Synthesis of Hydroxyapatite-Gelatin Composite Hydrogel for Bone Tissue Application
Abstract
1. Introduction
2. Results and Discussion
2.1. Gelatin/HAp Hydrogels Synthesized
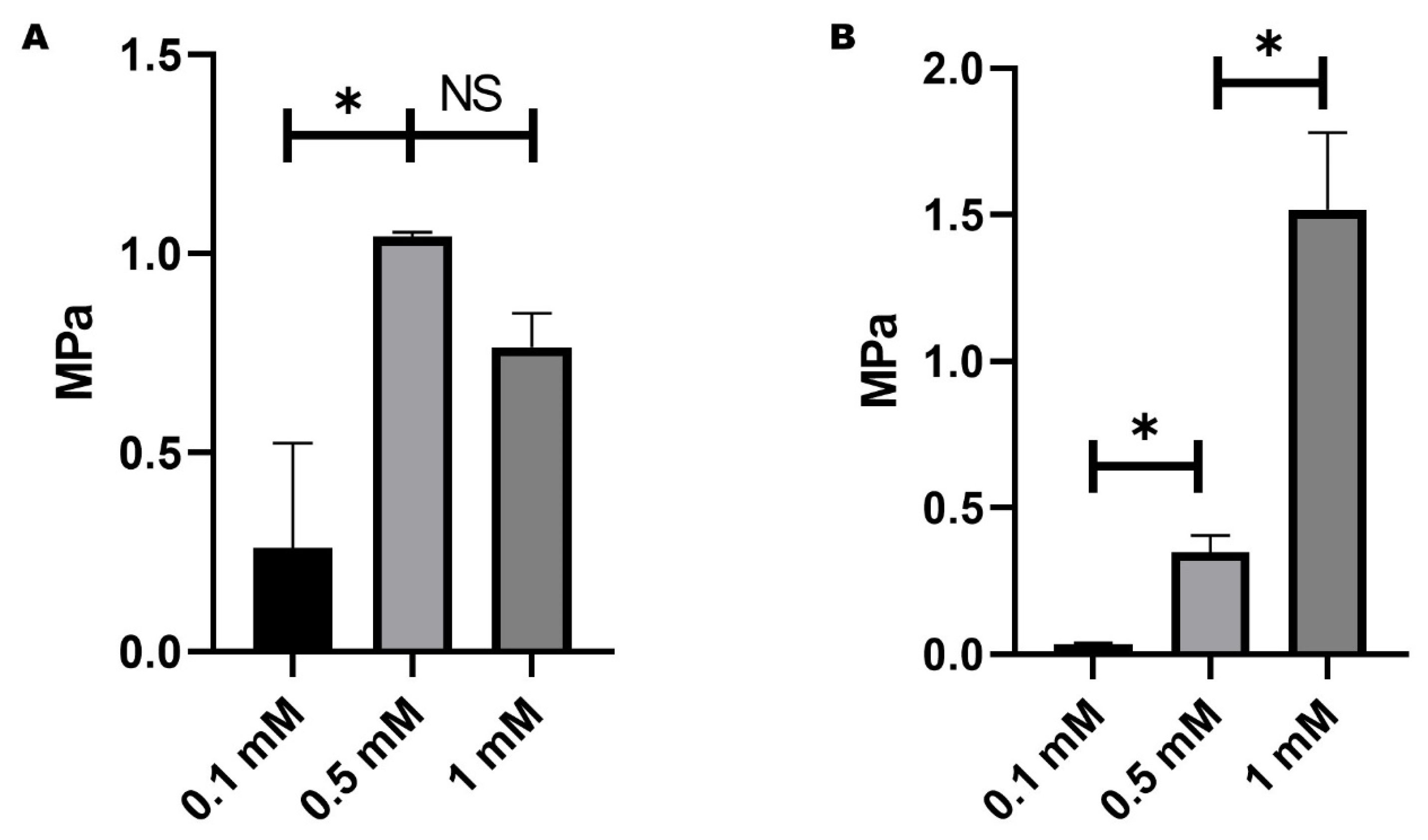
