Advances in Hydrogel-Based Delivery of RNA Drugs for Antitumor Therapy
Abstract
1. Introduction
2. Hydrogels
2.1. Classification of Materials for Hydrogel Preparation
2.1.1. Natural Hydrogels
2.1.2. Synthetic Hydrogels
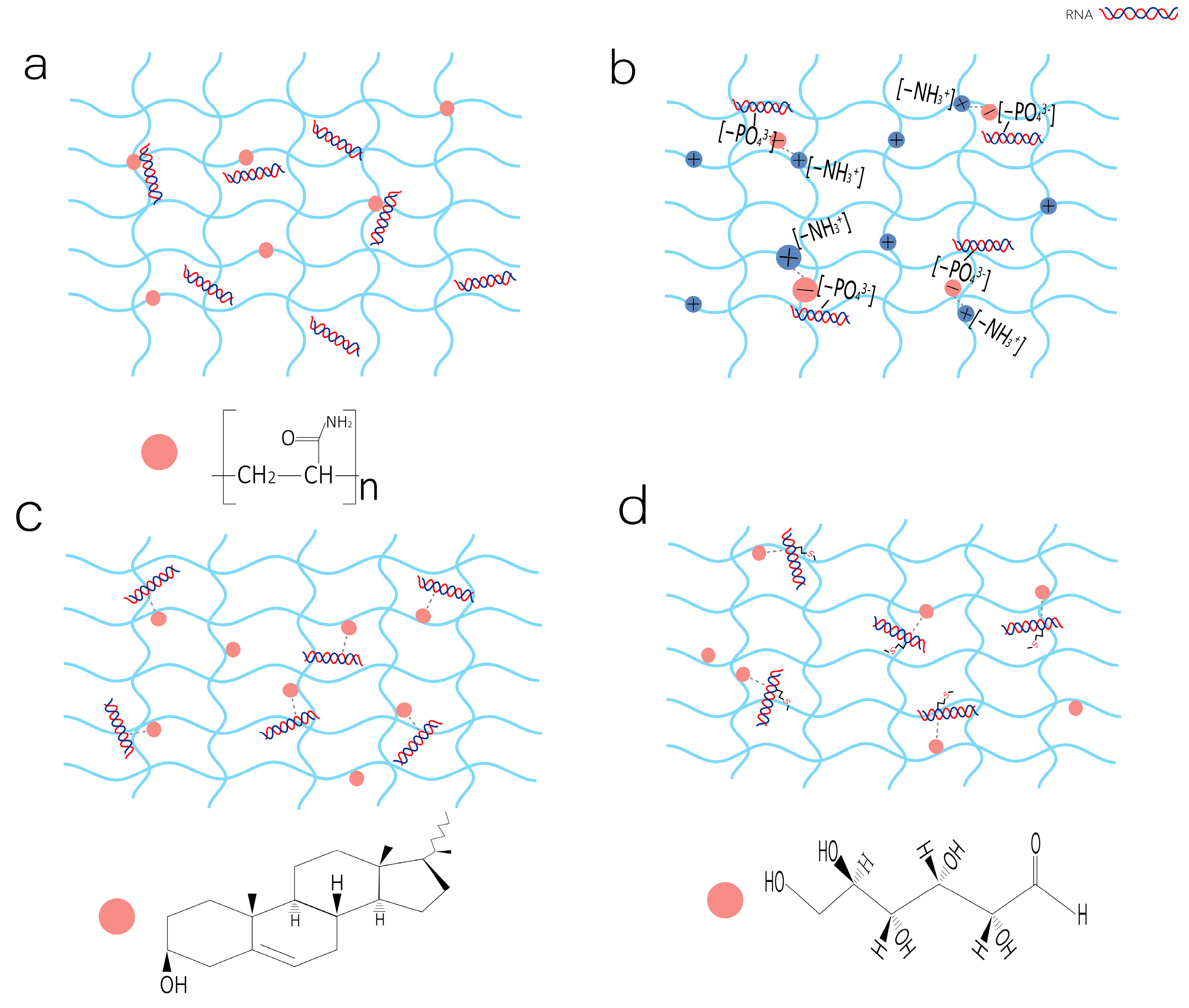
2.2. The Mechanisms of RNA Drug Loading in Hydrogels
2.2.1. Physical Embedding
2.2.2. Electrostatic Interaction
2.2.3. Hydrophobic Interactions
2.2.4. Covalent Coupling
2.3. Hydrogel Systems for RNA Delivery
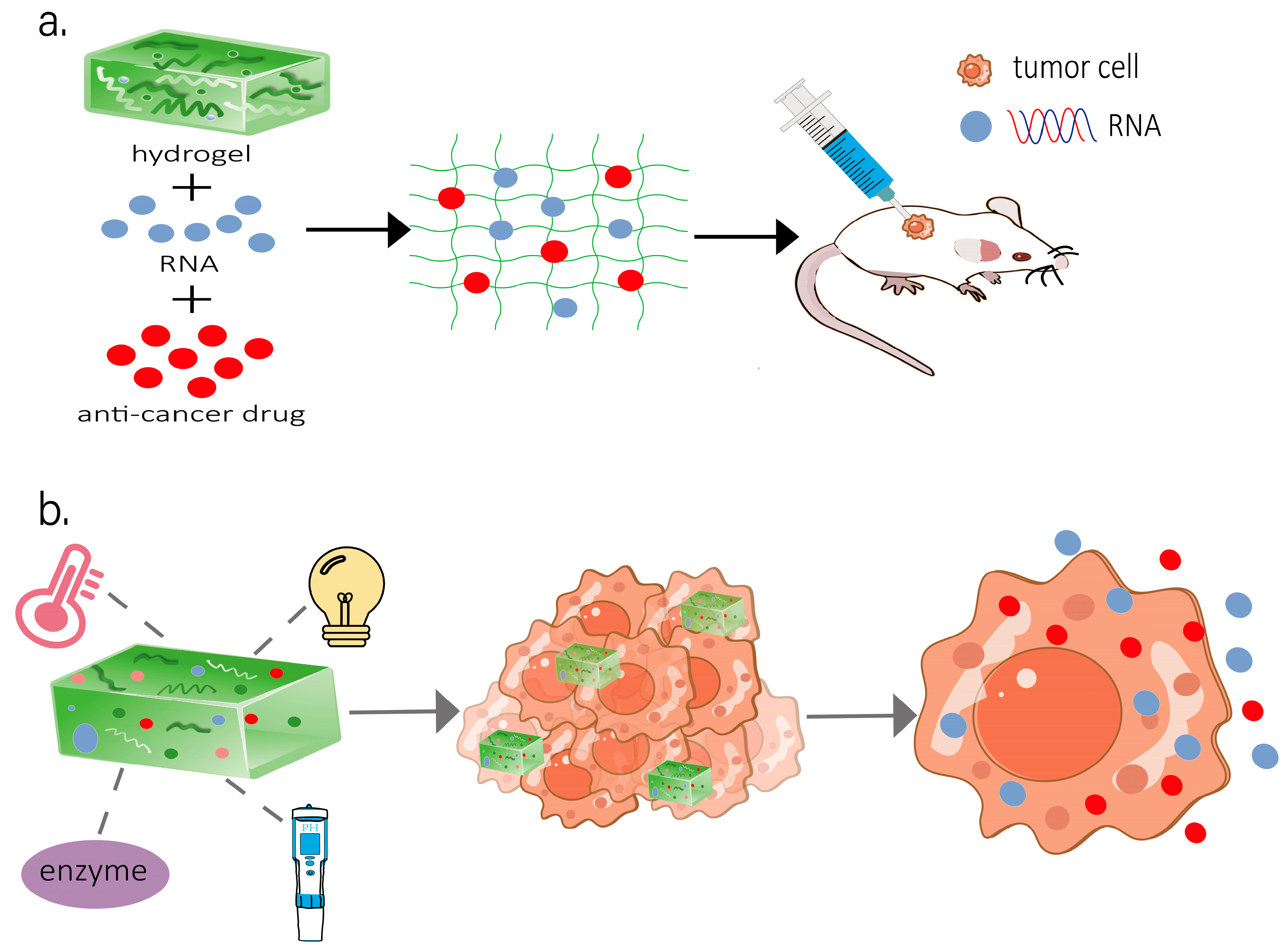
2.3.1. Stimuli-Responsive Hydrogel Systems for RNA Delivery
pH-Responsive Hydrogels
Thermosensitive Hydrogels
Photoresponsive Hydrogels
Magnetic Responsive Hydrogels
Multi-Stimulus Responsive Hydrogels
2.3.2. Non-Stimuli-Responsive Hydrogel Systems for RNA Delivery
3. Application of Hydrogel Delivery of RNA in Anti-Tumor Therapy
3.1. Breast Cancer
3.2. Melanoma
3.3. Colon Cancer
3.4. Ovarian Cancer
3.5. Glioblastoma
3.6. Hepatocellular Carcinoma
3.7. Lung Cancer
3.8. Osteosarcoma
3.9. Pancreatic Cancer
3.10. Other Cancers
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer Incidence and Mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef]
- Mitra, A.; Kumar, A.; Amdare, N.P.; Pathak, R. Current Landscape of Cancer Immunotherapy: Harnessing the Immune Arsenal to Overcome Immune Evasion. Biology 2024, 13, 307. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer Treatment Therapies: Traditional to Modern Approaches to Combat Cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Xiao, S.; Mu, M.; Feng, C.; Pan, S.; Chen, N. The Application of Bacteria-Nanomaterial Hybrids in Antitumor Therapy. J. Nanobiotechnol. 2024, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Lang, T.; Yan, W.; Zhu, X.; Huang, X.; Yin, Q.; Li, Y. Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy. Adv. Sci. 2021, 8, 2003542. [Google Scholar] [CrossRef]
- Hansen, S.N.; Holm, A.; Kauppinen, S.; Klitgaard, H. RNA Therapeutics for Epilepsy: An Emerging Modality for Drug Discovery. Epilepsia 2023, 64, 3113–3129. [Google Scholar] [CrossRef]
- Ciucci, G.; Braga, L.; Zacchigna, S. Discovery Platforms for RNA Therapeutics. Br. J. Pharmacol. 2025, 182, 281–295. [Google Scholar] [CrossRef]
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of Oligonucleotide-Based Therapeutics: Challenges and Opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, J.; Choi, Y.; Kim, K.; Yang, Y.; Kim, S.H.; Yoon, H.Y.; Kwon, I.C. Molecularly Engineered siRNA Conjugates for Tumor-Targeted RNAi Therapy. J. Control Release 2022, 351, 713–726. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. miRNA Biogenesis and Regulation of Diseases: An Updated Overview. Methods Mol. Biol. 2023, 2595, 1–12. [Google Scholar] [CrossRef]
- Fu, J.; Wu, S.; Bao, N.; Wu, L.; Qu, H.; Wang, Z.; Dong, H.; Wu, J.; Jin, Y. A Universal Strategy of Anti-Tumor mRNA Vaccine by Harnessing “Off-the-Shelf” Immunity. Adv. Sci. 2025, 12, e2401287. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic Acid Delivery for Therapeutic Applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.S.; Thelemann, T.; Klar, R.; Kallert, S.M.; Festag, J.; Buchi, M.; Hinterwimmer, L.; Schell, M.; Michel, S.; Jaschinski, F.; et al. Antisense Oligonucleotide Targeting CD39 Improves Anti-Tumor T Cell Immunity. J. Immunother. Cancer 2019, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, M.; Song, Y.; Kim, S.; Park, N. Recent Advances and Prospects of Nucleic Acid Therapeutics for Anti-Cancer Therapy. Molecules 2024, 29, 4737. [Google Scholar] [CrossRef]
- Su, L.-J.; Ji, Z.-H.; Xu, M.-X.; Zhu, J.-Q.; Chen, Y.-H.; Qiao, J.-F.; Wang, Y.; Lin, Y.-X. RNA-Based Nanomedicines and Their Clinical Applications. Nano Res. 2023, 16, 13182–13204. [Google Scholar] [CrossRef]
- Wang, J.; Tan, M.; Wang, Y.; Liu, X.; Lin, A. Advances in Modification and Delivery of Nucleic Acid Drugs. Zhejiang Da Xue Xue Bao Yi Xue Ban 2023, 52, 417–428. [Google Scholar] [CrossRef]
- Li, N.; Sun, Y.; Fu, Y.; Sun, K. RNA Drug Delivery Using Biogenic Nanovehicles for Cancer Therapy. Front. Pharmacol. 2021, 12, 734443. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Frazar, E.M.; X Klaus, M.V.; Paul, P.; Hilt, J.Z. Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Human and Environmental Treatment. Adv. Healthc. Mater. 2022, 11, e2101820. [Google Scholar] [CrossRef]
- Sun, J.; Yin, Z.; Wang, X.; Su, J. Exosome-Laden Hydrogels: A Novel Cell-Free Strategy for In-Situ Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 866208. [Google Scholar] [CrossRef]
