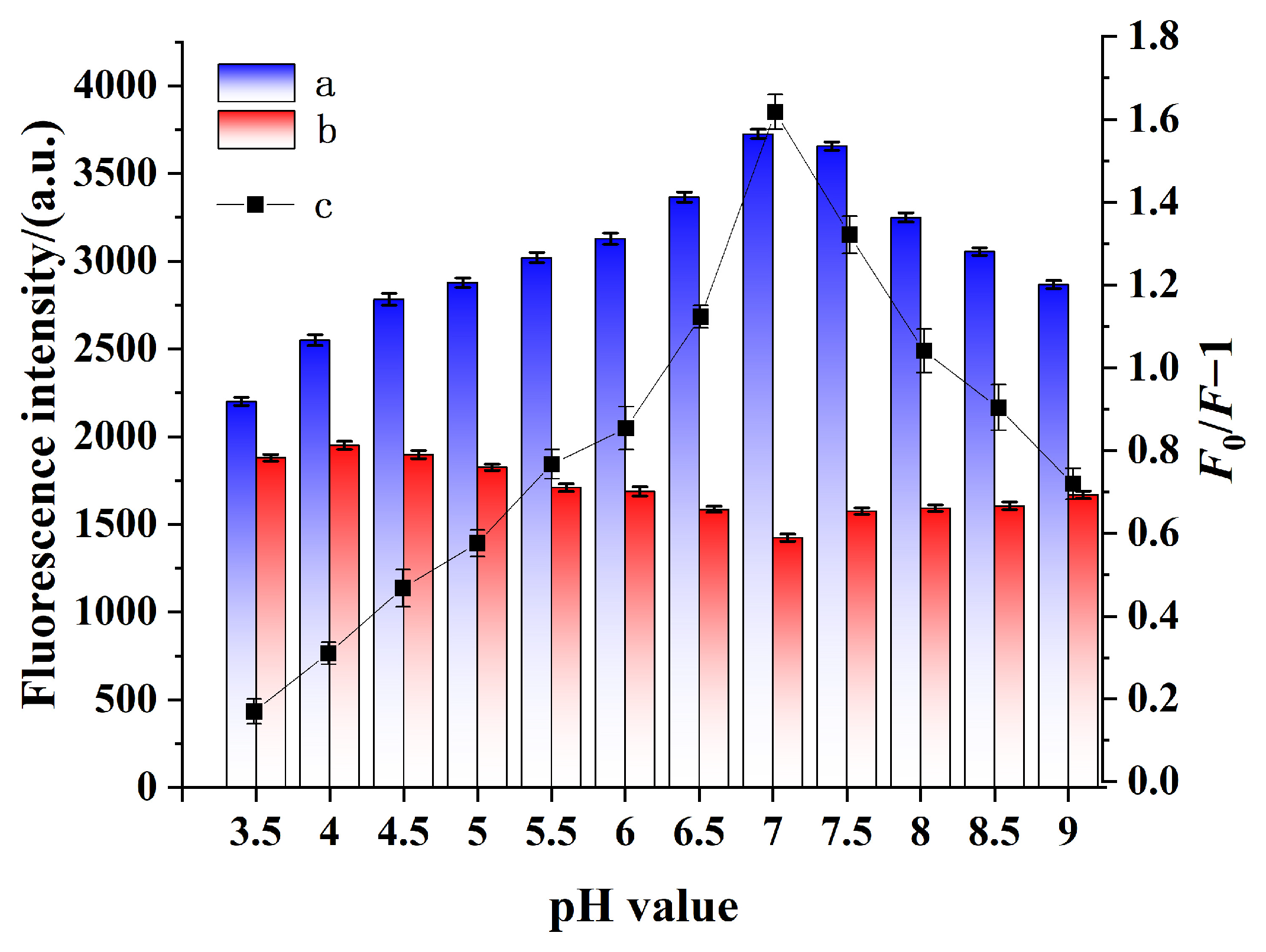
2.3. pH-Dependent Effects on QD@MIHs
2.4. Rebinding Experiments
2.4.1. Binding Kinetics
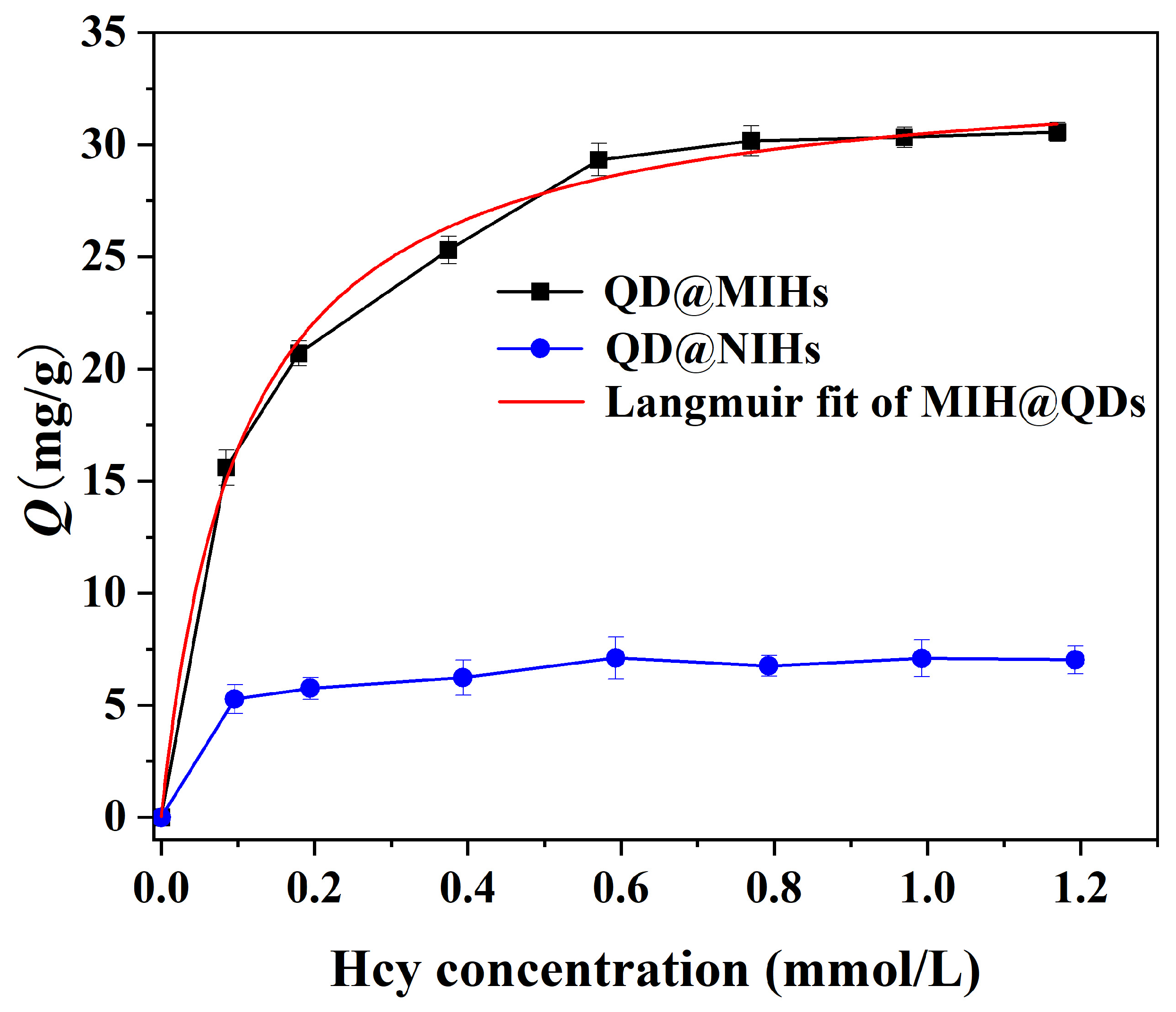
2.4.2. Adsorption Isotherm Experiments
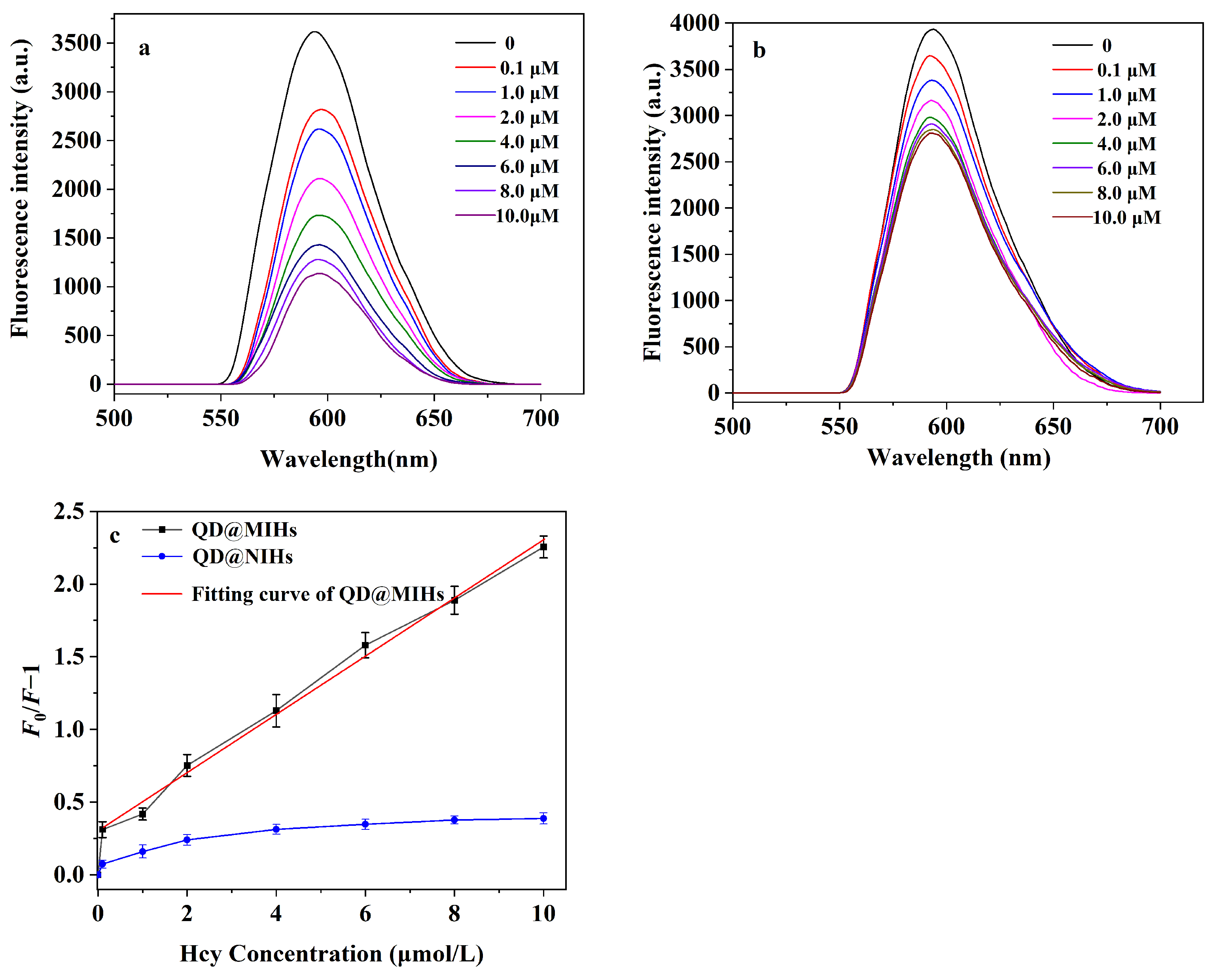
2.5. Fluorescence Sensing of Hcy Using QD@MIHs
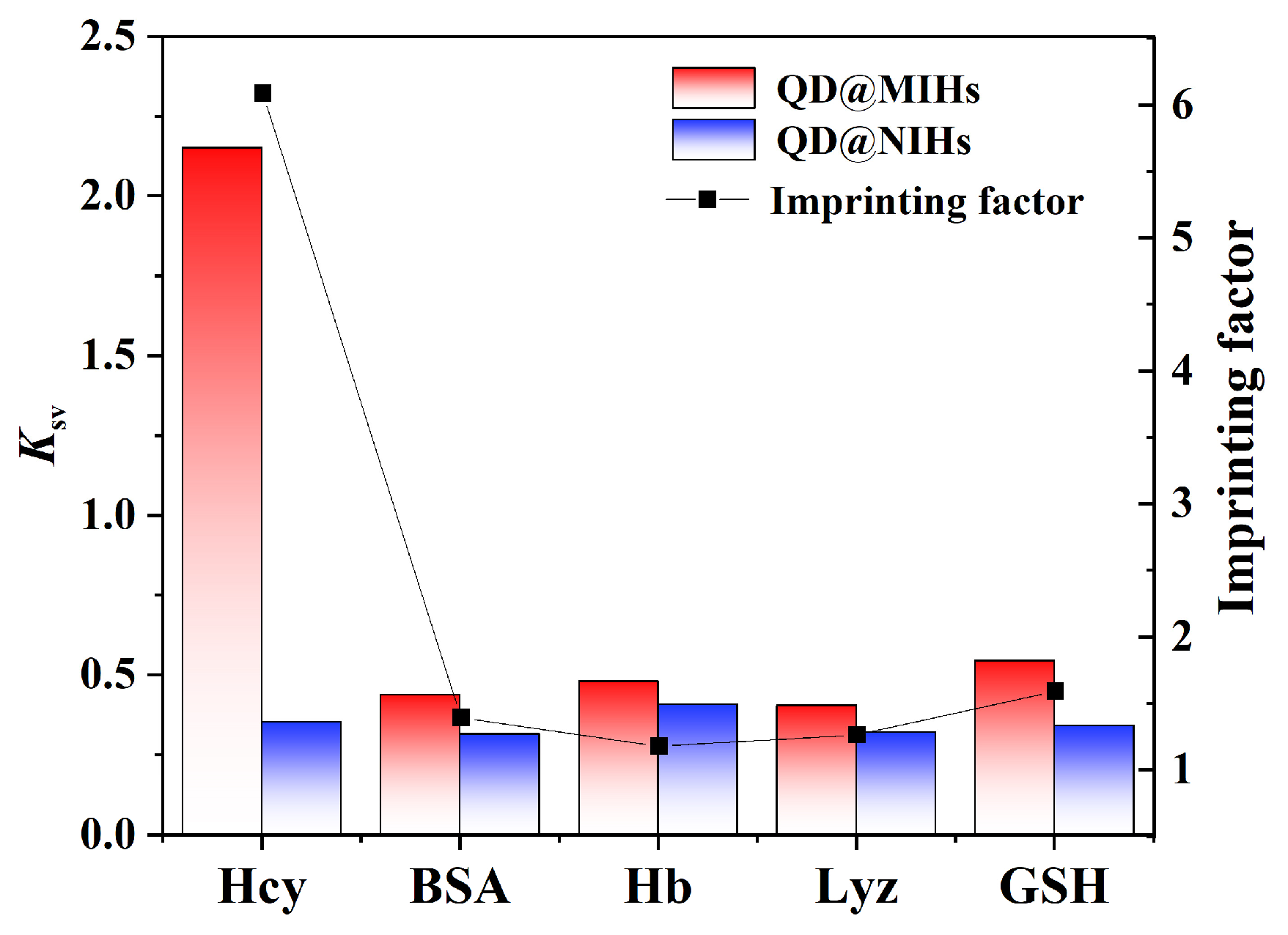
2.6. Selective Experiments
2.7. Applications
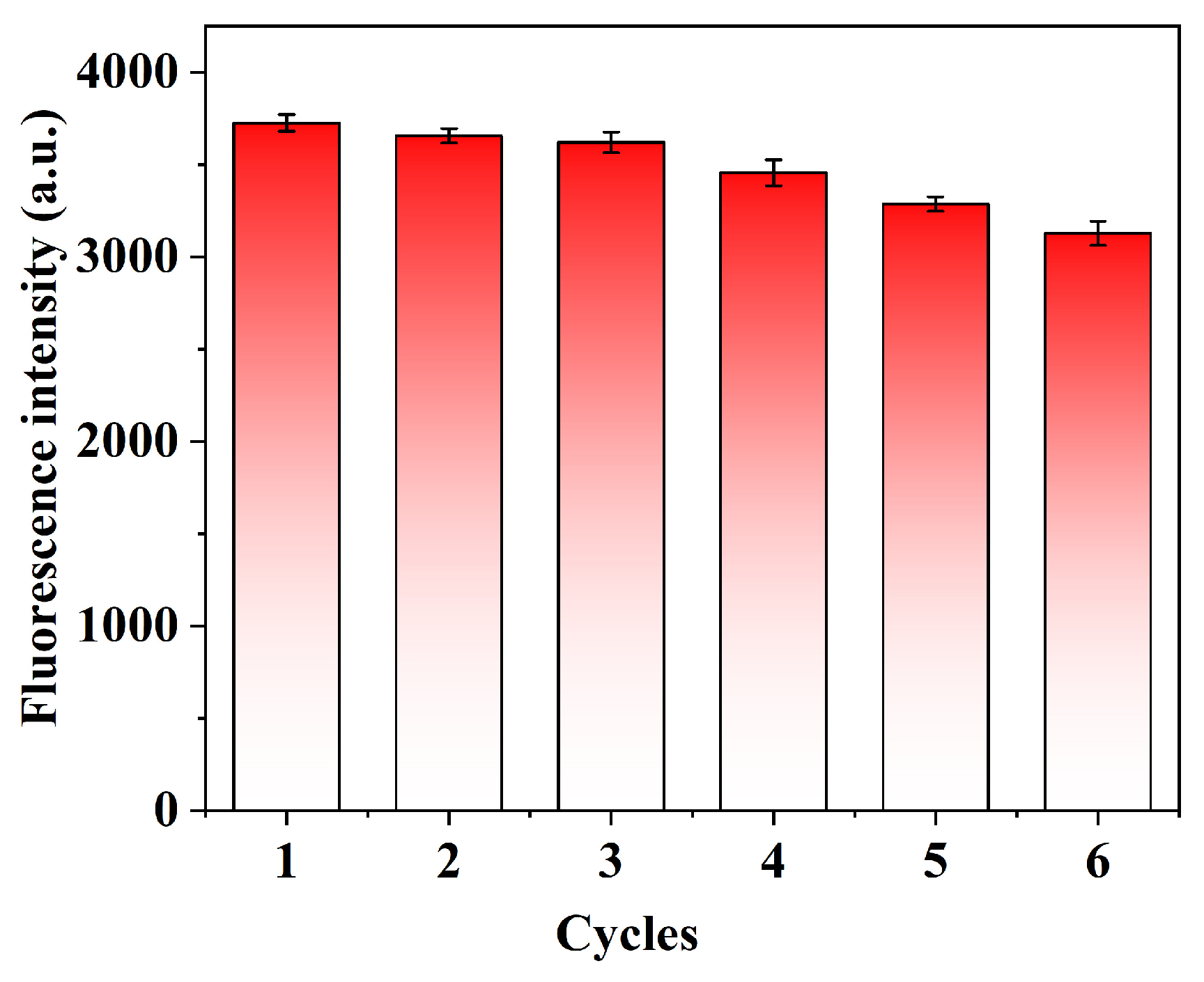
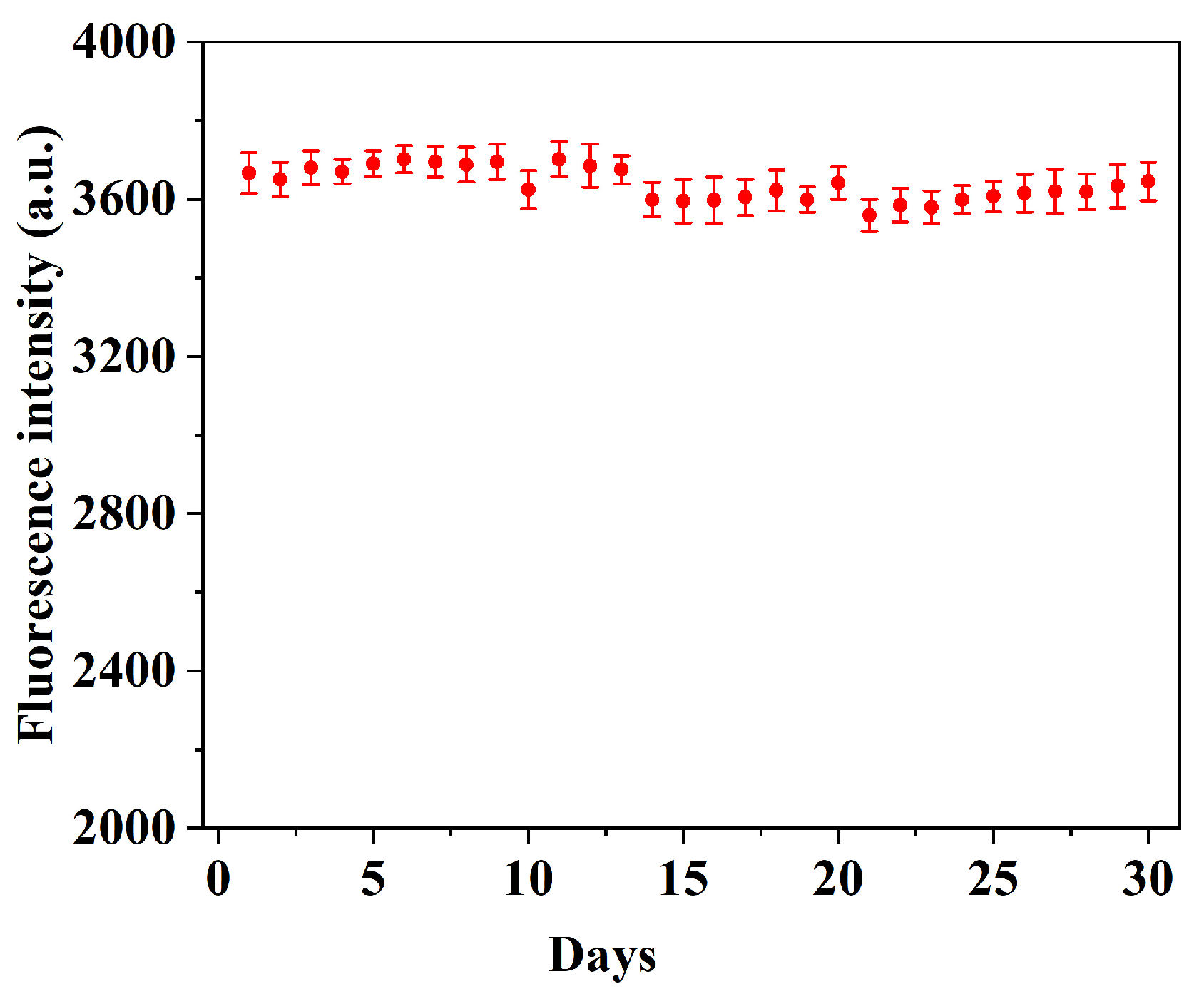
2.8. Reusability and Stability of QD@MIHs
3. Conclusions
4. Materials and Methods
4.1. Instruments and Reagents
4.2. Synthesis of L-Cysteine-Modified Quantum Dots
4.3. Fabrication of Molecularly-Imprinted-Hydrogel-Capped QDs
4.4. Effect of pH on the QD@MIHs
4.5. Adsorption Experiments
4.5.1. Adsorption Kinetics
4.5.2. Binding Isotherm Experiments
4.6. Determination of Homocysteine Using QD@MIHs
4.7. Selective Experiments Methods
4.8. Application in Real Samples
4.9. Stability and Reusability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QDs | Quantum dot |
| Hcy | Homocysteine |
| MIHs | Molecularly imprinted hydrogel |
| MIT | Molecular imprinting technology |
| GC | Gas chromatography |
| HPLC | High-performance liquid chromatography |
| IF | Imprinting factor |
| NIHs | Non-imprinted hydrogels |
| QD@MIHs | Quantum dot (QD)-based molecularly imprinted hydrogels |
References
- Wu, D.F.; Yin, R.X.; Deng, J.L. Homocysteine, hyperhomocysteinemia, and H-type hypertension. Eur. J. Prev. Cardiol. 2024, 31, 1092–1103. [Google Scholar] [CrossRef]
- Hu, D.F.; Yu, Y.; Wang, G.Y.; Wang, P.F.; Pu, J. Aberrant Human Epididymal Protein 4 in Heart Failure Patients’ Serum and its Association with C-Reactive Protein, Uric Acid and Homocysteine. Clin. Lab. 2024, 70, 740. [Google Scholar] [CrossRef]
