Preparation of Agrowaste-Based Nanocellulose by NaOH-Assisted Ball Milling Technique: Influence of Component Intervention
Abstract
1. Introduction
2. Results and Discussion
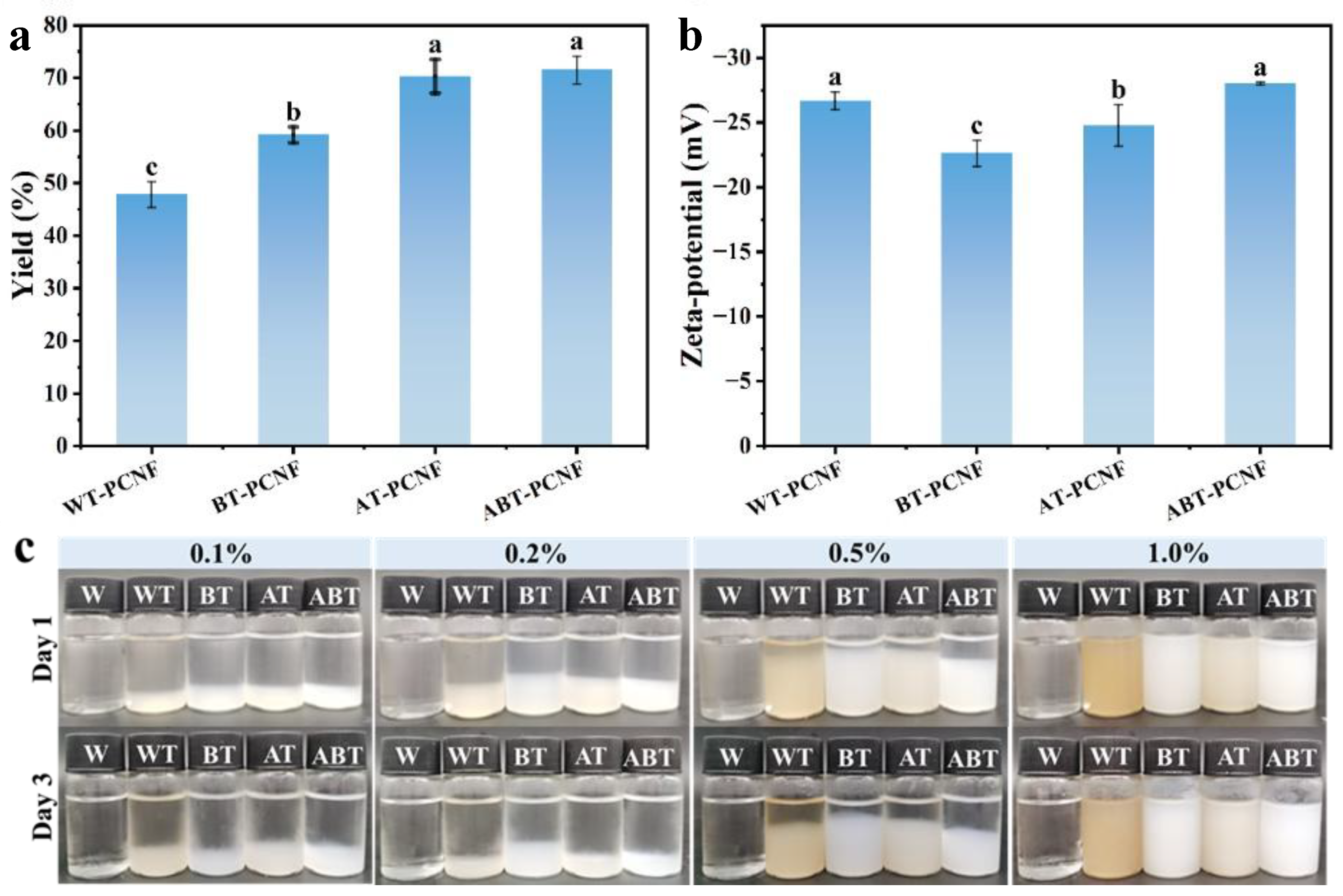
2.1. The Yield Analysis
2.2. Zeta Potential
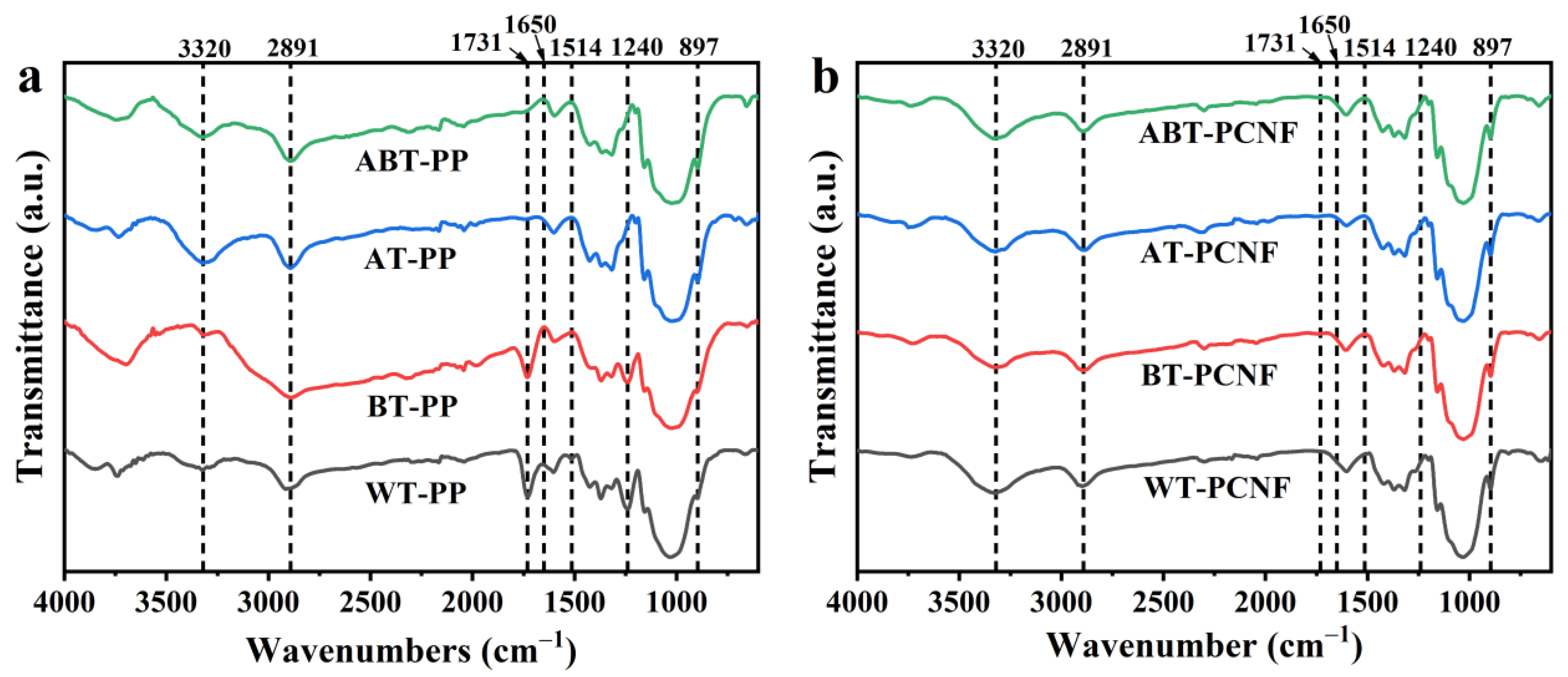
2.3. FTIR Analysis
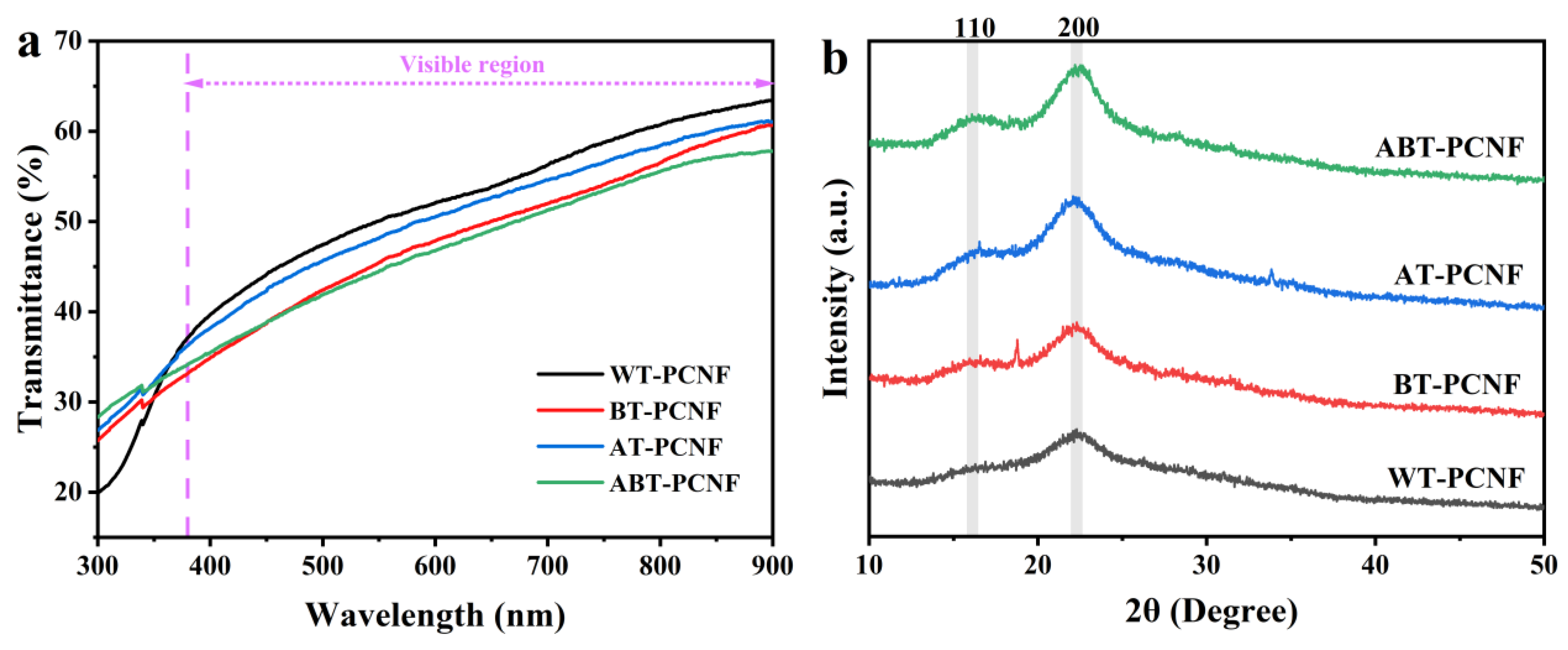
2.4. Light Transmittance Analysis
2.5. XRD Analysis
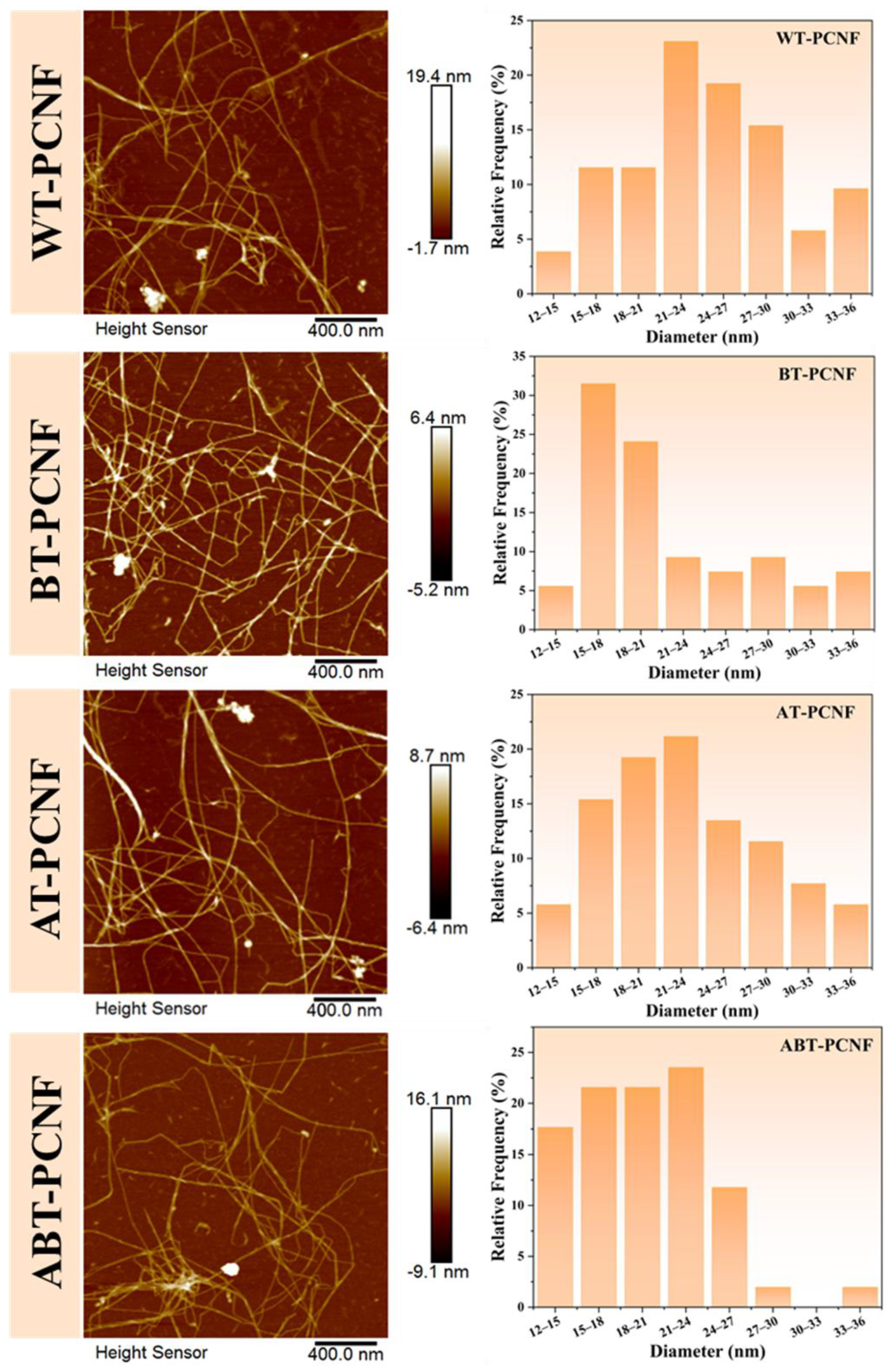
2.6. AFM Analysis
2.7. TGA Analysis
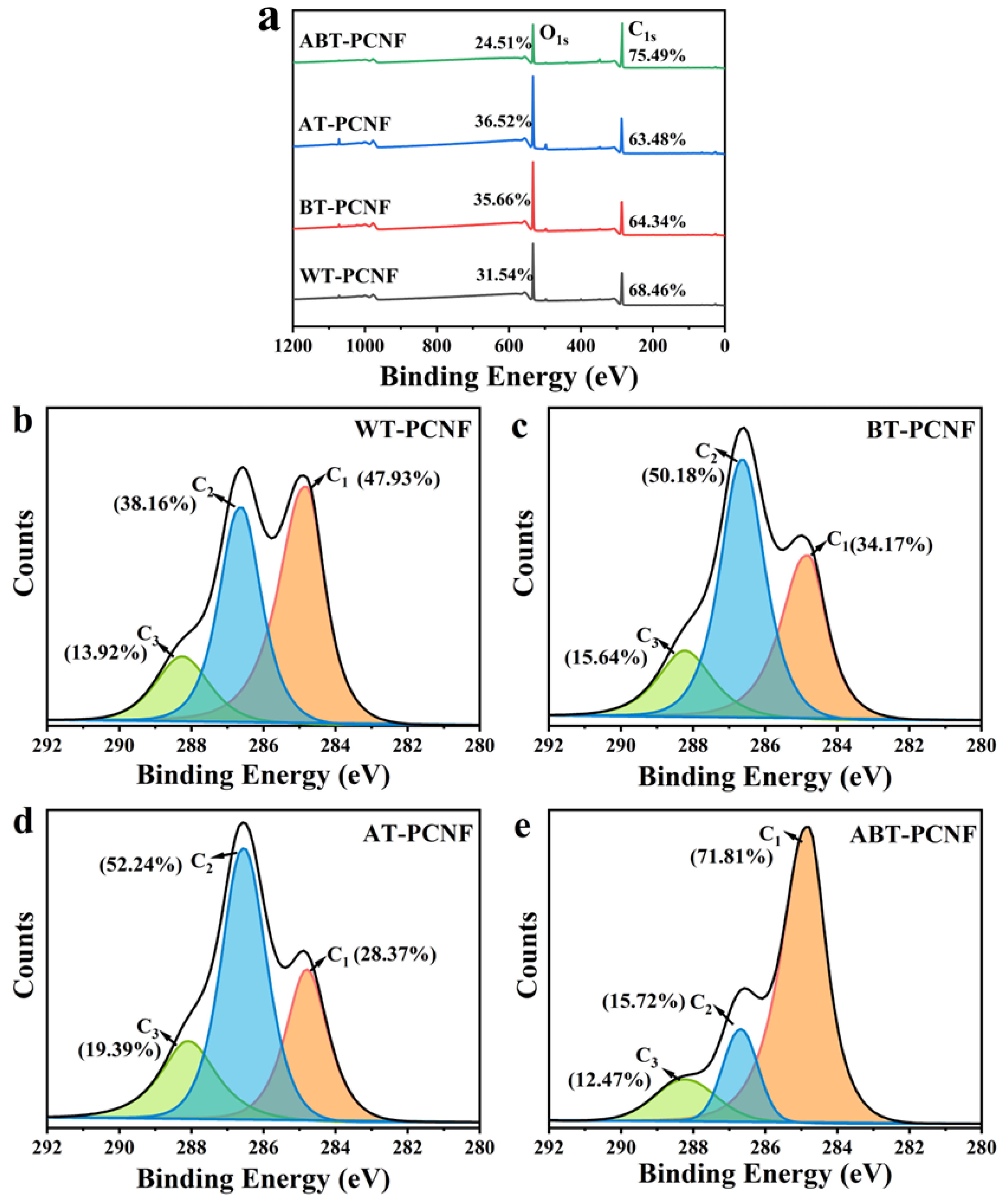
2.8. XPS Analysis
2.9. Rheological Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Component Regulation of Pineapple Peel Residue
4.3. Preparation of PCNF
4.4. Characterization of PCNF
4.4.1. Zeta Potential
4.4.2. Optical Transmittance
4.4.3. Atomic Force Microscope (AFM)
4.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.4.5. X-Ray Diffraction (XRD)
4.4.6. Thermogravimetric Analysis (TGA)
4.4.7. X-Ray Photoelectron Spectroscopy (XPS)
4.4.8. Rheological Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonu; Rani, G.M.; Pathania, D.; Abhimanyu; Umapathi, R.; Rustagi, S.; Huh, Y.S.; Gupta, V.K.; Kaushik, A.; Chaudhary, V. Agro-waste to sustainable energy: A green strategy of converting agricultural waste to nano-enabled energy applications. Sci. Total Environ. 2023, 875, 162667. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, Y.; Hu, M.; Wang, Y.; Dai, D.; Ma, L.; Zhang, Y.; Dai, H. Recent advances in nanocellulose based hydrogels: Preparation strategy, typical properties and food application. Int. J. Biol. Macromol. 2024, 277, 134015. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Luo, Y.; Zheng, X.; Hu, X.; Wang, H.; Ma, L.; Chen, H.; Zhang, Y. Nanocellulose-chitosan nanocomplex stabilized pickering emulsions with extremely low internal phase: Stabilization mechanism and application in yogurt. Food Hydrocoll. 2025, 163, 111088. [Google Scholar] [CrossRef]