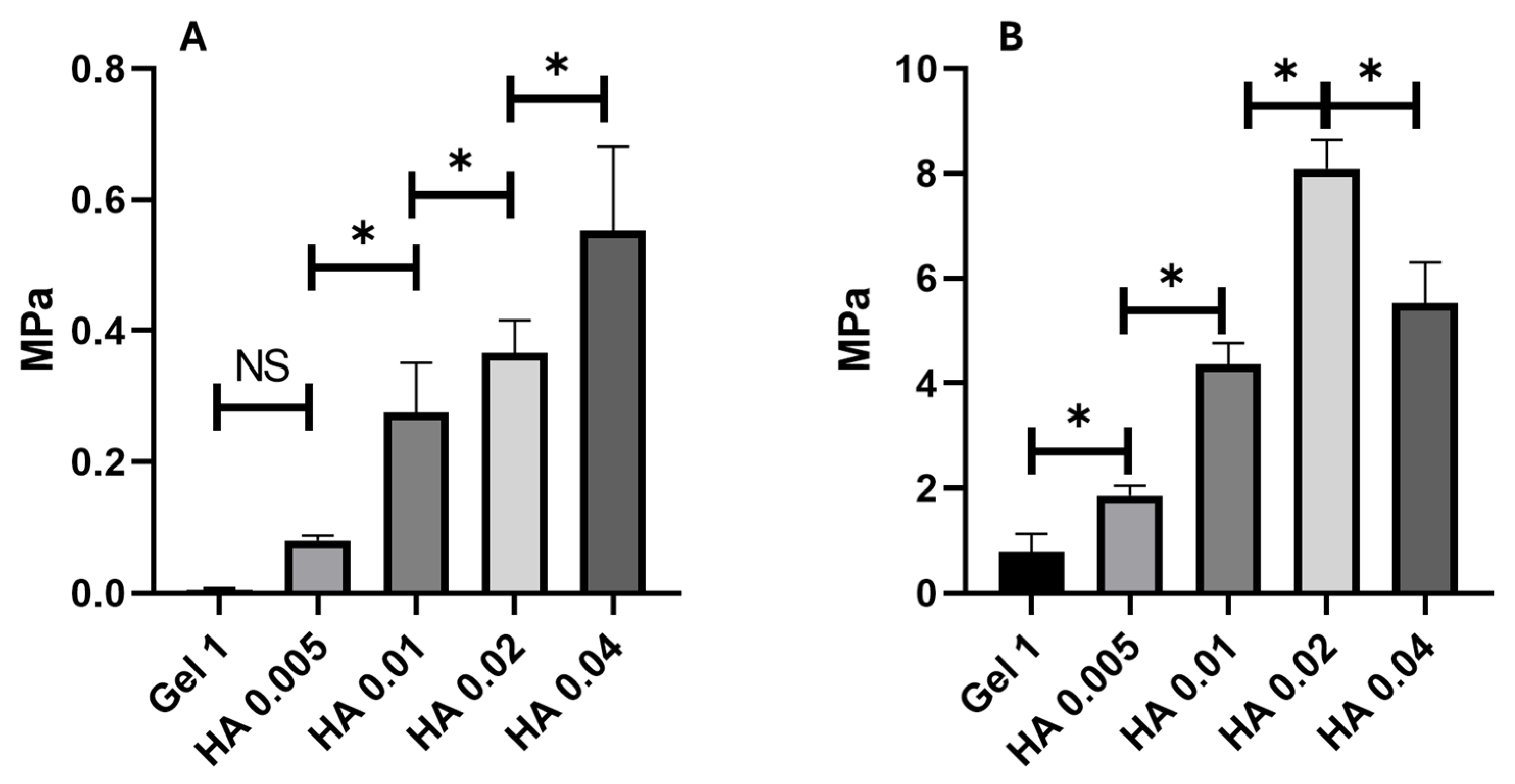
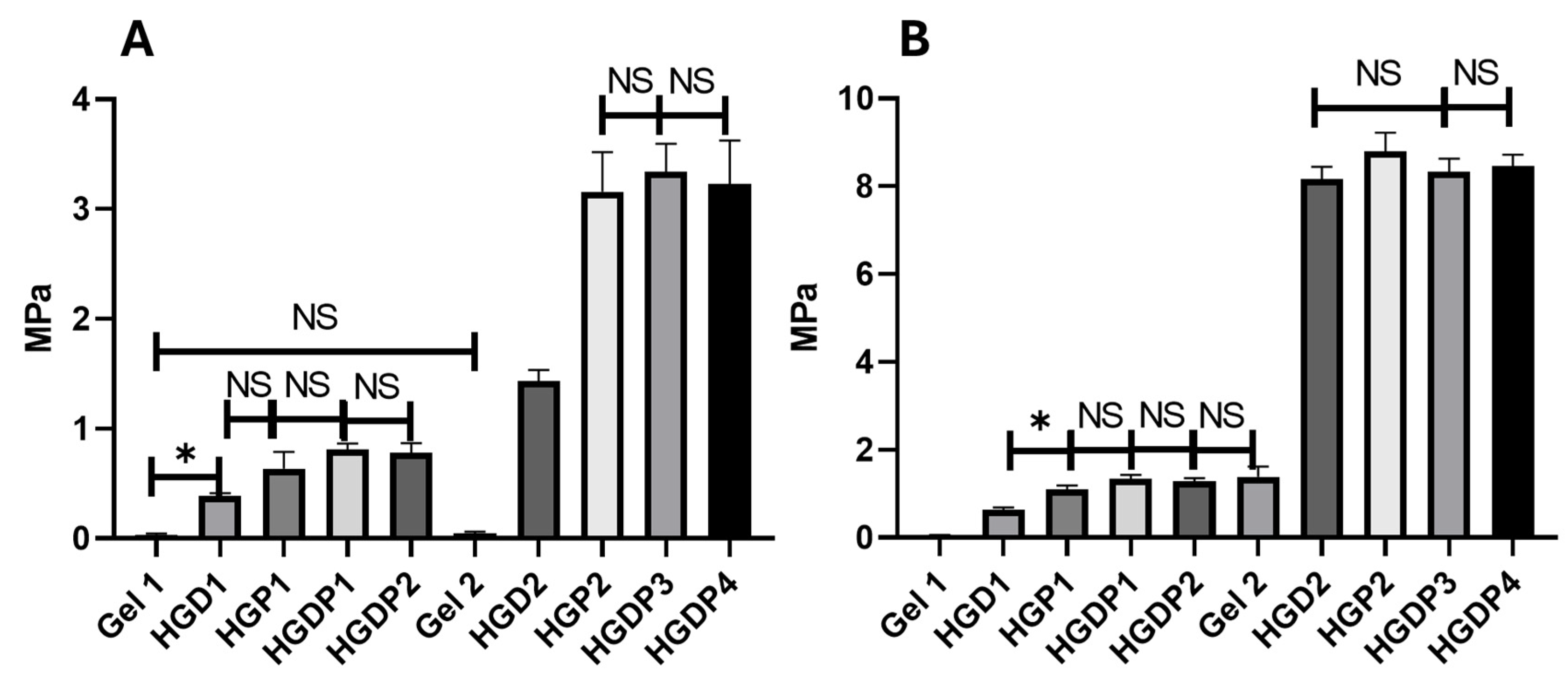
2.2. Mechanical Behaviour of Hydrogels
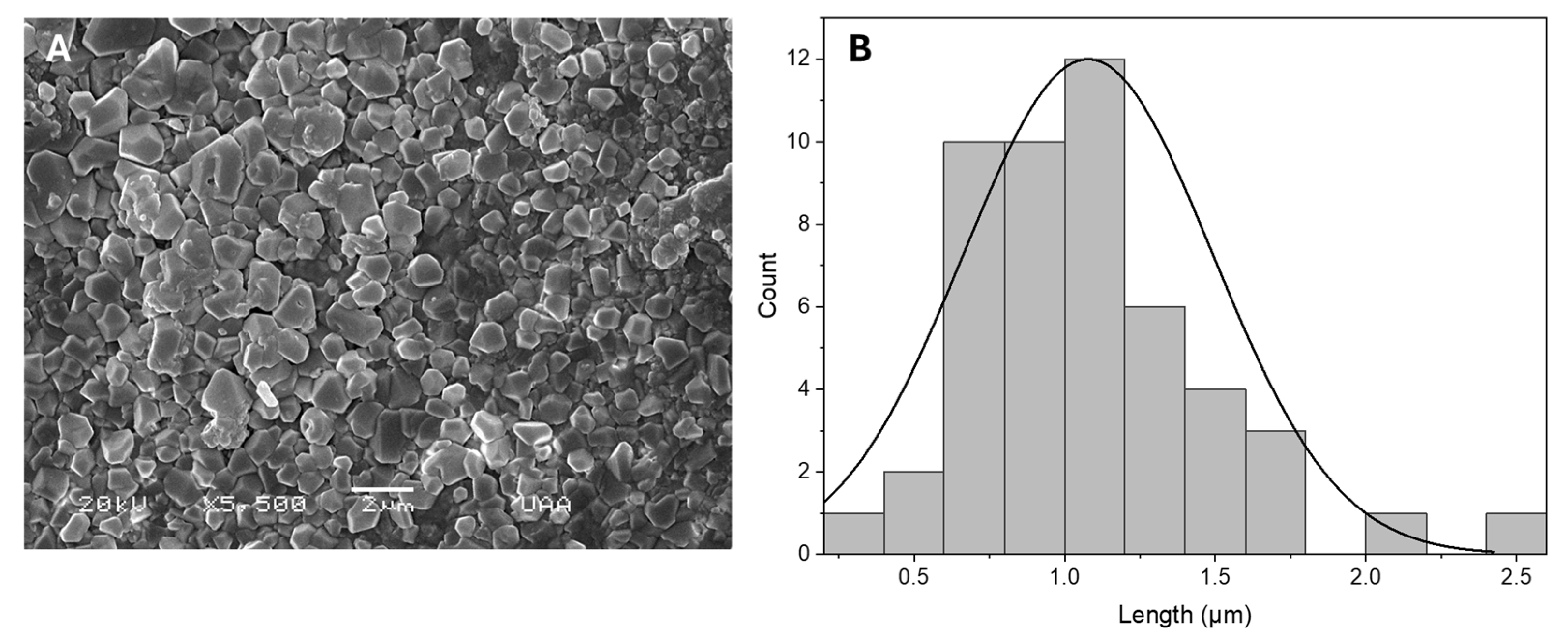
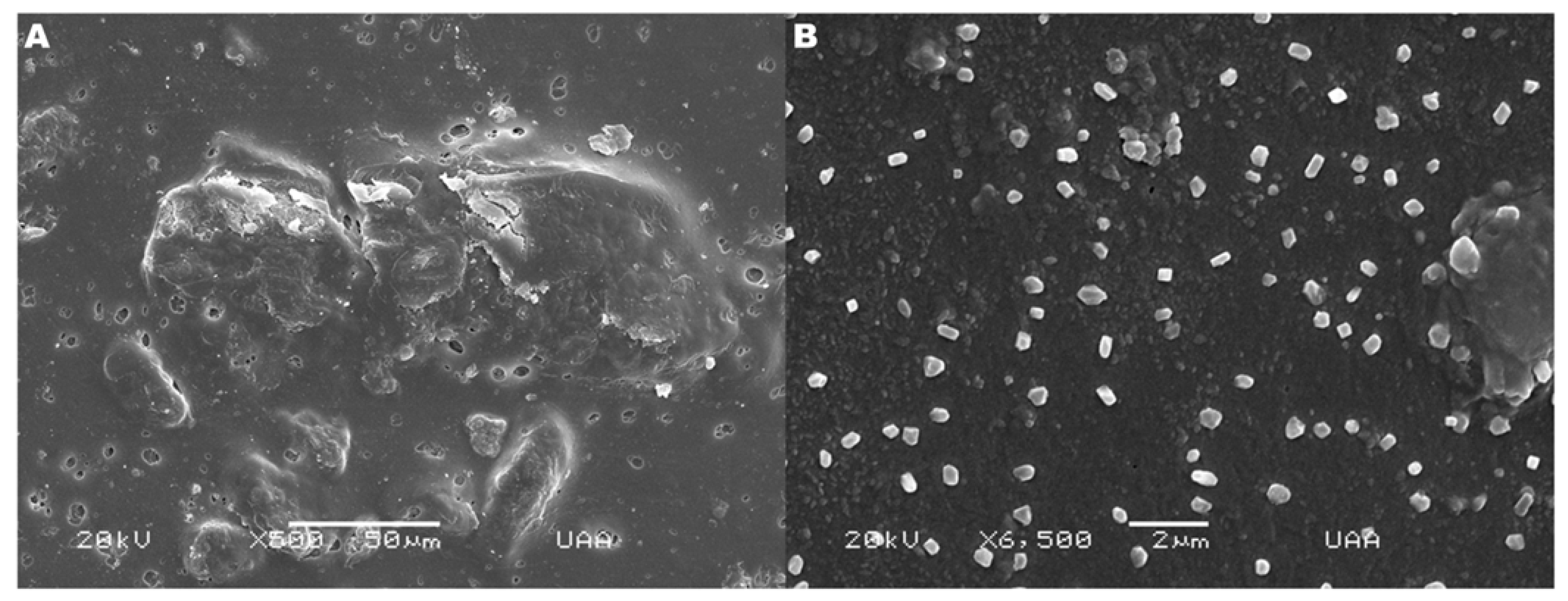
2.3. Hydrogel Structure and Morphology