- McCully, K.S. Homocysteine, vitamins, and vascular disease prevention. Am. J. Clin. Nutr. 2007, 86, 1563S–1568S. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine–from disease biomarker to disease prevention. J. Intern. Med. 2021, 290, 826–854. [Google Scholar] [CrossRef]
- Clarke, R.; Halsey, J.; Bennett, D.; Lewington, S. Homocysteine and vascular disease: Review of published results of the homocysteine-lowering trials. J. Inherit. Metab. Dis. 2021, 34, 83–91. [Google Scholar] [CrossRef]
- Hermann, A.; Sitdikova, G. Homocysteine: Biochemistry, molecular biology and role in disease. Biomolecules 2021, 11, 737. [Google Scholar] [CrossRef]
- Koklesova, L.; Mazurakova, A.; Samec, M.; Biringer, K.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Golubnitschaja, O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021, 12, 477–505. [Google Scholar] [CrossRef] [PubMed]
- Pervez, W.; Yin, C.; Huo, F. Homocysteine fluorescent probes: Sensing mechanisms and biological applications. Coord. Chem. Rev. 2025, 522, 216202. [Google Scholar] [CrossRef]
- Zhou, L. Homocysteine and Parkinson’s disease. CNS Neurosci. Ther. 2024, 30, e14420. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, G.; Brombo, G.; Polastri, M.; Romagnoli, T.; Mola, G.; Riccetti, R.; Seripa, G.; Trentini, A.; Cervellati, C. High plasma homocysteine levels predict the progression from mild cognitive impairment to dementia. Neurochem. Int. 2024, 177, 105763. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, H.; Cai, Y.; Shen, M.; Li, X.; Han, Y.; Zong, L.; Zhang, Y. Hydrogen sulfide-mediated persulfidation regulates homocysteine metabolism and enhances ferroptosis in non-small cell lung cancer. Mol. Cell. 2024, 84, 4016–4030. [Google Scholar] [CrossRef]