- Ghosh, G.; Barman, R.; Sarkar, J.; Ghosh, S. pH-Responsive Biocompatible Supramolecular Peptide Hydrogel. J. Phys. Chem. B 2019, 123, 5909–5915. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Wei, H.; Yu, C.-Y. Injectable Hydrogels as Emerging Drug-Delivery Platforms for Tumor Therapy. Biomater. Sci. 2024, 12, 1151–1170. [Google Scholar] [CrossRef]
- Wu, G.; Zhong, C.; Tian, X.; Zha, L.; Hou, L.; Feng, X. Emerging Roles of Hyaluronic Acid Hydrogels in Cancer Treatment and Wound Healing: A Review. Int. J. Biol. Macromol. 2025, 303, 140442. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Minculescu, M.A.; Niculescu, A.-G.; Hudiță, A.; Holban, A.M.; Alberts, A.; Grumezescu, A.M. Nanoparticle-Enhanced Collagen Hydrogels for Chronic Wound Management. J. Funct. Biomater. 2025, 16, 91. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Qin, M.; Lan, W.; Wang, L.; Liang, Z.; Li, X.; Wei, Y.; Hu, Y.; Zhao, L.; et al. Abundant Tannic Acid Modified Gelatin/Sodium Alginate Biocomposite Hydrogels with High Toughness, Antifreezing, Antioxidant and Antibacterial Properties. Carbohydr. Polym. 2023, 309, 120702. [Google Scholar] [CrossRef]
- Chellathurai, M.S.; Chung, L.Y.; Hilles, A.R.; Sofian, Z.M.; Singha, S.; Ghosal, K.; Mahmood, S. Pharmaceutical Chitosan Hydrogels: A Review on Its Design and Applications. Int. J. Biol. Macromol. 2024, 280, 135775. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, G.; Zhang, X.; Zheng, Y.; Lee, S.; Wang, D.; Yang, Y. Polyvinyl Alcohol/Chitosan and Polyvinyl Alcohol/Ag@MOF Bilayer Hydrogel for Tissue Engineering Applications. Polymers 2021, 13, 3151. [Google Scholar] [CrossRef]
- Chen, X.; Yan, H.; Bao, C.; Zhu, Q.; Liu, Z.; Wen, Y.; Li, Z.; Zhang, T.; Lin, Q. Fabrication and Evaluation of Homogeneous Alginate/Polyacrylamide–Chitosan–Gelatin Composite Hydrogel Scaffolds Based on the Interpenetrating Networks for Tissue Engineering. Polym. Eng. Sci. 2022, 62, 116. [Google Scholar] [CrossRef]
- Bagheri, E.; Ramezani, M.; Mohammadi, M.; Alibolandi, M. Injectable Hydrogels for Intratumoral Administration Against Breast Cancer. J. Polym. Environ. 2024, 32, 5468–5498. [Google Scholar] [CrossRef]
- Kass, L.E.; Nguyen, J. Nanocarrier-Hydrogel Composite Delivery Systems for Precision Drug Release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic Acid in the Treatment of Dry Eye Disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Zhang, Y.; Hu, J.; Ikegami, Y.; Aishima, S.; Ijima, H. Fast Wound Healing with a New Functional Hyaluronic Acid Dual Network Hydrogel. Gels 2025, 11, 266. [Google Scholar] [CrossRef]
- Sohani, Z.; Jamshidi, S.; Koohi, M.K.; Malakootikhah, J.; Abarkar, M.; Golchin, D.; Roshani, S.; Naghdi, H.; Aghajanpour-moghaddam-gazafroudi, N.; Gazafroudi; et al. Novel Ophthalmic Hyaluronic Acid-Hydrogel with Curcumin Nanoparticles for Enhanced Healing of Ulcerative Keratitis in Rabbit Model. Sci. Rep. 2024, 14, 23046. [Google Scholar] [CrossRef]
- Watson, A.L.; Eckhart, K.E.; Wolf, M.E.; Sydlik, S.A. Hyaluronic Acid-Based Antibacterial Hydrogels for Use as Wound Dressings. ACS Appl. Bio Mater. 2022, 5, 5608–5616. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.; Han, Y.; Cui, J.; Jing, Z.; Li, D.; Liu, J.; Xiao, C.; Li, D.; Cai, B. Collagen-Based Hydrogels for Cartilage Regeneration. Orthop. Surg. 2023, 15, 3026–3045. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Hashem, A.H.; Khattab, T.A.; Kamel, S. New Antibacterial Hydrogels Based on Sodium Alginate. Int. J. Biol. Macromol. 2023, 248, 125872. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-Based Hydrogel Wound Dressing: From Mechanism to Applications, a Review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Xia, W.; Cao, D.; Wang, X.; Kuang, Y.; Luo, Y.; Yuan, C.; Lu, J.; Liu, X. Application of Hydrogels as Carrier in Tumor Therapy: A Review. Chem. Asian J. 2022, 17, e202200740. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Do, N.H.N.; Lac, H.D.; Nguyen, P.L.N.; Le, P.K. Synthesis, Properties, and Applications of Chitosan Hydrogels as Anti-Inflammatory Drug Delivery System. J. Porous Mater. 2022, 30, 655–670. [Google Scholar] [CrossRef]
- Yao, J.; Song, S.; Zhao, H.; Yuan, Y. Platinum-Based Drugs and Hydrogel: A Promising Anti-Tumor Combination. Drug Deliv. 2023, 30, 2287966. [Google Scholar] [CrossRef] [PubMed]
- Chhetry, G.; Pattader, P.S.G. Effect of Patterns on Polyacrylamide Hydrogel Surface towards Enhancement of Water Retention. Polymer 2024, 308, 127362. [Google Scholar] [CrossRef]
- Poyraz, Y.; Baltacı, N.; Hassan, G.; Alayoubi, O.; Uysal, B.Ö.; Pekcan, Ö. Composite Hydrogel of Polyacrylamide/Starch/Gelatin as a Novel Amoxicillin Delivery System. Gels 2024, 10, 625. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Zeng, Q.; Wang, N.; Li, W.; Chen, B.; Guan, Q.; Li, C.; Li, W. Fabricating Oxygen Self-Supplying 3D Printed Bioactive Hydrogel Scaffold for Augmented Vascularized Bone Regeneration. Bioact. Mater. 2024, 40, 227–243. [Google Scholar] [CrossRef]