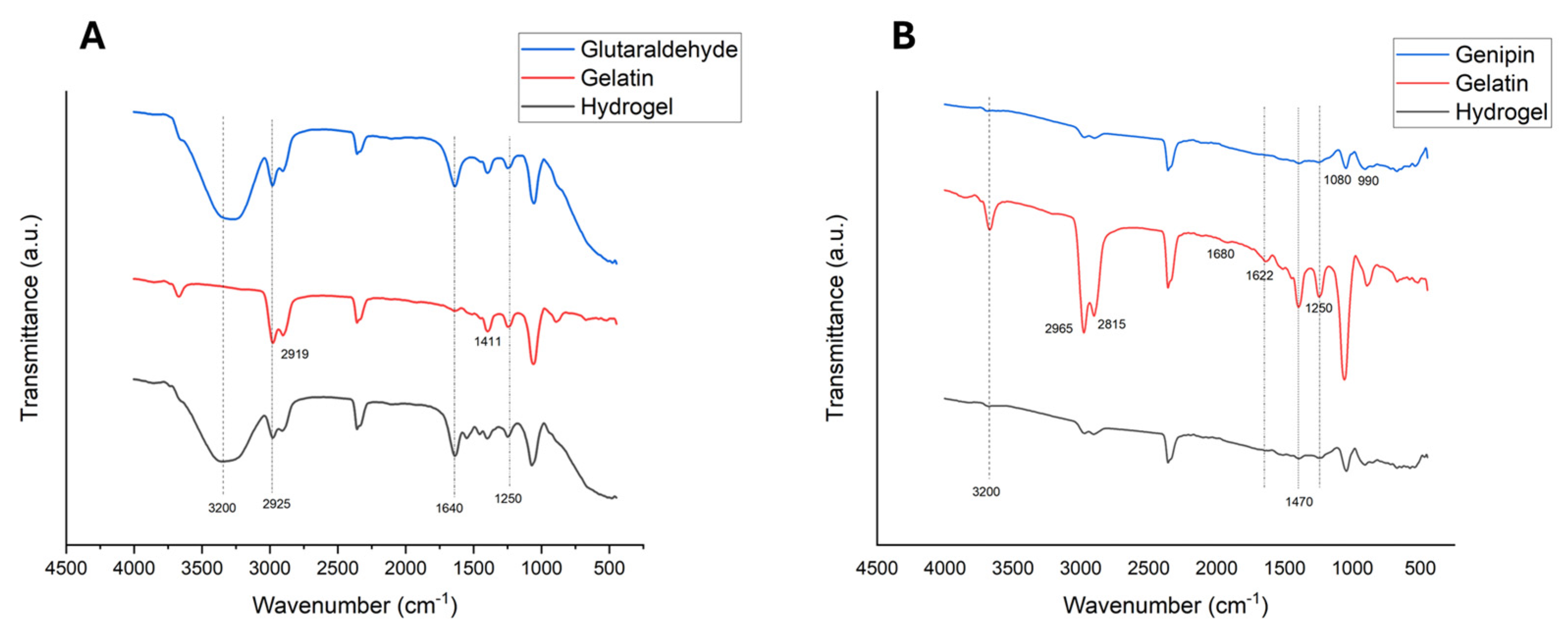
2.4. Characterization
2.4.1. FTIR Analysis Findings
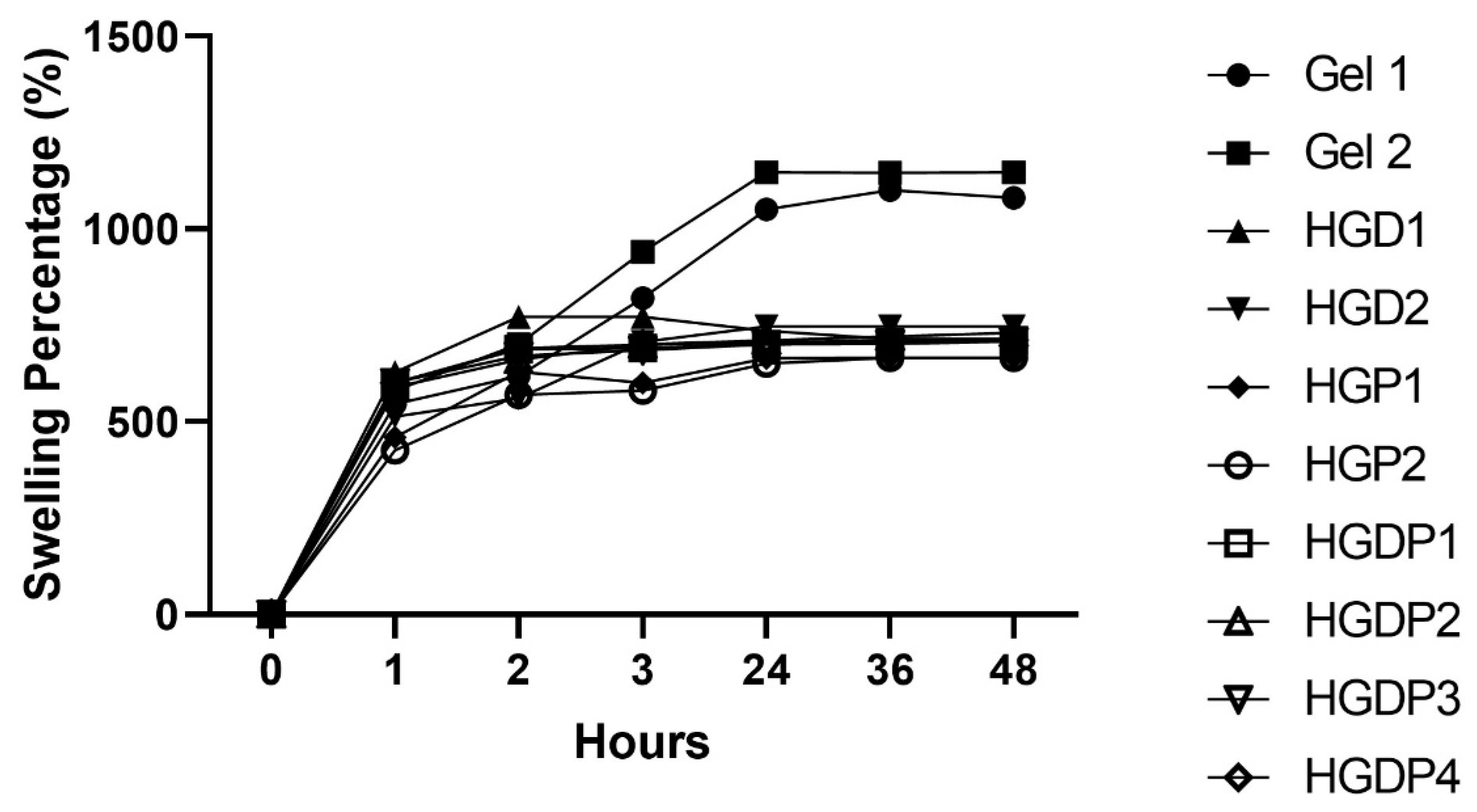
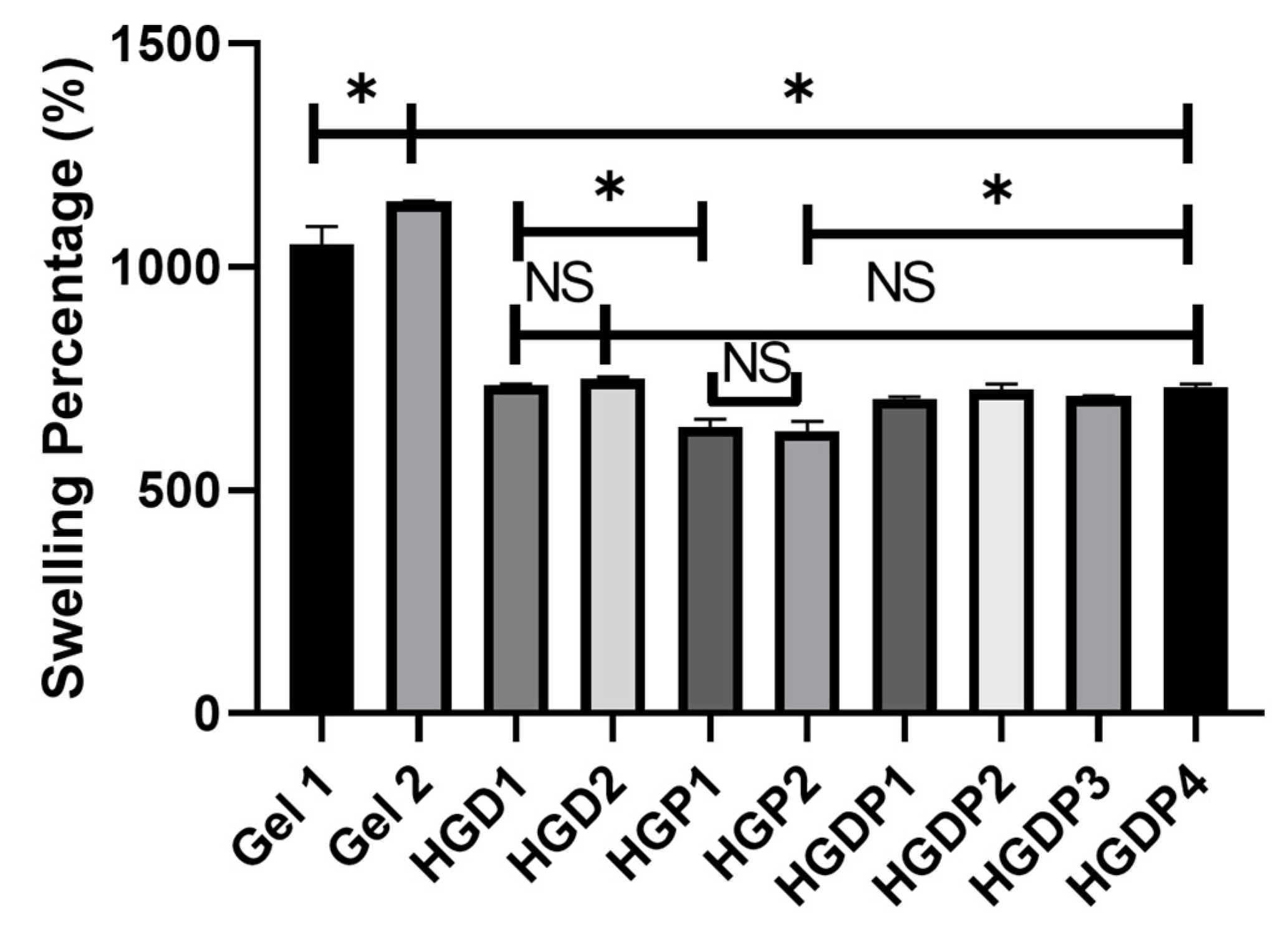
2.4.2. Equilibrium Swelling Ratio Results