- Ji, Q.; Zhou, C.; Li, Z.; Boateng, I.D.; Liu, X. Is nanocellulose a good substitute for non-renewable raw materials? A comprehensive review of the state of the art, preparations, and industrial applications. Ind. Crop. Prod. 2023, 202, 117093. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Gao, M.; Guo, Y.; Xu, T.; Jiang, H.; Zhang, Z.; Ji, X.; Si, C. Nanocellulose-based functional materials for physical, chemical, and biological sensing: A review of materials, properties, and perspectives. Ind. Crop. Prod. 2024, 212, 118326. [Google Scholar] [CrossRef]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced nanocellulose-based composites for flexible functional energy storage devices. Adv. Mater. 2021, 33, e2101368. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Wan, C.; Zhang, Z.; Cao, W.; Wang, G.; Wu, Y. A comprehensive review of cellulose nanomaterials for adsorption of wastewater pollutants: Focus on dye and heavy metal Cr adsorption and oil/water separation. Collagen Leather 2024, 6, 1–25. [Google Scholar] [CrossRef]
- Albornoz-Palma, G.; Ching, D.; Valerio, O.; Mendonça, R.T.; Pereira, M. Effect of lignin and hemicellulose on the properties of lignocellulose nanofibril suspensions. Cellulose 2020, 27, 10631–10647. [Google Scholar] [CrossRef]
- Hu, M.; Lv, X.; Wang, Y.; Zhang, Y.; Dai, H. Guideline for the extraction of nanocellulose from lignocellulosic feedstocks. Food Biomacromolecules 2024, 1, 9–17. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef]
- Albornoz-Palma, G.; Ortega-Sanhueza, I.; Teruel-Juanes, R.; Henríquez-Gallegos, S.; Ribes-Greus, A.; Pereira, M. Understanding the effect of lignin on the production process and characteristics of lignocellulose nanofibrils from Eucalyptus nitens. Cellulose 2023, 30, 6811–6831. [Google Scholar] [CrossRef]