- Tang, S.; Gong, Z.; Wang, Z.; Gao, X.; Zhang, X. Multifunctional Hydrogels for Wound Dressings Using Xanthan Gum and Polyacrylamide. Int. J. Biol. Macromol. 2022, 217, 944–955. [Google Scholar] [CrossRef]
- Saleh, R.I.; Park, J.-H.; Choi, C.; Jo, W.; Cha, C. Harnessing the Versatility and Precision of Room-Temperature Multiferroics for Dual-Mode Nanocomposite Hydrogel Biosensor. Chem. Eng. J. 2025, 513, 162896. [Google Scholar] [CrossRef]
- Ishikawa, S.; Yasuda, T.; Iwanaga, Y.; Sakai, T. Gel–Gel Phase Separation in Clustered Poly(Ethylene Glycol) Hydrogel with Enhanced Hydrophobicity. ACS Macro Lett. 2024, 13, 1369–1375. [Google Scholar] [CrossRef]
- Schweizer, S.; Monteiro, I.; Oliveira, A.S.; Nolasco, P.; Colaço, R.; Serro, A.P. Physically Crosslinked Polyvinyl Alcohol Hydrogels as Synthetic Cartilage Materials. Ann. Med. 2021, 53, S33. [Google Scholar] [CrossRef]
- Ferozekhan, S.; Umashankar, M.S.; Narayanasamy, D. A Comprehensive Review of Nanogel-Based Drug Delivery Systems. Cureus 2024, 16, e68633. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Y.; Li, J.; Liu, L.; Zhang, X.; Bai, Z.; Zhang, C.; Gu, T.; Yang, J. Advanced Therapeutic Strategy: A Single-Dose Injection of a Dual-Loaded 6-Mercaptopurine Gelatin-Based Hydrogel for Effective Inhibition of Tumor Growth. Int. J. Biol. Macromol. 2025, 303, 140528. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ding, J.; Li, Y.; Wei, P.; Liu, S.; Yang, R. The Formation of Protein–Chitosan Complexes: Their Interaction, Applications, and Challenges. Foods 2024, 13, 3572. [Google Scholar] [CrossRef]
- Rao, S.; Liu, Q.; Li, B.; Sun, Y.; Wang, Y.; Gao, G.; Sun, Z.; Yang, J. Enhanced the Dispersibility and Permeability of Silver Nanoparticles over rGO Grafted by Positively-Charged Polyethyleneimine toward Broad-Spectrum Sterilization. Ceram. Int. 2023, 49, 20351–20356. [Google Scholar] [CrossRef]
- Radmanesh, F.; Sadeghi Abandansari, H.; Ghanian, M.H.; Pahlavan, S.; Varzideh, F.; Yakhkeshi, S.; Alikhani, M.; Moradi, S.; Braun, T.; Baharvand, H. Hydrogel-Mediated Delivery of microRNA-92a Inhibitor Polyplex Nanoparticles Induces Localized Angiogenesis. Angiogenesis 2021, 24, 657–676. [Google Scholar] [CrossRef]
- Chun, Y.Y.; Yap, Z.L.; Seet, L.F.; Chan, H.H.; Toh, L.Z.; Chu, S.W.L.; Lee, Y.S.; Wong, T.T.; Tan, T.T.Y. Positive-Charge Tuned Gelatin Hydrogel-siSPARC Injectable for siRNA Anti-Scarring Therapy in Post Glaucoma Filtration Surgery. Sci. Rep. 2021, 11, 1470. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Yang, B.; Lai, L.; Chen, N.; Hu, J.; Lu, Q. Dual Physically Cross-Linked Hydrogels Incorporating Hydrophobic Interactions with Promising Repairability and Ultrahigh Elongation. Adv. Funct. Mater. 2020, 31, 2008187. [Google Scholar] [CrossRef]
- Takei, T.; Yoshihara, R.; Danjo, S.; Fukuhara, Y.; Evans, C.; Tomimatsu, R.; Ohzuno, Y.; Yoshida, M. Hydrophobically-Modified Gelatin Hydrogel as a Carrier for Charged Hydrophilic Drugs and Hydrophobic Drugs. Int. J. Biol. Macromol. 2020, 149, 140–147. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Huynh, C.T.; Gilewski, A.; Wilner, S.E.; Maier, K.E.; Kwon, N.; Levy, M.; Alsberg, E. Covalently Tethering siRNA to Hydrogels for Localized, Controlled Release and Gene Silencing. Sci. Adv. 2019, 5, eaax0801. [Google Scholar] [CrossRef]
- Zhao, D.; Qian, L.; Yang, Q.; Li, X.; Ye, C.; Shi, T.; Wang, Y. Microfluidic Synthesis of Stimuli-Responsive Hydrogel Particles. Appl. Mater. Today 2025, 42, 102571. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, W.; Ni, S.; Zhu, X.; Wu, M. A Review of Stimuli-Responsive Hydrogels for RNA Delivery: From Material Innovations to Clinical Barriers. Int. J. Biol. Macromol. 2025, 318, 144862. [Google Scholar] [CrossRef]
- Li, Q.; Ma, W.; Ma, H.; Shang, H.; Qiao, N.; Sun, X. Synthesis and Characterization of Temperature-/pH-Responsive Hydrogels for Drug Delivery. ChemistrySelect 2023, 8, e202204270. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Han, H.; Xu, Z.; Li, S.; Zhu, Y.; Chen, Y.; Ge, L.; Zhang, Y. Short and Simple Peptide-Based pH-Sensitive Hydrogel for Antitumor Drug Delivery. Chin. Chem. Lett. 2022, 33, 1936–1940. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, R.; Sun, Y.; Gao, C.; Song, X.; Shi, F. A Drug-Carrying Composite Hydrogel with pH Sensitivity for Bone Tissue Engineering. Mater. Today Commun. 2025, 42, 111127. [Google Scholar] [CrossRef]