2.4.3. Swelling Percentage
2.5. Biological Characterization Results
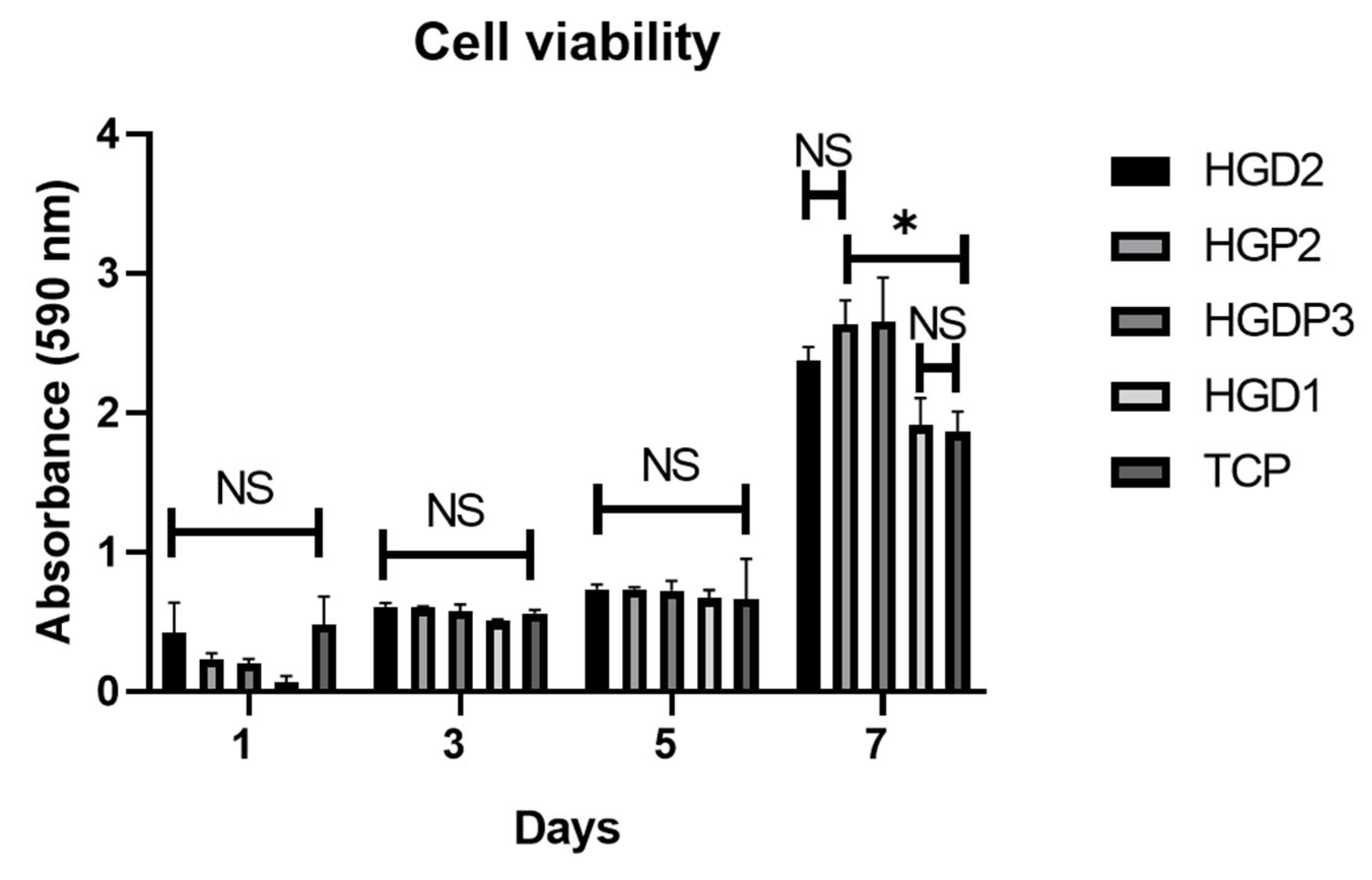
2.5.1. Cell Viability Tests
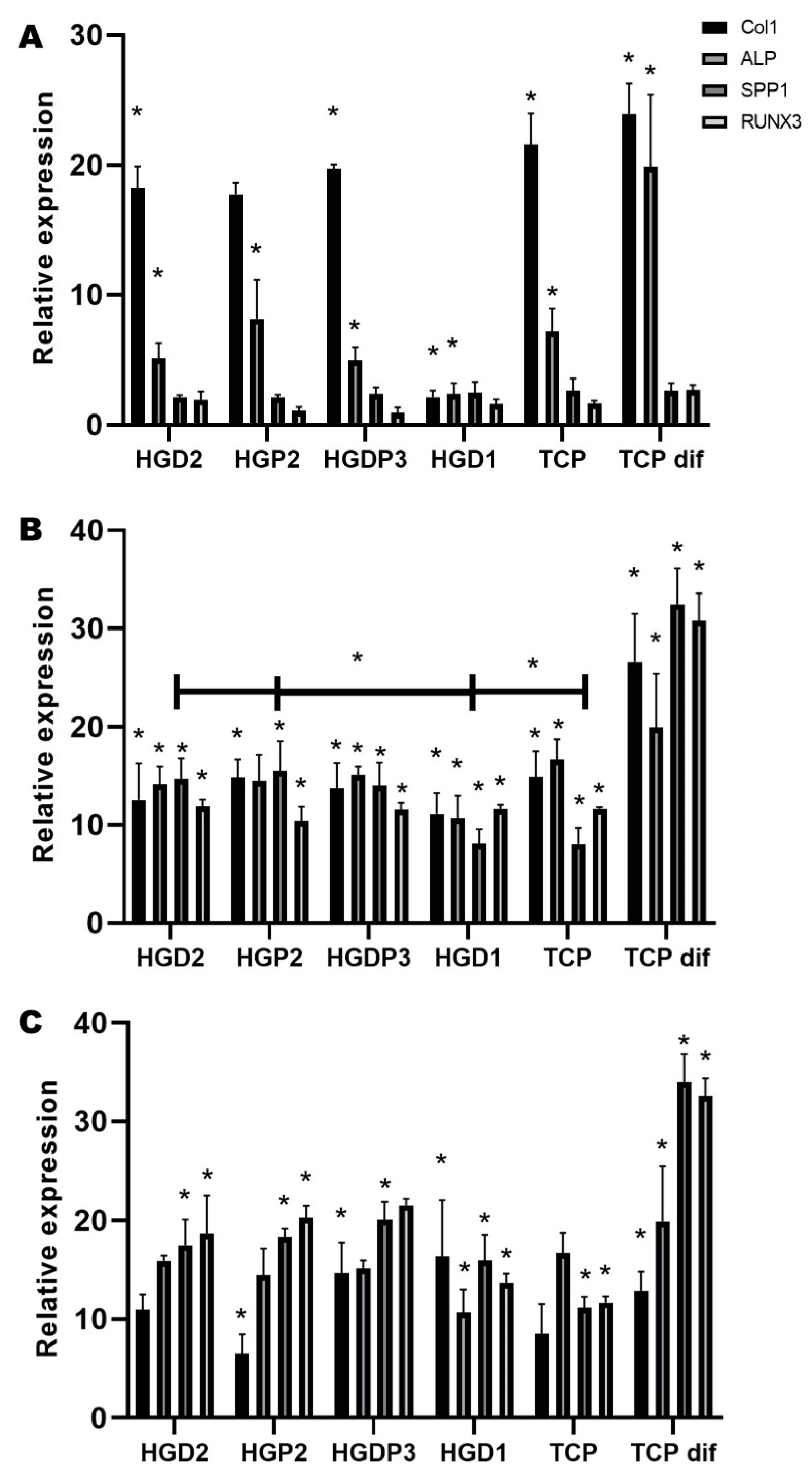
2.5.2. QRT-PCR Test
3. Conclusions
4. Materials and Methods
4.1. Preparation of the Different Gelatin/HAp Hydrogels
4.2. Mechanical Tests
4.3. Hydrogel Structure and Morphology
4.4. Characterization
4.4.1. FTIR Analysis
4.4.2. Equilibrium Swelling Ratio
- Ws is the swelling hydrogel (hydrogel weight/10 min in deionized water).
- Wd is the dry weight (initial weight).
4.4.3. Swelling Percentage
- We is the weight after 72 h of immersion in double-distilled water.
- Wd is the constant dry weight.