- Hu, M.; Lv, X.; Wang, Y.; Ma, L.; Zhang, Y.; Dai, H. Recent advance on lignin-containing nanocelluloses: The key role of lignin. Carbohydr. Polym. 2024, 343, 122460. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Gu, J.; Jiang, F.; Hsieh, Y. Holistic rice straw nanocellulose and hemicelluloses/lignin composite films. ACS Sustain. Chem. Eng. 2016, 4, 728–737. [Google Scholar] [CrossRef]
- Naderi, A. Nanofibrillated cellulose: Properties reinvestigated. Cellulose 2017, 24, 1933–1945. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef]
- Fu, C.; Li, Y.; Lin, Y.; Zhang, W.; Yang, J.; Liu, Y.; He, Z.; Hong, Y.; Shen, J.; Ni, Y.; et al. Crucial role of fiber swelling in microfibrillated cellulose extraction via ball milling. Ind. Crop. Prod. 2024, 218, 118899. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Othman, M.H.D. Enhanced production of ionized nano-fibrillated cellulose from sugar palm fiber via wet disk milling disintegration. Biomass Convers. Biorefinery 2024, 1–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Liu, J.; Zhao, Y.; Fan, F. Characterization of bamboo shoot cellulose nanofibers modified by TEMPO oxidation and ball milling method and its application in W/O emulsion. LWT 2024, 205, 116563. [Google Scholar] [CrossRef]
- Lv, T.; Luo, Y.; Chen, Y.; Dai, D.; Feng, X.; Chen, H.; Yu, Y.; Ma, L.; Zhang, Y.; Dai, H. Tuning the properties of pineapple peel cellulose nanofibrils by TEMPO-mediated oxidation and ball milling. Cellulose 2022, 29, 9609–9625. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Z.; Ji, X.; Ma, M. Preparation and modification of nanocellulose using deep eutectic solvents and their applications. Cellulose 2024, 31, 2175–2205. [Google Scholar] [CrossRef]