- Ding, C.; Li, J.; Wei, Y.; Fan, W.; Cao, T.; Chen, Z.; Shi, Y.; Yu, C.; Yuan, T.; Zhao, P.; et al. Associations of total homocysteine and kidney function with all-cause and cause-specific mortality in hypertensive patients: A mediation and joint analysis. Hypertens. Res. 2024, 47, 1500–1511. [Google Scholar] [CrossRef]
- Tawfik, A.; Mohamed, R.; Elsherbiny, N.M.; DeAngelis, M.M.; Bartoli, M.; Al-Shabrawey, M. Homocysteine: A potential biomarker for diabetic retinopathy. J. Clin. Med. 2019, 8, 121. [Google Scholar] [CrossRef]
- Wang, W.; Rusin, O.; Xu, X.; Kim, K.K.; Escobedo, J.O.; Fakayode, S.O.; Fletcher, K.A.; Lowry, M.; Schowalter, C.M.; Lawrence, C.M.; et al. Detection of homocysteine and cysteine. J. Am. Chem. Soc. 2005, 127, 15949–15958. [Google Scholar] [CrossRef]
- Piechocka, J.; Wieczorek, M.; Głowacki, R. Gas chromatography–mass spectrometry based approach for the determination of methionine-related sulfur-containing compounds in human saliva. Int. J. Mol. Sci. 2020, 21, 9252. [Google Scholar] [CrossRef]
- Li, J.S.; Huang, D.; Qiu, H.M.; Jiang, Q.S.; Liu, Y.H.; Du, T.T.; Jiang, X.H. A study on the determination of two related aminothiols in the brain of diabetes rats by HPLC-ECD. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 725–732. [Google Scholar] [CrossRef]
- Mikaliunaite, L.; Green, D.B. Using a 3-hydroxyflavone derivative as a fluorescent probe for the indirect determination of aminothiols separated by ion-pair HPLC. Anal. Methods 2021, 13, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