4.5. Biological Characterization
4.5.1. Cell Viability Tests
4.5.2. QRT-PCR Test
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, E.A.; Ritchie, R.O. Bone as a Structural Material. Adv. Healthc. Mater. 2015, 4, 1287–1304. [Google Scholar] [CrossRef]
- Buss, D.J.; Kröger, R.; McKee, M.D.; Reznikov, N. Hierarchical organization of bone in three dimensions: A twist of twists. J. Struct. Biol. X 2022, 6, 100057. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma. 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Polo, L.; Latoree-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Rezvaninejad, R. Biomaterials in bone and mineralized tissue engineering using 3D printing and bioprinting technologies. Biomed. Phys. Eng. Express 2021, 7, 062001. [Google Scholar] [CrossRef]
- Zhu, J.; Merchant, R. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2012, 8, 607–626. [Google Scholar] [CrossRef]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite Hydrogels for Bone Regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef]
- Hokugo, A.; Ozeki, M.; Kawakami, O.; Sugimoto, K.; Mushimoto, K.; Morita, S.; Tabata, Y. Augmented Bone Regeneration Activity of Platelet-Rich Plasma by Biodegradable Gelatin Hydrogel. Tissue Eng. 2005, 11, 1224–1233. [Google Scholar] [CrossRef]
- Lantigua, D.; Wu, X.; Suvarnapathaki, S.; Nguyen, M.A.; Camci-Unal, G. Composite scaffolds from gelatin and bone meal powder for tissue engineering. Bioengineering 2021, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.R.; Patel, S.; Singh, D. Natural polymer-based hydrogels as scaffolds for tissue engineering. In Nanobiomaterials in Soft Tissue Engineering: Applications of Nanobiomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 231–260. [Google Scholar] [CrossRef]
- Zustiak, S.; Leach, J. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 2011, 11, 1348–1357. [Google Scholar] [CrossRef]
- Truong, V.X.; Tsang, K.M.; Forsythe, J.S. Nonswelling Click-Cross-Linked Gelatin and PEG Hydrogels with Tunable Properties Using Pluronic Linkers. Biomacromolecules 2017, 18, 757–766. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive Modification of Poly(ethylene glycol) Hydrogels for Tissue Engineering. Biomaterials 2011, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Xu, X.; Deng, T.; Yin, M.; Zhang, X.; Wang, J. Fabrication of positively charged poly (ethylene glycol)-diacrylate hydrogel as a bone tissue engineering scaffold. Biomed. Mater. 2012, 7, 055009. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Wang, Y.; Duan, R.; Liu, L. Gelatin/Hyaluronic Acid Photocrosslinked Double Network Hydrogel with Nano-Hydroxyapatite Composite for Potential Application in Bone Repair. Gels 2023, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, P.; Lu, X.; Fang, L.; Lü, X.; Ren, F. A strong, tough, and osteoconductive hydroxyapatite mineralized polyacrylamide/dextran hydrogel for bone tissue regeneration. Acta Biomater. 2019, 88, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J Biomed Mater Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug. Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Pal, K.; Paulson, A.T.; Rousseau, D. Biopolymers in Controlled-Release Delivery Systems. Modern Biopolymer Science: Bridging the Divide between Fundamental Treatise and Industrial Application. In Modern Biopolymer Science; Academic Press: Cambridge, MA, USA, 2009; pp. 519–557. [Google Scholar]
- Wang, R.; Hu, H.; Guo, J.; Wang, Q.; Cao, J.; Wang, H.; Li, G.; Mao, J.; Zou, X.; Chen, D.; et al. Nano-hydroxyapatite modulates osteoblast differentiation through autophagy induction via mTOR signaling pathway. J. Biomed. Nanotechnol. 2019, 15, 405–415. [Google Scholar] [CrossRef]
- Byun, H.; Jang, G.N.; Jeong, H.; Lee, J.; Huh, S.J.; Lee, S. Development of a composite hydrogel incorporating anti-inflammatory and osteoinductive nanoparticles for effective bone regeneration. Biomater. Res. 2023, 27, 132. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, M.; Li, L.; Sun, S.; Yu, H.; Cheng, Y.; Yang, Y.; Fan, Y.; Sun, Y. Preparation and Properties of Double-Crosslinked Hydroxyapatite Composite Hydrogels. Int. J. Mol. Sci. 2022, 23, 9962. [Google Scholar] [CrossRef]
- Obando-Suárez, M.A.; Oliva Ridríguez, R.; González-Ortega, O.; Komabayashi, T.; Flores-Arriaga, J.C.; Cerda-Cristerna, B.I. BMP-7 loaded PEG-d, gelatin type-A hydrogels for mineral-tissue regeneration. Dent. Mater. 2015, 31, e61. [Google Scholar] [CrossRef]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef] [PubMed]
- Dimida, S.; Barca, A.; Cancelli, N.; De Benedictis, V.; Raucci, M.; Demitri, C. Effects of Genipin Concentration on Cross-Linked Chitosan Scaffolds for Bone Tissue Engineering: Structural Characterization and Evidence of Biocompatibility Features. Int. J. Polym. Sci. 2017, 2017, 8410750. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Brown, E. Development and Characterization of Genipin Cross-linked Gelatin Based Composites Incorporated with Vegetable-tanned Collagen Fiber (VCF)*. J. Am. Leather Chem. Assoc. 2017, 112, 410–419. [Google Scholar]