- Cebreiros, F.; Seiler, S.; Sánchez, G.; Lareo, C. Sequential ball milling as a promising method for the isolation of cellulose nanofibers (CNF) from enzyme-treated eucalyptus kraft pulp. Ind. Crop. Prod. 2024, 220, 119293. [Google Scholar] [CrossRef]
- Copenhaver, K.; Li, K.; Wang, L.; Lamm, M.; Zhao, X.; Korey, M.; Neivandt, D.; Dixon, B.; Sultana, S.; Kelly, P.; et al. Pretreatment of lignocellulosic feedstocks for cellulose nanofibril production. Cellulose 2022, 29, 4835–4876. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. Technol. 2018, 82, 60–70. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, T.A.; Singh, N.J.; Shadangi, K.P.; Srivastava, R.K.; Singh, A.K.; Chandel, A.K.; Pareek, N.; Vivekanand, V. Sustainable utilization of pineapple wastes for production of bioenergy, biochemicals and value-added products: A review. Bioresour. Technol. 2022, 351, 127085. [Google Scholar] [CrossRef]
- Torgbo, S.; Quan, V.M.; Sukyai, P. Cellulosic value-added products from sugarcane bagasse. Cellulose 2021, 28, 5219–5240. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S.; Gupta, S. Innovative strategy for rice straw valorization into nanocellulose and nanohemicellulose and its application. Ind. Crop. Prod. 2022, 179, 114695. [Google Scholar] [CrossRef]
- Sartika, D.; Firmansyah, A.P.; Junais, I.; Arnata, I.W.; Fahma, F.; Firmanda, A. High yield production of nanocrystalline cellulose from corn cob through a chemical-mechanical treatment under mild conditions. Int. J. Biol. Macromol. 2023, 240, 124327. [Google Scholar] [CrossRef]
- Mishra, S.; Prabhakar, B.; Kharkar, P.S.; Pethe, A.M. Banana peel waste: An emerging cellulosic material to extract nanocrystalline cellulose. ACS Omega 2022, 8, 1140–1145. [Google Scholar] [CrossRef]