- Scarpa, E.; Mastronardi, V.M.; Guido, F.; Algieri, L.; Qualtieri, A.; Fiammengo, R.; Rizzi, F.; De Vittorio, M. Wearable Piezoelectric Mass Sensor Based on pH Sensitive Hydrogels for Sweat pH Monitoring. Sci. Rep. 2020, 10, 10854. [Google Scholar] [CrossRef]
- Wen, J.; Lei, J.; Chen, J.; Gou, J.; Li, Y.; Li, L. An Intelligent Coating Based on pH-Sensitive Hybrid Hydrogel for Corrosion Protection of Mild Steel. Chem. Eng. J. 2020, 392, 123742. [Google Scholar] [CrossRef]
- Rungsima, C.; Boonyan, N.; Klorvan, M.; Kusuktham, B. Hydrogel Sensors with pH Sensitivity. Polym. Bull. 2021, 78, 5769–5787. [Google Scholar] [CrossRef]
- Salahuddin, A.; Ashraf, A.; Ahmad, K.; Hou, H. Recent Advances in Chitosan-Based Smart Hydrogel for Drug Delivery Systems. Int. J. Biol. Macromol. 2024, 280, 135803. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-Crosslinked Mussel-Inspired Smart Hydrogels with Enhanced Antibacterial and Angiogenic Properties for Chronic Infected Diabetic Wound Treatment via pH-Responsive Quick Cargo Release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, P. pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-Co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers 2019, 11, 285. [Google Scholar] [CrossRef]
- Fu, X.; Chen, T.; Song, Y.; Feng, C.; Chen, H.; Zhang, Q.; Chen, G.; Zhu, X. mRNA Delivery by a pH-Responsive DNA Nano-Hydrogel. Small 2021, 17, e2101224. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Wang, J. A Temperature-Responsive Supramolecular Hydrogel: Preparation, Gel-Gel Transition and Molecular Aggregation. Soft Matter 2018, 14, 3090–3095. [Google Scholar] [CrossRef]
- Elsherbeny, A.; Bayraktutan, H.; Gumus, N.; McCrorie, P.; Garcia-Sampedro, A.; Parmar, S.; Ritchie, A.A.; Meakin, M.; Oz, U.C.; Rahman, R.; et al. Pentablock Thermoresponsive Hydrogels for Chemotherapeutic Delivery in a Pancreatic Cancer Model. Biomater. Sci. 2025, 13, 1831–1848. [Google Scholar] [CrossRef] [PubMed]
- Sala, R.L.; Kwon, M.Y.; Kim, M.; Gullbrand, S.E.; Henning, E.A.; Mauck, R.L.; Camargo, E.R.; Burdick, J.A. Thermosensitive Poly(N-Vinylcaprolactam) Injectable Hydrogels for Cartilage Tissue Engineering. Tissue Eng. Part A 2017, 23, 935–945. [Google Scholar] [CrossRef]
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of Phase Transition in Thermoresponsive Hydrogel PNIPAM: Role in Drug Delivery and Tissue Engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Bennett, C.F.; Swayze, E.E. RNA Targeting Therapeutics: Molecular Mechanisms of Antisense Oligonucleotides as a Therapeutic Platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef]
- Fliervoet, L.A.L.; Zhang, H.; van Groesen, E.; Fortuin, K.; Duin, N.J.C.B.; Remaut, K.; Schiffelers, R.M.; Hennink, W.E.; Vermonden, T. Local Release of siRNA Using Polyplex-Loaded Thermosensitive Hydrogels. Nanoscale 2020, 12, 10347–10360. [Google Scholar] [CrossRef]
- Wang, C.; Willner, B.; Willner, I. Redox-Responsive and Light-Responsive DNA-Based Hydrogels and Their Applications. React. Funct. Polym. 2021, 166, 104983. [Google Scholar] [CrossRef]
- Luo, R.; Xiang, X.; Jiao, Q.; Hua, H.; Chen, Y. Photoresponsive Hydrogels for Tissue Engineering. ACS Biomater. Sci. Eng. 2024, 10, 3612–3630. [Google Scholar] [CrossRef]
- Chau, A.L.; Karnaukh, K.M.; Maskiewicz, I.; Read de Alaniz, J.; Pitenis, A.A. Photoresponsive Hydrogel Friction. Soft Matter 2024, 20, 7227–7236. [Google Scholar] [CrossRef]
- Pandey, T.; Pandey, V. A Mechanistic Understanding to Photophysical Phenomenon in Development of Near-Infrared (NIR) Responsive Hydrogels: Advancements in Precision Drug Delivery. J. Drug Deliv. Sci. Technol. 2025, 106, 106682. [Google Scholar] [CrossRef]
- Yang, J.; Tan, Q.; Li, K.; Liao, J.; Hao, Y.; Chen, Y. Advances and Trends of Photoresponsive Hydrogels for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2024, 10, 1921–1945. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xu, S.; Ma, H.; Li, C.; Huang, Z. Photoresponsive Hydrogel-Based Soft Robot: A Review. Mater. Today Bio 2023, 20, 100657. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Huynh, C.T.; Nguyen, M.K.; Tonga, G.Y.; Longé, L.; Rotello, V.M.; Alsberg, E. Photocleavable Hydrogels for Light-Triggered siRNA Release. Adv. Healthc. Mater. 2016, 5, 305–310. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-Responsive Hydrogels: From Strategic Design to Biomedical Applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Sharma, S.; Singh, K.; Kumar, A. Magnetoresponsive Biocomposite Hydrogels Comprising Gelatin and Valine Based Magnetic Ionic Liquid Surfactant as Controlled Release Nanocarrier for Drug Delivery. Mater. Adv. 2022, 3, 484–492. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-Stimuli-Responsive Hydrogels and Their Medical Applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Cai, J.; Zhong, H.; Zhou, W.; Zhu, Z.; Yu, K.; Li, P. Near-Infrared/pH Dual-Responsive Triggered on-Demand Release of Curcumin from Ammonium Alginate/Polyvinyl Alcohol Hydrogel for Bacterial Infected Cutaneous Wound Healing. Eur. Polym. J. 2025, 225, 113712. [Google Scholar] [CrossRef]