- Chen, R.; Chen, H.B.; Xue, P.P.; Yang, W.G.; Luo, L.Z.; Tong, M.Q.; Zhong, B.; Xu, H.-L.; Zhao, Y.-Z.; Yuan, J.-D. HA/MgO nanocrystal-based hybrid hydrogel with high mechanical strength and osteoinductive potential for bone reconstruction in diabetic rats. J. Mater. Chem. B 2021, 9, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Kurosawa, H.; Haga, H. Stiffness-Modulation of Collagen Gels by Genipin-Crosslinking for Cell Culture. Gels 2023, 9, 148. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, K.; Zhou, W.; Gu, J.; Liu, Y.; Han, C.C.; Xu, S. Factors Influencing the Interactions in Gelatin/Hydroxyapatite Hybrid Materials. Front. Chem. 2020, 8, 489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Výborný, K.; Vallová, J.; Kočí, Z.; Kekulová, K.; Jiráková, K.; Jendelová, P.; Hodan, J.; Kubinová, Š. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci. Rep. 2019, 9, 10674. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A. Could glutaric acid (GA) replace glutaraldehyde in the preparation of biocompatible biopolymers with high mechanical and thermal properties? J. Chem. Sci. 2014, 126, 127–140. [Google Scholar] [CrossRef]
- Kowalski, G.; Witczak, M.; Kuterasiński, Ł. Structure Effects on Swelling Properties of Hydrogels Based on Sodium Alginate and Acrylic Polymers. Molecules 2024, 29, 1937. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, X. Comparison of characteristics between glutaraldehyde- and genipin-crosslinked gelatin microspheres. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009, 23, 87–91. (In Chinese) [Google Scholar] [PubMed]
- Wang, S.; Li, K.; Zhou, Q. High strength and low swelling composite hydrogels from gelatin and delignified wood. Sci. Rep. 2020, 10, 17842. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release. Biomater. Sci. 2016, 4, 405–411. [Google Scholar] [CrossRef]
- Setzer, B.; Bächle, M.; Metzger, M.C.; Kohal, R.J. The gene-expression and phenotypic response of hFOB 1.19 osteoblasts to surface-modified titanium and zirconia. Biomaterials 2009, 30, 979–990. [Google Scholar] [CrossRef]
- De Araújo Batista, H.; Cardoso, M.J.B.; Vinicius, A.S.; Fook, M.V.L.; Barbero, M.A.R.; Carrodeguas, R.G. Manufacturing of calcium phosphate scaffolds by pseudomorphic transformation of gypsum. Boletín Soc. Española Cerámica Y Vidr. 2016, 55, 105–113. [Google Scholar] [CrossRef][Green Version]
- Chanes-Cuevas, O.A.; Barrera-Bernai, J.L.; Gaitán, I.; Masuoka, D. Calcium Phosphate and Bioactive Glasses. In Current Advances in Oral and Craniofacial Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2020; pp. 48–60. [Google Scholar]
- Virdi, J.K.; Pethe, P. Biomaterials Regulate Mechanosensors YAP/TAZ in Stem Cell Growth and Differentiation. Tissue Eng. Regen. Med. 2021, 18, 199–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ASTM F2150-13; Standard Guide for Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products. ASTM International: West Conshohocken, PA, USA, 2013.
- De la Rosa-Ruiz, M.D.P.; Álvarez-Pérez, M.A.; Cortés-Morales, V.A.; Monroy-García, A.; Mayani, H.; Fragoso-González, G.; Caballero-Chacón, S.; Diaz, D.; Candanedo-González, F.; Montesinos, J.J. Mesenchymal Stem/Stromal Cells Derived from Dental Tissues: A Comparative In Vitro Evaluation of Their Immunoregulatory Properties Against T cells. Cells 2019, 8, 1491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Components and Proportions | |||||||
|---|---|---|---|---|---|---|---|
| Hydrogels | PBS | Gelatin | PEG-D | PEG-PPG-PEG | Glutaraldehyde | Genipin | HAp |
| Gel 1 | 7.8 mL | 1.6 g | - | - | 1.2 mL | - | - |
| Gel 2 | 7.8 mL | 1.6 g | - | - | - | 5 mL | - |
| HGD1 | 7.8 mL | 1.6 g | 0.07 g | - | 1.2 mL | - | 0.04 g |
| HGP1 | 7.8 mL | 1.6 g | - | 0.07 g | 1.2 mL | - | 0.04 g |
| HGD2 | 7.8 mL | 1.6 g | 0.07 g | - | - | 5 mL | 0.04 g |
| HGP2 | 7.8 mL | 1.6 g | - | 0.07 g | - | 5 mL | 0.04 g |
| HGDP1 | 7.8 mL | 1.6 g | 0.035 g | 0.07 g | 1.2 mL | - | 0.04 g |
| HGDP2 | 7.8 mL | 1.6 g | 0.07 g | 0.035 g | 1.2 mL | - | 0.04 g |
| HGDP3 | 7.8 mL | 1.6 g | 0.035 g | 0.07 g | - | 5 mL | 0.04 g |
| HGDP4 | 7.8 mL | 1.6 g | 0.07 g | 0.035 g | - | 5 mL | 0.04 g |
| Name | 5’→3’ |
|---|---|
| Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Fw | gcatcctgggctacactgag |
| Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Rv | tgctgtagccaaattcgttg |
| Collagen 1 (Col 1) Fw | gagagcatgaccgatggatt |
| Collagen 1 (Col 1) Rv | atgtaggccacgctgttctt |
| Phosphatase alkaline (ALP) Fw | cgaccagacgtgaatgagag |
| Phosphatase alkaline (ALP) Rv | gctacgaagctctgctcctg |
| Osteopontin (SPP1) Fw | cgaggtgatagtgtggtttatgg |
| Osteopontin (SPP1) Rv | gcaccattcaactcctcgctttc |
| Runt-domain transcription factor (RUNX 3) Fw | tcagcaccacaagccactt |
| Runt-domain transcription factor (RUNX 3) Rv | aatgggttcagttccgaggt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera Bernal, J.L.; Gaytán Salvatella, Í.; del Campo, B.I.M.; Alvarez Perez, M.A.; Masuoka-Ito, D. Synthesis of Hydroxyapatite-Gelatin Composite Hydrogel for Bone Tissue Application. Gels 2025, 11, 630. https://doi.org/10.3390/gels11080630
Barrera Bernal JL, Gaytán Salvatella Í, del Campo BIM, Alvarez Perez MA, Masuoka-Ito D. Synthesis of Hydroxyapatite-Gelatin Composite Hydrogel for Bone Tissue Application. Gels. 2025; 11(8):630. https://doi.org/10.3390/gels11080630
Chicago/Turabian StyleBarrera Bernal, José Luis, Íñigo Gaytán Salvatella, Bryan Iván Martín del Campo, Marco Antonio Alvarez Perez, and David Masuoka-Ito. 2025. "Synthesis of Hydroxyapatite-Gelatin Composite Hydrogel for Bone Tissue Application" Gels 11, no. 8: 630. https://doi.org/10.3390/gels11080630
APA StyleBarrera Bernal, J. L., Gaytán Salvatella, Í., del Campo, B. I. M., Alvarez Perez, M. A., & Masuoka-Ito, D. (2025). Synthesis of Hydroxyapatite-Gelatin Composite Hydrogel for Bone Tissue Application. Gels, 11(8), 630. https://doi.org/10.3390/gels11080630