- Lin, H.; Guo, X.; Ding, K.; Li, D.; Zhang, H.; Jia, X.; Zhang, H.; Zhou, L.; Han, L.; Liu, X.; et al. Effect of alkali and ball milling on enhancing biomass depolymerization in the combined mechanochemical catalysis. Ind. Crop. Prod. 2024, 222, 119819. [Google Scholar] [CrossRef]
- Gao, C.; Yang, J.; Han, L. Systematic comparison for effects of different scale mechanical-NaOH coupling treatments on lignocellulosic components, micromorphology and cellulose crystal structure of wheat straw. Bioresour. Technol. 2021, 326, 124786. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Noël, M.; Aitomäki, Y.; Öman, T.; Oksman, K. Production potential of cellulose nanofibers from industrial residues: Efficiency and nanofiber characteristics. Ind. Crop. Prod. 2016, 92, 84–92. [Google Scholar] [CrossRef]
- Amiralian, N.; Annamalai, P.K.; Garvey, C.J.; Jiang, E.; Memmott, P.; Martin, D.J. High aspect ratio nanocellulose from an extremophile spinifex grass by controlled acid hydrolysis. Cellulose 2017, 24, 3753–3766. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Malucelli, L.C.; Matos, M.; Jordão, C.; Lomonaco, D.; Lacerda, L.G.; Filho, M.A.S.C.; Magalhães, W.L.E. Influence of cellulose chemical pretreatment on energy consumption and viscosity of produced cellulose nanofibers (CNF) and mechanical properties of nanopaper. Cellulose 2019, 26, 1667–1681. [Google Scholar] [CrossRef]
- Sun, F.; Xu, J.; Wang, Z.; Cheng, T.; Wang, D.; Liu, J.; Guo, Z.; Wang, Z. Effect of glycosylation on soy protein isolate–sodium carboxymethyl cellulose conjugates heat-induced gels and their applications as carriers of riboflavin. Food Hydrocoll. 2024, 153, 110072. [Google Scholar] [CrossRef]