- Greño, M.; Salgado, A.; Castro-Puyana, M.; Marina, M.L. Nuclear magnetic resonance to study the interactions acting in the enantiomeric separation of homocysteine by capillary electrophoresis with a dual system of γ-cyclodextrin and the chiral ionic liquid EtCholNTf2. Electrophoresis 2019, 40, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, R.; Khan, M.A.; Mushtaq, M.H.; Nasir, M.; Khan, A.; Haq, I.U.; Muhammad, J. Hyperhomocysteinemia as an independent risk factor for coronary heart disease. Comparison with conventional risk factors. Braz. J. Biol. 2021, 83, e249104. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, M.; Głowacki, R.; Piechocka, J. First HPLC-UV method for the determination of homocysteine thiolactone in human urine after derivatization with 1-benzyl-2-chloropyridinium bromide. Sci. Rep. 2025, 15, 7310. [Google Scholar] [CrossRef]
- Piechocka, J.; Głowacki, R. Comprehensive studies on the development of HPLC-MS/MS and HPLC-FL based methods for routine determination of homocysteine thiolactone in human urine. Talanta 2024, 272, 125791. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T.S. Molecularly imprinted polymers. Mol. Imprint. 2011, 325, 1–28. [Google Scholar]
- Liu, Y.; Wang, L.; Li, H.; Zhao, L.; Ma, Y.; Zhang, Y.; Liu, J.; Wei, Y. Rigorous recognition mode analysis of molecularly imprinted polymers—Rational design, challenges, and opportunities. Prog. Polym. Sci. 2024, 150, 101790. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef]
- Byrne, M.E.; Park, K.; Peppas, N.A. Molecular imprinting within hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 149–161. [Google Scholar] [CrossRef]
- Fan, J.P.; Lai, J.H.; Huang, C.B.; Lai, Z.T.; Xie, C.F.; Chen, H.P.; Liu, Y.D. Synthesis of a gelatin based molecularly imprinted hydrogel with high selectivity on adsorbing bovine serum albumin. Sep. Purif. Technol. 2024, 328, 124999. [Google Scholar] [CrossRef]