- Itakura, S.; Hama, S.; Matsui, R.; Kogure, K. Effective Cytoplasmic Release of siRNA from Liposomal Carriers by Controlling the Electrostatic Interaction of siRNA with a Charge-Invertible Peptide, in Response to Cytoplasmic pH. Nanoscale 2016, 8, 10649–10658. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Kong, L.; He, Y.; Li, H. Injectable and Microporous Microgel-Fiber Granular Hydrogel Loaded with Bioglass and siRNA for Promoting Diabetic Wound Healing. Small 2024, 20, 2309599. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Wu, C.; Yan, F.; Li, X.; Zhang, S. A Self-Assembled RNA-Triple Helix Hydrogel Drug Delivery System Targeting Triple-Negative Breast Cancer. J. Mater. Chem. B 2020, 8, 3527–3533. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Ding, L.; Jin, H.J.; Li, X. RNA Hydrogel Combined with MnO2 Nanoparticles as a Nano-Vaccine to Treat Triple Negative Breast Cancer. Front. Chem. 2021, 9, 797094. [Google Scholar] [CrossRef]
- Chen, L.-H.; Liang, N.-W.; Huang, W.-Y.; Liu, Y.-C.; Ho, C.-Y.; Kuan, C.-H.; Huang, Y.-F.; Wang, T.-W. Supramolecular Hydrogel for Programmable Delivery of Therapeutics to Cancer Multidrug Resistance. Biomater. Adv. 2023, 146, 213282. [Google Scholar] [CrossRef] [PubMed]
- Segovia, N.; Pont, M.; Oliva, N.; Ramos, V.; Borrós, S.; Artzi, N. Hydrogel Doped with Nanoparticles for Local Sustained Release of siRNA in Breast Cancer. Adv. Healthc. Mater. 2015, 4, 271–280. [Google Scholar] [CrossRef]
- Han, H.D.; Mora, E.M.; Roh, J.W.; Nishimura, M.; Lee, S.J.; Stone, R.L.; Bar-Eli, M.; Lopez-Berestein, G.; Sood, A.K. Chitosan Hydrogel for Localized Gene Silencing. Cancer Biol. Ther. 2011, 11, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Sun, W.; Zhang, S.; Xing, Y.; Wang, C.; Wei, M.; Li, Q.; Ji, G.; Yang, G. Modular Hydrogel Vaccine for Programmable and Coordinate Elicitation of Cancer Immunotherapy. Adv. Sci. 2023, 10, 2301789. [Google Scholar] [CrossRef]
- Khaled, S.Z.; Cevenini, A.; Yazdi, I.K.; Parodi, A.; Evangelopoulos, M.; Corbo, C.; Scaria, S.; Hu, Y.; Haddix, S.G.; Corradetti, B.; et al. One-Pot Synthesis of pH-Responsive Hybrid Nanogel Particles for the Intracellular Delivery of Small Interfering RNA. Biomaterials 2016, 87, 57–68. [Google Scholar] [CrossRef]
- Fernandez-Alarcon, J.; Cladera, M.A.; Rodriguez-Camenforte, N.; Sitia, G.; Guerra-Rebollo, M.; Borros, S.; Fornaguera, C. Regulation of Mitochondrial Apoptosis via siRNA-Loaded Metallo-Alginate Hydrogels: A Localized and Synergistic Antitumor Therapy. Biomaterials 2025, 318, 123164. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; Ma, H.; Zhang, J.; Yu, D.; Zhao, R.; Yu, S.; Nie, G.; Wang, H. In Situ Transforming RNA Nanovaccines from Polyethylenimine Functionalized Graphene Oxide Hydrogel for Durable Cancer Immunotherapy. Nano Lett. 2021, 21, 2224–2231. [Google Scholar] [CrossRef]
- Lu, J.; Song, J.; Zhang, P.; Huang, Y.; Lu, X.; Dai, H.; Xi, J. Biomineralized Polydopamine Nanoparticle-Based Sodium Alginate Hydrogels for Delivery of Anti-Serine/Threonine Protein Kinase B-Rapidly Accelerated Fibrosarcoma siRNA for Metastatic Melanoma Therapy. ACS Nano 2023, 17, 18318–18331. [Google Scholar] [CrossRef]
- Wang, F.; Xie, M.; Huang, Y.; Liu, Y.; Liu, X.; Zhu, L.; Zhu, X.; Guo, Y.; Zhang, C. In Situ Vaccination with An Injectable Nucleic Acid Hydrogel for Synergistic Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2024, 63, e202315282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Z.-C.; Li, S.; Ma, J.; Wei, C.; Yu, D.; Stelzel, J.L.; Ni, B.Y.X.; Miao, Y.; Van Batavia, K.; et al. mRNA Lipid Nanoparticle-Incorporated Nanofiber-Hydrogel Composite Generates a Local Immunostimulatory Niche for Cancer Immunotherapy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Lei, S.; Gao, Y.; Li, J.; Chen, X.; Zhou, W.; Wu, J.; Ma, P.; Men, K.; Duan, X. Dual-RNA Controlled Delivery System Inhibited Tumor Growth by Apoptosis Induction and TME Activation. J. Control Release 2022, 344, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Nalbadis, A.; Trutschel, M.-L.; Lucas, H.; Luetzkendorf, J.; Meister, A.; Mäder, K. Selection and Incorporation of siRNA Carrying Non-Viral Vector for Sustained Delivery from Gellan Gum Hydrogels. Pharmaceutics 2021, 13, 1546. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Zhang, Y.; Artzi, N. Local Triple-Combination Therapy Results in Tumour Regression and Prevents Recurrence in a Colon Cancer Model. Nat. Mater. 2016, 15, 1128–1138. [Google Scholar] [CrossRef]