- Barbosa, B.M.; Vaz, S.; Colodette, J.L.; de Aguiar, A.R.; Cabral, C.P.T.; de Freitas Homem De Faria, B. Structural and chemical characterization of lignin and hemicellulose isolated from corn fibers toward agroindustrial residue valorization. Cellulose 2022, 29, 8117–8132. [Google Scholar] [CrossRef]
- Galiwango, E.; Abdel Rahman, N.S.; Al-Marzouqi, A.H.; Abu-Omar, M.M.; Khaleel, A.A. Isolation and characterization of cellulose and α-cellulose from date palm biomass waste. Heliyon 2019, 5, e02937. [Google Scholar] [CrossRef]
- Bilatto, S.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Lignocellulose nanocrystals from sugarcane straw. Ind. Crop. Prod. 2020, 157, 112938. [Google Scholar] [CrossRef]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of lignin contents from infrared spectroscopy: Chemical digestion and lignin/biomass ratios of Cryptomeria japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Fonseca, A.d.S.; Panthapulakkal, S.; Konar, S.K.; Sain, M.; Bufalinof, L.; Raabe, J.; Miranda, I.P.d.A.; Martins, M.A.; Tonoli, G.H.D. Improving cellulose nanofibrillation of non-wood fiber using alkaline and bleaching pre-treatments. Ind. Crop. Prod. 2019, 131, 203–212. [Google Scholar] [CrossRef]
- Kaschuk, J.J.; Al Haj, Y.; Garcia, J.V.; Kamppinen, A.; Rojas, O.J.; Abitbol, T.; Miettunen, K.; Vapaavuori, J. Processing factors affecting roughness, optical and mechanical properties of nanocellulose films for optoelectronics. Carbohydr. Polym. 2024, 332, 121877. [Google Scholar] [CrossRef] [PubMed]
- Abou-Yousef, H.; Kamel, S. Physico-mechanical properties of all-cellulose composites prepared by different approaches from micro-fibrillated bagasse pulp fibers. Mater. Today Commun. 2023, 35, 105672. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, H.; Ma, L.; Huang, H.; Wu, J.; Zhang, Y. Direct fabrication of hierarchically processed pineapple peel hydrogels for efficient Congo red adsorption. Carbohydr. Polym. 2020, 230, 115599. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Ren, S.; Dong, L.; Ai, Y.; Lei, T.; Wu, Q. Lignin containing cellulose nanofiber based nanopapers with ultrahigh optical transmittance and haze. Cellulose 2023, 30, 5967–5985. [Google Scholar] [CrossRef]