- Ivaskiene, T.; Kaspute, G.; Ramanavicius, A.; Prentice, U. Molecularly imprinted polymer advanced hydrogels as tools for gastrointestinal diagnostics. Gels 2025, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kubo, T.; Otsuka, K. Specific recognition of a target protein, cytochrome c, using molecularly imprinted hydrogels. J. Mater. Chem. B 2022, 10, 6800–6807. [Google Scholar] [CrossRef]
- Pan, M.; Gao, M.; Cui, J.; Gao, R.; Li, H.; Sun, J.; Chen, W.; Wang, S. Fluorescent molecularly imprinted hydrogel sensing strip based on nitrogen-doped carbon dots and inverse opal photonic crystals applying for effective detection for imidacloprid in fruits and vegetables. Food Chem. 2025, 477, 143497. [Google Scholar] [CrossRef] [PubMed]
- Benito-Pena, E.; Gonzalez-Vallejo, V.; Rico-Yuste, A.; Barbosa-Pereira, L.; Cruz, J.M.; Bilbao, A.; Alvarez-Lorenzo, C.; Moreno-Bondi, M.C. Molecularly imprinted hydrogels as functional active packaging materials. Food Chem. 2016, 190, 487–494. [Google Scholar] [CrossRef]
- Kakkar, V.; Narula, P. Role of molecularly imprinted hydrogels in drug delivery—A current perspective. Int. J. Pharm. 2022, 625, 121883. [Google Scholar] [CrossRef] [PubMed]
- Völlmecke, K.; Afroz, R.; Bierbach, S.; Brenker, L.J.; Frücht, S.; Glass, A.; Giebelhaus, R.; Hoppe, A.; Kanememaru, K.; Lazarek, M.; et al. Hydrogel-based biosensors. Gels 2022, 8, 768. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, Z.; Lin, Y.; Wei, X.; Zhao, K.; Yang, Z. Bovine serum albumin molecularly imprinted electrochemical sensors modified by carboxylated multi-walled carbon nanotubes/CaAlg hydrogels. Gels 2023, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Kershaw, S.V.; Li, Y.; Huang, X.; Li, Y.; Rogach, A.L.; Gao, M. Aqueous based semiconductor nanocrystals. Chem. Rev. 2016, 116, 10623–10730. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in quantum dots photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Azeez, K.F.; Salimi, A.; Hallaj, R. MOF and CdTe quantum dots entrapped hydrogel film as dual-signal ratiometric fluorometric-colorimetric sensing platform for erythromycin detection: Portable smartphone-assisted visual sensing in milk and water samples. Food Chem. 2025, 482, 143972. [Google Scholar] [CrossRef]
- Yang, S.; Sarkar, S.; Xie, X.; Li, D.; Chen, J. Application of Optical Hydrogels in Environmental Sensing. Energy Environ. Mater. 2024, 7, e12646. [Google Scholar] [CrossRef]
- Piloto, A.M.L.; Ribeiro, D.S.; Santos, J.L.; Sales, G. Development of a sensitive ratiometric imprinted hydrogel for the detection of matrix metalloproteinase 7 (MMP7) biomarker. ACS Appl. Opt. Mater. 2023, 2, 57–67. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-responsive hydrogels: Intelligent materials for biosensing and drug delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, Y.; Kawamura, A.; Takashima, Y.; Miyata, T. Design of molecularly imprinted hydrogels with thermoresponsive drug binding sites. J. Mater. Chem. B 2022, 10, 6644–6654. [Google Scholar] [CrossRef]
- Tan, L.; Kang, C.; Xu, S.; Tang, Y. Selective room temperature phosphorescencesensing of target protein using Mn-doped ZnS QDs-embedded molecularlyimprinted polymer. Biosens. Bioelectron. 2013, 48, 216–223. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, H.; Wang, L. Composite QDs@MIP nanospheres for specific recognition and direct fluorescent quantification of pesticides in aqueous media. Anal. Chem. 2012, 84, 386–395. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Peng, Y.; Yang, Q.; Chen, X.; Wu, W.; Zhu, Y.; Zhuang, S. Quantitative analysis of homocysteine in liquid by terahertz spectroscopy. Biomed. Opt. Express 2020, 11, 2570–2577. [Google Scholar] [CrossRef]