- Xiao, B.; Viennois, E.; Chen, Q.; Wang, L.; Han, M.K.; Zhang, Y.; Zhang, Z.; Kang, Y.; Wan, Y.; Merlin, D. Silencing of Intestinal Glycoprotein CD98 by Orally Targeted Nanoparticles Enhances Chemosensitization of Colon Cancer. ACS Nano 2018, 12, 5253–5265. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, X.; Xiao, J.; He, X.; Liu, C.; Xu, X.; Viitala, T.; Feng, J.; Zhang, H. Exploiting the Warburg Effect: Co-Delivery of Metformin and FOXK2 siRNA for Ovarian Cancer Therapy. Small Sci. 2024, 4, 2300192. [Google Scholar] [CrossRef]
- Casadidio, C.; Fens, M.H.A.M.; Fliervoet, L.A.L.; Censi, R.; Vermonden, T. Injectable Thermosensitive Hydrogel for Local and Controlled Delivery of siRNA-STAT3 Polyplexes to Treat Advanced-Stage Ovarian Cancer. J. Control Release 2025, 384, 113890. [Google Scholar] [CrossRef]
- Peng, D.; Gao, H.; Huang, P.; Shi, X.; Zhou, J.; Zhang, J.; Dong, A.; Tang, H.; Wang, W.; Deng, L. Host-Guest Supramolecular Hydrogel Based on Nanoparticles: Co-Delivery of DOX and siBcl-2 for Synergistic Cancer Therapy. J. Biomater. Sci. Polym. Ed. 2019, 30, 877–893. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lan, Y.-H.; Chuang, C.-C.; Lu, W.-T.; Chan, L.-Y.; Hsu, P.-W.; Chen, J.-P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 shRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, H.; Zhang, Y.; Shao, Y.; Li, D.; Li, Z.; Ma, Y.; Zhang, Y.; Xi, K.; Xue, Z.; et al. Lymph Node-Inspired Immunoregulatory Hydrogel with siRNA Delivery Property for Postoperative Glioblastoma Treatment. Chem. Eng. J. 2023, 476, 146343. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Wang, D.; Wang, J.; Zhou, J.; Zhang, Z.; Wang, J.; Hu, Y.; Xu, Q.; Xie, C.; et al. Hydrogel Loading Functionalized PAMAM/shRNA Complex for Postsurgical Glioblastoma Treatment. J. Control Release 2021, 338, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhuge, X.; Lin, W.; Yu, W.; Zhu, Y.; Shi, C.; Shi, Z. Hydrogel-Based miR-192 Delivery Inhibits the Development of Hepatocellular Carcinoma by Suppressing the GSK3β/Wnt/β-Catenin Pathway. Neoplasma 2023, 70, 555–565. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, B.; Shi, K.; Zhang, R.; Dong, C.; Xie, D.; Tang, L.; Tian, Y.; Qian, Z.; Yang, L. Sustained and Targeted Delivery of siRNA/DP7-C Nanoparticles from Injectable Thermosensitive Hydrogel for Hepatocellular Carcinoma Therapy. Cancer Sci. 2021, 112, 2481–2492. [Google Scholar] [CrossRef]
- Kaps, L.; Leber, N.; Klefenz, A.; Choteschovsky, N.; Zentel, R.; Nuhn, L.; Schuppan, D. In Vivo siRNA Delivery to Immunosuppressive Liver Macrophages by α-Mannosyl-Functionalized Cationic Nanohydrogel Particles. Cells 2020, 9, 1905. [Google Scholar] [CrossRef]
- Li, J.; Yuan, D.; Zheng, X.; Zhang, X.; Li, X.; Zhang, S. A Triple-Combination Nanotechnology Platform Based on Multifunctional RNA Hydrogel for Lung Cancer Therapy. Sci. China Chem. 2020, 63, 546–553. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Y.; Gu, Z.; Zang, H.; Li, L.; Wang, Q.; Wang, Y.; Zhao, X.; Wu, H.; Qiu, S.; et al. Immunotherapeutic Hydrogel for Co-Delivery of STAT3 siRNA Liposomes and Lidocaine Hydrochloride for Postoperative Comprehensive Management of NSCLC in a Single Application. Asian J. Pharm. Sci. 2024, 19, 100925. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Cheng, Y.; Li, D.; Gong, Y.; Liu, J.; Tian, H.; Chen, X. PLK1shRNA and Doxorubicin Co-Loaded Thermosensitive PLGA-PEG-PLGA Hydrogels for Osteosarcoma Treatment. Biomaterials 2014, 35, 8723–8734. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, G.; Fu, X.; Qiu, S.; Zhang, Y.; Chen, X.; Liu, Y.; Wan, X.; Li, Z.; Li, Y.; et al. ZIF-8-Modified Multifunctional Hydrogel Loading siRNA and DOX for Postoperative Therapy of Maxillofacial Osteosarcoma and Bone Repair. ACS Appl. Mater. Interfaces 2025, 17, 17990–18002. [Google Scholar] [CrossRef]
- Gao, C.; Cheng, K.; Li, Y.; Gong, R.; Zhao, X.; Nie, G.; Ren, H. Injectable Immunotherapeutic Hydrogel Containing RNA-Loaded Lipid Nanoparticles Reshapes Tumor Microenvironment for Pancreatic Cancer Therapy. Nano Lett. 2022, 22, 8801–8809. [Google Scholar] [CrossRef]