- Yuan, T.; Zeng, J.; Wang, B.; Cheng, Z.; Chen, K. Lignin containing cellulose nanofibers (LCNFs): Lignin content-morphology-rheology relationships. Carbohydr. Polym. 2021, 254, 117441. [Google Scholar] [CrossRef]
- Corrêa, A.C.; de Morais Teixeira, E.; Pessan, L.A.; Mattoso, L.H.C. Cellulose nanofibers from curaua fibers. Cellulose 2010, 17, 1183–1192. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Teo, H.L.; Wahab, R.A. Towards an eco-friendly deconstruction of agro-industrial biomass and preparation of renewable cellulose nanomaterials: A review. Int. J. Biol. Macromol. 2020, 161, 1414–1430. [Google Scholar] [CrossRef]
- Anari, E.S.; Soltanizadeh, N.; Fathi, M. The potential of DBD plasma pretreatment for the isolation of micro- and nano-cellulose fibers from the walnut shells. Carbohydr. Polym. 2024, 327, 121692. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Thakur, V.K. Impact of physico-chemical properties of nanocellulose on rheology of aqueous suspensions and its utility in multiple fields: A review. J. Vinyl Addit. Technol. 2023, 29, 617–648. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Yue, Y.; Chen, M.; Wu, Q. Structure and rheology of nanocrystalline cellulose. Carbohydr. Polym. 2011, 84, 316–322. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, X.; Huang, Y.; Wang, Y.; Chen, H.; Yu, Y.; Ma, L.; Zhang, Y.; Dai, H. Facile isolation of cellulose nanofibrils from agro-processing residues and its improved stabilization effect on gelatin emulsion. Int. J. Biol. Macromol. 2022, 216, 272–281. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-assembling behavior of cellulose nanoparticles during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Nelson, M.L.; Oconnor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Gond, R.K.; Gupta, M.K.; Jawaid, M. Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym. Compos. 2021, 42, 5400–5412. [Google Scholar] [CrossRef]
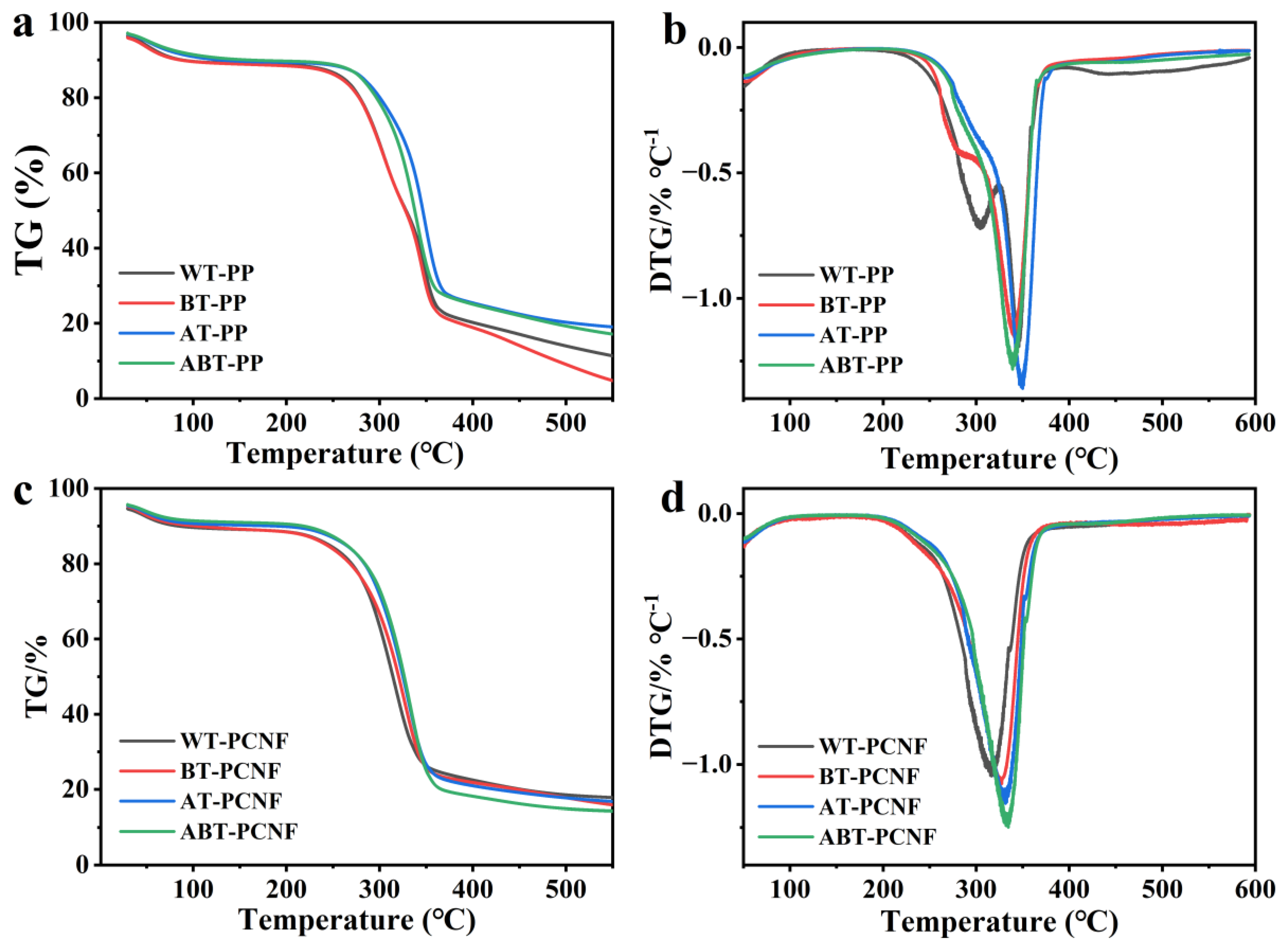

| Sample | Tonset (°C) | Tmax (°C) | Char Residue at 600 °C (%) |
|---|---|---|---|
| WT-PP | 223.5 | 345.7 | 11.5 |
| BT-PP | 234.3 | 340.5 | 4.8 |
| AT-PP | 236.5 | 349.3 | 19.4 |
| ABT-PP | 239.9 | 339.2 | 17.4 |
| WT-PCNF | 232.0 | 316.3 | 17.9 |
| BT-PCNF | 237.3 | 326.4 | 16.0 |
| AT-PCNF | 240.5 | 330.5 | 17.1 |
| ABT-PCNF | 245.1 | 333.2 | 14.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, Y.; Hu, S.; Yu, J.; Huang, Y.; Dai, H. Preparation of Agrowaste-Based Nanocellulose by NaOH-Assisted Ball Milling Technique: Influence of Component Intervention. Gels 2025, 11, 631. https://doi.org/10.3390/gels11080631
Wang Y, Yu Y, Hu S, Yu J, Huang Y, Dai H. Preparation of Agrowaste-Based Nanocellulose by NaOH-Assisted Ball Milling Technique: Influence of Component Intervention. Gels. 2025; 11(8):631. https://doi.org/10.3390/gels11080631
Chicago/Turabian StyleWang, Yuxi, Yong Yu, Shuhan Hu, Jinyao Yu, Yue Huang, and Hongjie Dai. 2025. "Preparation of Agrowaste-Based Nanocellulose by NaOH-Assisted Ball Milling Technique: Influence of Component Intervention" Gels 11, no. 8: 631. https://doi.org/10.3390/gels11080631
APA StyleWang, Y., Yu, Y., Hu, S., Yu, J., Huang, Y., & Dai, H. (2025). Preparation of Agrowaste-Based Nanocellulose by NaOH-Assisted Ball Milling Technique: Influence of Component Intervention. Gels, 11(8), 631. https://doi.org/10.3390/gels11080631