- Wen, X.H.; Zhao, X.F.; Peng, B.F.; Yuan, K.P.; Li, X.X.; Zhu, L.Y.; Lu, H.L. Facile preparation of an electrochemical aptasensor based on Au NPs/graphene sponge for detection of homocysteine. Appl. Surf. Sci. 2021, 556, 149735. [Google Scholar] [CrossRef]
- Wei, F.; Ding, Y.; Ou, J.; Chen, X.; Li, L.; Zhou, Q.; Chen, Q.; Wang, Q.; Feng, Y.; Meng, X. Accurate detection of hcy in human serum and two-photon visualization of atherosclerosis using a highly specific fluorescent probe. Anal. Chem. 2023, 95, 9173–9181. [Google Scholar] [CrossRef] [PubMed]
- Kongintr, U.; Lertanantawong, B.; Promptmas, C. A label-free electrochemical biosensor for homocysteine detection using molecularly imprinted polymer and nanocomposite-modified electrodes. Polymers 2023, 15, 2241. [Google Scholar] [CrossRef] [PubMed]
- da Conceição, P.; dos Santos Neto, A.G.; Khan, S.; Tanaka, A.A.; Santana, A.E.G.; del Pilar Taboada-Sotomayor, M.; Goulart, M.O.F.; Santos, A.C.F. Extraction-assisted voltammetric determination of homocysteine using magnetic nanoparticles modified with molecularly imprinted polymer. Microchim. Acta 2023, 190, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, M.; Zhang, Y.; Zhao, P.; Cai, J.; Yao, Y.; Liang, J. Preparation of Molecularly Imprinted Cysteine Modified Zinc Sulfide Quantum Dots Based Sensor for Rapid Detection of Dopamine Hydrochloride. Molecules 2023, 28, 3646. [Google Scholar] [CrossRef]


| Analysis Technique | Linearity (μM) | LOD | Detection Time (min) | References |
|---|---|---|---|---|