- Gong, K.; Lin, J.; Chen, X.; Duan, Y.; Zhang, J.; Yu, J.; Wang, J.; Sun, R.; Li, J.; Duan, Y. Thermosensitive Gel-Nano System against Esophageal Cancer via Restoring P53 Activity and Boosting T-Cell Immunity. J. Control Release 2024, 371, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, Y.; Yang, Y.; Jiang, L.; Xing, C.; Li, J.; Lu, C.; Yang, H. Functional Self-Assembled DNA Nanohydrogels for Specific Telomerase Activity Imaging and Telomerase-Activated Antitumor Gene Therapy. Anal. Chem. 2020, 92, 15179–15186. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Luo, J.; Pei, M.; Liu, S.; Gao, Y.; Zhou, H.; Nueraihemaiti, Y.; Zhan, X.; Xie, T.; Yao, X.; et al. Biomimetic Hydrogel-Mediated Mechano-Immunometabolic Therapy for Inhibition of ccRCC Recurrence After Surgery. Adv. Sci. 2024, 11, e2308734. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, Y.; Hu, Q.; Guo, Z.; Liu, T.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. Injectable Supramolecular Hydrogel Formed from α-Cyclodextrin and PEGylated Arginine-Functionalized Poly(l-Lysine) Dendron for Sustained MMP-9 shRNA Plasmid Delivery. Acta Biomater. 2017, 49, 456–471. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy. Bioengineering 2024, 11, 830. [Google Scholar] [CrossRef]
- Shinde, S.S.; Ahmed, S.; Malik, J.A.; Hani, U.; Khanam, A.; Ashraf Bhat, F.; Ahmad Mir, S.; Ghazwani, M.; Wahab, S.; Haider, N.; et al. Therapeutic Delivery of Tumor Suppressor miRNAs for Breast Cancer Treatment. Biology 2023, 12, 467. [Google Scholar] [CrossRef]
- Blount, S.L.; Liu, X.; McBride, J.D. The Utilization of PRAME in the Diagnosis, Prognosis, and Treatment of Melanoma. Cells 2024, 13, 1740. [Google Scholar] [CrossRef]
- Montuori, E.; Capalbo, A.; Lauritano, C. Marine Compounds for Melanoma Treatment and Prevention. Int. J. Mol. Sci. 2022, 23, 10284. [Google Scholar] [CrossRef]
- Quintanilla-Dieck, M.J.; Bichakjian, C.K. Management of Early-Stage Melanoma. Facial Plast. Surg. Clin. N. Am. 2019, 27, 35–42. [Google Scholar] [CrossRef]
- Massand, S.; Neves, R.I. Emerging Therapies in the Treatment of Advanced Melanoma. Clin. Plast. Surg. 2021, 48, 713–733. [Google Scholar] [CrossRef]
- Grunewald, T.; Ledermann, J.A. Targeted Therapies for Ovarian Cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, J. Advancements in Hydrogel-Based Therapies for Ovarian Cancer: A Review. Cell Biochem. Biophys. 2025, 83, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Mahfooz, S.; Elbasan, E.B.; Karacam, B.; Oztanir, M.N.; Hatiboglu, M.A. Targeting Glioblastoma: The Current State of Different Therapeutic Approaches. Curr. Neuropharmacol. 2021, 19, 1701–1715. [Google Scholar] [CrossRef]
- Sethi, P.; Ghosh, S.; Singh, K.K.; Han, S.S.; Bhaskar, R.; Sinha, J.K. Nanoparticle-Based Therapeutics for Glioblastoma Multiforme Treatment. Adv. Ther. 2025, 8, 2400546. [Google Scholar] [CrossRef]
- Hao, L.; Li, S.; Ye, F.; Wang, H.; Zhong, Y.; Zhang, X.; Hu, X.; Huang, X. The Current Status and Future of Targeted-Immune Combination for Hepatocellular Carcinoma. Front. Immunol. 2024, 15, 1418965. [Google Scholar] [CrossRef]
- Matuszyk, J. MALAT1-miRNAs Network Regulate Thymidylate Synthase and Affect 5FU-Based Chemotherapy. Mol. Med. 2022, 28, 89. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Hajiesmaeili, M.; Shoorei, H.; Bahroudi, Z.; Taheri, M.; Sharifi, G. The Impact of lncRNAs and miRNAs in Regulation of Function of Cancer Stem Cells and Progression of Cancer. Front. Cell Dev. Biol. 2021, 9, 696820. [Google Scholar] [CrossRef]
- Yang, Y.; Razak, S.R.A.; Ismail, I.S.; Ma, Y.; Yunus, M.A. Molecular Mechanisms of miR-192 in Cancer: A Biomarker and Therapeutic Target. Cancer Cell Int. 2025, 25, 94. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Huang, Z.; Reng, M.; Ma, Y.; Gao, B.; Song, S.; Liu, F.; Zhang, X.; Huang, J.; He, Z.; et al. Integration of Temperature-Sensitive Hydrogels Loaded with Realgar and Magnetic Particles for Lung Cancer Diagnosis and Treatment. Cancer Nanotechnol. 2025, 16, 8. [Google Scholar] [CrossRef]
- Wang, C.; Lu, D.; Cronin-Fenton, D.; Huang, C.; Liew, Z.; Wei, D.; Qin, G.; Yu, Y.; Li, J. Cardiovascular Disease and Risk of Lung Cancer Incidence and Mortality: A Nationwide Matched Cohort Study. Front. Oncol. 2022, 12, 950971. [Google Scholar] [CrossRef]
- Vinod, S.K.; Hau, E. Radiotherapy Treatment for Lung Cancer: Current Status and Future Directions. Respirology 2020, 25 (Suppl. S2), 61–71. [Google Scholar] [CrossRef]
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the Immune Microenvironment of Osteosarcoma: The Outlook for Osteosarcoma Treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef]