| HPLC | 0.15–22.2 | 0.037 | 30.0 | [16] |
| Terahertz spectroscopy | 10.0–150.0 | 10.0 | 7.0 | [44] |
| Electrochemical biosensor | 5.0–150 | 1.2 | 10.0 | [45] |
| Two-photon fluorescent probe | 0–10 | 0.018 | 60.0 | [46] |
| Aptasensor based on aptamer/Au NPs | 1–100 | 1.0 | 30.0 | [47] |
| Magnetic imprinted polymer | 1.0–2.0 | 0.03 | 90.0 | [48] |
| QD@MIHs | 0.1–10 | 0.027 | 5.0 | This work |
| Sample | Detected (μM) | Added (μM) | Measured (μM) | Recovery Rates (%) | RSD (n = 3, %) |
|---|---|---|---|---|---|
| urine sample 1 | Nd 1 | 0.1 | 0.0952 | 95.2 | 4.25 |
| 0.5 | 0.5128 | 102.6 | 5.67 | ||
| 1.0 | 0.9838 | 98.4 | 3.56 | ||
| urine sample 2 | nd | 0.1 | 0.0974 | 97.4 | 5.72 |
| 0.5 | 0.5204 | 104.1 | 6.19 | ||
| 1.0 | 1.015 | 101.5 | 4.23 | ||
| urine sample 3 | nd | 0.1 | 0.0943 | 94.34 | 7.17 |
| 0.5 | 0.5178 | 103.6 | 5.21 | ||
| 1.0 | 1.012 | 101.2 | 5.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liang, J.; Zheng, B.; Jiao, P.; Xu, Q. Quantum-Dot-Based Molecularly Imprinted Hydrogel for Rapid Detection of Homocysteine. Gels 2025, 11, 632. https://doi.org/10.3390/gels11080632
Zhang X, Liang J, Zheng B, Jiao P, Xu Q. Quantum-Dot-Based Molecularly Imprinted Hydrogel for Rapid Detection of Homocysteine. Gels. 2025; 11(8):632. https://doi.org/10.3390/gels11080632
Chicago/Turabian StyleZhang, Xin, Jiarong Liang, Binglei Zheng, Pengfei Jiao, and Qian Xu. 2025. "Quantum-Dot-Based Molecularly Imprinted Hydrogel for Rapid Detection of Homocysteine" Gels 11, no. 8: 632. https://doi.org/10.3390/gels11080632
APA StyleZhang, X., Liang, J., Zheng, B., Jiao, P., & Xu, Q. (2025). Quantum-Dot-Based Molecularly Imprinted Hydrogel for Rapid Detection of Homocysteine. Gels, 11(8), 632. https://doi.org/10.3390/gels11080632