- Ji, L.; Huang, J.; Yu, L.; Jin, H.; Hu, X.; Sun, Y.; Yin, F.; Cai, Y. Recent Advances in Nanoagents Delivery System-Based Phototherapy for Osteosarcoma Treatment. Int. J. Pharm. 2024, 665, 124633. [Google Scholar] [CrossRef]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic Developments in Pancreatic Cancer: Current and Future Perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Merotto, E.; Pavan, P.G.; Piccoli, M. Three-Dimensional Bioprinting of Naturally Derived Hydrogels for the Production of Biomimetic Living Tissues: Benefits and Challenges. Biomedicines 2023, 11, 1742. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable Hydrogels: Design Mechanisms and Versatile Applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Willner, I. Stimuli-Responsive Biomolecule-Based Hydrogels and Their Applications. Angew. Chem. Int. Ed. Engl. 2020, 59, 15342–15377. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Chen, X. Injectable Hydrogels as Local Depots at Tumor Sites for Antitumor Immunotherapy and Immune-Based Combination Therapy. Macromol. Biosci. 2021, 21, e2100039. [Google Scholar] [CrossRef]
- Wei, L.; Chen, J.; Zhao, S.; Ding, J.; Chen, X. Thermo-Sensitive Polypeptide Hydrogel for Locally Sequential Delivery of Two-Pronged Antitumor Drugs. Acta Biomater. 2017, 58, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Kim, Y.-M.; Song, S.-C. Injectable and Quadruple-Functional Hydrogel as an Alternative to Intravenous Delivery for Enhanced Tumor Targeting. ACS Appl. Mater. Interfaces 2019, 11, 34634–34644. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Liu, S.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Wang, F.; Nie, J.; Ma, C.; Gao, M. POSS Hybrid Hydrogels: A Brief Review of Synthesis, Properties and Applications. Eur. Polym. J. 2021, 143, 110180. [Google Scholar] [CrossRef]
| Cancer | RNA | Hydrogel | References |
|---|---|---|---|
| Breast Cancer | miRNA, siRNA | RNA-triple helix hydrogel | [91] |
| miRNA | Photosensitive hydrogel | [92] | |
| siRNA | Supramolecular hydrogel of PEG | [93] | |
| siRNA | Polyamide-based amine hydrogel | [94] | |
| siRNA | Chitosan thermosensitive hydrogel | [95] | |
| mRNA | Gelatin methacryloyl hydrogel | [96] | |
| siRNA | pH-responsive hydrogel | [97] | |
| Melanoma | siRNA | Alginates hydrogel | [98] |
| mRNA | Polyethyleneimine hydrogel | [99] | |
| siRNA | Injectable sodium alginate hydrogels with pH-responsive and light-responsive properties | [100] | |
| PD-L1 siRNA | Injectable hydrogel | [101] | |
| mRNA | Nanofiber–water gel composite | [102] | |
| Colon Cancer | siRNA, mRNA | Photosensitive methacrylated gelatin hydrogel | [103] |
| siRNA | Gellan glue gelatin | [104] | |
| siRNA | Photosensitive hydrogel | [105] | |
| siRNA | Chitosan and alginate pH-responsive hydrogels | [106] | |
| Ovarian Cancer | FOXK2 siRNA | Gelatin methacryloyl-based photo-responsive hydrogel | [107] |
| siRNA | Injectable thermosensitive hydrogel | [108] | |
| siRNA | PEG injectable hydrogel | [109] | |
| Glioblastoma | SLP2 shRNA | Chitosan-Poly(N-isopropylacrylamide) thermosensitive hydrogel | [110] |
| siRNA | Polyethylene glycol acid-responsive hydrogel | [111] | |
| shRNA | Thermosensitive hydrogel | [112] | |
| Hepatocellular Carcinoma | miRNA 192 | Sodium alginate and polyethyleneimine hydrogel | [113] |
| Pin1 siRNA | PDLLA-PEG-PDLLA thermosensitive hydrogel | [114] | |
| siRNA | α-Mannosylated nanogel | [115] | |
| Lung Cancer | miRNA | Photo-responsive RNA nano hydrogel | [116] |
| STAT3 siRNA | Hybrid hydrogel of alginate | [117] | |
| Osteosarcoma | PLK1 shRNA | PLGA-PEG-PLGA thermosensitive hydrogel | [118] |
| PD-L1 siRNA | pH-responsive hydrogel | [119] | |
| Pancreatic Cancer | mRNA, siRNA | Chitosan thermosensitive hydrogel | [120] |
| Esophagus Cancer | siRNA4 | mPEG-PLGA thermosensitive hydrogel | [121] |
| Cervical Cancer | siRNA | Enzyme-responsive DNA nanogel | [122] |
| Kidney Cancer | siRNA targeting IDO1 | Biomimetic hydrogel of fibrinogen | [123] |
| Nasopharyngeal Cancer | shRNA | PEG hydrogel | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Fei, Y.; Wang, X.; Jiao, W.; Jin, Y. Advances in Hydrogel-Based Delivery of RNA Drugs for Antitumor Therapy. Gels 2025, 11, 633. https://doi.org/10.3390/gels11080633
Xu H, Fei Y, Wang X, Jiao W, Jin Y. Advances in Hydrogel-Based Delivery of RNA Drugs for Antitumor Therapy. Gels. 2025; 11(8):633. https://doi.org/10.3390/gels11080633
Chicago/Turabian StyleXu, Hui, Yang Fei, Xueya Wang, Wenfeng Jiao, and Yong Jin. 2025. "Advances in Hydrogel-Based Delivery of RNA Drugs for Antitumor Therapy" Gels 11, no. 8: 633. https://doi.org/10.3390/gels11080633
APA StyleXu, H., Fei, Y., Wang, X., Jiao, W., & Jin, Y. (2025). Advances in Hydrogel-Based Delivery of RNA Drugs for Antitumor Therapy. Gels, 11(8), 633. https://doi.org/10.3390/gels11080633







